Abstract
The Rh D antigen is the most clinically important protein blood group antigen of the erythrocyte. It is expressed as a collection of at least 37 different epitopes. The external domains of the Rh D protein involved in epitope presentation have been predicted based on the analysis of variant Rh D protein structures inferred from their cDNA sequences and their D epitope expression. This analysis can never be absolute because (1) most partial D phenotypes involve multiple amino acid changes in the Rh D protein and (2) deficiency for 1 or more epitopes may be due to gross structural alteration in the variant Rh D protein structure. We report here the amino acid requirements for the majority of D epitopes. They have been defined by generating a series of novel Rh mutant constructs by mutagenesis using an Rh cE cDNA as template and mutagenic oligonucleotide primers. When transfected into K562 cells, the D epitope expression of the derived mutant clones was then assessed by flow cytometry. The introduction of 9 externally predicted Rh D-specific amino acids on the Rh cE protein was sufficient to express 80% of all tested D epitopes, whereas other clones expressed none. We concluded from our data that the D epitope expression is consistent with at least 6 different epitope clusters localized on external regions of the Rh D protein, most involving overlapping regions within external loops 3, 4, and 6.
THE HUMAN Rh ANTIGENS are of high clinical relevance in transfusion medicine and can cause hemolytic disease resulting from fetomaternal blood group incompatibility and hemolytic transfusion reactions. Of the antigens of the Rh system, D is the most clinically significant, because it is highly immunogenic. This high degree of immunogenicity stems from the fact that the entire Rh D protein is absent from the erythrocyte membranes of persons expressing D-negative phenotypes.1 2 Thus, in contrast to most blood group antigens that arise through allelic genes, most often caused by single point mutation, the absence of an entire protein produces a far greater potential for immunological stimuli.
The human erythrocyte Rh D protein complex is most probably an α2β2 type heterotetramer that is composed of 2 Rh-associated glycoprotein (RhAG) and 2 Rh D protein monomers. The Rh CcEe antigens are expressed by a similar complex, but on Rh CcEe protein monomers. The Rh D and CcEe proteins arise from the RHDand RHCE genes, respectively, whereas the RhAG component arises from the RHAG gene (see Cartron et al3 and Avent and Reid4 for reviews). Between 30 and 35 amino acid substitutions define the Rh CcEe and D proteins, dependent on the CcEe phenotype of the individual (point mutations in the RHCE gene generate the C/c and E/e polymorphisms, located within exons 2 and 5, respectively5 6). These amino acid substitutions are those that generate the Rh D antigen; and the predicted membrane topology of the protein indicates that only 9 or 10 of them are externally located and hence involved in anti-D binding.
Because the D+/D-polymorphism is due to the complete absence of the Rh D protein, it is not surprising that there are many D epitopes recognized during a polyclonal anti-D response. These have been studied using human monoclonal anti-D and rare D variants that lack unique patterns of these epitopes. D variant (partial D phenotypes) individuals, if exposed to D-positive erythrocytes, may make anti-D to those epitopes they lack. The study of D variants at the serological and molecular level has resulted in the prediction of regions of the D protein involved in epitope presentation.7 8 However, these predictions are hindered by the fact that most D variants arise through multiple changes in the Rh D protein sequence, some of which may have no effect on D antigenicity. Furthermore, ablation of D epitopes observed in partial D phenotypes may be an indirect indication of regions of the protein involved in epitope presentation as the changes may introduce local disruption to structure.
In this and our previous study,9 we have used site-directed mutagenesis (SDM) to study the expression of individual D epitopes and, as a consequence, have predicted the external loop-loop interactions of the Rh D protein, which govern their expression. In contrast to a hypothesis presented in a recent study,10 our findings indicate that, although most D epitopes overlap, many are spatially distinct and may involve just 1 external loop of the protein. Our hypothesis is consistent with the consideration of both the dimensions of antibody paratopes (combining sites) and the predicted size of the external face of an Rh D protein monomer.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs).
Monoclonal anti-D, anti-E, and anti-c antibodies were available through the Third International Workshop on MoAbs against human red blood cells and related antigens. Others were obtained as follows: anti-Ds, P3X249 from Diagast Laboratories (Lille, France); HAM-A and MAD-2 from IBGRL (Bristol, UK); REG-A from N. Hughes-Jones (Cambridge, UK); and anti-D, H41 from H. Sonneborn (Biotest AG, Dreieche, Germany). Mouse monoclonal BRIC 69 was from IBGRL. K562 cells were obtained from the European Collection of Animal Cell Cultures (Porton Down, Salisbury, Wilts, UK). Amphotropic retroviral packaging cells (AM12 fibroblasts) were obtained from Genetix Pharmaceuticals (Rye, NY). Retroviral vector, Pbabe-puro, was provided by Dr H. Land (ICRF, London, UK). K562/D and K562/cE cell lines are as previously described.11
Mutagenic constructs.
RHCE→RHD mutants were constructed using the inverse polymerase chain reaction (PCR) method. Rh cE cDNA and plasmid Rh cE-Asp350His-Gly353Trp-Ala354Asn DNA were EcoRI-cut, gel-purified (Qiaex; Qiagen, Crawley, West Sussex, UK), and recircularized by dilute ligation. These DNAs were used as templates for inverse PCR. Mutagenic oligonucleotides were as follows.
For RhcEloop3mut, forward primer (L169M/R170M/F172I) had the sequence 5′-CATGATGCACATCTACGTGTTCGCAGCCTA-3′ (corresponding to nts 504-533 of the Rh30A/D cDNA sequence; mutagenic nucleotides underlined and boldfaced). Corresponding reverse primers MMI-rev had the sequence 5′-TTCATGTGGTAGTCTGTGTTGAAGATATT rev (corresponding to nts 503-474). Plasmid DNA from an Rh cE clone that contained the L169M/R170M/F172I incorporations wasEcoRI-digested and gel-purified. For the generation of SDM mutant constructs containing Met169, Met170, and Ile172, this product was recircularized to serve as a template, and other mutated residues were incorporated in a stepwise fashion.
For RhcEloop4mut, forward primer (Q233E) had the sequence 5′-TCCAATCGAAAGGAAGAATGCCATGTT-3′ (corresponding to nts 690-716). Inverse primers (V223F/P226A) had the sequence 5′-CTTCTCAGCAGAGCAGAGTTGAAACTTGGC-3′ (corresponding to nts 689-660). The manufacturer (GIBCO BRL, Paisley, Renfrewshire, Scotland) phosphorylated all primers. Inverse PCR was performed as follows: 94°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes for 35 cycles with Pfu DNA polymerase (Stratagene Inc, Palo Alto, CA).
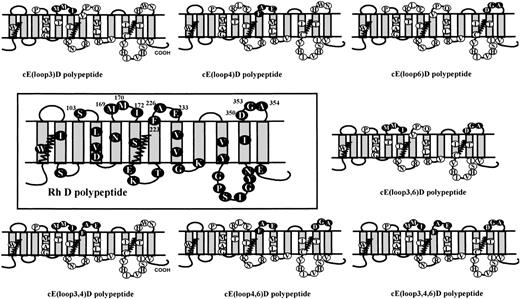
Six new mutant RhcE cDNA constructs were obtained: (1) Rh cE(loop3)D (Rh cE+Leu169Met; Arg170Met; Phe172Ile); (2) Rh cE(loop3,6)D (Rh cE+ Leu169Met; Arg170Met; Phe172Ile; Asp350His; Gly353Trp; Ala354Asn); (3) Rh cE(loop4)D (Rh cE+Val223Phe; Pro226Ala; Gln233Glu); (4) Rh cE(loop4,6)D (Rh cE+Val223Phe; Pro226Ala; Gln233Glu; Asp350His; Gly353Trp; Ala354Asn); (5) Rh cE(loop3,4)D (RhcE+Leu169Met; Arg170Met; Phe172Ile; Val223Phe; Pro226Ala; Gln233Glu); and (6) Rh cE(loop3,4,6)D (RhcE+Leu169Met; Arg170Met; Phe172Ile; Val223Phe; Pro226Ala; Gln233Glu; Asp350His; Gly353Trp; Ala354Asn).
The predicted structures of the mutant Rh proteins encoded by their corresponding cDNAs are depicted in Fig 1.
Predicted membrane topology and amino acid sequences of mutant Rh polypeptide species encoded by SDM constructs. This figure depicts the hypothetical membrane orientation of the Rh D polypeptide (large central panel) in which predicted externalized amino acids are numbered. The wild-type Rh D protein is then compared with SDM mutants that were derived initially from a cDNA encoding an Rh cE cDNA. The mutants were generated by inverse PCR using mutagenic primers, and the predicted extracellular positions of the targeted, Rh D-specific amino acids are shown as solid circles on each of the 7 different mutants. The polymorphic amino acid residues that differentiate the Rh D and Rh CcEe are shown as solid and open circles, respectively, with the single letter amino acid code inside each circle.
Predicted membrane topology and amino acid sequences of mutant Rh polypeptide species encoded by SDM constructs. This figure depicts the hypothetical membrane orientation of the Rh D polypeptide (large central panel) in which predicted externalized amino acids are numbered. The wild-type Rh D protein is then compared with SDM mutants that were derived initially from a cDNA encoding an Rh cE cDNA. The mutants were generated by inverse PCR using mutagenic primers, and the predicted extracellular positions of the targeted, Rh D-specific amino acids are shown as solid circles on each of the 7 different mutants. The polymorphic amino acid residues that differentiate the Rh D and Rh CcEe are shown as solid and open circles, respectively, with the single letter amino acid code inside each circle.
PCR products were gel-purified (Qiaex; Qiagen), blunt-end ligated,EcoRI-cut, and recloned into the EcoRI site of phosphatase-treated pBabe-puro retroviral expression vector. All clones were sequenced fully to confirm orientation, to ensure that no PCR misincorporations occurred, and to confirm that clones contained mutagenic residues.
Transfection of amphotropic AM12 packaging cell line.
Approximately 40 μg of Sca I-linearized mutant pBabe/Rh DNA was used to transfect 1 × 106 cultured AM12 cells by electroporation using a Bio-Rad Gene pulser II (Bio-Rad, Hemel Hempstead, UK). Transfected cells were cultured in Iscove's modified Eagle's medium supplemented with 10% vol/vol fetal calf serum (IMEM/FCS) at 37°C, which was replaced with IMEM/FCS containing 3 μg/mL puromycin (Sigma Chemical Co) after 2 days. Puromycin-resistant colonies were cultured in fresh plates and retroviral supernatants were collected as described previously.
Retroviral transduction of K562 cells.
K562 cells (2 × 105) were transduced with 1 mL of retrovirus and allowed to incubate at 37°C for 4 hours before cultivation in IMEM/FCS for an additional 2 days. Cultures were transferred to a 96-well microplate once diluted in IMEM/FCS supplemented with 3 μg/mL puromycin. Puromycin-resistant K562 clones were transferred to fresh plates and cultured further until sufficient cells were available for flow cytometric analysis.
Flow cytometric analysis.
Cultivated clones were initially screened with BRIC 69 to identify those expressing Rh polypeptides before expanding the cultures to be screened with a panel of human monoclonal anti-D antibodies with anti-epD1 to -epD37 (37 epitope model) specificities and with a panel of monoclonal anti-c and anti-E antibodies.
Cells were resuspended in phosphate-buffered saline with 1% wt/vol bovine serum albumin (PBSA) and 1 × 105 cells were used for each antibody reaction as described previously.9 11 Cells were washed once with PBSA and incubated with 50 μL of Fab monomer rabbit antimouse IgG or rabbit antihuman IgG (or IgM) affinity-purified fluorescein isothiocyanate (FITC)-labeled (Dako, Copenhagen, Denmark) for 1 hour at room temperature and washed once, and the sample volume was adjusted with PBSA for flow cytometric analysis performed on a Becton Dickinson FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA). Mean fluorescence intensity (FLI) values were used as a measure of antibody binding. Background binding (negative control) was assessed by using K562/pBabe mutant cells incubated with relevant primary antibody. Positive antibody binding was demonstrated by FLI values of 2× background, because this level was found to be positive binding to control lines.
mRNA isolation from K562 cells and reverse transcriptase-PCR (RT-PCR).
mRNA was extracted from 1 × 106 cells of the K562/mutant Rh lines using Oligo dT12-18 magnetic beads, prepared according to manufacturer's instructions (Dynal, Oslo, Norway). Synthesis of cDNA was from approximately 1 μg of mRNA using oligo dT12-18 (Pharmacia, Uppsala, Sweden), as described previously. The primers corresponded to pBabe sequence 5′ of the insert site, 5′-GCC TCG ATC CTC CCT TTA TCC-3′ sense and the antisense primer to the Rh cE cDNA 3′ noncoding region, exon 10 antisense 5′-GTACAAATGCAGGCAACAGTG-3′. PCR conditions were as follows: 94°C for 1 minute, 60°C for 1 minute, and 72°C for 2.5 minutes for 30 cycles.
RESULTS
Mutagenic constructs and mutant K562 cell lines.
The different mutant K562 lines were generated essentially as described previously9 using the K562/cE(loop6)D plasmid as starting template. The approach adopted was a D-epitope construction approach to analyze the creation of D epitopes, rather than a deconstruction approach in which the loss of D epitope expression may be observed. This method had proved successful in the previous study to delineate D epitopes expressed on loop 6 of the Rh D protein. Thus, it was anticipated that this method would be successful when other Rh D protein external loops were analyzed.
Each mutagenic PCR was performed, using SDM primers, as described in Materials and Methods, and then sequenced fully on both strands of the DNA to ensure that no PCR misincorporations or primer truncations had occurred. All 6 mutant constructs had the expected alterations introduced by the mutagenic primers. Each plasmid DNA was then transfected in AM12 fibroblasts, and the monoclonal AM12 lines generated after puromycin selection used to retrovirally transduce K562 cells as described in Materials and Methods. Monoclonal K562 cells were then selected by limited dilution and then screened for the level of Rh expression using BRIC 69, a murine anti-Rh MoAb. To confirm that K562 clones were expressing the expected mutant Rh protein, mRNA was isolated and reverse transcribed. PCR was then performed using a forward vector-specific primer and an Rh-specific reverse primer (located within exon 10; see Materials and Methods). Full-length pBabe-Rh transcripts were obtained in all cell lines (Fig 2), PCR products were gel-purified and sequenced directly on both strands of DNA. All K562 clones contained the expected mutant pBabe-Rh transcript. K562 lines appear to express high levels of endogenous RhAG. Thus, cotransfection experiments using a RhAG cDNA are not necessary.
RT-PCR generation of mutant Rh cDNA from the 6 SDM K562 cell lines. PCR products of 1,497 bp were obtained from each monoclonal K562 line after reverse transcription of purified mRNA from each line. To confirm that the K562 line expressed the anticipated Rh mutant, PCR products were gel-purified and subjected to direct sequencing. All lines were found to express the expected mutant Rh mRNAs. The cE(loop6)D K562 line had been characterized in a previous study.9 NTC, no template control.
RT-PCR generation of mutant Rh cDNA from the 6 SDM K562 cell lines. PCR products of 1,497 bp were obtained from each monoclonal K562 line after reverse transcription of purified mRNA from each line. To confirm that the K562 line expressed the anticipated Rh mutant, PCR products were gel-purified and subjected to direct sequencing. All lines were found to express the expected mutant Rh mRNAs. The cE(loop6)D K562 line had been characterized in a previous study.9 NTC, no template control.
Flow cytometry using monoclonal anti-D.
After the initial screening with BRIC 69, the 6 different K562/cE→D mutant clones were expanded and a total of 50 different monoclonal anti-D were used to analyze the different expression pattern of D epitopes. To assess background binding of FITC-conjugated secondary antibodies to the K562 cell lines, tissue culture medium (TCM) was used as a negative control. Positive and negative controls for antibody binding included K562 clones transfected with Rh D and Rh cE (wild-type) cDNAs described previously.11 Mean fluorescence intensity was used as a measure of antibody binding (Table 1), in preference to median or mode channel fluorescence intensity, because often fluorescence intensity does not give a bell-shaped curve. The figures given in Table 1 for fluorescence intensity are logarithmic values.
Flow Cytometric Analysis of 7 Different Rh SDM Mutant K562 Lines Using Monoclonal Anti-D
| Anti- EpD1-9 . | Anti-EpD1-37 . | ISBT No. . | K562/ pBabe . | K562/ cE . | K562/ D . | K562/ lp3 . | K562/ lp3 + 6 . | K562/ lp4 . | K562/ lp4 + 6 . | K562/ lp3 + 4 . | K562/ lp3 + 4 + 6 . | K562/ lp6 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EpD1 | EpD1 GAD 2 | — | 3.2 | 3.6 | 9.1 | 3.9 | 2.9 | 3.5 | 3.4 | 4.1 | 4.6 | 3.1 |
| EpD1 | EpD1 Reg A | — | 3.3 | 3.4 | 9.3 | 3.6 | 2.8 | 3.3 | 3.4 | 3.9 | 3.8 | 2.9 |
| EpD1 | EpD1 LHM169/81 | 82 | 4.2 | 4.1 | 17.1 | 4.1 | 3.2 | 3.6 | 5.3 | 4.2 | 7.8 | 3.6 |
| EpD2 | EpD3 D6D-02 | 123 | 3.5 | 3.7 | 13.3 | 3.2 | 3.1 | 3.3 | 3.6 | 3.9 | 6.3 | 3.3 |
| EpD2 | EpD3 L87 1G7 | 108 | 4.1 | 4.2 | 22.9 | 4.2 | 3.4 | 3.7 | 6.7 | 4.1 | 24.1 | 4.2 |
| EpD2 | EpD3 P3X249 | 5.7 | 6.1 | 24.4 | 5.2 | 5.6 | 4.9 | 6.8 | 7.1 | 19.1 | 5.1 | |
| EpD3 | EpD5 LOR29 FF749 | 49 | 3.9 | 4.2 | 20.1 | 4.4 | 5.6 | 3.7 | 6.7 | 4.1 | 23.3 | 3.7 |
| EpD3 | EpD5 LHM 76/55 | 75 | 4.3 | 4.3 | 29.4 | 4.3 | 5.9 | 4.0 | 30.8 | 4.2 | 32.3 | 3.4 |
| EpD3 | EpD5 LHM 76/59 | 76 | 5.3 | 5.9 | 25.3 | 5.4 | 27.7 | 5.0 | 51.7 | 5.0 | 27.0 | 14.9 |
| EpD3 | EpD5 H41-11B7 | 106 | 4.4 | 4.2 | 23.3 | 3.9 | 35.2 | 3.9 | 31.1 | 4.2 | 31.1 | 32.7 |
| EpD3 | EpD5/35 P3X290 | 4.1 | 4.2 | 21.6 | 4.2 | 3.7 | 3.5 | 5.2 | 4.3 | 22.2 | 3.4 | |
| EpD4 | EpD6 LOR 176C7 | 45 | 3.4 | 3.7 | 6.6 | 3.8 | 3.1 | 3.2 | 8.0 | 4.1 | 10.5 | 3.6 |
| EpD5 | EpD7 CAZ 7 4C5 | 42 | 3.8 | 3.5 | 8.6 | 2.3 | 6.0 | 2.6 | 6.1 | 3.8 | 10.1 | 2.5 |
| EpD5 | EpD8 D10 | 114 | 5.7 | 5.6 | 35.7 | 5.3 | 3.7 | 4.6 | 6.8 | 5.0 | 21.7 | 3.5 |
| EpD5 | EpD10 BS229 | 109 | 4.1 | 4.2 | 21.2 | 4.0 | 3.4 | 3.4 | 3.9 | 7.1 | 12.9 | 3.4 |
| EpD5 | EpD10 MAR1/F8 | 52 | 4.5 | 4.9 | 22.1 | 3.6 | 2.9 | 5.4 | 5.6 | 15.5 | 35.1 | 5.2 |
| EpD5 | EpD10 BS231 | 110 | 3.4 | 3.8 | 13.1 | 3.6 | 3.1 | 3.2 | 3.6 | 4.9 | 6.1 | 3.3 |
| EpD5 | EpD11 HAM-A | 3.8 | 4.1 | 17.1 | 2.4 | 2.8 | 3.2 | 3.0 | 20.2 | 25.0 | 2.6 | |
| EpD6/7 | EpD12 LOR178D3 | 46 | 4.7 | 4.5 | 27.1 | 4.6 | 3.5 | 4.3 | 5.3 | 25.2 | 33.5 | 3.6 |
| EpD6/7 | EpD12 P3AF6 | 102 | 5.2 | 6.5 | 30.9 | 6.5 | 6.6 | 5.1 | 7.8 | 26.2 | 30.6 | 5.2 |
| EpD6/7 | EpD12 175-2 | 35 | 3.4 | 3.0 | 13.4 | 2.8 | 6.2 | 5.7 | 24.4 | 15.8 | 17.5 | 2.3 |
| EpD6/7 | EpD12 MS201 | 113 | 7.2 | 10.0 | 26.9 | 10.1 | 13.5 | 11.9 | 45.3 | 32.9 | 35.6 | 18.3* |
| EpD6/7 | EpD12 P3X61 | ND | 8.3 | 45.4 | 5.3 | 13.1 | 11.7 | 12.7 | 66.1 | 58.9 | 8.4 | |
| EpD6/7 | EpD13 F5S | 90 | 6.5 | 6.4 | 23.9 | 6.1 | 4.5 | 3.9 | 4.8 | 22.0 | 18.4 | 4.2 |
| EpD6/7 | EpD13 BRAD3 | 105 | ND | 4.1 | 21.4 | 2.6 | 6.7 | 2.9 | 3.3 | 7.5 | 21.9 | 3.0 |
| EpD6/7 | EpD15 BTSN4 | 71 | 5.8 | 4.9 | 32.9 | 4.7 | 7.3 | 4.6 | 41.0 | 12.5* | 28.9 | 3.3 |
| EpD6/7 | EpD15 D-90/7 | 31 | 5.5 | 5.1 | 35.2 | 5.5 | 3.8 | 4.5 | 5.2 | 4.7 | 20.6 | 3.4 |
| EpD6/7 | EpD15 L87 1G7 | 4.2 | 22.9 | 4.2 | 3.4 | 3.7 | 5.7 | 4.1 | 24.1 | 4.2 | ||
| EpD6/7 | EpD17 NaTh28-C11 | 37 | 3.5 | 4.4 | 22.0 | 5.9 | 6.1 | 3.5 | 9.4 | 22.9 | 29.2 | 4.3 |
| EpD6/7 | EpD17 P3 187 | 98 | 3.0 | 3.3 | 18.8 | 4.1 | 4.7 | 9.9 | 30.6 | 22.6 | 21.2 | 3.8 |
| EpD6/7 | EpD18 HG/92 (G) | 30 | 5.3 | 4.3 | 36.0 | 4.6 | 3.5 | 4.2 | 4.9 | 4.3 | 19.0 | 3.1 |
| EpD6/7 | EpD18 T3A2F6 (G) | ND | 6.4 | 32.6 | 3.5 | 11.6 | 6.4 | 2.4 | 54.5 | 50.7 | 7.5 | |
| EpD6/7 | EpD18 HM10 (M) | ND | 10.2 | 45.0 | 8.5 | 16.6 | 8.9 | 10.0 | 58.2 | 65.2 | 7.6 | |
| EpD6/7 | EpD19 MAD2 (M) | ND | 5.1 | 20.2 | 3.1 | 3.6 | 3.4 | 3.9 | 47.5 | 51.6 | 3.1 | |
| EpD6/7 | EpD21 HIRO-2 | 122 | 6.7 | 7.6 | 20.2 | 6.4 | 6.6 | 5.0 | 5.1 | 16.5 | 18.9 | 7.4 |
| EpD8 | EpD22 LHM76/58 | 74 | 4.8 | 5.2 | 26.5 | 4.4 | 3.4 | 3.7 | 4.4 | 5.9 | 5.9 | 3.6 |
| EpD8 | EpD22 LHM59/19 | 78 | 4.4 | 4.3 | 25.5 | 4.3 | 3.5 | 3.9 | 4.4 | 5.4 | 6.5 | 3.3 |
| EpD9 | EpD23 BIRMA D6 | 96 | 5.0 | 4.6 | 30.4 | 4.2 | 32.7 | 3.9 | 52.4 | 4.2 | 29.0 | 13.1 |
| EpD9 | EpD23 P3G6 | 101 | 4.3 | 4.2 | 26.6 | 3.9 | 5.0 | 3.5 | 15.8 | 4.0 | 20.4 | 2.9 |
| EpD9 | EpD23 HIRO-4 | 118 | 3.8 | 3.8 | 15.6 | 3.7 | 3.5 | 3.4 | 5.2 | 3.8 | 11.3 | 3.2 |
| EpD9 | EpD23 MS26 | 112 | 5.7 | 6.3 | 27.0 | 3.7 | 18.7 | 4.6 | 20.2 | 4.7 | 32.7 | 12.6 |
| EpD9 | EpD23 P3X21223B | 5.8 | 6.9 | 27.6 | 3.2 | 56.3 | 4.7 | 37.3 | 4.3 | 33.4 | 58.5 | |
| EpD30 8D8 | 4.6 | 4.9 | 25.3 | 5.8 | 9.3 | 5.4 | 40.8 | 4.9 | 25.7 | 8.4 | ||
| EpD32 ZIG189 | 62 | ND | 5.9 | 14.1 | 3.1 | 4.2 | 3.5 | 3.6 | 7.8 | 5.0 | 3.6 | |
| EpD34 LORA/F | 50 | 4.7 | 5.4 | 14.2 | 5.7 | 3.8 | 4.7 | 5.1 | 5.1 | 11.1 | 4.1 | |
| EpD35 SALSA | 60 | 5.1 | 7.8 | 28.1 | 7.7 | 4.5 | 5.6 | 6.1 | 6.1 | 10.3* | 6.1 | |
| EpD36 NAV3 2E8 | 54 | 4.7 | 5.7 | 26.0 | 6.7 | 5.9 | 6.0 | 8.5 | 5.6 | 24.0 | 7.6 | |
| EpD36 NAV6/F 4D5 | 55 | 5.2 | 7.4 | 31.7 | 6.8 | 6.4 | 6.1 | 30.4 | 5.8 | 26.5 | 10.8* | |
| EpD37 BTSN10 | 73 | 5.7 | 5.6 | 32.7 | 6.4 | 4.2 | 6.6 | 11.3* | 5.9 | 11.9* | 8.6 | |
| EpD37 MCAD6 | 124 | 5.8 | 6.3 | 33.2 | 6.6 | 5.9 | 5.4 | 7.9 | 11.7* | 32.7 | 4.8 |
| Anti- EpD1-9 . | Anti-EpD1-37 . | ISBT No. . | K562/ pBabe . | K562/ cE . | K562/ D . | K562/ lp3 . | K562/ lp3 + 6 . | K562/ lp4 . | K562/ lp4 + 6 . | K562/ lp3 + 4 . | K562/ lp3 + 4 + 6 . | K562/ lp6 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EpD1 | EpD1 GAD 2 | — | 3.2 | 3.6 | 9.1 | 3.9 | 2.9 | 3.5 | 3.4 | 4.1 | 4.6 | 3.1 |
| EpD1 | EpD1 Reg A | — | 3.3 | 3.4 | 9.3 | 3.6 | 2.8 | 3.3 | 3.4 | 3.9 | 3.8 | 2.9 |
| EpD1 | EpD1 LHM169/81 | 82 | 4.2 | 4.1 | 17.1 | 4.1 | 3.2 | 3.6 | 5.3 | 4.2 | 7.8 | 3.6 |
| EpD2 | EpD3 D6D-02 | 123 | 3.5 | 3.7 | 13.3 | 3.2 | 3.1 | 3.3 | 3.6 | 3.9 | 6.3 | 3.3 |
| EpD2 | EpD3 L87 1G7 | 108 | 4.1 | 4.2 | 22.9 | 4.2 | 3.4 | 3.7 | 6.7 | 4.1 | 24.1 | 4.2 |
| EpD2 | EpD3 P3X249 | 5.7 | 6.1 | 24.4 | 5.2 | 5.6 | 4.9 | 6.8 | 7.1 | 19.1 | 5.1 | |
| EpD3 | EpD5 LOR29 FF749 | 49 | 3.9 | 4.2 | 20.1 | 4.4 | 5.6 | 3.7 | 6.7 | 4.1 | 23.3 | 3.7 |
| EpD3 | EpD5 LHM 76/55 | 75 | 4.3 | 4.3 | 29.4 | 4.3 | 5.9 | 4.0 | 30.8 | 4.2 | 32.3 | 3.4 |
| EpD3 | EpD5 LHM 76/59 | 76 | 5.3 | 5.9 | 25.3 | 5.4 | 27.7 | 5.0 | 51.7 | 5.0 | 27.0 | 14.9 |
| EpD3 | EpD5 H41-11B7 | 106 | 4.4 | 4.2 | 23.3 | 3.9 | 35.2 | 3.9 | 31.1 | 4.2 | 31.1 | 32.7 |
| EpD3 | EpD5/35 P3X290 | 4.1 | 4.2 | 21.6 | 4.2 | 3.7 | 3.5 | 5.2 | 4.3 | 22.2 | 3.4 | |
| EpD4 | EpD6 LOR 176C7 | 45 | 3.4 | 3.7 | 6.6 | 3.8 | 3.1 | 3.2 | 8.0 | 4.1 | 10.5 | 3.6 |
| EpD5 | EpD7 CAZ 7 4C5 | 42 | 3.8 | 3.5 | 8.6 | 2.3 | 6.0 | 2.6 | 6.1 | 3.8 | 10.1 | 2.5 |
| EpD5 | EpD8 D10 | 114 | 5.7 | 5.6 | 35.7 | 5.3 | 3.7 | 4.6 | 6.8 | 5.0 | 21.7 | 3.5 |
| EpD5 | EpD10 BS229 | 109 | 4.1 | 4.2 | 21.2 | 4.0 | 3.4 | 3.4 | 3.9 | 7.1 | 12.9 | 3.4 |
| EpD5 | EpD10 MAR1/F8 | 52 | 4.5 | 4.9 | 22.1 | 3.6 | 2.9 | 5.4 | 5.6 | 15.5 | 35.1 | 5.2 |
| EpD5 | EpD10 BS231 | 110 | 3.4 | 3.8 | 13.1 | 3.6 | 3.1 | 3.2 | 3.6 | 4.9 | 6.1 | 3.3 |
| EpD5 | EpD11 HAM-A | 3.8 | 4.1 | 17.1 | 2.4 | 2.8 | 3.2 | 3.0 | 20.2 | 25.0 | 2.6 | |
| EpD6/7 | EpD12 LOR178D3 | 46 | 4.7 | 4.5 | 27.1 | 4.6 | 3.5 | 4.3 | 5.3 | 25.2 | 33.5 | 3.6 |
| EpD6/7 | EpD12 P3AF6 | 102 | 5.2 | 6.5 | 30.9 | 6.5 | 6.6 | 5.1 | 7.8 | 26.2 | 30.6 | 5.2 |
| EpD6/7 | EpD12 175-2 | 35 | 3.4 | 3.0 | 13.4 | 2.8 | 6.2 | 5.7 | 24.4 | 15.8 | 17.5 | 2.3 |
| EpD6/7 | EpD12 MS201 | 113 | 7.2 | 10.0 | 26.9 | 10.1 | 13.5 | 11.9 | 45.3 | 32.9 | 35.6 | 18.3* |
| EpD6/7 | EpD12 P3X61 | ND | 8.3 | 45.4 | 5.3 | 13.1 | 11.7 | 12.7 | 66.1 | 58.9 | 8.4 | |
| EpD6/7 | EpD13 F5S | 90 | 6.5 | 6.4 | 23.9 | 6.1 | 4.5 | 3.9 | 4.8 | 22.0 | 18.4 | 4.2 |
| EpD6/7 | EpD13 BRAD3 | 105 | ND | 4.1 | 21.4 | 2.6 | 6.7 | 2.9 | 3.3 | 7.5 | 21.9 | 3.0 |
| EpD6/7 | EpD15 BTSN4 | 71 | 5.8 | 4.9 | 32.9 | 4.7 | 7.3 | 4.6 | 41.0 | 12.5* | 28.9 | 3.3 |
| EpD6/7 | EpD15 D-90/7 | 31 | 5.5 | 5.1 | 35.2 | 5.5 | 3.8 | 4.5 | 5.2 | 4.7 | 20.6 | 3.4 |
| EpD6/7 | EpD15 L87 1G7 | 4.2 | 22.9 | 4.2 | 3.4 | 3.7 | 5.7 | 4.1 | 24.1 | 4.2 | ||
| EpD6/7 | EpD17 NaTh28-C11 | 37 | 3.5 | 4.4 | 22.0 | 5.9 | 6.1 | 3.5 | 9.4 | 22.9 | 29.2 | 4.3 |
| EpD6/7 | EpD17 P3 187 | 98 | 3.0 | 3.3 | 18.8 | 4.1 | 4.7 | 9.9 | 30.6 | 22.6 | 21.2 | 3.8 |
| EpD6/7 | EpD18 HG/92 (G) | 30 | 5.3 | 4.3 | 36.0 | 4.6 | 3.5 | 4.2 | 4.9 | 4.3 | 19.0 | 3.1 |
| EpD6/7 | EpD18 T3A2F6 (G) | ND | 6.4 | 32.6 | 3.5 | 11.6 | 6.4 | 2.4 | 54.5 | 50.7 | 7.5 | |
| EpD6/7 | EpD18 HM10 (M) | ND | 10.2 | 45.0 | 8.5 | 16.6 | 8.9 | 10.0 | 58.2 | 65.2 | 7.6 | |
| EpD6/7 | EpD19 MAD2 (M) | ND | 5.1 | 20.2 | 3.1 | 3.6 | 3.4 | 3.9 | 47.5 | 51.6 | 3.1 | |
| EpD6/7 | EpD21 HIRO-2 | 122 | 6.7 | 7.6 | 20.2 | 6.4 | 6.6 | 5.0 | 5.1 | 16.5 | 18.9 | 7.4 |
| EpD8 | EpD22 LHM76/58 | 74 | 4.8 | 5.2 | 26.5 | 4.4 | 3.4 | 3.7 | 4.4 | 5.9 | 5.9 | 3.6 |
| EpD8 | EpD22 LHM59/19 | 78 | 4.4 | 4.3 | 25.5 | 4.3 | 3.5 | 3.9 | 4.4 | 5.4 | 6.5 | 3.3 |
| EpD9 | EpD23 BIRMA D6 | 96 | 5.0 | 4.6 | 30.4 | 4.2 | 32.7 | 3.9 | 52.4 | 4.2 | 29.0 | 13.1 |
| EpD9 | EpD23 P3G6 | 101 | 4.3 | 4.2 | 26.6 | 3.9 | 5.0 | 3.5 | 15.8 | 4.0 | 20.4 | 2.9 |
| EpD9 | EpD23 HIRO-4 | 118 | 3.8 | 3.8 | 15.6 | 3.7 | 3.5 | 3.4 | 5.2 | 3.8 | 11.3 | 3.2 |
| EpD9 | EpD23 MS26 | 112 | 5.7 | 6.3 | 27.0 | 3.7 | 18.7 | 4.6 | 20.2 | 4.7 | 32.7 | 12.6 |
| EpD9 | EpD23 P3X21223B | 5.8 | 6.9 | 27.6 | 3.2 | 56.3 | 4.7 | 37.3 | 4.3 | 33.4 | 58.5 | |
| EpD30 8D8 | 4.6 | 4.9 | 25.3 | 5.8 | 9.3 | 5.4 | 40.8 | 4.9 | 25.7 | 8.4 | ||
| EpD32 ZIG189 | 62 | ND | 5.9 | 14.1 | 3.1 | 4.2 | 3.5 | 3.6 | 7.8 | 5.0 | 3.6 | |
| EpD34 LORA/F | 50 | 4.7 | 5.4 | 14.2 | 5.7 | 3.8 | 4.7 | 5.1 | 5.1 | 11.1 | 4.1 | |
| EpD35 SALSA | 60 | 5.1 | 7.8 | 28.1 | 7.7 | 4.5 | 5.6 | 6.1 | 6.1 | 10.3* | 6.1 | |
| EpD36 NAV3 2E8 | 54 | 4.7 | 5.7 | 26.0 | 6.7 | 5.9 | 6.0 | 8.5 | 5.6 | 24.0 | 7.6 | |
| EpD36 NAV6/F 4D5 | 55 | 5.2 | 7.4 | 31.7 | 6.8 | 6.4 | 6.1 | 30.4 | 5.8 | 26.5 | 10.8* | |
| EpD37 BTSN10 | 73 | 5.7 | 5.6 | 32.7 | 6.4 | 4.2 | 6.6 | 11.3* | 5.9 | 11.9* | 8.6 | |
| EpD37 MCAD6 | 124 | 5.8 | 6.3 | 33.2 | 6.6 | 5.9 | 5.4 | 7.9 | 11.7* | 32.7 | 4.8 |
Values are expressed as the mean fluorescence intensity. Values twice that of background antibody binding (ie, that observed binding to a negative control K562 line) are considered positive and are shaded gray and boldfaced. The epitope specificity of each anti-D is shown using either the 1-9 model12,13 or the 1-37 model.14 The identification number allocated to each anti-D in the recent third workshop on MoAbs against red blood cell antigens is also shown in the column labeled ISBT No.
Weakly positive samples.
The data obtained as a result of screening the 7 mutant K562 lines are summarized in Table 1. Distinct patterns of reactivity were observed, ranging from the complete absence of D epitope expression [cE(loop3)D and cE(loop4)D K562 lines] to reaction of 40 of 50 tested anti-D [cE(loop3+4+6)D K562 line]. The cE(loop3+4)D and cE(loop4+6) K562 lines were intermediary in their reaction profiles with anti-D, indicating that these Rh D-specific loops are strongly implicated in D epitope expression. The cE(loop3+6)D K562 line gave an identical serological profile to the cE(loop6)D K562 line, suggesting that no anti-D require an interaction between loops 3 and 6 of the Rh D protein monomer.
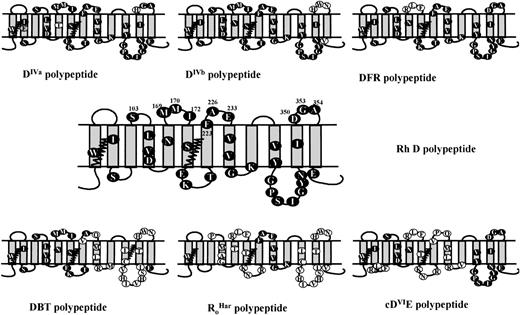
Comparison of serological reactivity of mutant K562 lines with partial D phenotype erythrocytes.
We have considered in detail the potential amino acid requirements for D antigenicity by comparing (and contrasting) our data with that of the serological reactivity of partial D phenotype erythrocytes. This assessment is complex, and our assumptions are considered in detail in the Discussion. Table 2 summarizes the epD1-37 reaction profile of the K562 lines described here and that of known partial D phenotype erythrocytes. The topologies of hybrid Rh proteins responsible for expression of partial D phenotypes are indicated in Fig 3. By comparing the predicted structures of the SDM constructs to naturally occurring partial D variants, it can be seen that there are structural similarities. Our analysis has resulted in the prediction of a model for the loop-loop interactions of the Rh D protein and is based on an assimilation of the reaction patterns of both partial D phenotype red blood cells and the mutant K562 lines described here.
Summary of D Epitopes Expressed by Some Common Partial D Phenotypes and the K562 SDM Mutants
| Partial D . | Predicted External AA Altered . | Loop Involved . | EpD Present . | EpD Absent . |
|---|---|---|---|---|
| DII | 354 | loop6 | 1-5, 7-15, 17, 18, 19, 21, 22, 24, 12-22, 24, 27, 28, 3-37 | 6, 23, 25, 26, 29 |
| DIIIb | 103 | loop2 | 1-26; 28-37 | 27 |
| DIVa | 350 | loop3, loop6 | 6-15, 17-19, 21, 22, 25, 27, 28, 31-37 | 1-5, 23, 24, 26, 29, 30 |
| DIVb | 350, 353, 354 | loop6 | 7-15, 17-19, 21, 22, 25, 32, 33, 37 | 1-6, 23, 24, 26, 29, 30, 31, 34-36 |
| DVa | 223, 233 | loop4 | 3-6, 12-15, 17-19, 21-25, 27-30, 33-37 | 1, 2, 7-11, 26, 31, 32 |
| DVI | 169, 170, 172, 223, 233 | loop3, loop4 | 5, 6, 23-25, 30, 36, 37 | 1, 2, 3, 4, 7-22, 26-29, 31-35 |
| DVII | 110 | loop2 | 1-15, 17-19, 21, 23-26, 29, 30, 33-37 | 22, 27, 28, 31, 32 |
| DFR | 169, 170, 172 | loop3 | 1, 3, 5-8, 12-15, 23, 25, 29, 30, 34-37 | 2, 4, 9-11, 17-19, 21, 22, 24, 26-28, 31-33 |
| DBT | 223, 226, 233 350, 353, 354 | loop4 loop6 | 12, 13, 17, 18, 22, 33, 37 | 1-11, 14, 15, 19, 21, 23-32, 34-36 |
| RoHAR | 103, 169, 170, 172, 350, 353, 354 | loop2, 3, 6 | 7, 9, 12, 14, 17, 19 | 1-6, 8, 10, 11, 13, 15, 18, 21-37 |
| DMH | 1, 3-6, 10, 12, 13, 15, 17, 19, (20), 21, 23, 35-37 | 2, 7, 11, 18, (20), 22, 31, 32, 34 | ||
| HMi | 283 | loop5 | 1-3, 5-10, 12-15, 17-19, 23, 35-37 | 4, 11, 21, 22, 31-34 |
| K562/cE (loop3,4)D | 169, 170, 172, 223, 226, 233 | loop3, loop4 | (10), 11, 12, 13, 17, (18), 19, 21 | 1-7, (10), 13, 15, (18), 22-37 |
| K562/cE (loop4,6)D | 223, 226, 233 350, 353, 354 | loop4 loop6 | (5), 6, (12), (15), (17), (23), 30, (36) | 1, 3, (5), 7-11, (12), 13, (15), (17), 18-22, (23), 32, 33, 34, 35, (36), 37 |
| K562/cE (loop3,4,6) D | 169, 170, 172 223, 226, 233 350, 353, 354 | loop3 loop4 loop6 | 3-21, 23, 30, 34, 36, 37 | 1, 22, 32, 34 |
| K562/cE (loop 6)D | 350, 353, 354 | (5), (23) | 1-4, (5), 6-22, (23), 24-37 |
| Partial D . | Predicted External AA Altered . | Loop Involved . | EpD Present . | EpD Absent . |
|---|---|---|---|---|
| DII | 354 | loop6 | 1-5, 7-15, 17, 18, 19, 21, 22, 24, 12-22, 24, 27, 28, 3-37 | 6, 23, 25, 26, 29 |
| DIIIb | 103 | loop2 | 1-26; 28-37 | 27 |
| DIVa | 350 | loop3, loop6 | 6-15, 17-19, 21, 22, 25, 27, 28, 31-37 | 1-5, 23, 24, 26, 29, 30 |
| DIVb | 350, 353, 354 | loop6 | 7-15, 17-19, 21, 22, 25, 32, 33, 37 | 1-6, 23, 24, 26, 29, 30, 31, 34-36 |
| DVa | 223, 233 | loop4 | 3-6, 12-15, 17-19, 21-25, 27-30, 33-37 | 1, 2, 7-11, 26, 31, 32 |
| DVI | 169, 170, 172, 223, 233 | loop3, loop4 | 5, 6, 23-25, 30, 36, 37 | 1, 2, 3, 4, 7-22, 26-29, 31-35 |
| DVII | 110 | loop2 | 1-15, 17-19, 21, 23-26, 29, 30, 33-37 | 22, 27, 28, 31, 32 |
| DFR | 169, 170, 172 | loop3 | 1, 3, 5-8, 12-15, 23, 25, 29, 30, 34-37 | 2, 4, 9-11, 17-19, 21, 22, 24, 26-28, 31-33 |
| DBT | 223, 226, 233 350, 353, 354 | loop4 loop6 | 12, 13, 17, 18, 22, 33, 37 | 1-11, 14, 15, 19, 21, 23-32, 34-36 |
| RoHAR | 103, 169, 170, 172, 350, 353, 354 | loop2, 3, 6 | 7, 9, 12, 14, 17, 19 | 1-6, 8, 10, 11, 13, 15, 18, 21-37 |
| DMH | 1, 3-6, 10, 12, 13, 15, 17, 19, (20), 21, 23, 35-37 | 2, 7, 11, 18, (20), 22, 31, 32, 34 | ||
| HMi | 283 | loop5 | 1-3, 5-10, 12-15, 17-19, 23, 35-37 | 4, 11, 21, 22, 31-34 |
| K562/cE (loop3,4)D | 169, 170, 172, 223, 226, 233 | loop3, loop4 | (10), 11, 12, 13, 17, (18), 19, 21 | 1-7, (10), 13, 15, (18), 22-37 |
| K562/cE (loop4,6)D | 223, 226, 233 350, 353, 354 | loop4 loop6 | (5), 6, (12), (15), (17), (23), 30, (36) | 1, 3, (5), 7-11, (12), 13, (15), (17), 18-22, (23), 32, 33, 34, 35, (36), 37 |
| K562/cE (loop3,4,6) D | 169, 170, 172 223, 226, 233 350, 353, 354 | loop3 loop4 loop6 | 3-21, 23, 30, 34, 36, 37 | 1, 22, 32, 34 |
| K562/cE (loop 6)D | 350, 353, 354 | (5), (23) | 1-4, (5), 6-22, (23), 24-37 |
The table summarizes the reaction profiles of partial D phenotype red blood cells and the K562 lines described in this report using the 37 epitope model.14 Some antibodies of 1 epitope cluster fail to react with one partial D or K562 clone, whereas others react. These epitope clusters are indicated by parentheses.
Membrane topology of some variant D proteins associated with partial D phenotypes. The serological reactivity of the mutant K562 cell lines was compared with that of some naturally occurring partial D phenotype red cells. These phenotypes are predominantly associated with hybrid Rh proteins. The predicted structures of these hybrids are depicted: RHCE-derived amino acids are shown as open circles, whereas RHD-derived amino acids as shown as solid circles. The molecular bases of the different partial D phenotypes shown have been described previously: DIVa; DIVb and DFR24; DBT20; RoHar19; and cDVIE.17
Membrane topology of some variant D proteins associated with partial D phenotypes. The serological reactivity of the mutant K562 cell lines was compared with that of some naturally occurring partial D phenotype red cells. These phenotypes are predominantly associated with hybrid Rh proteins. The predicted structures of these hybrids are depicted: RHCE-derived amino acids are shown as open circles, whereas RHD-derived amino acids as shown as solid circles. The molecular bases of the different partial D phenotypes shown have been described previously: DIVa; DIVb and DFR24; DBT20; RoHar19; and cDVIE.17
Flow cytometry using monoclonal anti-c and anti-E.
Because the initial cDNA used to generate the mutant constructs encodes Rh c and E specificities,5,6,11 15 the effects of incorporating Rh D-specific residues in various combinations on c and E antigen expression was investigated by flow cytometry (Table 3). K562 clones transfected with Rh cE and D cDNAs were used as positive and negative controls, respectively. Some of the K562 lines exhibit partial c and E phenotypes, indicating that c and E antigenicity can be disrupted by incorporating D-specific amino acids.
Flow Cytometric Analysis of 7 Different Rh SDM Mutant K562 Lines Using Monoclonal Anti-c and E
| Anti-c/Anti-E . | K562/D . | K562/cE . | K562/lp3 . | K562/lp4 . | K562/lp3 + 6 . | K562/lp4 + 6 . | K562/lp3 + 4 . | K562/lp3 + 4 + 6 . | K562/lp6 . |
|---|---|---|---|---|---|---|---|---|---|
| Anti-c MS33 | 8.7 | 39.9 | 32.9 | 43.1 | 37.3 | 31.3 | 11.7 | 17.7 | 39.9 |
| Anti-c BS240 | 3.6 | 11.2 | 14.3 | 18.4 | 3.8 | 3.0 | 3.1 | 3.8 | 6.0 |
| Anti-c MS45 | 10.6 | 42.1 | 30.1 | 19.3 | 54.8 | 30.0 | 34.7 | 45.3 | 20.7 |
| Anti-c 951 | 8.4 | 16.1 | 5.2 | 6.8 | 7.3 | 5.3 | 3.3 | 3.7 | 10.3 |
| Anti-c MS40 | 4.4 | 10.0 | 3.7 | 3.3 | 4.9 | 4.6 | 3.5 | 3.7 | 5.3 |
| Anti-c MS51 | 9.1 | 42.1 | 27.8 | 34.7 | 30.8 | 20.7 | 10.8 | 14.1 | 24.4 |
| Anti-c MS37 | 6.4 | 30.0 | 16.0 | 32.7 | 52.0 | 24.1 | 10.6 | 11.2 | 19.2 |
| Anti-E 906 | 2.9 | 10.9 | 8.7 | 4.1 | 2.3 | 2.8 | 2.7 | 2.9 | 12.7 |
| Anti-E H98 | 3.9 | 26.0 | 2.8 | 2.8 | 2.5 | 2.9 | 2.8 | 3.0 | 2.7 |
| Anti-E H4-1 | 3.6 | 32.0 | 15.7 | 27.4 | 3.2 | 3.2 | 3.3 | 3.3 | 47.2 |
| Anti-E HIRO-18 | 3.6 | 50.1 | 22.5 | 40.8 | 3.6 | 4.3 | 3.6 | 4.2 | 41.1 |
| Anti-c/Anti-E . | K562/D . | K562/cE . | K562/lp3 . | K562/lp4 . | K562/lp3 + 6 . | K562/lp4 + 6 . | K562/lp3 + 4 . | K562/lp3 + 4 + 6 . | K562/lp6 . |
|---|---|---|---|---|---|---|---|---|---|
| Anti-c MS33 | 8.7 | 39.9 | 32.9 | 43.1 | 37.3 | 31.3 | 11.7 | 17.7 | 39.9 |
| Anti-c BS240 | 3.6 | 11.2 | 14.3 | 18.4 | 3.8 | 3.0 | 3.1 | 3.8 | 6.0 |
| Anti-c MS45 | 10.6 | 42.1 | 30.1 | 19.3 | 54.8 | 30.0 | 34.7 | 45.3 | 20.7 |
| Anti-c 951 | 8.4 | 16.1 | 5.2 | 6.8 | 7.3 | 5.3 | 3.3 | 3.7 | 10.3 |
| Anti-c MS40 | 4.4 | 10.0 | 3.7 | 3.3 | 4.9 | 4.6 | 3.5 | 3.7 | 5.3 |
| Anti-c MS51 | 9.1 | 42.1 | 27.8 | 34.7 | 30.8 | 20.7 | 10.8 | 14.1 | 24.4 |
| Anti-c MS37 | 6.4 | 30.0 | 16.0 | 32.7 | 52.0 | 24.1 | 10.6 | 11.2 | 19.2 |
| Anti-E 906 | 2.9 | 10.9 | 8.7 | 4.1 | 2.3 | 2.8 | 2.7 | 2.9 | 12.7 |
| Anti-E H98 | 3.9 | 26.0 | 2.8 | 2.8 | 2.5 | 2.9 | 2.8 | 3.0 | 2.7 |
| Anti-E H4-1 | 3.6 | 32.0 | 15.7 | 27.4 | 3.2 | 3.2 | 3.3 | 3.3 | 47.2 |
| Anti-E HIRO-18 | 3.6 | 50.1 | 22.5 | 40.8 | 3.6 | 4.3 | 3.6 | 4.2 | 41.1 |
Values in the table are expressed as the mean fluorescence intensity. Values twice that of background antibody binding (ie, that observed binding to a negative control K562 line) are considered positive and are shaded gray and boldfaced. The results indicate that some K562 SDM mutant clones exhibit partial c phenotypes in that they bind some anti-c but not all.
DISCUSSION
The intention of this study was to exploit the recently described successes9 11 of in vitro Rh antigen expression using retroviral transduction of K562 cells to study precisely the molecular requirements for D, c, and E antigenicity. Previously, this has only been possible by studying naturally occurring Rh variants, which are difficult to obtain and do not allow specific targeting of predicted epitope-critical amino acids. This study has initially analyzed groups of 3 amino acids located at external positions on the Rh D protein. We have also used these investigations to predict a model of topography of the external loop-loop interactions of the Rh D protein (and thus Rh CcEe protein); this model will be useful until high-resolution crystal structures of Rh protein complexes become available, an exercise that is likely to be technically difficult.
The definition of regions of the Rh D protein complex involved in D epitope presentation has been analyzed directly in these studies using SDM methodology. SDM provides a powerful means with which to investigate epitope-paratope interactions; predictions made by SDM have been proved extremely accurate after subsequent analysis by crystallography when investigating the Hen-egg lysozyme (HEL)-antibody complex,16 human growth hormone receptor complex, andEscherichia coli histidine-containing phosphocarrier protein immune complex.17 Our studies were designed to address several important questions regarding the nature of the human D antigen, including the number and identities of externally facing D-specific amino acids, and their potential interactions as various D epitopes when located on spatially different loop domains of the polytopic Rh D protein monomer. To achieve this objective, we have designed a series of constructs initially starting with an Rh cE cDNA and altering amino acids predicted to be exofacial and to lie on extracellular loops 3, 4, and 6 of the protein (Fig 1). By retroviral transduction of K562 erythroleukemia cells with these mutant constructs, we were then able to analyze the effects of the introduction of Rh D-specific amino acid combinations on essentially an Rh cE polypeptide backbone. Our results clearly demonstrate that 9 amino acids predicted to lie in extracellular positions are directly involved in D epitope expression, and our data provide the most accurate assessment to date as to D epitope localization on the D polypeptide. Furthermore, our evidence directly refutes the recent hypothesis of Chang and Siegel10 that all D epitopes are overlapping, and encompass the entire externalized region of the molecule. We consider that the D antigen is composed of distinct local epitope clusters, some of which are indeed overlapping, but predominantly are located on the RHD-specific loops 3, 4, and 6 of the protein. We consider each epitope cluster in turn and rationalize which Rh D-specific amino acids are critical for antibody binding, based on the data presented here and comparison to the molecular bases of known partial D phenotypes.
Loop 6 clusters.
Our findings here confirm our earlier study that concluded thatRHD amino acids 350 (His), 353 (Gly), and 354 (Ala), located on the sixth external loop of the D protein, are essential for epD3 (5 in 37 epitope model) and 9 (23 in 37 epitope model) expression.9,18 Therefore, in this instance, no other D-specific amino acids are required for D epitope expression, strongly suggesting that the anti-D that recognize these epitopes do not bind to any other external domain. However, the previous study found that not all epD3 and epD9 could be regenerated, indicating that other D-specific amino acids may be necessary for correct epitope presentation. In this study, we have regenerated all epD3 and 9 tested (with the exception of the epitope recognized by monoclonal anti-D BS231, which showed only marginal expression). In some cases, expression was shown on cE(loop4,6)D K562 lines, but the majority were detected on the cE(loop3,4,6)D K562 lines (Table 1). This finding was surprising, because we had anticipated, based on the molecular structure of hybrid Rh proteins found in DVIphenotypes,19-21 that all of these epitopes were located on the sixth loop of the wild-type Rh D protein. This structure is the only D-specific domain found on all DVI proteins; thus, it has been thought that epD3,5 4,6 and 923 reside on this loop.7 8 Our data suggest that the incorporation of D-like amino acids on loops 4 and 3 of the molecule alter the conformation, probably by altering transmembrane side chain interactions on the mutant proteins. This may result in the stabilization and subsequent expression of all epD3,4, and 9 (5, 6, and 23 in 37-epitope model) on the mutant Rh proteins expressed by the K562 lines. It is highly improbable that the D-like residues located on loops 3 and 4 of these mutants play any direct part in antibody binding, because these residues are not present on DVIhybrid proteins, and DVI red blood cells express epD3, 4, and 9 (5, 6, and 23 in 37-epitope model).
Loops 3 and 4 cluster and loops 3, 4, and 6 cluster.
Most antibodies known to be of the major clinically significant epD6/7 (12 through 21 in the 37-epitope model) and also the epD5 (7 through 11 in the 37-epitope model) epitope clusters were found to react with the cE(loop3,4)D, cE(loop4,6)D, and cE(loop3,4,6)D K562 mutant cell lines. On considering the minimal requirements for some epD6/7, it would appear that just 6 amino acids (Met169, Met170, and Ile172 from loop 3; and Val223, Ala226, and Glu233 from loop 4) are required for expression, because some antibodies react with the cE(loop3,4)D line (eg, T3A2F6, HM10, MAD-2, and HIRO-2; see Table 1). In this instance, loop 6 D-specific residues are not involved in antibody binding, and it is unlikely that this loop is involved in these epitopes at all. Some anti-epD6/7 (eg, D90/7, D10, and L871G7; see Table 1) were found to bind only to the cE(loop3,4,6) K562 line, which may either suggest that all 3 D-specific loops 3, 4, and 6 are required for antibody binding or that the introduction of D-like residues on the sixth external domain altered the conformation of the mutant protein so that antibody binding could be achieved to an epitope located on the third and fourth loops of the protein.
Loop 3 or 4 cluster.
Interestingly, no D epitopes were regenerated on the cE(loop3)D and cE(loop4)D K562 lines. The hybrid Rh proteins associated with the DBT and RoHar phenotypes express the amino acids that are derived from the third loop (DBT) and fourth loop (RoHar)22 23 (Fig 3). The red blood cells of individuals with these variant phenotypes express a unique pattern of D epitopes, but, paradoxically, some epitopes are found on both DBT and RoHar phenotype red blood cells. Two antibodies that recognize this epitope (epD17) were found to react with RoHar (Rh D-like loop 4) and DBT (Rh D-like loop 3) hybrid proteins (see Fig 3) and the cE(loop3,4)D K562 line, but not the cE(loop3)D and cE(loop4)D K562 lines. This suggests that these 2 antibodies have the potential to bind to either loop 3 or loop 4 of the wild-type Rh D protein. The inability of these antibodies to react with the cE(loop3)D and cE(loop4)D K562 lines may be explained by the fact that transmembrane and/or cytoplasmic-localized amino acids play a role in the conformation of these epitopes. The amino acids required to induce such a conformational change may be present on the RoHar and DBT hybrid Rh proteins, but absent from the cE(loop3)D and cE(loop4)D mutant proteins.
Loops 1, 2, 3, and 5 cluster.
The external regions of the Rh D polypeptide that share complete sequence identity with the Rh CcEe proteins play only a minor role in D antigenicity (ie, loops 1, 2, and 5).
We predict that the paratope of monoclonal anti-D with epD8 specificity (epD22, 37-epitope model) includes loops 1, 2, 3, and 5 of the D protein. EpD8 is deficient from DVII cells (altered second loop, L110P24), DMH cells (altered first loop, L54P25), HMi cells (altered fifth loop, I283T26), and DFR and DOL cells25 27 (altered third loop). Because none of our mutant lines expressed epD8, we concluded that the presence of P103 and not S103, as found on the wild-type protein, was responsible for the lack of its expression.
D epitopes are likely to be localized and do not encompass the entire external region of the D protein.
A recent report by Chang and Siegel10 argued that the conventional model for D epitopes, proposed by many serologists over a number of years, might not be correct. Curiously, their hypothesis was based on the high degree of sequence similarity of anti-D Fvs and not on analysis of the molecular nature of the D antigen. Direct expression of D epitopes on K562 cells described by our work here strongly indicates that previous epitope prediction models were reasonably accurate. It must also be stressed that the approach adopted in the work described within this report results in the creation of D epitopes, rather than observing the deficiency of D epitopes from partial D phenotype red blood cells. Our previous SDM study9 used a cDNA clone (called Dmut) that mimicked a partial D phenotype red blood cell (DIVb).27 The intention of this experiment was to assess how similar the serological reactivity of K562 cells was to DIVb red blood cells and not to explore directly epitope deficiencies; this had already been performed in the natural variant. The disruption of D epitope expression (as seen on naturally occurring mutants) is not a good indicator of the role of a specific amino acid, because the changes undoubtedly introduce local disruptions in structure with undefined effects of D protein orientation.28 It is clear that the reactivity of some mutant lines indicate that certain regions of the Rh D protein are not important for epitope expression: loops 3 and 4 are not required for binding to cE(loop6)D line and loop 6 is not required for binding to cE(loop3,4)D lines, for example. The fact that nonpolymorphic loops of the Rh D protein play a relatively minor role in antigenicity (as judged by the reactivity of naturally occurring mutants; see above) would suggest that other regions that share sequence homology with the Rh CcEe proteins have little role (if any) in antibody binding. Mutagenesis may now directly address this issue.
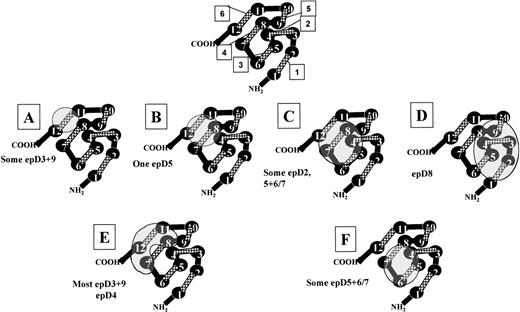
Predicted loop-loop interactions of the Rh D protein monomer and possible locations of the D epitope clusters.
We have considered our data in the context of how different anti-D bind D epitopes with respect to their predicted positions on the Rh D protein. Before this was attempted, we considered some important physical characteristics of both anti-D Ig and the Rh D protein. Many crystal structures of Igs have been defined, and the area of paratope (ie, residues from the 6 different light and heavy chain complementary determining regions [CDRs]) interaction involves between 15 and 20 amino acids, which corresponds to a binding site of approximately 15 to 20 Å in diameter, which is considered binding to a structural epitope.29 On consideration of the extent of binding to the Rh D protein an anti-D paratope may have, we considered the best structurally characterized human erythrocyte membrane protein, aquaporin 1.30,31 The structure of this integral membrane protein has been defined by 2-dimensional crystallography and is composed of 6 α helical membrane spans that are arranged in an annular configuration surrounding an aqueous vestibule. The distance between each externally facing loop domain (of which there are 3) is between 20 and 30 Å, which is almost identical to the maximum binding diameter of an Ig paratope. Although it is difficult to estimate the distance between each of the 6 Rh D external facing loops (Rh proteins may not be porins), it is likely that 30 Å may encompass no more than 3 such loops. It is thus this physical constraint on which we proposed the relative positions of the D protein external loops and the epitopes they present (Fig 4). The Rh protein monomer (416 amino acids, 6 external domains) is likely to exceed 50 Å in diameter (the aquaporin 1 monomer [269 amino acids, 3 external domains] is 31 to 34 Å in diameter); thus, it is a physical improbability for the entire external surface of the D protein to encompass 1 single binding site, as suggested previously.10Our assumption that the Rh D protein monomer exceeds 50 Å in diameter is further substantiated by the 3-dimensional prediction of the structure of the dimeric AE-1 (band 3) membrane domain, which has dimensions of 60 × 110 Å and has a thickness of 80 Å.32 Each monomeric membrane domain comprises approximately 500 amino acids, which is similar to the membrane domains of Rh D (∼400).
Predicted localization of different D epitope clusters and hypothetical model of loop-loop interactions of the Rh D polypeptide. Based on the serological reaction of the different mutant K562 lines, a model for the loop-loop interactions that govern D epitope expression is presented. The model is a plan view of an Rh D protein monomer in which each membrane-spanning segment is represented by a circle (numbered 1 through 12) and each externalized loop as a cross-hatched line (numbered 1 through 6). Cytoplasmic-localized loops are represented as black lines. Each external loop is positioned such that adjacent membrane-spanning segments may interact, altering the confirmation and expression of D epitopes (see Discussion). The model considers that each epitope cluster is no more than 25 Å in diameter, based on the fact that an antibody paratope is of this dimension. It could be anticipated that the diameter of an Rh D protein monomer is greater than 50 Å if compared with the determined dimensions of 2 other erythrocyte membrane proteins aquaporin 130,31 and the anion transport protein.32 The locations of the predicted 6 Rh D epitope clusters are indicated by letters A through F, and the D epitopes expressed by each cluster (1-9 model) is indicated by each panel. (A) Loop 6-dependent epitopes require only anRHD loop 6 for antibody binding. (B) Loop 4+6-dependent epitopes require amino acids located on both these loops. (C) Loop 3, 4, and 6-dependent epitopes have contact points with all 3 of theseRHD-specific loops. (D) Loop 1, 2, 3, and 5-dependent epitopes have a relatively large contact area and are not expressed on partial D proteins with mutations in the Rh CcEe/D common loops 1, 2, and 5 (DMH, DVII, and DHMi, respectively). Contact with loop 3RHD-specific residues gives the antibodies anti-D specificity, because partial D phenotypes with mutations in this loop lack epD8 (DFR and DOL). (E) Loop 6-dependent epitopes. These epitopes requireRHD-specific cytoplasmic/transmembrane residues to stabilize their configuration. (F) Loop 3+4- and loop 3 or 4-dependent epitopes. This group includes antibodies that require RHDresidues on both loops 3 and 4 and also those antibodies that can bind to both RoHar and DBT red blood cells.
Predicted localization of different D epitope clusters and hypothetical model of loop-loop interactions of the Rh D polypeptide. Based on the serological reaction of the different mutant K562 lines, a model for the loop-loop interactions that govern D epitope expression is presented. The model is a plan view of an Rh D protein monomer in which each membrane-spanning segment is represented by a circle (numbered 1 through 12) and each externalized loop as a cross-hatched line (numbered 1 through 6). Cytoplasmic-localized loops are represented as black lines. Each external loop is positioned such that adjacent membrane-spanning segments may interact, altering the confirmation and expression of D epitopes (see Discussion). The model considers that each epitope cluster is no more than 25 Å in diameter, based on the fact that an antibody paratope is of this dimension. It could be anticipated that the diameter of an Rh D protein monomer is greater than 50 Å if compared with the determined dimensions of 2 other erythrocyte membrane proteins aquaporin 130,31 and the anion transport protein.32 The locations of the predicted 6 Rh D epitope clusters are indicated by letters A through F, and the D epitopes expressed by each cluster (1-9 model) is indicated by each panel. (A) Loop 6-dependent epitopes require only anRHD loop 6 for antibody binding. (B) Loop 4+6-dependent epitopes require amino acids located on both these loops. (C) Loop 3, 4, and 6-dependent epitopes have contact points with all 3 of theseRHD-specific loops. (D) Loop 1, 2, 3, and 5-dependent epitopes have a relatively large contact area and are not expressed on partial D proteins with mutations in the Rh CcEe/D common loops 1, 2, and 5 (DMH, DVII, and DHMi, respectively). Contact with loop 3RHD-specific residues gives the antibodies anti-D specificity, because partial D phenotypes with mutations in this loop lack epD8 (DFR and DOL). (E) Loop 6-dependent epitopes. These epitopes requireRHD-specific cytoplasmic/transmembrane residues to stabilize their configuration. (F) Loop 3+4- and loop 3 or 4-dependent epitopes. This group includes antibodies that require RHDresidues on both loops 3 and 4 and also those antibodies that can bind to both RoHar and DBT red blood cells.
From our data, we have proposed a possible configuration of the Rh D protein that considers the spatial arrangements of the different exofacial loops. The model argues for a close spatial relationship between loops 3, 4, and 6 of the protein and argues that loops 3 and 6 are nonadjacent [no D epitopes were found to react with cE(loop3,6)D K562 lines as a minimal requirement]. Loops 1, 2, 3, and 5 are considered to comprise the location of epD8 (epD22 using 37-epitope model). It is also possible that side chain interaction in membrane domains affect c epitope expression in mutants that have alterations to loop 4 (see next section and Table 3); we assume that loops 2 and 4 of the Rh CcEe protein and their associated membrane domains are close. Our model of Rh protein topography is depicted in Fig 4, which also speculates on the positions of the 6 D epitope clusters. The model is consistent with each epitope cluster having an approximate diameter of 20 Å.
Clearly, at present, it is impossible to speculate on the magnitude and precise involvement of amino acids on the Rh D protein that are directly involved in the binding of anti-D. This assessment can only accurately be achieved by x-ray crystallography of Rh D protein-antibody complexes, which will be extremely difficult to obtain. Many anti-D sequences have been defined, and in some instances the interactions of their paratopes with corresponding epitopes have been speculated upon.10 The prediction of epitope configuration and/or interactions of the 6 Ig CDRs based on antibody sequences alone is likely to be unproductive. For example, the interactions of 2 different MoAbs against the influenza virus neuraminidase have been studied by x-ray crystallography.33 34 One antibody, NC41, contacts the N9 neuraminidase with 5 of 6 CDRs. However, another antibody (NC10) contacts whale N9 neuraminidase with only 4 of its 6 CDRs (H1 and L2 do not make contact). The H1 CDR, although it is of identical sequence in both antibodies and occupies a similar position during antigen-antibody binding, does not make contact with the whale neuraminidase. By analogy, the framework regions and perhaps many of the CDRs of anti-Rh antibodies may be of similar structures (and thus primary amino acid sequence) to enable binding to the Rh D or Rh CcEe protein complexes, which are likely to have similar overall conformation. However, only very small differences between the CDR sequences may be sufficient to invoke interaction of Ig with the Rh D protein and not with the Rh CcEe protein. What is clear from our studies is that we have defined D-epitope critical amino acids that have served to localize sites of anti-D binding. These residues are therefore those that are either directly involved in antibody binding or, as we have suggested, may induce structural alteration in the D protein complex to allow binding to occur.
Requirements for c and E antigenicity.
Because the initial cDNA used in mutagenesis has the potential to express c and E antigens, we assessed anti-c and anti-E binding to the mutant lines. From our data (Table 2), it is clear that the expression of RHD residues on a cE polypeptide disrupts c and E antigenicity. Some of the mutant K562 lines express partial c and E phenotypes and indicate that there are indeed different amino acid requirements for antibody binding between different anti-c, with some binding to all cell lines (MS45), whereas others bind to only one [cE(loop6)D; MoAb 951]. One binds to none of the mutants, but binds normally to the control K562/cE line (MS40). These lines therefore exhibit novel examples of partial c phenotypes. Naturally occurring partial E phenotypes have recently been defined at the molecular level35; and different E epitopes have been proposed. Of the 4 different anti-E tested, 3 different reaction patterns can be distinguished (Table 3). Our findings suggest that loops 2 and 4 of the Rh CcEe protein (and/or surrounding membrane spanning domains) may be structurally quite close (ie, within 20 Å of each other). This would explain the existence of compound Rh CE antigens [eg, Rh f (RH6; ce), Rh Ce (RH7), Rh CE (RH22), and Rh cE (RH27)].
Concluding remarks.
In these studies, SDM has been applied to study one of the most clinically significant antigens in transfusion medicine. Our findings strongly suggest that there are at least 6 different groups of epitopes (clusters) on the surface of the Rh D protein. Which of these promote highest immunogenicity is open to debate, but those absent from DVI individuals appear to be the most clinically significant, because DVI phenotype mothers have made anti-D to their D-positive babies with sometimes fatal consequences. Therefore, for this reason, we suggest that epitope clusters C and F (involving the third and fourth loops of the Rh D protein; Fig 4) are likely to be the most immunogenic that arise from the Rh D protein. It is hoped that, using technology explored in these studies, more direct answers concerning the different manner in which certain individuals respond to the D antigen can be addressed. Such information may be useful in designing different therapeutic anti-D or defining diagnostic tests to highlight high-risk pregnancies in which hemolytic disease is likely to occur. A recent report has confirmed our earlier study that epD3 and epD9 (5 and 23 in the 37-epitope model) have a critical requirement for Rh D amino acids 350, 353, and 354.36 These investigators suggest that their K562 cells have a relatively high endogenous expression of D epitopes and that our previous demonstration of low-level D expression on our K562 line was due to suboptimal flow cytometry. Our data presented here clearly show that this is not the case; Table 1 (see columns showing FL1 values for anti-D binding to K562/pBabe and K562/cE clones compared with K562/D and mutant K562 clones) shows that endogenous D epitope expression on our K562 line is low. We suggest, therefore, that there is a difference in the Bristol and New York K562 lines in terms of the levels of endogenous D expression and that our line is more favorable to analyze the effects of D epitope expression using SDM.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal