Abstract
Solvent/detergent (S/D)-treated plasma is currently marketed by the American Red Cross as a virally inactivated alternative to fresh-frozen plasma (FFP). The serpin-type serine proteinase inhibitors have a flexible reactive site loop (RSL) that can convert from the active conformation to the inactive latent or polymerized conformations when exposed to heat and/or detergents. We have compared the conformational stability and inhibitory activity of 3 plasma serpins—antithrombin, antitrypsin, and antiplasmin—in S/D plasma and FFP. In S/D plasma, virtually 100% of the antiplasmin and approximately 50% of the antitrypsin are in either the latent or polymerized conformation and lack inhibitory activity, while in FFP only the active conformation is present. Interestingly, antithrombin is not affected by S/D treatment and remains fully active. These data demonstrate that S/D plasma is not simply a virally inactivated equivalent of FFP. The lack of antiplasmin activity and decreased antitrypsin activity in S/D plasma suggest that it may not be as effective as FFP for the treatment of bleeding in patients with systemic activation of proteolytic cascades, such as disseminated intravascular coagulation and sepsis, acquired fibrinolytic states, and large-volume transfusion. Although there has been extensive use of S/D plasma in several European countries with no reports of adverse effects, clinical studies directly comparing the efficacy of these 2 plasma products are needed to directly evaluate the relative therapeutic efficacy of FFP and S/D plasma for the treatment of these diseases.
MEMBERS OF THE SERPIN superfamily of serine proteinase inhibitors act to control major plasma proteolytic cascades, including blood clotting (antithrombin and heparin cofactor II), fibrinolysis (antiplasmin and plasminogen activator inhibitor-1 [PAI-1]), inflammation (antitrypsin and antichymotrypsin), and complement activation (C1-inhibitor). A key structural feature of the serpins is the reactive site loop (RSL) that connects the “A” and “C” β-sheets.1 The RSL can adopt several different conformations by flexing in and out of the center of the “A” β-sheet. In the active conformation, most of the RSL is exposed to surrounding solvent, accessible for binding to the active site of a target proteinase with which it forms a tight equimolar complex.1-3 In the latent conformation, the RSL is buried as the center strand of the “A” β-sheet and the serpin is inactive.4 In the polymerized conformation, the RSL of one molecule inserts intermolecularly into the “A” β-sheet of another, resulting in the formation of noncovalent, loop-sheet polymers that lack inhibitory activity.2 The latent and polymerized conformations are thermodynamically stabilized and do not readily convert back to the active conformation.2,5 6
In May 1998, the United States Food and Drug Administration approved solvent/detergent (S/D)-treated plasma as a virally inactivated alternative to fresh-frozen plasma (FFP) for the replacement of coagulation factors in deficiencies, the reversal of warfarin effect, and the treatment of thrombotic thrombocytopenic purpura. S/D plasma is prepared from pooled FFP by heating at 31°C for 4 hours in the presence of 1% tri(n-butyl)phosphate (TNBP) and 1% Triton-X-100 (Rohm and Haas Co, Philadelphia, PA). The TNBP and Triton-X-100 are then removed by vegetable oil extraction and C-18 reverse-phase chromatography. The final product is sterilized by filtration through a 0.2-μm filter.7 S/D plasma has been shown to effectively replace clotting factors in deficient patients.8-11 However, reports indicating that the inhibitory activity of antiplasmin in S/D plasma is reduced to approximately half of that present in FFP12,13 suggest that it may have adopted either the latent or polymerized conformation during the viral inactivation process. This hypothesis is supported by studies of the effect of detergents on purified serpins, which have demonstrated that heat-induced polymerization of antitrypsin is greatly enhanced by 0.025% Nonidet P-40,2 that antithrombin polymerizes in the presence of 0.6% deoxycholate,14 and that PAI-1 can be converted from the active to the latent conformation by incubation with trace amounts of Triton-X-100 or sodium dodecyl sulfate (SDS).15
To identify and characterize conformational changes in serpins induced by S/D treatment of plasma, we measured the conformational stability and inhibitory activity of 3 serpins—antithrombin, antitrypsin, and antiplasmin—in FFP and S/D plasma. The results indicate that S/D treatment of plasma causes a significant degree of conformational change and inactivation of antiplasmin and antitrypsin, but not antithrombin.
MATERIALS AND METHODS
Reagents.
The chromogenic substrate for porcine pancreatic elastase (PPE) (N-succinyl-Ala-Ala-Pro-Val-p-nitroanilide), methylamine, Triton-X-100, and TNBP were obtained from Sigma Chemical, St Louis, MO. The plasmin chromogenic substrate (D-But-CHT-Lys-pNA.2AcOH) was obtained from Biopool, Burlington, Canada.
Proteins.
PPE, purified antitrypsin, bovine serum albumin (A-6793), polyclonal antibodies directed against human antitrypsin, human antithrombin, human fibronectin, and goat antirabbit or antimouse IgG linked to horseradish peroxidase were obtained from Sigma. Polyclonal antibody directed against human antiplasmin was purchased from Enzyme Research, South Bend, IN. Plasmin was obtained from Biopool. The monoclonal 2H8 antibody directed against tissue factor pathway inhibitor was a gift of Dr George Broze, Washington University, St Louis, MO. Purified antiplasmin was a gift of Dr Jan Enghild, Duke University. Normal pooled plasma was obtained from George King Biomedical, Overland Park, KS. FFP was obtained from the Memphis Veterans Affairs Hospital Blood Bank. S/D plasma was purchased from the American Red Cross, Washington, DC. The FFP and S/D plasma were thawed at 37°C, aliquoted, and refrozen at −20°C. A new aliquot was thawed for each experiment and then discarded. Thus, in most experiments, both the FFP and the S/D plasma were subjected to 1 additional freeze/thaw cycle over that of a product that is transfused into a patient.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were analyzed using 7.5% gels cast in a Tris/glycine buffer system described by Laemmli.16 All samples were boiled for 3 minutes in the presence of 50 mmol/L dithiothreitol and 1% SDS before electrophoresis.
Nondenaturing-PAGE.
Proteins were analyzed using the same gel and buffer system described for SDS-PAGE except that SDS was omitted from all buffers and the samples were not boiled or treated with dithiothreitol.
Transverse urea gradient-PAGE (TUG-PAGE).
Batches of 10 × 9 × 0.15-cm 7% polyacrylamide gels containing a continuous linear 0 to 8 mol/L urea gradient were cast in the Tris/glycine buffer system described for nondenaturing-PAGE.17 18 The gel was rotated 90 degrees, and the sample containing 5 μL plasma in a 150-μL total volume was loaded evenly across the top of the gel. Electrophoresis was performed at 23°C with a constant current of 15 mA for 2 hours.
Western blot analysis.
Plasma proteins were separated by gel electrophoresis, blotted onto nitrocellulose (Schleicher & Schuell, Keene, NH), and immunostained with appropriate primary and secondary antibodies. All proteins were visualized using ECL Western blotting detection reagents (Amersham, Buckinghamshire, UK).
Laboratory preparation of S/D plasma.
FFP was incubated for 4 hours at 31°C in the presence of 1% Triton-X-100 and 1% TNBP. The soy bean oil extraction, C-18 chromatography, and sterile filtration steps used during commercial preparation of S/D plasma were not performed.
Collection of insoluble aggregate in S/D plasma.
In an aliquot of S/D plasma containing insoluble aggregates after thawing at 37°C, the aggregates were removed with tweezers and transferred to a separate tube. The aggregates were washed 3 times with phosphate-buffered saline and homogenized in 3x gel sample loading buffer without SDS using a borosillicate glass pipette that had the tip blunted by holding over a flame. The homogenized aggregates and the remaining portion of the aliquot were then analyzed by nondenaturing-PAGE.
Antithrombin activity assay.
Antithrombin activity in S/D plasma was measured as a routine clinical sample in the Baptist Memorial Hospital Clinical Coagulation Laboratory, Memphis, TN using a Diagnostica Stago (Parsippany, NJ) STA analyzer and reagents.
Progress curve amidolytic assays.
The plasmin and PPE inhibitory activities of S/D plasma and normal pooled plasma were measured by mixing various dilutions of plasma with 500 μmol/L appropriate substrate in 50 mmol/L Tris-HCl pH 7.5, 100 mmol/L NaCl, 0.1% bovine serum albumin. The reaction was initiated by the addition of either 0.4 μmol/L PPE or 0.2 μmol/L plasmin and the formation of the colored reaction product was measured at A405 at 20- or 30-second intervals at 23°C. In PPE inhibition assays, the plasma samples were treated with 0.2 mol/L methylamine at 23°C for 4 hours before assay to specifically inactivate α2-macroglobulin.19 In plasmin inhibition assays, the plasma samples were diluted into the sample dilution buffer provided with the Biopool Spectrolyse α2-antiplasmin Chromogenic Assay Kit as directed by the manufacturer. This buffer contains methylamine to minimize interference by α2-macroglobulin.
RESULTS
Nondenaturing and SDS-PAGE analysis of antithrombin and antitrypsin in FFP and S/D plasma.
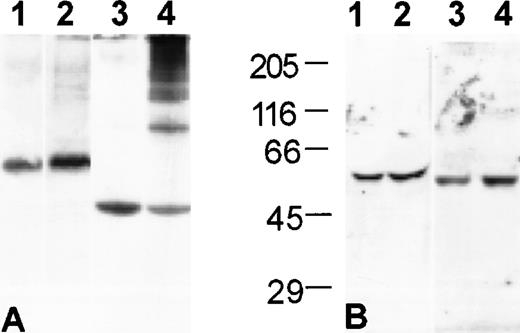
When S/D plasma is analyzed under nondenaturing conditions, a small portion (<5%) of the antithrombin and a large portion (>50%) of the antitrypsin migrate as a series of bands of decreasing mobility that are not present in FFP (Fig 1A). The presence of the high-molecular-weight bands is consistent with formation of noncovalent, loop-sheet polymers during the production of S/D plasma. The polymers are not present in Western blots following denaturation in SDS, confirming that they are noncovalently assembled (Fig 1B).
Noncovalent polymers of antitrypsin and, to a much lesser extent, antithrombin, are present in S/D plasma. Western blot analysis of plasma proteins. (A) Nondenaturing-PAGE and (B) SDS-PAGE with molecular weight standards as indicated: (1) antithrombin in FFP; (2) antithrombin in S/D plasma; (3) antitrypsin in FFP; (4) antitrypsin in S/D plasma.
Noncovalent polymers of antitrypsin and, to a much lesser extent, antithrombin, are present in S/D plasma. Western blot analysis of plasma proteins. (A) Nondenaturing-PAGE and (B) SDS-PAGE with molecular weight standards as indicated: (1) antithrombin in FFP; (2) antithrombin in S/D plasma; (3) antitrypsin in FFP; (4) antitrypsin in S/D plasma.
Nondenaturing and SDS-PAGE analysis of antiplasmin in FFP and S/D plasma.
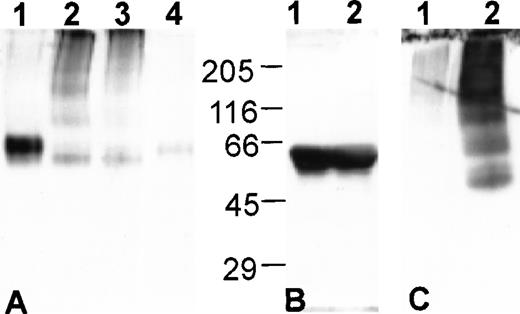
When analyzed by nondenaturing-PAGE, antiplasmin in S/D plasma migrates as a series of bands of decreasing mobility that are not present in FFP (Fig 2A), consistent with the formation of loop-sheet polymers as observed for antitrypsin and antithrombin. In addition to the polymers, a band migrating slightly faster than the active antiplasmin in FFP is present (compare lanes 3 and 4, Fig 2A). When analyzed by SDS-PAGE, antiplasmin from S/D plasma migrates as a single band of molecular weight identical to antiplasmin in FFP (Fig2B). This suggests that the rapidly migrating band seen in S/D plasma under nondenaturing conditions is not a partially degraded form of antiplasmin. Instead, it is most likely the latent conformation of antiplasmin which has a more compact structure than the active conformation and, therefore, has increased electrophoretic mobility in nondenaturing-PAGE.
The latent and polymerized conformations of antiplasmin are present in S/D plasma. Western blot analysis of plasma proteins. (A) Nondenaturing-PAGE: (1) antiplasmin in FFP; (2) antiplasmin in FFP following S/D treatment in our laboratory (see Materials and Methods); (3) antiplasmin in S/D plasma; (4) antiplasmin in FFP diluted 10-fold more than lane 1. (B) SDS-PAGE with molecular weight standards as indicated: (1) antiplasmin in FFP; (2) antiplasmin in S/D plasma. (C) Nondenaturing-PAGE of S/D plasma containing aggregates: (1) antiplasmin in S/D plasma after removal of aggregates; (2) antiplasmin in aggregates isolated as described in Materials and Methods.
The latent and polymerized conformations of antiplasmin are present in S/D plasma. Western blot analysis of plasma proteins. (A) Nondenaturing-PAGE: (1) antiplasmin in FFP; (2) antiplasmin in FFP following S/D treatment in our laboratory (see Materials and Methods); (3) antiplasmin in S/D plasma; (4) antiplasmin in FFP diluted 10-fold more than lane 1. (B) SDS-PAGE with molecular weight standards as indicated: (1) antiplasmin in FFP; (2) antiplasmin in S/D plasma. (C) Nondenaturing-PAGE of S/D plasma containing aggregates: (1) antiplasmin in S/D plasma after removal of aggregates; (2) antiplasmin in aggregates isolated as described in Materials and Methods.
Each purchased unit of S/D plasma was thawed and separated into aliquots that were refrozen for later use. Samples from a freshly thawed unit of S/D plasma and an aliquot of S/D plasma that had undergone an additional freeze/thaw cycle had identical migration patterns for antithrombin, antitrypsin, and antiplasmin in nondenaturing PAGE indicating that the conformational changes observed are not a result of the freeze/thaw cycle used to aliquot the plasma (data not shown). Although all were treated identically, the antiplasmin in approximately 10% of the aliquots precipitated completely out of solution as small aggregates at 37°C (Fig 2C). Their formation could not be reliably reproduced by subjecting the plasma to multiple freeze-thaw cycles. The aggregates formed in aliquots from two separate commercial lots (type O and type A) of S/D plasma indicating that their formation is not lot-specific. Additionally, they were not found in aliquots of laboratory prepared S/D plasma not subjected to soy bean oil extraction and C-18 chromatography (Fig 2A), which suggests they form only after the Triton-X-100 and TNBP are removed during the manufacturing process. The formation and subsequent removal of these aggregates when the S/D plasma is sterilized by filtration may explain reports of low antiplasmin antigen concentration in S/D plasma.12
Nondenaturing-PAGE analysis of nonserpin plasma proteins in FFP and S/D plasma.
Fibronectin and tissue factor pathway inhibitor were analyzed by Western blot following nondenaturing-PAGE of FFP or S/D plasma. Tissue factor pathway inhibitor was chosen for study as it is a Kunitz-type serine proteinase inhibitor present in plasma. Kunitz-type inhibitors are structurally distinct from the serpins and have a rigid RSL that undergoes only a slight conformational change upon proteolytic cleavage. S/D treatment has no effect on the migration of either protein (data not shown). These data, along with previous reports demonstrating that the activity of the plasma coagulation factors is not affected by S/D treatment,12 20 indicate that the type of conformational changes described here are likely a serpin-specific phenomenon due to the effects of the S/D treatment on their unique loop-sheet structure.
TUG-PAGE analysis of antithrombin, antitrypsin, and antiplasmin in FFP and S/D plasma.
The conformational stability of serpins can be readily determined by analyzing unfolding transitions in TUG-PAGE.18 The structure of the serpin “A” β-sheet is thought to be the primary determinant of the unfolding transition observed in these gels. When additional RSL residues are inserted into the “A” β-sheet, the stability of the protein increases and higher concentrations of urea are necessary to induce unfolding.2 Active serpins tend to unfold between 2 and 3 mol/L urea; however, latent and polymerized serpins, which have an RSL fully inserted as the center strand of the “A” β-sheet, do not unfold in 8 mol/L urea.2,5 6 Analysis of antithrombin, antitrypsin, and antiplasmin in FFP and S/D plasma by TUG-PAGE (Fig3) further characterizes the conformational changes observed in nondenaturing-PAGE (Figs 1A and 2A). In S/D plasma, virtually all of the antithrombin undergoes an unfolding transition similar to that observed in FFP, indicating that S/D treatment does not significantly alter its conformation. As observed in nondenaturing PAGE (Fig 1A), a significant portion of the antitrypsin in S/D plasma is polymerized. As expected, the polymers are conformationally stable and do not unfold in 8 mol/L urea. The nonpolymerized antitrypsin undergoes an unfolding transition similar to that seen in FFP, indicating that it is in the active conformation. None of the antiplasmin in S/D plasma undergoes the unfolding transitions observed for the active conformation present in FFP, which indicates that it is totally inactivated by S/D treatment. Most of the antiplasmin is polymerized. The remaining protein appears monomeric, but does not undergo the unfolding transitions of active antiplasmin, consistent with adoption of the latent conformation.
Conformational stability of antithrombin, antitrypsin, and antiplasmin in FFP and S/D plasma. Analysis is performed by TUG-PAGE. Each panel represents Western blot analysis of plasma proteins separated on a single gel with the direction of migration from top to bottom and the urea gradient (0 to 8 mol/L) from left to right.
Conformational stability of antithrombin, antitrypsin, and antiplasmin in FFP and S/D plasma. Analysis is performed by TUG-PAGE. Each panel represents Western blot analysis of plasma proteins separated on a single gel with the direction of migration from top to bottom and the urea gradient (0 to 8 mol/L) from left to right.
Inhibition of thrombin, PPE, and plasmin activity by FFP and S/D plasma in amidolytic assays.
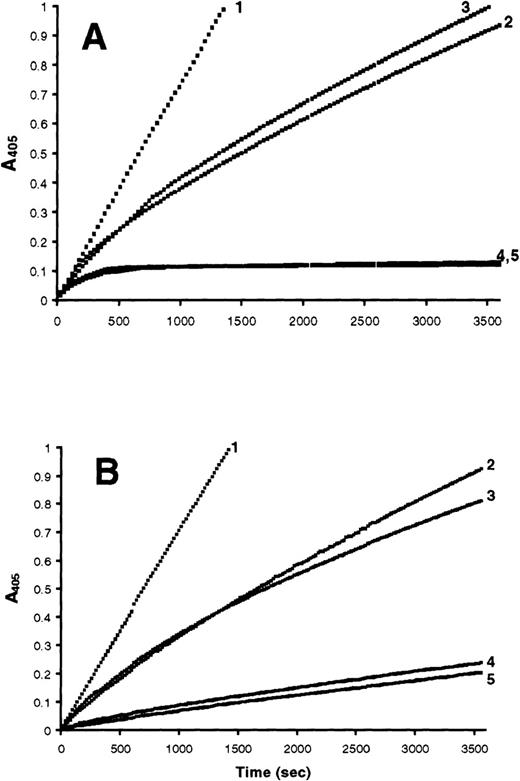
Amidolytic assays comparing S/D plasma and normal pooled plasma were performed to determine whether the conformational changes observed in the nondenaturing and TUG-PAGE correlated with a loss of proteinase inhibitory activity. Antithrombin activity can be measured directly in plasma using thrombin as the assay proteinase. In antitrypsin and antiplasmin activity assays, plasma samples are treated with methylamine to specifically inactivate α2-macroglobulin, which interferes with the assays by rapidly inhibiting the PPE or plasmin.21,22 Antithrombin activity in S/D plasma was measured with a commercial assay used for clinical samples and found to be 100% active (data not shown). This result is consistent with the nondenaturing and TUG–PAGE data, as well as the results of others,12 13 and was not pursued further. Antitrypsin and antiplasmin activity were measured in progress curve amidolytic assays. These assays allow proteinase inhibition to be followed continuously over time, as opposed to end-point amidolytic assays, which measure residual proteinase activity at only one time point. As demonstrated in Fig 4A, a 1:2 dilution of FFP has approximately the same PPE inhibition progress curve as nondiluted S/D plasma. When purified antitrypsin is added to S/D plasma at half the normal plasma concentration (12.5 μmol/L), the progress curve approximates that of nondiluted FFP. While it is difficult to precisely quantify the percentage of antitrypsin polymerized, the amidolytic assays are in general agreement with a visual evaluation of the nondenaturing and TUG-PAGE, which indicate that at least 50% of the antitrypsin is in the inactive, polymerized conformation (Figs 1A and3). The plasmin inhibitory activity present in S/D plasma is slightly less than that present in a 1:10 dilution of FFP. When the normal plasma concentration (0.95 μmol/L) of purified antiplasmin is added to S/D plasma, the plasmin inhibition progress curve approximates that of non-diluted FFP (Fig 4B). These data indicate that greater than 90% of the antiplasmin is inactivated by S/D treatment, consistent with predictions made from the conformational data obtained in the nondenaturing and TUG-PAGE experiments.
Progress curve amidolytic assays measuring inhibition of PPE or plasmin by FFP and S/D plasma. Reactions were initiated by the addition of either 0.4 μmol/L PPE or 0.2 μmol/L plasmin to a mixture of plasma and 500 μmol/L of the appropriate substrate. (A) Reactions initiated with PPE: (1) noninhibited control; (2) S/D plasma; (3) 1:2 dilution of FFP; (4) FFP; (5) S/D plasma after addition of 12.5 μmol/L purified antitrypsin. (B) Reactions initiated with plasmin: (1) noninhibited control; (2) S/D plasma; (3) 1:10 dilution of FFP; (4) FFP; (5) S/D plasma after addition of 0.9 μmol/L purified antiplasmin.
Progress curve amidolytic assays measuring inhibition of PPE or plasmin by FFP and S/D plasma. Reactions were initiated by the addition of either 0.4 μmol/L PPE or 0.2 μmol/L plasmin to a mixture of plasma and 500 μmol/L of the appropriate substrate. (A) Reactions initiated with PPE: (1) noninhibited control; (2) S/D plasma; (3) 1:2 dilution of FFP; (4) FFP; (5) S/D plasma after addition of 12.5 μmol/L purified antitrypsin. (B) Reactions initiated with plasmin: (1) noninhibited control; (2) S/D plasma; (3) 1:10 dilution of FFP; (4) FFP; (5) S/D plasma after addition of 0.9 μmol/L purified antiplasmin.
DISCUSSION
The serpin superfamily of serine proteinase inhibitors represents the largest class of proteinase inhibitors in plasma where they downregulate all of the major plasma proteolytic cascades. Serpins constitute approximately 3% of the total plasma protein and 17% of the nonalbumin, nonimmunoglobulin protein. It is well established that heat and/or detergents can induce purified serpins to undergo large conformational changes that result in irreversible loss of inhibitory activity.2,6,14 15 To determine the effects of the S/D viral inactivation process on plasma serpins, we analyzed the inhibitory activity and conformational stability of antithrombin, antitrypsin and antiplasmin in FFP and S/D plasma purchased from the American Red Cross.
In the presence of heparin, antithrombin rapidly inhibits factor Xa and thrombin. Its significance as an inhibitor of coagulation in vivo is demonstrated by the apparent usefulness of antithrombin concentrates in the treatment of some patients with disseminated intravascular coagulation23 and the increased incidence of venous thrombosis in patients with low plasma antithrombin concentration.24 Our studies of antithrombin in S/D plasma indicate that it is fully active by both conformational analysis and activity assays, in agreement with previous reports.12,13The resistance of plasma antithrombin to conformational change is somewhat surprising because purified antithrombin will readily either polymerize or adopt the latent conformation when exposed to heat or detergents,6 14 suggesting that there may be a component in plasma that conformationally stabilizes this serpin.
The plasma concentration of antitrypsin is 25 μmol/L, which makes it the most abundant proteinase inhibitor in plasma. It is the primary tissue inhibitor of neutrophil elastase, a highly destructive proteinase that is released into the tissues during the normal turnover of neutrophils at a rate of approximately 65 mg/d (based on a calculation assuming 1 pg of neutrophil elastase per neutrophil and a turnover of 750,000 neutrophils per second). Unregulated neutrophil elastase activity has been implicated as a mediator of disseminated intravascular coagulation and septic shock.25 Additionally, chronic exposure of the lungs to neutrophil elastase activity, as occurs in patients with low circulating concentrations of antitrypsin, results in the development of emphysema.26 Our studies of antitrypsin in S/D plasma demonstrate that approximately 50% is polymerized and inactive. Previous studies of antitrypsin in S/D plasma documented that the antigen concentration was within normal limits. However, antitrypsin activity was not measured and, therefore, the loss of inhibitory activity was not detected.12 13
Antiplasmin is the primary inhibitor of the fibrinolytic proteinase plasmin. Its importance in vivo is demonstrated in patients undergoing liver transplantation who commonly develop low plasma antiplasmin activity by the end of the anhepatic phase of the operation, contributing to the development of a fibrinolytic state and subsequent intraoperative bleeding.27-29 Additionally, rare patients identified with congenital deficiency of antiplasmin have severe bleeding diatheses.30-32 Our studies demonstrate that essentially 100% of the antiplasmin in S/D plasma is either polymerized or in the latent conformation. In the progress curve amidolytic assays, S/D plasma has less than 10% of the plasmin inhibitory capacity of normal pooled plasma. Since none of the antiplasmin is in the active conformation, it is likely that the residual plasmin inhibitory activity in S/D plasma is caused by other plasma proteinase inhibitors such as antitrypsin, C1-inhibitor, or inter-α-trypsin inhibitor that slowly inhibit plasmin.33,34 The discrepancy between the total loss of antiplasmin activity reported here and the 50% loss previously reported12 13 is likely due to the presence of these inhibitors, which can produce falsely elevated antiplasmin activity in end-point amidolytic assays depending on the amount of time the plasmin is allowed to incubate with the plasma before residual proteinase activity is measured.
The antitrypsin and antiplasmin polymers formed during S/D treatment most likely are the well-characterized loop-sheet type that form when the RSL of one protein molecule inserts intermolecularly as the center of strand of the “A” β-sheet of another.2 This type of polymerization occurs both during the in vitro viral inactivation of plasma-derived products and also in vivo, where several mutations that cause spontaneous serpin polymerization under physiological conditions have been described. The most common of these is the Z-antitrypsin phenotype, where the antitrypsin polymerizes within hepatocytes and is not secreted, resulting in low plasma antitrypsin concentrations and increased risk for the development of emphysema.2,35,36 Other, less common mutations of antitrypsin,37 antithrombin,38,39 and C1-inhibitor40 41 also adopt the latent or polymerized conformations under physiologic conditions and result in disease. Therefore, strategies for the prevention of serpin conformational change will be helpful to both commercial manufacturers of plasma derived blood products and patients with diseases caused by polymerization of abnormal serpins.
The analysis of serpins in S/D plasma provides the beginnings of a database that may lead to new understanding of the structural features of serpins important for altering their susceptibility to conformational change. It is interesting that S/D treatment causes extensive conformational change of antiplasmin, while antitrypsin is only partially affected and antithrombin is essentially not altered. Structural features of antiplasmin that are not present in antitrypsin or antithrombin include a 51-residue C-terminal extension, a deletion in the region of the D-helix (nomenclature of Loebermann et al1), and a unique disulfide bond structure.42Additionally, in mouse plasma-elimination studies, antiplasmin-proteinase complexes are removed from the circulation by a hepatic receptor separate from the one that removes antitrypsin, antithrombin, antichymotrypsin, and heparin cofactor II–proteinase complexes.43 At present, little is known which, if any, of these structural features make antiplasmin more susceptible to conformational change under the conditions of S/D treatment. Further characterization of conformational changes that the various plasma serpins undergo when exposed to different combinations of detergent, solvent and heat may identify specific structural regions or features of the serpin superfamily that alter the susceptibility for conversion to the latent or polymerized conformations.
The S/D treatment process used for plasma is similar to that used for viral inactivation of coagulation factor concentrates and virtually eliminates the risk of transfusion associated transmission of enveloped viruses including hepatitis B, hepatitis C, and human immunodeficiency virus.7,10,11 However, S/D treatment of plasma does not leave all plasma proteins intact. In addition to the decreased antitrypsin and absent antiplasmin activity described here, the high-molecular-weight forms of von Willebrand factor are absent and the protein S concentration is less than 50% that found in FFP.12,13 It is likely that other plasma serpins, including antichymotrypsin, C1-inhibitor, PAI-1, and heparin cofactor II, are also conformationally altered and inactivated to various degrees in S/D plasma. While replacement of coagulation factors, which are present in normal concentrations in S/D plasma, is generally considered the primary purpose of plasma therapy, there are several conditions where the antitrypsin and antiplasmin activity present in FFP may be beneficial for the patient. These include disseminated intravascular coagulation and sepsis, where systemic, unregulated activation of plasma proteolytic cascades occurs, acquired fibrinolytic states, such as occur during liver transplantation, and acutely bleeding patients, where the entire blood volume is rapidly replaced. Although it has been used extensively in several European countries with no adverse reports, the effectiveness of S/D plasma for the treatment of these diseases is not established44 and additional clinical studies are needed to develop guidelines for the use of FFP and S/D plasma in these clinical situations.
ACKNOWLEDGMENT
We thank Carolyn Chesney, David Gailani, Morey Blinder, Doug Blackall, James Travis, Guy Salvesen, and Karen Hasty for helpful discussions.
Supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alan E. Mast, MD, PhD, Research Service-151, Veterans Affairs Hospital, 1030 Jefferson Ave, Memphis, TN 38104.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal