Abstract
Hereditary hemochromatosis (HH) is a common autosomal-recessive disorder of iron metabolism. More than 80% of HH patients are homozygous for a point mutation in a major histocompatibility complex (MHC) class I type protein (HFE), which results in a lack of HFE expression on the cell surface. A previously identified interaction of HFE and the transferrin receptor suggests a possible regulatory role of HFE in cellular iron absorption. Using an HeLa cell line stably transfected with HFE under the control of a tetracycline-sensitive promoter, we investigated the effect of HFE expression on cellular iron uptake. We demonstrate that the overproduction of HFE results in decreased iron uptake from diferric transferrin. Moreover, HFE expression activates the key regulators of intracellular iron homeostasis, the iron-regulatory proteins (IRPs), implying that HFE can affect the intracellular “labile iron pool.” The increase in IRP activity is accompanied by the downregulation of the iron-storage protein, ferritin, and an upregulation of transferrin receptor levels. These findings are discussed in the context of the pathophysiology of HH and a possible role of iron-responsive element (IRE)-containing mRNAs.
HEREDITARY HEMOCHROMATOSIS (HH) is an autosomal-recessive disorder of iron metabolism, affecting approximately 1 in 400 people of Northern European descent.1 With a gene frequency of about 1 in 10, it represents one of the most common inherited diseases overall.2 The cause is a malregulation of dietary iron absorption in the duodenum. Under physiologic conditions, body iron concentrations are balanced through absorption in the duodenum, since there are no specific excretion mechanisms for iron. Approximately 0.5 to 2.0 mg of iron is lost per day, mainly through exfoliation of gastrointestinal mucosal cells, with smaller amounts lost through bile, urine, and skin.3,4 As body iron stores decline, absorption increases and vice versa. In hemochromatosis, a 2- to 4-fold increase of iron absorption causes the continuous accumulation of body iron, mainly in the liver.4 The reason for this misregulation remained unclear for a long time. Although the close association of the hemochromatosis gene with the major histocompatibility complex (MHC) I locus on chromosome 6 was known since 1976,5 it took until 1996 before the hemochromatosis gene, now namedhfe, was identified.6
The HFE protein is closely related to MHC class I proteins. More than 80% of hemochromatosis patients are homozygous for a cysteine to tyrosine exchange in position 282 of the HFE protein. This mutation was predicted to disrupt a disulfide bond in the protein, known to be essential for the interaction of MHC class I proteins with β2-microglobulin.6 This complex formation is a prerequisite for insertion of MHC class I proteins into the cell membrane. In fact, it was demonstrated that the Cys282 Tyr mutated HFE is not transported to the cell surface.7,8 In addition, β2-microglobulin deficient mice, as well as HFE knockout mice, develop iron overload.9-11 However, in contrast to classical MHC class I proteins, HFE lacks a peptide-binding domain and thus does not seem to be involved in antigen peptide presentation.12
Low-level expression of HFE occurs in many tissues, but transcripts are most abundant in small intestine and liver. In the liver, HFE is present in Kupffer cells and endothelium, but absent from parenchyma.13 In the duodenum, the organ that regulates iron absorption, HFE protein is only detectable in cells of the deep crypts, with a mainly intracellular and perinuclear distribution pattern.14
So far, the role of a nonclassical MHC class I protein in cellular iron homeostasis is poorly understood. First indications come from the finding that HFE forms specific complexes with the transferrin receptor to decrease the affinity for its substrate, transferrin.15These findings link the HFE protein to the regulation of iron uptake. Furthermore, the transferrin receptor may play a role in HFE trafficking as newly synthesized HFE forms stable complexes with transferrin receptor, whereas excess and uncomplexed HFE is rapidly degraded.16
Investigations into the mechanism(s) of how HFE expression controls the complex network of cellular iron homeostasis will be important for the understanding of its role in HH. The key regulators of intracellular iron metabolism are the iron-regulatory proteins (IRP)-1 and IRP-2.17 In iron-deficient cells, they bind to iron-responsive elements (IREs) located in the untranslated regions of mRNAs involved in iron uptake, storage, and utilization to posttranscriptionally regulate their expression. IRP binding inhibits translation of ferritin L- and H-chain mRNA and stabilizes the transferrin receptor transcript. It also controls the synthesis of eALAS, an enzyme involved in erythroid heme synthesis. Recently, an IRE has been detected in the iron transporter Nramp2, but its role has not been functionally characterized so far.18 Interestingly, the binding of IRP is not only regulated by the intracellular iron level, but also in response to oxidative stress and nitric oxide.19
In this report, we demonstrate an effect of HFE expression on cellular iron uptake and IRP activity. The activation of IRP is accompanied by the downregulation of the iron-storage protein ferritin and an upregulation of transferrin receptor levels. These findings are discussed in the context of the pathophysiology of HH and a possible role of IRE-containing mRNAs.
MATERIALS AND METHODS
Cloning of the human hfe cDNA.
The wild-type HFE cDNA was cloned from human peripheral blood mononuclear cells (PBMC), using the reverse-transcriptase polymerase chain reaction (RT-PCR) technique. PBMC were isolated from the blood of a healthy donor following standard protocols20 and RNA was purified using the Fastprep RNA kit green (Bio 101, Vista, CA). First-strand cDNA synthesis was performed using the oligo dT/Not primed first-strand cDNA synthesis kit (Pharmacia, Freiburg, Germany) and the HFE cDNA was amplified by PCR with gene-specific primers and high-fidelity Taq polymerase (Boehringer Mannheim, Mannheim, Germany). The primer pair used was 5’-attatatcccgggatATC-TCCTGAGCCTAGGCAATAGCTG-3’ (position 111-135 of the hfe mRNA) and 5’-ttataggatccgagcTCCTGAGCACCTGCCCTTCAAGAC-3’ (position 2439-2415). Uppercase letters and positions refer to the nucleotide sequence of the hfe cDNA; lowercase letters refer to nucleotides added to the hfe-specific primer sequences in order to introduce additional, singular restriction sites. The restriction sites chosen were SmaI for the 5’-primer and BamHI for the 3-primer, respectively.
The plasmid pSGH-1 was generated by cloning the 2.3-kb PCR product, containing the entire hfe coding sequence (position 222-1268) into the EcoRV site of plasmid pBI-5.21 It was fully sequenced and found to be identical with the previously published hfegene.6 Clones were further tested for in vitro expression of HFE in a reticulocyte transcription and translation system (Promega, Mannheim, Germany). For this purpose, inserts were subcloned into plasmid pSG-5,22 carrying a T7 promotor. When subcloned into pSG-5, the inserts give rise to expression of a 43-kD protein that is specifically recognized by HFE antisera.
Generation of the cell line HtTA-HFE.
To generate HtTA-hfe, pSGH-1 was stably transfected into the HeLa cell line HtTA, which constitutively expresses the tetracycline-dependent tTA repressor (tet-off system).23 Since pSGH-1 does not contain a selectable marker, plasmid pSV-2 (kindly provided by S. Freundlieb, ZMBH, Heidelberg, Germany) conferring hygromycine resistance was cotransfected in a 1:50 molar ratio. Hygromycine-resistant clones were tested for regulated expression of HFE by Western blot analysis. To suppress the expression of recombinant HFE, cells are treated with 1 μg/mL of doxycycline for the indicated times.
Generation of rabbit antisera directed against HFE peptides.
Rabbits were immunized with 2 HFE-specific peptides. The N-terminal peptide (cgRRVEPRTPWVSSRISSQ) is located in the extracellular domain and the C-terminal peptide (cgRKRQGSRGAMGHYVLAERE) in the intracellular domain of the HFE protein. The first 2 amino acids of each peptide, given in lowercase letters, were added for coupling to the carrier protein. Peptides were coupled to keyhole limpet hemocyanin (KLH) using the imgen-activated immunogen conjugation kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. The immunization procedure was performed by Eurogentec, Seraing, Belgium, according to standard procedures.
Western analysis.
The regulated expression of the HFE protein was tested by Western blot analysis using an anti–C-terminal peptide antiserum (1:10,000) as primary antibody and alkaline phosphatase–conjugated sheep antirabbit antibody (1:3,000 dilution) as a secondary antibody. Chemiluminescence was detected using CDP-Star reagent (Tropix, Bedford, MA).
Iron uptake experiments.
For transferrin uptake, 59Fe-labeled diferric transferrin (holotransferrin) was prepared exactly as described.24Uptake experiments were performed in 6-cm dishes at 37°C for the indicated times in 1 mL of buffer A (140 mmol/L NaCl, 5 mmol/L KCL, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 5 mmol/L NaH2PO4, and 5 mmol/L HEPES pH 7.4). Cells were washed 5 times with ice-cold phosphate-buffered saline (PBS) and then lysed with 1 mL 1-mol/L NaOH. Radioactivity in the lysate was determined using a Cobra II Auto Gamma counter (Canberra Packard, Meriden, CT). For all uptake experiments, controls were incubated on ice to determine nonspecific binding of holotransferrin to the cell membrane. The 0°C values were subtracted from the 370°C values to determine net uptake rates.
Immunofluorescence staining.
Subconfluent HtTA-HFE or HtTA parental cells were grown on coverslips (11 mm; Menzel Glas, Merck, Bruchsal, Germany) and washed twice with PBS (pH 7.4). Cells were fixed in 3.7% paraformaldehyde in CSK-buffer (100 mmol/L NaCl, 300 mmol/L sucrose, 10 mmol/L Pipes pH 6.8, 3 mmol/L MgCl2, 1 mmol/L EGTA) for 10 minutes at room temperature. Where indicated, cells were subsequently permeabilized with 0.5% Triton X-100 in CSK-buffer for 15 minutes at room temperature. Following fixation or fixation and permeabilization, cells were washed 3 times for 5 minutes with PBS and rinsed once with PBS/0.05% Tween 20. The coverslips were then incubated for 90 minutes with antiserum directed against the C-terminal (1:250 dilution) or the N-terminal domain (1:100 dilution) of HFE, and then washed 3 times (5 minutes each) with PBS/0.05% Tween. Subsequently, coverslips were incubated for 60 minutes with fluorescein isothiocyanate (FITC)-conjugated goat antirabbit antibodies (Boehringer Mannheim), washed 4 times for 5 minutes with PBS, and then analyzed by immunofluorescence microscopy.
Gel retardation assays.
Gel retardation assays were performed exactly as described earlier. Briefly, detergent extracts of HtTA or HtTA-HFE cells were prepared25 and incubated with a 32P-labeled IRE probe, transcribed in vitro from the XbaI-linearized plasmid I-12 CAT.26 The IRE/IRP complexes were analyzed by sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) as described.27
Metabolic labeling and immunoprecipitation.
Cells were labeled for 2 hours with (35S)-methionine (1,85 Mbq/mL) in methionine-free medium. Quantitative immunoprecipitation from equal amounts of trichloroacetic acid–insoluble radioactivity was performed with polyclonal antiferritin antibodies (Boehringer Mannheim), monoclonal antitransferrin receptor antibodies (Boehringer Mannheim), or U1A antiserum (kindly provided by Iain Mattaj, EMBL, Heidelberg, Germany). Immunoprecipitated proteins were analyzed by SDS-PAGE followed by autoradiography.28 Quantitative analysis of the autoradiographs was done by phosphorimaging.
RESULTS
Generation of the HeLa cell line HtTA-HFE that expresses human HFE under the control of a tetracycline-regulated promoter.
To examine the effect of HFE expression on cellular iron uptake and IRP activity, we generated stable HeLa cell transfectants in which HFE expression is controlled via a tetracycline-responsive promoter.23 The regulated expression HFE was tested by Western blot analysis. In the induced state, ie, in the absence of doxycycline, 2 predominant protein bands with molecular weights of approximately 43 and 48 kD, respectively, are detected by the HFE antiserum. Upon addition of 1 μg/mL of doxycycline, synthesis of HFE is almost completely inhibited (Fig 1). Since the highest range of regulated HFE expression was afforded using clone 17, all subsequently described experiments were performed with this cell line. To estimate the regulation efficiency of this system, Western blot experiments were performed with extracts from induced and noninduced cells. These preliminary experiments indicated that HFE expression was reduced in the presence of doxycycline by at least 2 orders of magnitude (not shown). Thus, these cells represent a suitable model system for studying the effects of the HFE expression.
Regulated expression of HFE in stably transfected HtTA-HFE cells. HtTA cells, stably transfected with the HFE-expressing plasmid pSGH-1, were grown for 4 days in the presence (+) or absence (−) of 1 μg/mL doxycycline. Protein lysates (corresponding to ≈1 × 105 cells per lane) were prepared and subjected to SDS-PAGE followed by Western blotting with HFE antisera. Results are for 4 individual HtTA-HFE clones, all showing expression of a 43/48-kD HFE protein double band. No HFE expression is detected in HtTA controls.
Regulated expression of HFE in stably transfected HtTA-HFE cells. HtTA cells, stably transfected with the HFE-expressing plasmid pSGH-1, were grown for 4 days in the presence (+) or absence (−) of 1 μg/mL doxycycline. Protein lysates (corresponding to ≈1 × 105 cells per lane) were prepared and subjected to SDS-PAGE followed by Western blotting with HFE antisera. Results are for 4 individual HtTA-HFE clones, all showing expression of a 43/48-kD HFE protein double band. No HFE expression is detected in HtTA controls.
The cellular distribution of the induced HFE protein was analyzed by immunofluorescence, using HFE antisera directed against an N-terminal or C-terminal HFE peptide. In permeabilized cells, most of the staining occurred intracellular in a perinuclear location (Fig2A). This distribution is similar to the one described for the transferrin receptor.16 29 Using the antiserum directed against the N-terminal peptide, membrane staining was clearly detectable with nonpermeabilized cells, demonstrating that a distinct fraction of HFE is associated with the cell membrane (Fig2C). The specificity of the immunostaining experiments is demonstrated by the lack of fluorescence in cells repressed for HFE synthesis, under otherwise identical experimental conditions (Fig 2B and D).
Immunostaining of HFE. HtTA-HFE cells were grown on coverslips for 4 days in the absence (A,C) or presence (B,D) of 1 μg/mL doxycycline. Immunostaining was perfromed as described in the methods. Permeabilzed cells were incubated with the antiserum directed against the C-terminal peptide (A,B), unpermeabilized cells were incubated with the antiserum directed against the N-terminal peptide (C,D).
Immunostaining of HFE. HtTA-HFE cells were grown on coverslips for 4 days in the absence (A,C) or presence (B,D) of 1 μg/mL doxycycline. Immunostaining was perfromed as described in the methods. Permeabilzed cells were incubated with the antiserum directed against the C-terminal peptide (A,B), unpermeabilized cells were incubated with the antiserum directed against the N-terminal peptide (C,D).
HFE expression decreases iron uptake from diferric transferrin.
Complex formation of HFE with the transferrin receptor has been reported.12,15 This interaction results in a lower affinity of the transferrin receptor for transferrin.15 We therefore investigated the effect of HFE expression on iron uptake from diferric transferrin.
HtTA-HFE cells were cultivated to approximately 50% to 75% confluence in the presence or absence of doxycycline. The cells were washed and increasing concentrations of 59Fe-diferric transferrin were added. Uptake was determined for 30 minutes at 37°C. For low transferrin concentrations between 1 and 10 μg/mL, there was approximately 50% inhibition of iron uptake in HFE-induced cells, ie, in the absence of doxycycline (Fig 3). For higher transferrin concentrations, these differences diminished, and no HFE effect was seen with supraphysiologic diferric transferrin concentrations above 50 μg/mL. These data suggest that saturation of receptor-mediated transferrin uptake occurs at higher transferrin concentrations in the presence than in the absence of HFE, which is in good agreement with the finding that HFE lowers the affinity of the transferrin receptor for transferrin.15
Concentration-dependent uptake of iron from59Fe-diferric transferrin in HtTA-HFE cells. Cells were grown for 4 days in the presence (•) or absence (○) of 1 μg/mL doxycycline. Uptake of 59Fe from diferric transferrin was determined as outlined in Materials and Methods. Cells were incubated for 30 minutes at 37°C. Transferrin concentrations are indicated. Data are expressed as means of triplicate samples.
Concentration-dependent uptake of iron from59Fe-diferric transferrin in HtTA-HFE cells. Cells were grown for 4 days in the presence (•) or absence (○) of 1 μg/mL doxycycline. Uptake of 59Fe from diferric transferrin was determined as outlined in Materials and Methods. Cells were incubated for 30 minutes at 37°C. Transferrin concentrations are indicated. Data are expressed as means of triplicate samples.
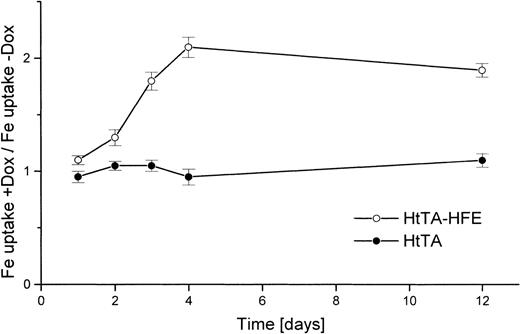
To clearly demonstrate that the reduction of transferrin uptake is in fact due to HFE expression and not to nonspecific stimulation of transferrin uptake by doxycycline treatment, several control experiments were performed. HtTA-HFE cells and cells of the maternal cell line HtTA were kept for 1 to 12 days in the absence or presence of doxycycline. Uptake of transferrin was determined, and the uptake ratios of treated versus untreated cells were determined. With the nontransfected HtTA cells, no significant doxycycline effect was observed, whereas for the HtTA-HFE cells, uptake of transferrin slowly increased in the doxycycline-treated cells, with the ratio reaching its maximum approximately 4 days after addition of the antibiotic (Fig4). Reciprocal to this increase in iron uptake, the amount of HFE protein decreased within these 4 days of doxycycline treatment to control levels usually found in permanently repressed cells (not shown).
The regulated iron uptake from transferrin is HFE-expression–dependent. HtTA cells (•) and HtTA-HFE cells (○) were grown in the absence of doxycycline. Cells were divided and either left untreated or doxycycline (1 μg/mL) was added. At different times after onset of doxycycline-mediated HFE repression, iron uptake was examined in doxycycline-treated and untreated cells. Uptake was determined for 30 minutes at 37°C with 5 μg/mL59Fe-diferric transferrin as substrate. The results are given as quotient of uptake rates of doxycyline treated vuntreated cells. All data are derived from triplicate samples.
The regulated iron uptake from transferrin is HFE-expression–dependent. HtTA cells (•) and HtTA-HFE cells (○) were grown in the absence of doxycycline. Cells were divided and either left untreated or doxycycline (1 μg/mL) was added. At different times after onset of doxycycline-mediated HFE repression, iron uptake was examined in doxycycline-treated and untreated cells. Uptake was determined for 30 minutes at 37°C with 5 μg/mL59Fe-diferric transferrin as substrate. The results are given as quotient of uptake rates of doxycyline treated vuntreated cells. All data are derived from triplicate samples.
Both findings, (1) that doxycycline has no effect on diferric transferrin uptake in untransfected cells and (2) that altered rates of transferrin uptake reciprocally correspond to changes to the amount of expressed HFE in transfected cells, demonstrate that HFE protein expression leads to diminished iron uptake via the transferrin/transferrin receptor system.
HFE expression increases IRP activity.
The overexpression of HFE protein results in decreased transferrin iron uptake, which may result in relative intracellular iron deficiency. As low intracellular iron levels increase IRP activity, we next investigated whether HFE expression affects the key regulators of intracellular iron homeostasis, the IRPs.
To study possible effects of HFE expression on IRP activity, HtTA-HFE and HtTA cells were grown in the presence and absence of doxycycline, and detergent extracts were prepared at various times after the onset of doxycycline treatment. The IRE-binding activity of the IRPs was monitored by electrophoretic mobility shift assays. IRP activity increases in HFE-expressing cells, when compared with either repressed HFE-HtTA cells or the nontransfected HtTA control cells (Fig5A, lanes 1 to 4). IRP activity in the doxycycline treated HtTa-HFE cell line (Fig 5A, lane 2) is comparable to the activity in nontransfected HtTA cells (Fig 5A, lanes 3 and 4). This again demonstrates the ability of the tet-regulated HFE expression system to almost completely shut off HFE expression. The HFE-dependent increase in IRP-binding activity is due to posttranslational activation and not to de novo synthesis, since total amounts of IRP, as demonstrated by complete activation of IRP by 2-mercaptoethanol (2-ME),25 remain constant under all conditions tested (Fig5A, lanes 5 to 8). These results suggest that the HFE-induced decrease of diferric transferrin iron uptake affects the intracellular “labile iron pool” and thus results in the activation of the IRPs.
HFE expression stimulates IRP activity. (A) Cytoplasmic extracts of HtTA or HtTA-HFE cells grown with or without doxycycline (1 μg/mL for 12 days) were prepared and subjected to gel retardation assays. Where indicated (lanes 5 to 9), 2-mercaptoethanol (2%) was added to the sample (25 μg of protein/lane) before the RNA probe. Positions of IRE/IRP complexes and unbound RNA (free probe) are indicated by arrows. (B) Gel retardation assays as above, except that doxycycline treatment of the cells was performed for the times indicated. For each time point, the amount of IRP/IRE complexes formed in the absence of doxycyline was set at 100%; the percentages of IRP/IRE complexes in the presence of doxycycline are given below the lanes. Quantitative determination of IRP/IRE complexes was done by phosphorimaging.
HFE expression stimulates IRP activity. (A) Cytoplasmic extracts of HtTA or HtTA-HFE cells grown with or without doxycycline (1 μg/mL for 12 days) were prepared and subjected to gel retardation assays. Where indicated (lanes 5 to 9), 2-mercaptoethanol (2%) was added to the sample (25 μg of protein/lane) before the RNA probe. Positions of IRE/IRP complexes and unbound RNA (free probe) are indicated by arrows. (B) Gel retardation assays as above, except that doxycycline treatment of the cells was performed for the times indicated. For each time point, the amount of IRP/IRE complexes formed in the absence of doxycyline was set at 100%; the percentages of IRP/IRE complexes in the presence of doxycycline are given below the lanes. Quantitative determination of IRP/IRE complexes was done by phosphorimaging.
As shown above, transferrin uptake increases within 4 days after addition of doxycycline to HFE-expressing cells (Fig 4). Thus, cells can restore normal iron levels within this time and IRP-binding activity should decrease to control cell levels. In fact, IRP activity was found to decrease to approximately 50% within the first 3 days of doxycycline treatment and to about 30% after 12 days (Fig 5B). Interestingly, 10 to 14 days were required before repressed HtTA-HFE cells completely returned to control cell (HtTA) characteristics (Figs3 and 5).
Regulation of IRP target mRNAs in HFE-expressing HtTA cells.
The expression of several mRNAs involved in iron metabolism is regulated posttranscriptionally by the binding of IRPs to IREs. IRP binding to a single IRE in the 5′-untranslated region inhibits the translation of ferritin mRNAs, while IRP binding to multiple IREs in the 3′-untranslated region of transferrin receptor mRNAs stabilizes these transcripts to increase TfR expression.17
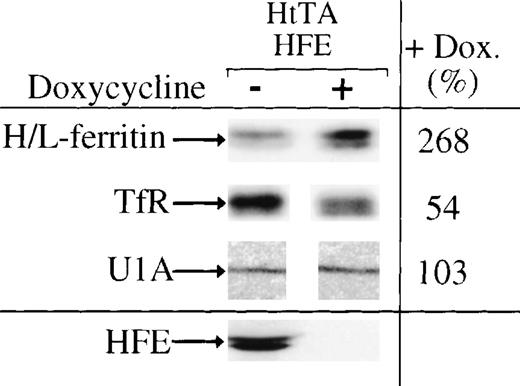
Since HFE synthesis stimulates IRP activity, we investigated the effects of HFE expression on ferritin and transferrin receptor synthesis in HtTA-HFE cells. Cells grown for 12 days with or without doxycycline were labeled for 2 hours with (35S)-methionine. Equal amounts of trichloroacetic acid–precipitable radioactivity were subjected to immunoprecipitation for a quantitative determination of transferrin receptor and ferritin expression. In good agreement with the increase of IRP activity in HFE-expressing cells, ferritin biosynthesis is downregulated and transferrin receptor expression is upregulated under conditions of HFE overexpression. Quantitation of the immunoprecipitation products shows that ferritin synthesis decreases by approximately 50% to 70%, whereas transferrin receptor expression approximately doubles (Fig 6). Thus, the effects of HFE expression on transferrin iron uptake and IRP activity cause changes in the expression of IRP target mRNAs.
Regulation of IRE-containing mRNAs by HFE expression. Ferritin (molecular weight, 19/21 kD), transferrin receptor (94 kD), U1A (32 kD), and HFE (43/47 kD) were immunoprecipitated from extracts of doxycycline-untreated (−) or -treated (+) cells, respectively. Precipitated proteins were seperated by SDS-PAGE and quantitated by phosphorimaging. The amounts of proteins immunoprecipitated from cells not treated with doxycycline were set to 100%.
Regulation of IRE-containing mRNAs by HFE expression. Ferritin (molecular weight, 19/21 kD), transferrin receptor (94 kD), U1A (32 kD), and HFE (43/47 kD) were immunoprecipitated from extracts of doxycycline-untreated (−) or -treated (+) cells, respectively. Precipitated proteins were seperated by SDS-PAGE and quantitated by phosphorimaging. The amounts of proteins immunoprecipitated from cells not treated with doxycycline were set to 100%.
DISCUSSION
To investigate the role of HFE in cellular iron homeostasis, we studied the effects of HFE expression on transferrin iron uptake, IRP activity, and the expression of IRP target mRNAs. As a tool, we generated a stably transfected HeLa cell line that expresses HFE under control of a tet-sensitive promotor. With this cell line (HtTA-HFE), HFE synthesis can be almost completely turned off upon addition of doxycycline and be regulated by more than 2 orders of magnitude (Fig 1). The nontransfected cell line (HtTA) expresses only little HFE: (1) in Western blot experiments, no HFE-specific protein band was detected with either of the 2 peptide antisera; and (2) highly sensitive RT-PCR showed only faint HFE-specific signals after 50 amplification cycles (unpublished data, February 1998). Therefore, HFE synthesis can be strictly regulated by addition of doxycycline, making these cells a suitable model for studying HFE function. Consistent with observations in other cell systems,7,14 in the HtTA-HFE cell line most of the detectable HFE is localized intracellularly, with a distinct fraction localized at the plasma membrane (Fig 2). The staining pattern for HFE closely resembles the one obtained for the transferrin receptor (unpublished data, August 1998), in good agreement with the finding that it forms stable complexes with HFE.12 15
Using the HtTA-HFE cell line, we demonstrate that expression of HFE results in decreased iron uptake from diferric transferrin. This effect is specific for HFE expression as prolonged exposure of HtTA cells to doxycycline did not alter transferrin uptake rates (Fig 4). In HFE-expressing cells, a saturation of transferrin uptake occurs at an approximately 3-fold higher concentration in comparison to control cells (Fig 3). However, the maximum uptake rates seen for induced and repressed cells are comparable. These findings are consistent with a previous report that HFE lowers the affinity of transferrin for the transferrin receptor.15
Moreover, HFE expression leads to activation of the IRPs. In iron-deficient cells, IRPs bind to IREs, palindromic sequences in the untranslated regions of mRNAs, to posttranscriptionally regulate their expression. An approximately 3-fold increase of IRP-binding activity to IRE was detected under HFE-expressing conditions in HtTA-HFE cells (Fig5). To our knowledge, this is the first direct demonstration of the regulation of IRP activity by the hemochromatosis gene product HFE. Due to the comigration of complexes between IREs and human IRP-1 and IRP-2, respectively, it remains open whether both IRP-1 and IRP-2 are affected by HFE expression. Both IRP-1 and IRP-2 act as sensors of the intracellular labile iron pool, suggesting that HFE expression profoundly affects iron levels in the cells.
The increase in IRP activity is accompanied by the downregulation of the iron-storage protein ferritin (2- to 3-fold) and an upregulation of transferrin receptor levels (2-fold). These findings show that HFE expression can affect the regulation of IRE-containing mRNAs, including proteins involved in cellular iron uptake.
HH is characterized by increased body iron load, caused by dysregulation of iron uptake in the duodenum. Occurrence of the disease is closely linked to a point mutation in the HFE protein that prevents interaction of HFE with β2-microglobulin, resulting in a failure of HFE insertion into the cell membrane.7 HFE is thought to play an important role in responding to body iron needs and controlling iron uptake in the duodenal mucosal cell. In intestinal epithelial cells of HH patients, ferritin levels have been found to be reduced.30 Moreover, it has been reported that IRP activity is increased in duodenal enterocytes of HH patients compared with healthy individuals.30 These findings are in sharp contrast to the results that we have obtained with our cellular model system. This suggests that HFE expression and/or IRP activity in enterocytes is under the control of tissue-specific and/or systemic regulatory influences that have not been uncovered so far.
On the other hand, in tissues other than the duodenum, HH patients exhibit elevated ferritin levels and decreased transferrin receptor expression.31 Currently, it is not understood whether the mutated HFE protein plays a role in causing tissue iron overload (eg, hepatocytes) in HH patients. Cellular iron deposition could therefore either be determined solely by the elevated plasma iron concentration (in particular by the non–transferrin-bound iron concentration), as a consequence of an increase in iron uptake in the duodenum. It has been reported that patients with secondary iron overload can show similar tissue iron loading as patients suffering from classical HFE hemochromatosis.32 Thus, tissue iron overload can occur in the context of an apparently functional HFE protein. On the other hand, the majority of patients suffering from secondary iron overload display a different pattern of iron distribution, with maximal iron accumulation in cells of the reticuloendothelial system rather than parenchymal iron deposition.33 This suggests the possibility that the hfe mutation could contribute to tissue iron overload in HH patients.
The HeLa-derived cell line may represent a good model for studying the functions of HFE in regulation of cellular iron uptake in body cells other than the enterocyte. We show that HFE downregulates cellular iron uptake from diferric transferrin, which is in good agreement with previously reported results that HFE interacts with the transferrin receptor, thereby lowering its affinity for transferrin.15We also show that IRP activity is increased in HFE-expressing cells and, consistent with this finding, ferritin concentration is lowered, whereas transferrin receptor concentration is elevated compared with control cells in which HFE synthesis is suppressed.
In hemochromatosis patients, the lack of a functional HFE protein results in pathologic iron overload in multiple body tissues. IRP activity in these tissues is downregulated according to the iron overload situation, also indicating that sensing of intracellular iron concentration by the IRP regulatory system is unaffected in hemochromatosis.
In summary, our findings implicate that HFE expression leads to a decrease in intracellular iron concentration, most probably by limiting cellular iron uptake via the transferrin receptor pathway. This regulatory function of HFE at least partly explains why body cells that do not express a functional HFE protein, as in hemochromatosis, exhibit iron overload.
ACKNOWLEDGMENT
The invaluable help of S. Freundlieb (ZMBH, Heidelberg, Germany) regarding the tet-system is greatly acknowledged.
H.D.R. and M.U.M. contributed equally to this work.
Supported by Grant No. SFB 601 of the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to H.D. Riedel, PhD, Universitaetsklinik Heidelberg, Innere Medizin IV/Gastrolabor, Bergheimerstraβe 58, 69115 Heidelberg, Germany.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal