Abstract
We investigated the involvement of NF-κB/Rel transcription factors that reportedly can inhibit apoptosis in various cell types in the antiapoptotic mechanism of the cytoprotectant amifostine. In the nontumorigenic murine myeloid progenitor 32D cells incubated with amifostine, we detected a reduction of the IκB cytoplasmic levels by Western blotting and a raising of nuclear NF-κB/Rel complexes by electrophoretic mobility shift assay. Amifostine inhibited by more than 30% the growth factor deprivation-induced apoptosis, whereas its effect failed when we blocked the NF-κB/Rel activity with an NF-κB/Rel-binding phosphorothioate decoy oligodeoxynucleotide. In human cord blood CD34+ cells, the NF-κB/Rel p65 subunit was detectable (using immunofluorescence analysis) mainly in the cytoplasm in the absence of amifostine, whereas its presence was appreciable in the nuclei of cells incubated with the cytoprotectant. In 4 CD34+ samples incubated for 3 days in cytokine-deficient conditions, cell apoptosis was reduced by more than 30% in the presence of amifostine (or amifostine plus a control oligo); the effect of amifostine was abolished in cultures with the decoy oligo. These findings indicate that the inhibition of hematopoietic progenitor cell apoptosis by amifostine requires the induction of NF-κB/Rel factors and that the latter can therefore exert an antiapoptotic activity in the hematopoietic progenitor cell compartment. Furthermore, the identification of this specific mechanism underlying the survival-promoting activity of amifostine lends support to the possible use of this agent in apoptosis-related pathologies, such as myelodysplasias.
THE ORGANIC THIOPHOSPHATE, amifostine, has been characterized for its cytoprotective activity against the toxic effects of radiation therapy1 or antineoplastics, including cyclophosphamide,2,3 alkylating and platinum agents,4-6 paclitaxel,7 etc.8-10Cytoprotection has been shown to rely on at least 3 different activities of amifostine: binding to and protection of DNA, neutralization of antineoplastic drugs, and antioxidant properties.11,12 Because normal tissues, due to their alkaline phosphatase content, can more efficiently transform amifostine in its active metabolite compared with a majority of tumor cells,11 the cytoprotectant can apparently diminish the treatment-related toxicity (myelotoxicity, nephrotoxicity, neurotoxicity, mucositis, and esophagitis) of radiation and chemotherapeutic agents while preserving their antineoplastic effects.8-12
Amifostine appears also to possess a broader survival-stimulating activity, based on its effects in myelodysplasias.13 A number of studies indicate that amifostine can stimulate the hematopoietic growth both in vitro and in vivo in cells from patients with myelodysplastic syndromes (reviewed in Capizzi14). In normal bone marrow progenitors, the drug has been shown to stimulate the growth of granulocyte, erythroid, macrophage, megakaryocyte colony-forming units (CFU-GEMM) and erythroid bursts (BFU-E) and to inhibit apoptosis in cytokine-deficient conditions.15 The mechanisms underlying the survival-enhancing activity of amifostine in hematopoietic progenitors are still poorly investigated.
Cell survival can be enhanced in various cell systems by the activity of NF-κB/Rel transcription factors.16-24 These are dimers of proteins (p50/p105 or NF-κB1, p52/p100 or NF-κB2, p65 or RelA, c-Rel, and RelB) containing an approximately 300 amino acid REL homology region. The NF-κB/Rel complexes are retained in the cytoplasm of several cell types by inhibitors of the IκB (α-ε) family; cytokines, hormones, and other stimuli can induce the phosphorylation and ubiquitin-mediated degradation of the IκB proteins, allowing the NF-κB/Rel dimers to reach the nucleus (reviewed in Ghosh et al23). Besides their effects on the expression of a vast number of genes involved in inflammatory processes or cell adhesion,23 NF-κB/Rel activities can also inhibit the apoptosis induced by tumor necrosis factor-α (TNF-α), ionizing radiations, and chemotherapeutic agents.16-24
We investigated the effect of amifostine on NF-κB/Rel activation in the murine myeloid progenitor cell line 32D or in human cord blood CD34+ cells and the involvement of these factors in the amifostine-induced inhibition of cell apoptosis. This study was aimed at elucidating the biological activities of amifostine and exploring the antiapoptotic properties of NF-κB/Rel factors in hematopoietic progenitors.
MATERIALS AND METHODS
Cells, cytokines, and amifostine.
Cells of the murine myeloid progenitor line 32D were maintained in 10% fetal calf serum (FCS)-RPMI 1640 medium, supplemented with the 10% of conditioned medium (CM) obtained from WEHI-3 cells.25CD34+ cells were isolated from cord blood samples by binding to anti-CD34–conjugated beads (miniMACS system QBEND/10; Miltenyi, Biotech GmbH, Milan, Italy). The cells (>96% CD34+ in immunofluorescence) were cultured26 in 10% FCS-RPMI 1640 medium, without or with 50 ng/mL stem cell factor (SCF), 50 ng/mL interleukin-3 (IL-3), 100 ng/mL granulocyte colony-stimulating factor (G-CSF), 50 ng/mL IL-6, and 3 U/mL erythropoietin (Epo26; Becton Dickinson, Mountain View, CA). Amifostine was kindly provided by US Bioscience (Conshohocken, PA).
Analysis of apoptosis.
Apoptosis was measured by flow cytometry as described.27Briefly, the cells (2 × 105) were washed in phosphate-buffered saline (PBS) and resuspended in 1 mL of a solution containing 0.1% sodium citrate, 0.1% Triton X-100, and 50 μg/mL propidium iodide (Sigma Chemical Co, Gallarate, Italy). After an incubation at 4°C for 30 minutes in the dark, cell nuclei were analyzed with a Becton Dickinson FACScan flow cytometer using the Lysis 1 program. Cellular debris was excluded from analysis by raising the forward scatter threshold, and the DNA content of the nuclei was registered on a logarithmic scale. The percentage of the cells in the hypodiploid region was calculated.
Assay of caspase 3 activity.
Caspase 3 activity was determined in cell extracts by analyzing the release of 7-amino-4-methylcoumarin (AMC) from N-acetyl-DEVD-AMC.28 Briefly, cells (2 × 106) were lysed in a buffer containing 10 mmol/L Tris (pH 7.5), 130 mmol/L NaCl, 1% Triton-X-100, 10 mmol/L NaPi, and 10 mmol/L NaPPi; 100 μg of protein was then incubated with 20 μmol/L Ac-DEVD-AMC (Becton Dickinson) in a buffer containing 20 mmol/L HEPES (pH 7.5), 10% glycerol, and 2 mmol/L dithiothreitol (DTT) at 37°C for 2 hours. The release of AMC was monitored in a spectrofluorometer with an excitation wavelength of 380 nm and emission wavelength range of 430 to 460 nm.
Nuclear extracts and electrophoretic mobility shift assays (EMSA).
Nuclear extracts were prepared29 from 20 × 106 32D or 7.5 × 105 CD34+cells by cell pellet homogenization in 2 vol of 10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L phenylmethyl sulfonyl fluoride (PMSF), and 10% glycerol. Nuclei were centrifuged at 1,000g for 5 minutes, washed, and resuspended in 2 vol of the solution specified above. KCl (3 mol/L) was added to reach 0.39 mol/L KCl. Nuclei were extracted at 4°C for 1 hour and centrifuged at 10,000g for 30 minutes. The supernatants were clarified by centrifugation and stored at −80°C. Protein concentration was determined using the Bradford method. The double-stranded oligonucleotides corresponding to, respectively, the 5′-CAACGGCAGGGGAATCTCCCTCTCCTT-3′ NF-κB/Rel28 and the Ets-1 consensus sequences (Promega, Madison, WI) were end-labeled with [γ-32P] ATP (Amersham International plc, Milano, Italy) using a polynucleotide kinase (Roche, Germany) to a specific activity of 2 to 5 × 104 cpm/μg. End-labeled DNA fragments (2 × 104 cpm) were incubated at room temperature for 20 minutes with 3 to 5 μg of nuclear protein in the presence of 1 μg poly(dI-dC) in 20 μL of a buffer consisting of 10 mmol/L Tris-HCl, pH 7.5, 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, and 5% glycerol. Protein-DNA complexes were separated from free probe on a 6% polyacrilamide gel and run in 0.25× Tris borate buffer at 200 V for 3 hours at room temperature. The gels were dried and exposed to x-ray film (Kodak AR, Milan, Italy).
Cytosolic extracts and Western blot analysis.
Cells (3 × 107) were harvested, washed twice in cold PBS, and resuspended in 100 μL of lysing buffer (10 mmol/L HEPES buffer, pH 7.9, 1 mmol/L EDTA, 60 mmol/L KCl, 1 mmol/L DTT, 1 mmol/L PMSF, 50 μg/mL antipain, 40 μg/mL APMSF, 10 μg/mL aprotinin, 40 μg/mL bestatin, 20 μg/mL chymostatin, and 0.2% vol/vol Nonidet P-40) for 5 minutes. Samples were centrifuged at 400g for 5 minutes. The supernatants (cytosolic fractions) were stored at −80°C until use. Protein concentration was determined using the Bradford method. Cytosolic proteins (15 μg) were separated by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis, transferred onto a membrane filter (Cellulosenitrate; Schleicter & Schuell, Germany), and incubated with an antihuman IκBα rabbit polyclonal serum (kindly provided by Dr N. Rice, Frederick Cancer Research and Development Center, Frederick, MD) in PBS plus 5% dry milk for 2 hours at room temperature. The blots were washed with PBS, incubated with peroxidase-conjugated goat antirabbit IgG (Boehringer Mannheim) for 1 hour, and visualized by using an enhanced chemiluminescence system (Amersham International, Milan, Italy). The specificity of detection was verified by the absence of immunostaining in the presence of the second antibody alone or anti-IκBα antibodies.
NF-κB/Rel decoy and scrambled oligonucleotides.
The phosphorothioate oligodeoxynucleotides, corresponding to the κB consensus sequence (5′-CCTTGAAGGGATTTCCCTCC-3′)21 or its mutated (scrambled) form (5′-CCTTGTACCATTGTTAGCC-3′), were purchased from Primm Srl (Milan, Italy).
Intracellular immunofluorescence.
Cells (1 × 104) were pelletted and resuspended in 200 μL PBS containing 20% bovine serum albumin (BSA). Resuspended cells were then deposited on a slide (cytoslide Shandon) by spinning in a cyto-centrifuge (Cytospin 3 Shandon) at 600 rpm for 10 minutes, fixed in 2% paraformaldehyde in PBS for 20 minutes, and permeabilized with 0.2% Triton X-100 in PBS. After 3 washes in PBS, the cells were incubated with an antihuman NF-κB/p65 rabbit polyclonal serum for 1 hour, washed, incubated with fluorescein isothiocyanate (FITC)-conjugated goat antirabbit Ig antibodies for 1 hour, and finally stained with bisbenzimide (HOECHST 33258, 0.25 μg/mL; Sigma) for 20 minutes. After rinsing with PBS, a drop of a 50% solution of glycerol in PBS was added to the slide before mounting with a glass coverslip.
Statistical analysis.
Analysis (Student's paired t-test) was performed using GB-Stat 5.0 for Macintosh (Dynamic Microsystem Inc, Silver Spring, MD).
RESULTS
Raising of NF-κB/Rel nuclear levels in 32D cells by amifostine.
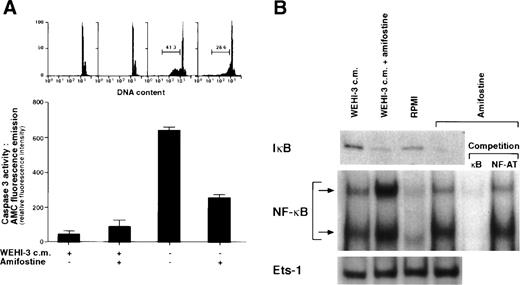
The addition of amifostine to hematopoietic progenitors cultured without cytokines reportedly counteracted cell death.15Because in various systems, including hematopoietic cells, apoptosis can be inhibited by agents that activate NF-κB/Rel factors,16-24 we analyzed whether the survival-enhancing effect of amifostine might be related to a raising of NF-κB/Rel nuclear levels in cells stimulated with the cytoprotectant. To this end, we investigated in parallel the effects of amifostine on apoptosis and NF-κB/Rel nuclear activity in the nontumorigenic murine myeloid progenitor cell line 32D that requires stromal cells (WEHI-3) CM, containing IL-3, to survive and grow.25 Cells incubated without WEHI-3 CM or amifostine for 18 hours displayed greater than 40% hypodiploid elements detected by propidium iodide incorporation in cell DNA. In the presence of amifostine, such a percentage was reduced by more than one third in 5 different experiments (P = .0004; representative results are shown in Fig1A). Also, the activity of caspase 3, which was induced in CM-deprived cells, was much less (P = .03) in cells incubated with the cytoprotectant (Fig 1A). Amifostine displayed its half-maximal activity at a concentration of approximately 50 μg/mL (results not shown). In parallel, we analyzed the nuclear presence of NF-κB/Rel complexes in 32D cells incubated with amifostine. Results are shown in Fig 1B. Using EMSA of cell nuclear extracts incubated with a 32P-labeled κB oligonucleotide, we detected appreciable amounts of NF-κB/Rel complexes in cells cultured with WEHI-3-CM and reduced levels in cells incubated with RPMI without CM for 3 hours. Amifostine appeared to enhance the nuclear presence of NF-κB/Rel, either in the presence or absence of CM. By competition of the 32P-labeled κB oligonucleotide with excess (50×) amounts of unlabeled κB or a different (NF-AT) oligonucleotide, we verified that the complexes detected by EMSA specifically corresponded to NF-κB/Rel factors. Furthermore, the levels of a different transcription factor, Ets-1, did not significantly change in cultures with or without the cytoprotectant. Finally, by Western blot analysis of cell cytoplasmic extracts, we detected reduced levels of IκBα in cells incubated with amifostine (Fig 1B). This was consistent with a stimulatory effect of the molecule on the nuclear translocation of NF-κB/Rel complexes.
Inhibition of apoptosis and enhancement of NF-κB/Rel nuclear levels in 32D cells by amifostine. (A) 32D cells (2 × 105/mL) were incubated in the presence or the absence of WEHI-3 CM and with or without amifostine (100 μg/mL). Propidium iodide incorporation in cell DNA and caspase 3 activity were measured after 18 and 12 hours, respectively. (B) 32D cells (2 × 105/mL) were incubated in the presence or absence of WEHI-3 CM and with or without amifostine (100 μg/mL) for 3 hours. The levels of IκB were analyzed in cytoplasmic extracts by Western blotting. The NF-κB/Rel complexes were examined by EMSA of nuclear extracts incubated with a 32P-labeled κB oligonucleotide. Where indicated, the nuclear extracts were incubated with the32P-labeled κB oligonucleotide in the presence of excess (50×) amounts of unlabeled κB or a different (NF-AT) oligonucleotide.
Inhibition of apoptosis and enhancement of NF-κB/Rel nuclear levels in 32D cells by amifostine. (A) 32D cells (2 × 105/mL) were incubated in the presence or the absence of WEHI-3 CM and with or without amifostine (100 μg/mL). Propidium iodide incorporation in cell DNA and caspase 3 activity were measured after 18 and 12 hours, respectively. (B) 32D cells (2 × 105/mL) were incubated in the presence or absence of WEHI-3 CM and with or without amifostine (100 μg/mL) for 3 hours. The levels of IκB were analyzed in cytoplasmic extracts by Western blotting. The NF-κB/Rel complexes were examined by EMSA of nuclear extracts incubated with a 32P-labeled κB oligonucleotide. Where indicated, the nuclear extracts were incubated with the32P-labeled κB oligonucleotide in the presence of excess (50×) amounts of unlabeled κB or a different (NF-AT) oligonucleotide.
We concluded that amifostine stimulated the activation of NF-κB/Rel complexes, thereby sustaining the nuclear levels of these factors in 32D cells.
The amifostine-activated NF-κB/Rel factors are required for the inhibition of apoptosis in 32D cells.
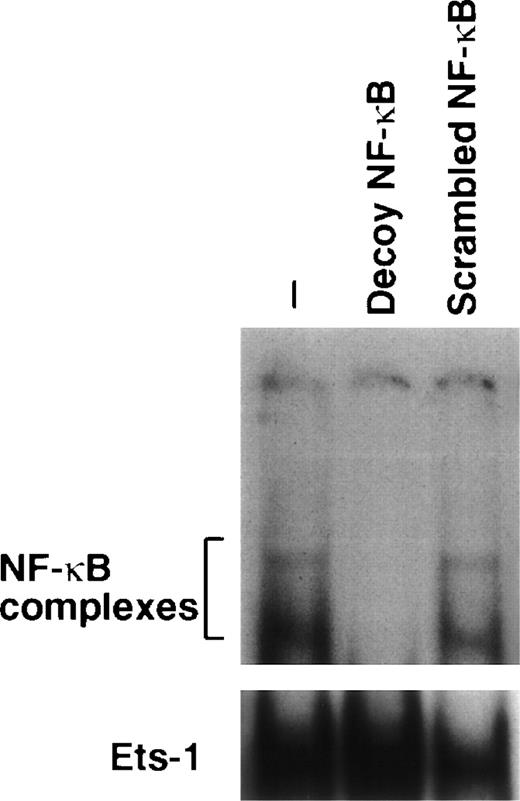
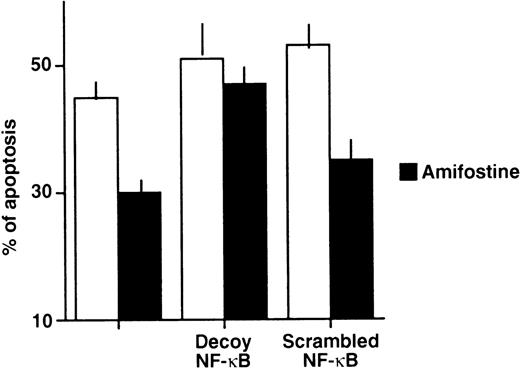
We verified whether amifostine could inhibit 32D cell apoptosis when NF-κB/Rel complexes were subtracted. To this end, we used a decoy phosphorothioate oligodeoxynucleotide carrying the NF-κB/Rel consensus sequence.21 In several cell types, this approach allowed us to specifically block the translocation and activity of NF-κB/Rel complexes.21 30-33 Similarly, in the murine cell line 32D, the incubation with the decoy oligonucleotide resulted in abating the nuclear levels of NF-κB/Rel complexes. These were not affected; instead, in cells incubated with a mutated (scrambled) form of oligonucleotide, they were used as a control (Fig 2). We analyzed the effects of these oligonucleotides on the growth factor deprivation-induced apoptosis in 32D cells incubated with or without amifostine. The results of 4 separate experiments are shown in Fig 3. The effect of amifostine was specifically abolished by the decoy oligo. Indeed, amifostine that inhibited apoptosis in the absence of oligos or in the presence of the scrambled oligonucleotide (P = .0004) failed to exert a significant inhibition (P > .11) in the presence of the decoy oligonucleotide. Therefore, the NF-κB/Rel factors were apparently required for the antiapoptotic effect of amifostine.
Downmodulation of NF-κB/Rel nuclear complexes by a κB decoy oligodeoxynucleotide in 32D cells. 32D cells (2 × 105/mL) were incubated in the presence or absence of 7.5 μmol/L κB decoy (5′-CCTTGAAGGGATTTCCCTCC-3′) or scrambled (5′-CCTTGTACCATTGTTAGCC-3′) oligodeoxynucleotide for 16 hours. Nuclear extracts were then obtained, incubated with a32P-labeled κB oligonucleotide, and analyzed by EMSA.
Downmodulation of NF-κB/Rel nuclear complexes by a κB decoy oligodeoxynucleotide in 32D cells. 32D cells (2 × 105/mL) were incubated in the presence or absence of 7.5 μmol/L κB decoy (5′-CCTTGAAGGGATTTCCCTCC-3′) or scrambled (5′-CCTTGTACCATTGTTAGCC-3′) oligodeoxynucleotide for 16 hours. Nuclear extracts were then obtained, incubated with a32P-labeled κB oligonucleotide, and analyzed by EMSA.
Impairment of the amifostine antiapoptotic effect in 32D cells by the κB decoy oligonucleotide. 32D cells (2 × 105/mL) were incubated without WEHI-3 CM and with or without amifostine (100 μg/mL), 7.5 μmol/L κB decoy, or scrambled oligodeoxynucleotide for 18 hours. Cell apoptosis was then analyzed by propidium iodide incorporation in cell DNA. The results represent the means ± SE of 4 separate experiments. In cells incubated with WEHI-3 CM, either with or without amifostine and/or oligonucleotides, apoptosis was less than 4%.
Impairment of the amifostine antiapoptotic effect in 32D cells by the κB decoy oligonucleotide. 32D cells (2 × 105/mL) were incubated without WEHI-3 CM and with or without amifostine (100 μg/mL), 7.5 μmol/L κB decoy, or scrambled oligodeoxynucleotide for 18 hours. Cell apoptosis was then analyzed by propidium iodide incorporation in cell DNA. The results represent the means ± SE of 4 separate experiments. In cells incubated with WEHI-3 CM, either with or without amifostine and/or oligonucleotides, apoptosis was less than 4%.
Involvement of NF-κB/Rel factors in the antiapoptotic activity of amifostine on human cord blood CD34+cells.
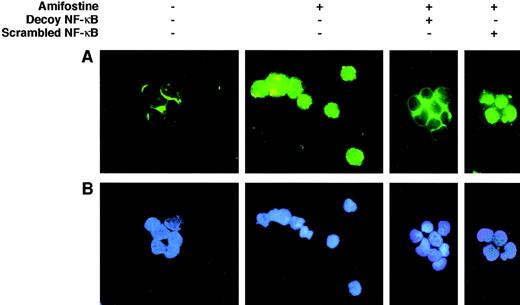
We verified whether, in analogy with its effect on 32D cells, amifostine could raise the NF-κB/Rel nuclear levels in CD34+ cells isolated from human cord blood. To this end, we incubated CD34+ cells with or without amifostine for 16 hours and then analyzed the nuclear presence of NF-κB/Rel by EMSA. As shown in Fig 4, amifostine apparently enhanced the nuclear amount of NF-κB/Rel factors. To verify whether the κB decoy oligonucleotide could diminish the NF-κB/Rel nuclear levels, we incubated CD34+ cells with or without amifostine, decoy, or scrambled oligo for 16 hours, followed by staining with an antibody recognizing the NF-κB/Rel p65 subunit. In cells incubated without amifostine, the antibody stained mainly the cytoplasmic area; on the other hand, a clear nuclear signal was detected in the cells incubated with amifostine. The decoy, but not the scrambled, oligo abolished the nuclear signal (Fig 5). Analogous results were obtained by analyzing 3 different CD34+ samples.
Enhancement of NF-κB/Rel nuclear levels in human cord blood CD34+ cells by amifostine. CD34+cells (purity, >96%, ≤98%; 105/mL) were incubated in 10% FCS-RPMI, with or without amifostine (8 μg/mL), for 16 hours. Then nuclear extracts were obtained, incubated with a32P-labeled κB oligonucleotide, and analyzed by EMSA. Where indicated, the nuclear extracts were incubated with the32P-labeled κB oligonucleotide in the presence of excess (50×) amounts of unlabeled κB or a different (NF-AT) oligonucleotide.
Enhancement of NF-κB/Rel nuclear levels in human cord blood CD34+ cells by amifostine. CD34+cells (purity, >96%, ≤98%; 105/mL) were incubated in 10% FCS-RPMI, with or without amifostine (8 μg/mL), for 16 hours. Then nuclear extracts were obtained, incubated with a32P-labeled κB oligonucleotide, and analyzed by EMSA. Where indicated, the nuclear extracts were incubated with the32P-labeled κB oligonucleotide in the presence of excess (50×) amounts of unlabeled κB or a different (NF-AT) oligonucleotide.
Effect of the κB decoy oligonucleotide on the NF-κB/Rel nuclear complexes in CD34+ cells. CD34+ cells (purity, >96%, ≤99%; 7.5 × 105/mL) were incubated in 10% FCS-RPMI, with or without amifostine (8 μg/mL), decoy, or scrambled oligo (5 μmol/L) for 16 hours. The cells were then analyzed in immunofluorescence with an antihuman NF-κB/p65 rabbit polyclonal serum and FITC-conjugated goat antirabbit Ig antibodies.
Effect of the κB decoy oligonucleotide on the NF-κB/Rel nuclear complexes in CD34+ cells. CD34+ cells (purity, >96%, ≤99%; 7.5 × 105/mL) were incubated in 10% FCS-RPMI, with or without amifostine (8 μg/mL), decoy, or scrambled oligo (5 μmol/L) for 16 hours. The cells were then analyzed in immunofluorescence with an antihuman NF-κB/p65 rabbit polyclonal serum and FITC-conjugated goat antirabbit Ig antibodies.
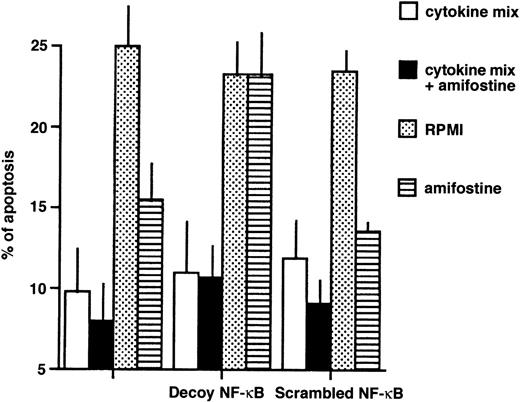
To investigate whether the NF-κB/Rel factors were required for the inhibition of apoptosis, we incubated 4 different samples of CD34+ cells with or without a mix of survival-sustaining cytokines26 and either in the absence or presence of amifostine; the κB decoy or scrambled oligonucleotides were added. An analysis of cell apoptosis is shown in Fig6. Whereas the cells incubated with the cytokine mix showed 9.8% ± 2.6% (mean ± SE) of hypodiploid elements, those incubated in medium without cytokines displayed 25.3% ± 2.7% of apoptosis; in cultures with amifostine, as expected,15 the cytoprotectant reduced (P = .03) the apoptosis percentage, which indeed was 15.5% ± 2.0%. Similarly, amifostine inhibited apoptosis (P = .03) in the presence of the scrambled oligo (13.0% ± 0.5%). On the other hand, in the presence of the κB decoy oligo, the effect of amifostine failed, because the apoptosis percentage (23.3% ± 2.6%) was not significantly different (P > .95) from that observed in cultures without the cytoprotectant.
Reversion of the amifostine antiapoptotic effect in CD34+ cells by the κB decoy oligonucleotide. CD34+ cells (purity, >96%, ≤98%; 105/mL) were incubated in 10% FCS-RPMI, with or without a cytokine mix (see Materials and Methods), amifostine (8 μg/mL), decoy, or scrambled oligo (5 μmol/L) for 16 hours. Apoptosis was then analyzed by propidium iodide incorporation in cell DNA. The results represent the means ± SE of 4 separate experiments.
Reversion of the amifostine antiapoptotic effect in CD34+ cells by the κB decoy oligonucleotide. CD34+ cells (purity, >96%, ≤98%; 105/mL) were incubated in 10% FCS-RPMI, with or without a cytokine mix (see Materials and Methods), amifostine (8 μg/mL), decoy, or scrambled oligo (5 μmol/L) for 16 hours. Apoptosis was then analyzed by propidium iodide incorporation in cell DNA. The results represent the means ± SE of 4 separate experiments.
We concluded that the NF-κB/Rel factors, activated by amifostine, were required for the inhibition of human cord blood CD34+cell apoptosis.
DISCUSSION
These results show a previously unknown biological property of amifostine that can contribute to explain its antiapoptotic effect on hematopoietic progenitors. Indeed, amifostine appeared to counteract apoptosis by raising the nuclear levels of NF-κB/Rel factors; this effect might be due to an activity of amifostine on IκBα-triggering kinases23 or possibly on the IκBα molecule itself. The NF-κB/Rel factors were required for the antiapoptotic activity of the cytoprotectant, strongly indicating that they mediate this property of amifostine. The identification of a specific mechanism, underlying the survival factor activity of amifostine, lends support to the possible use of this molecule in apoptosis-related pathologies, such as myelodysplasias, and can direct therapeutic efforts towards envisioning new molecules with NF-κB/Rel-stimulating properties.
On the other hand, these findings contribute an element of criticism in the evaluation of amifostine as cytoprotectant during antineoplastic therapies. Indeed, although drug neutralization could constitute a major effect of amifostine in some cases,12 in others, the NF-κB/Rel–mediated inhibition of apoptosis might provide a possible growth advantage for cells altered or selected by the antitumor agent. The respective roles of the drug-neutralizing11 12 and the antiapoptotic properties of amifostine in tumor cells triggered by different classes of antineoplastics should be worthy of specific investigation.
The NF-κB/Rel–mediated inhibition of apoptosis by amifostine provides a model that shows the antiapoptotic properties of these transcription complexes in hematopoietic progenitors. Furthermore, the described findings could contribute to the analysis of the NF-κB/Rel antiapoptotic properties. In this respect, a specific question concerns the requirement of NF-κB/Rel activity for basal cell survival.34,35 In analogy with other systems, including B-chronic lymphocytic leukemia (B-CLL) cells incubated with the κB-specific decoy oligonucleotide21 or fibrosarcoma-injected SCID mice infected with a superrepressor IκBα-carrying adenovirus,36 in hematopoietic progenitors the inhibition of NF-κB/Rel also did not by itself induce apoptosis (Fig 6). This finding has been confirmed in 8 different cord blood CD34+ cell preparations (data not shown). Therefore, the NF-κB/Rel activity did not appear to be indispensable for cell survival. Pyatt et al37 have recently reported that a nuclear localization signal (NLS)-carrying peptide, able to inhibit the nuclear translocation of NF-κB/Rel, induced apoptosis in CD34+ cells from human bone marrow. Differences between those and our results might be ascribable to the different origin of the CD34+ cells or possibly to the effect of the NLS-carrying peptide on NLS-containing factors different from NF-κB/Rel.38,39 Furthermore, in our hands, the inhibition of NF-κB/Rel did not induce apoptosis in CD34+ cells incubated with a survival-maintaining cytokine mixture (Fig 6). Although some of the cytokines contained in the mixture have been shown to induce NF-κB/Rel activity in hematopoietic precursor cell types,37,40,41 other antiapoptotic mechanisms, such as those involving Akt kinase activation, induced by the same or other cytokines of the mix,42-44 could be responsible for cell survival when NF-κB/Rel activity was inhibited.
Instead, when apoptosis was triggered by cytokine deprivation, the activation of NF-κB/Rel by amifostine appeared to counteract the execution of the apoptosis program. This is in line with the antiapoptotic activity of NF-κB/Rel triggered by physiological molecules such as TNFR or CD40.16-24 In fact, rather than sustaining the constitutive levels of housekeeping gene product(s) required for cell survival, the activation of NF-κB/Rel could be likely to regulate the expression of genes able to face apoptotic signals. These could include IAP caspase inhibitors,45,46the IEX-1L gene product,47 the Bcl2-homolog Blf-1/A1,48,49 and possibly others.22 50-52
In conclusion, the findings described here provide new information about the antiapoptotic mechanism induced by amifostine and show that the activation of NF-κB/Rel factors can counteract apoptosis in the hematopoietic compartment. This raises the possibility that, in addition to amifostine-related therapies, other NF-κB/Rel–stimulating strategies could improve the hematopoietic progenitor cell survival. Furthermore, because the NF-κB/Rel activity was not apparently indispensable for the CD34+ cell survival, this latter could be compatible with antineoplastic programs based on the inhibition of these transcription factors.34-36
ACKNOWLEDGMENT
The authors thank Dr F. Ferrara for help and discussion and Carmine Del Gaudio for his excellent technical help.
M.C.T. and S.V. contributed equally to this work.
Supported by AIRC, MURST (COFIN 98), and Regione Campania.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Maria Caterina Turco, MD, PhD, Dipartimento di Biochimica e Biotecnologie Mediche, Via Pansini, 5, 80131 Napoli, Italy; e-mail: turco@dbbm.unina.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal