Abstract
The two tyrosine kinase receptors, c-kit and flt3, and their respective ligands KL and FL, have been demonstrated to play key and nonredundant roles in regulating the earliest events in hematopoiesis. However, their precise roles and potential interactions in promoting early lymphoid commitment and development remain unclear. Here we show that most if not all murine Lin−/loSca1+c-kit+ bone marrow (BM) cells generating B220+CD19+proB-cells in response to FL and interleukin-7 (IL-7) also have a myeloid potential. In contrast to FL + IL-7, KL + IL-7 could not promote proB-cell formation from Lin−/loSca1+c-kit+ cells. However, KL potently enhanced FL + IL-7–stimulated proB-cell formation, in part through enhanced recruitment of FL + IL-7–unresponsive Lin−/loSca1+c-kit+progenitors, and in part by enhancing the growth of proB-cells. The enhanced recruitment (4-fold) in response to KL occurred exclusively from the Lin−/loSca1+c-kit+flt3−long-term repopulating stem cell population, whereas KL had no effect on FL + IL-7–stimulated recruitment of Lin−/loSca1+c-kit+flt3+short-term repopulating cells. The progeny of FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells lacked in vitro and in vivo myeloid potential, but efficiently reconstituted both B and T lymphopoiesis. In agreement with this FL, but not KL, efficiently induced expression of B220 and IL-7 receptor- on Lin−/loSca1+c-kit+flt3+cells. Thus, whereas KL appears crucial for recruitment of FL + IL-7–unresponsive candidate (c-kit+flt3−) murine stem cells, FL is essential and sufficient for development toward lymphoid restricted progenitors from a population of (c-kit+flt3+) multipotent short-term reconstituting progenitors.
THE PROCESS BY WHICH multipotent progenitors differentiate and commit into lymphoid restricted progenitors is currently poorly understood. An important requisite to be able to dissect the molecular mechanisms involved in this process is to identify and characterize the earliest stages of lymphoid development. Studies of mice deficient in Ikaros expression have demonstrated a selective loss of natural killer (NK)-, T- and B-lymphoid development implicating the potential presence of a common lymphoid progenitor cell (CLP).1 Recent data support the existence of such a CLP in adult murine bone marrow (BM).2 Specifically, single Lin−Sca1loc-kitloIL-7R+BM cells, lacking detectable myeloid potential, were demonstrated to give rise to both T and B cells. In addition, others have characterized in detail the phenotype of the earliest cells committed to the B-lymphoid lineage.3-7 However, our knowledge regarding the potential function of early acting cytokines in promoting development from candidate stem cells with a combined myeloid and lymphoid differentiation potential to these earliest stages of lymphoid restricted progenitors remains limited.
Lin−/loSca1+c-kit+ cells, although representing only approximately 0.05% of adult murine BM cells, contain virtually all long-term reconstituting stem cells (LTRC) and represent a pure population of multipotent progenitor cells.8-13 Whereas c-kit appears to be critically involved in promoting sustained self-renewal and maintenance of LTRC,14,15Lin−/loSca1+c-kit+ cells expressing flt3 have been shown to contain less LTRC than the flt3− subpopulation.16 Interestingly, whereas mice deficient in flt3-receptor expression have normal numbers of all mature cell lineages, they have a selective reduction in the number of pro and preB-cells.17 In addition, transplantation experiments with BM cells from flt3-deficient mice also showed a defect in the T-cell compartment, indicating a role for flt3, and its ligand, in early lymphoid development.17 Although specific lymphoid defects have not been reported in c-kit–deficient mice, this might be more difficult to show because c-kit–deficient mice have severe deficiencies in their stem cell pools.18,19 However, c-kit has been demonstrated to be expressed in the earliest stages of B- and T-cell progenitors as well as the CLP, and its ligand implicated in promoting early lymphoid development.2,19-25 Specifically, both c-kit–ligand (KL) and flt3-ligand (FL) have been shown to act as growth factors for early B-lymphoid progenitors, and it has been clearly demonstrated that KL can stimulate proliferation of proB, as well preB-cells, in synergy with interleukin-7 (IL-7).19,21,22,26-29 Much less effort has been devoted to establish to what degree KL and FL might also promote the transition from multipotent progenitors to CLP and subsequently to the earliest proB-cells. Whereas our previous studies have suggested that multipotent Lin−/loSca1+c-kit+ BM cells stimulated with FL + IL-7, (but not KL + IL-7) generate a virtually pure population of proB-cells,30 others have suggested that KL might promote such commitment of primitive progenitors in the BM, as well as fetal liver.6,27 31-34 Thus, the present studies were initiated to clarify and further dissect the roles of KL and FL in promoting lymphoid commitment.
Here we present data on the ability of FL and KL to promote early lymphoid development from multipotent progenitors, resolving previously seemingly contradicting results. Importantly, despite coexpression of flt3 and c-kit, FL is superior to KL at promoting growth, as well as lymphoid development of Lin−/loSca1+c-kit+flt3+cells. However, KL also has a distinct and dual effect on early lymphoid development by first allowing recruitment of FL-nonresponsive Lin−/loSca1+c-kit+flt3−cells (containing most, if not all, LTRC) to generate B-lymphoid progenitors and secondly by synergistically enhancing IL-7–dependent growth of committed proB-cell progenitors. Thus, FL and KL appear to have distinct, but complimentary roles, in promoting the earliest stages of murine lymphoid-restricted development.
MATERIALS AND METHODS
Hematopoietic growth factors.
Recombinant human (rh) IL-7 and recombinant murine (rm) IL-3 were from Peprotech (Rocky Hill, NJ). Recombinant rat (rr) KL (c-kit-ligand, stem cell factor), rhMGDF (megakaryocyte growth and development factor, thrombopoietin), rmGM-CSF (granulocyte-macrophage colony-stimulating factor) and rhG-CSF (granulocyte colony-stimulating factor) were generously provided by Amgen Corp (Thousand Oaks, CA), whereas rhFL was a kind gift from Immunex (Seattle, WA). RhEpo (erythropoietin) was provided by Boehringer Mannheim Corp (Mannheim, Germany). All growth factors were used at the following predetermined optimal concentrations: rhEpo 5 U/mL, rhFL 50 ng/mL, rhG-CSF 50 ng/mL, rmGM-CSF 20 ng/mL, rmIL-3 10 ng/mL, rhIL-7 100 ng/mL, rrKL 50 ng/mL, and rhMGDF 50 ng/mL.
Enrichment and purification of subpopulations of Lin−/loSca1+ BM cells based on expression of c-kit and flt3.
Lineage-depleted (Lin−/lo) BM cells were isolated from 6- to 10-week-old C57Bl/6 mice (Ly5.2) from M&B (Ry, Denmark) or B6SJL mice (Ly5.1) from Jackson Laboratories (Bar Harbor, ME). In some experiments cells were also isolated from transgenic mice expressing hCD25 under control of the λ5-promoter.35 Briefly, femurs and tibias were gently crushed in a mortar. Iscove’s Modified Dulbecco’s Medium (IMDM; BioWhittaker, Walkersville, MD) supplemented with 5% fetal calf serum (FCS; BioWhittaker) was used as medium throughout the isolation. The cell suspension was filtered through a 70-μm mesh filter (Falcon, Becton Dickinson, Lincon Park, NJ), white blood cells counted in a hemacytometer, and concentrated to 400 × 106 cells/mL. The cells were incubated at 4°C for 30 minutes in a cocktail of predetermined optimal concentrations of unconjugated antibodies: B220 (RA3-6B2), Gr-1 (RB6-8C5), Mac-1 (M1/70), CD8 (53-6.7), CD5 (53-7.3), CD4 (H129.19), and TER-119 (all from PharMingen, San Diego, CA).
Cells were washed once, resuspended to 250 × 106cells/mL, and sheep antirat IgG (Fc)-conjugated immunomagnetic beads (Dynal, Oslo, Norway) were added at a cell:bead ratio of 1:0.3 and incubated at 4°C for 45 minutes on a mixing wheel. Magnetic beads were removed with a magnetic particle concentrator (MPC-6, Dynal), and unattached cells transferred to a second tube containing the same absolute amount of magnetic beads, incubated for 30 minutes, and processed as in the first bead separation.
Lin−/lo cells recovered from the supernatant were further purified based on the expression of stem cell antigen-1 (Sca1) and c-kit as previously described.9,12,13 36 Briefly, Lin−/lo cells were resuspended at 100 to 400 × 106 cells/mL and incubated for 30 minutes on ice with a phycoerythrin (PE)-conjugated goat antirat antibody (Southern Biotechnology, Birmingham, AL). Subsequently, cells were washed and stained with a fluorescein isothiocyanate (FITC)-conjugated rat antimouse Ly-6A/E antibody (Sca1), and allophycocyanin (APC)-conjugated anti-CD117 (c-kit) antibody or isotype-matched control antibodies (all from PharMingen). The cells were washed and Lin−/loSca1+c-kit+ cells were sorted on a FACSVantage Cell Sorter (Becton Dickinson, San Jose, CA), equipped with an 488 nm argon and a 633 nm He-Ne laser, at a rate of 1,000 to 4,000 cells/second. Only cells expressing high, not low or intermediate, levels of c-kit were sorted. Practically all cells recovered after the magnetic bead isolation coexpressing Sca1 and c-kit fell within the Lin−/lo region (O.J.B. and S.E.W.J., unpublished observations). Reanalysis of sorted Lin−/loSca1+c-kit+ cells on a FACSCalibur (Becton Dickinson) showed reproducibly a purity of 95% to 99%. In some experiments Lin−/loSca1−c-kit−, Lin−/loSca1+c-kit− and Lin−/loSca1−c-kit+cells were sorted as well. Lin−/loSca1+c-kit+ cells were subdivided into flt3+ (20% to 25% highest expressing cells) and flt3− (those with anti-flt3 PE-fluorescence less than 20% of the maximum PE-fluorescence of the isotype control) using Sca1-FITC, c-kit-APC, and flt3-PE (PharMingen) antibodies.
Single-cell proliferation assay.
Lin−/loSca1+c-kit+ cells were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a concentration of 1 cell per well in 20 μL of serum-depleted medium, as previously described.37 The serum-depleted medium (X-vivo 15; BioWhittaker) was supplemented with 1% detoxified bovine serum albumin (BSA; StemCell Technologies, Vancouver, Canada), 100 U/mL penicillin (BioWhittaker), 100 U/mL streptomycin (BioWhittaker), 3 mg/mL L-glutamine (BioWhittaker), and freshly made 1 × 10−4 mol/L 2-mercaptoethanol (Sigma, St Louis, MO). In some experiments the presence of single cells was verified by microscopy 2 to 12 hours after plating and only wells containing 1 cell were included. Wells were scored for cell growth (≥3 cells) after 10 to 12 days of incubation at 37°C and 5% CO2 in humidified air. Individual colonies (covering more than 10% of the well) were sampled and either transferred to slides using a cytospin centrifuge (Shandon, Cheshire, UK) to be examined morphologically after Giemsa-staining (Sigma) or stained with antibodies and analyzed by flow cytometry (FACSCalibur, Becton Dickinson).
Semisolid clonogenic assays.
Colony-forming unit-granulocyte macrophage (CFU-GM) potential was evaluated using semisolid culture conditions. The cells were plated in duplicate in 1 mL IMDM with 20% FCS, 1.2% methylcellulose (MC; Methocel, Fluka Chemie, Switzerland), L-glutamine, penicillin/streptomycin, and 1 × 10−4 mol/L 2-mercaptoethanol, and supplemented with cytokines in 35-mm petri dishes. Cultures were incubated at 37°C and 5% CO2 in humidified air for 7 to 9 days, at which time colonies (>50 cells) were visualized and scored using an inverted microscope.
3H-thymidine incorporation assay was used to evaluate the proliferation potential of the progenitors derived from Lin−/loSca1+c-kit+ cells cultured for 7, 12, and 17 days in the presence of FL + IL-7 or KL + FL + IL-7. Cells were washed 3 times to remove cytokines present during the primary culture, counted, and plated in triplicate in round bottomed 96-well plates at 5 to 40 × 103cells per well in 100 μL serum-depleted medium, and cultured for 3 additional days before being pulsed with 1 μCi3H-thymidine (Nycomed Amersham, Buckinghamshire, UK). After an additional 12 hours incubation, the cells were harvested using a Scatron cell harvester (Drammen, Norway), and the amount incorporated 3H-thymidine determined using a scintillation counter (LKB Wallac, Turku, Finland).
Immunophenotyping by flow cytometry was performed as previously described30 with specified antibodies (all from PharMingen) conjugated directly to biotin, FITC, PE, or APC used at predetermined optimal concentrations. Antibodies conjugated to biotin were subsequently stained with streptavidin-PerCP (Becton Dickinson).
In vivo reconstitution experiments.
A total of 1,000 Lin−/loSca1+c-kit+ freshly isolated or in vitro–cultured cells originating from 1,000 Lin−/loSca1+c-kit+ cells was intravenously injected in the tail vein (0.5 mL per mice) of lethally irradiated (950 rad) C57Bl/6 (Ly5.2) mice. All mice were kept in individually ventilated cages throughout the experiment and given sterile food and autoclaved acidified water. Irradiated C57Bl/6 mice were cotransplanted with 150,000 unfractionated syngeneic (Ly5.2) BM cells to provide a competitor and survival population. Peripheral blood cells were collected after 6 and 10 weeks, red blood cells lyzed with ammonium chloride, and the white blood cells stained with antibodies against Ly5.1, Ly5.2, and lineage-specific antigens (all from PharMingen), and subsequently analyzed on a FACSCalibur.
Statistical analysis.
Student’s t-test was used for statistical analysis.
RESULTS
High-efficiency lymphoid-restricted development in response to FL and IL-7 is exclusively observed from the multipotent Lin−/loSca1+c-kit+ stem cell population.
Whereas multiple studies have demonstrated that FL when acting in combination with IL-7 can promote growth of early B- and T-cell progenitors,23,27-29,34,38,39 much less is known about the potential ability of FL + IL-7 to promote lymphoid commitment of progenitor/stem cells with a combined myeloid and lymphoid development potential. In previous studies we had demonstrated that FL + IL-7 could efficiently recruit approximately 10% of Lin−/loScal+ BM cells into proliferation.30 Furthermore, a high fraction of these FL + IL-7–recruited progenitor cells had myeloid potential, and although some of the resulting colonies were too small to analyze, most of them appeared to have B-lymphoid potential as well, suggesting that they at least in part were derived from cells with combined myeloid and lymphoid potential.30 Recent and further optimization of the conditions for optimal myeloid as well as B-lymphoid development from single Lin−/loSca1+c-kit+ BM cells now allowed us to demonstrate more unequivocally that FL + IL-7–responsive Lin−/loScal+c-kit+ BM cells have a combined lymphoid and myeloid lineage potential, and also establish to what extent they have such a mixed lineage potential. Toward this aim, a number of single-cell cloning experiments were initiated. In the first set of experiments, stimulation with a combination of myeloid growth factors (KL + FL + IL-3 + G-CSF + GM-CSF + MGDF + EPO) showed that as much as 95% (±2%) of Lin−/loSca1+c-kit+ cells (individually deposited and identified) had a high proliferative and myeloid potential (Table 1). In the same experiments, 42% (±1%) of the Lin−/loScal+c-kit+ progenitor cells proliferated in response to FL + IL-7, and resulting clones large enough to be analyzed by flow cytometry (63% ± 4%), all showed a proB-cell phenotype (Table 1). Accordingly, these studies clearly established that most if not all Lin−/loScal+c-kit+ progenitor cells that develop into proB-cells, in response to FL + IL-7, have a combined in vitro B-lymphoid and myeloid potential.
Single Lin−/loSca1+c-kit+ Cells Undergoing Lymphoid-Restricted Development in Response to FL + IL-7 Have a Combined Myeloid and Lymphoid Differentiation Potential
| . | Cloning Frequency . | Clones Containing Myeloid Cells . | Clones Containing ProB Cells . |
|---|---|---|---|
| Myeloid cocktail* | 95(2)% | 100(0)% | ND |
| FL + IL-7 | 42(1)% | ND | 63(4)% |
| . | Cloning Frequency . | Clones Containing Myeloid Cells . | Clones Containing ProB Cells . |
|---|---|---|---|
| Myeloid cocktail* | 95(2)% | 100(0)% | ND |
| FL + IL-7 | 42(1)% | ND | 63(4)% |
Lin−/loSca1+c-kit+ cells were plated individually in Terasaki plates, and the presence of a single cell per well was verified by microscopy less than 12 hours after initiation of culture to exclude wells containing no or more than 1 cell. After 12 days of culture, the cultures were assayed for the presence of clones (≥3 cells) and the presence of myeloid cells (macrophages and/or granulocytes) by Giemsa-staining. After a total of 15 to 25 days, colonies were analyzed by flow cytometry to investigate the presence of B220+CD19+ proB cells.
Abbreviation: ND, not determined.
Myeloid cocktail = KL + FL + IL-3 + G-CSF + GM-CSF + MGDF + Epo used at predetermined optimal concentrations. The data represent the mean (±SEM) from 4 individual experiments.
Previously, we had shown that FL + IL-7 are very efficient at promoting formation of high numbers of B-lymphoid restricted progenitors from Lin−/loSca1+c-kit+ progenitor cells.30 Because cells with this phenotype represent only approximately 0.05% of the total BM cells and have been demonstrated to contain most if not all pluripotent stem cells,9 11-13we now investigated whether FL + IL-7 also could promote such high-efficiency proB-cell formation from other early (Lin−/lo) progenitor cell populations. If so, it could not be excluded that the high output B-lymphoid production from the isolated Lin−/loScal+c-kit+ progenitor cells could be derived from contaminating cells of more abundant, but less primitive, progenitor cell populations. Interestingly, although representing only 2% of the total Lin−/lopopulation, the Lin−/loSca1+c-kit+ population was the only Lin−/lo population, which reproducibly could produce B220+CD19+ proB-cells in response to FL + IL-7. Thus, multipotent Lin−/loSca1+c-kit+ BM progenitor cells are the primary targets for FL + IL-7–stimulated in vitro proB-cell formation (Table 2).
FL + IL-7–Responsive Cells Are Exclusively Recruited From the Lin−/loSca1+c-kit+Stem Cell Population
| Cell Phenotype . | Percent of Total Lin−/low Population* . | Cell Production From 500 Starting Cells (×1,000) . | |||
|---|---|---|---|---|---|
| Lin . | Sca1 . | c-kit . | Exp 1 . | Exp 2 . | |
| −/lo | + | + | 2.0 (1.1) | 413 | 172 |
| −/lo | + | − | 4.1 (1.2) | 0 | 0 |
| −/lo | − | + | 14.2 (6.7) | 6 | 0 |
| −/lo | − | − | 79.7 (43.5) | 0 | 0 |
| Cell Phenotype . | Percent of Total Lin−/low Population* . | Cell Production From 500 Starting Cells (×1,000) . | |||
|---|---|---|---|---|---|
| Lin . | Sca1 . | c-kit . | Exp 1 . | Exp 2 . | |
| −/lo | + | + | 2.0 (1.1) | 413 | 172 |
| −/lo | + | − | 4.1 (1.2) | 0 | 0 |
| −/lo | − | + | 14.2 (6.7) | 6 | 0 |
| −/lo | − | − | 79.7 (43.5) | 0 | 0 |
In 2 separate experiments lineage-depleted BM cells were sorted into 4 phenotypically distinct subpopulations, which each were investigated for their ability to respond to FL + IL-7. The total cell production from each subpopulation was established after 12 days of culture and presented as number of cells produced from 500 starting cells.
The percentage of the entire population is shown, whereas the size of each of the sorted populations is shown within the parenthesis.
B220+CD19− cells derived from multipotent Lin−/loSca1+c-kit+ progenitor cells develop almost exclusively into B-lymphoid committed progenitor cells.
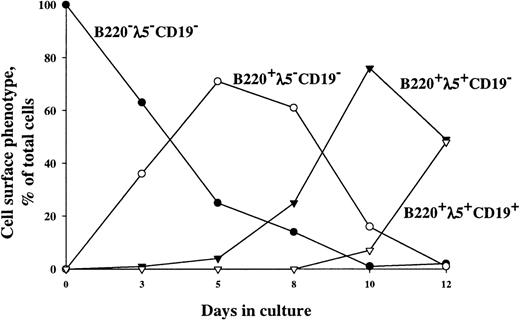
Stimulation of Lin−/loScal+c-kit+ cells by FL + IL-7 results in generation of 2 populations of B220+cells, 1 expressing CD19 and the other lacking CD19 expression (Fig 1).36 Whereas expression of CD19 is believed to be B-lymphoid–specific, B220 is not.5,40 Thus, although previous reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of Lin−/loSca1+c-kit+–derived B220+CD19− cells had indicated that these cells contained B-lymphoid–committed progenitor cells,36it remained possible that most of the B220+CD19− cells produced in response to FL + IL-7 were not yet committed to the B-lymphoid lineage. Thus, in the present studies, we used transgenic mice expressing hCD25 under control of the λ5-promoter to investigate the timed expression of hCD25 as an indicator of B-lymphoid–specific λ5-expression in B220+CD19+ and B220+CD19− cells derived from Lin−/loScal+c-kit+ (all hCD25/λ5−) progenitor cells in response to FL+IL-7. B220−CD19−λ5−cells dominated early in culture, but these were gradually replaced by B220+CD19− cells (almost 40% of cells at day 3), which initially were λ5−, but eventually (after 12 days) became almost exclusively λ5+, suggesting commitment to the B-lymphoid lineage. Finally, and as expected, B220+CD19+ cells appeared later (8 days) in culture and were all λ5+. Thus, FL+IL-7 promote formation of B220+CD19− as well as B220+CD19+-committed proB-cell progenitors from multipotent Lin−/loScal+c-kit+ progenitor cells.
Timed expression of λ5, B220, and CD19 on Lin−/loSca1+c-kit+ cells cultured in FL + IL-7. Cultures were initiated with Lin−/loSca1+c-kit+ (all hCD25−) cells isolated from transgenic mice expressing hCD25 under control of the λ5-promoter.35 Cells were supplemented with FL + IL-7, and at each indicated time point, cells were analyzed for the coexpression of B220, hCD25(λ5), and CD19 by flow cytometry. One representative experiment of 3 individual experiments is shown. In initial control experiments in which hCD25− and hCD25+ cells were sorted from FL + IL-7–stimulated cultures, endogenous λ5 mRNA was detected by RT-PCR in the hCD25+ fraction, but not in the hCD25− fraction, demonstrating the specificity and sensitivity of hCD25 expression as a marker for λ5 expression (data not shown).
Timed expression of λ5, B220, and CD19 on Lin−/loSca1+c-kit+ cells cultured in FL + IL-7. Cultures were initiated with Lin−/loSca1+c-kit+ (all hCD25−) cells isolated from transgenic mice expressing hCD25 under control of the λ5-promoter.35 Cells were supplemented with FL + IL-7, and at each indicated time point, cells were analyzed for the coexpression of B220, hCD25(λ5), and CD19 by flow cytometry. One representative experiment of 3 individual experiments is shown. In initial control experiments in which hCD25− and hCD25+ cells were sorted from FL + IL-7–stimulated cultures, endogenous λ5 mRNA was detected by RT-PCR in the hCD25+ fraction, but not in the hCD25− fraction, demonstrating the specificity and sensitivity of hCD25 expression as a marker for λ5 expression (data not shown).
c-kit activation in combination with FL + IL-7 is essential for efficient B-lymphoid development from candidate murine stem cells.
Our previous studies had suggested that FL was far superior to KL at promoting in vitro lymphoid development from Lin−/loSca1+c-kit+ BM progenitor cells.30 In seemingly contrast, a number of other studies suggested that KL had an ability to enhance growth of early B-lymphoid progenitors, and thus potentially also lymphoid development from candidate murine stem cells.6,19,21,22,27 31-34 However, the level of commitment of the KL-responsive progenitors remained unclear in these studies. In an effort to better uncover and resolve the effects of KL on the earliest stages of lymphoid commitment and development, in particular in relationship to the effects mediated by FL, we investigated to what degree KL might enhance the ability of FL + IL-7 to promote B-lymphoid development from Lin−/loSca1+c-kit+ progenitor cells.
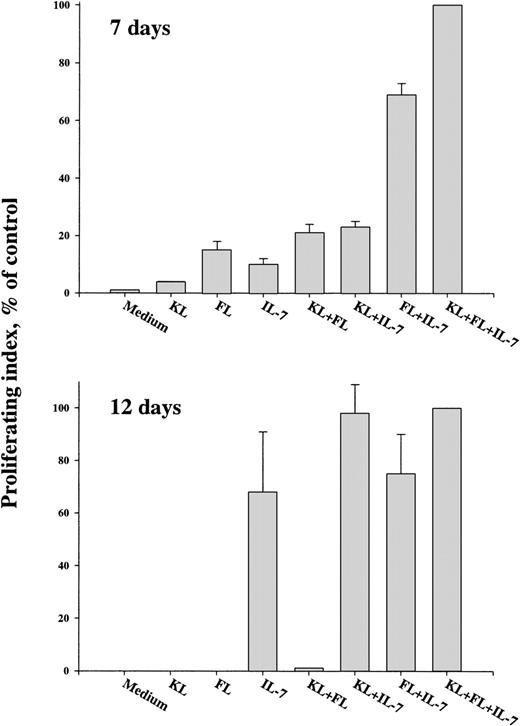
In agreement with our previous studies,30 a 292-fold cellular expansion was observed when Lin−/loSca1+c-kit+ BM cells were cultured for 12 days in the presence of FL + IL-7 (Fig 2) and 55% of these showed a proB-cell phenotype (B220+CD19+). In contrast, in cultures stimulated with KL + IL-7, none of the cells produced by day 12 had a B220+CD19+ phenotype and only 6% of the cells were B220+CD19−. Rather, most of the cellular expansion (293-fold) observed in response to KL + IL-7 resulted from production of myeloid cells (41% ± 11% Gr1/Mac1+). In agreement with this, single-cell cloning experiments with Lin−/loSca1+c-kit+ cells never showed any clonal formation (0% ± 0%) of B220+CD19+ cells in response to KL + IL-7, whereas as many as 26% of the Lin−/loSca1+c-kit+ cells generated large proB-cell clones in response to FL + IL-7 (Table 1). Combined stimulation with KL and FL resulted in a 148-fold cell expansion by 12 days, but as for KL + IL-7–stimulated cultures, none of these cells expressed the B-lymphoid–specific marker, CD19 (Fig 2), and were rather predominantly Gr1/Mac1+ (42% ± 8%). However, when combined with FL + IL-7, KL enhanced cell expansion 5.3-fold (Fig 2), and although 4% ± 3% of these cells expressed Gr1/Mac1, most were B220+CD19+, suggesting that the growth-promoting effect of KL was predominantly mediated through an increase in proB-cell formation. A more detailed cell surface phenotyping after 17 days of culture showed a similar proB-cell phenotype of cells generated in KL + FL + IL-7 as in FL + IL-7 (Table 3), with a fraction of the B220+CD19+ cells becoming BP-1+ by day 17, but with no cells expressing cytoplasmic (or cell surface) IgM.
KL enhances FL + IL-7–stimulated proB-cell formation from Lin−/loSca1+c-kit+progenitors. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of the indicated cytokines. After 12 days of culture, total cells were counted and analyzed for expression of B220 and CD19. The B220+ and CD19+ bars indicate the fraction of total cells represented by B220+and CD19+ cells, respectively. Data presented are the mean (+standard error of mean [SEM]) of 4 individual experiments.
KL enhances FL + IL-7–stimulated proB-cell formation from Lin−/loSca1+c-kit+progenitors. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of the indicated cytokines. After 12 days of culture, total cells were counted and analyzed for expression of B220 and CD19. The B220+ and CD19+ bars indicate the fraction of total cells represented by B220+and CD19+ cells, respectively. Data presented are the mean (+standard error of mean [SEM]) of 4 individual experiments.
Phenotypic Characterization of Day 17 Progeny of FL + IL-7 and KL + FL + IL-7–Stimulated Lin−/loSca1+c-kit+ Cells
| Antigen . | % Expression . | |
|---|---|---|
| FL + IL-7 . | KL + FL + IL-7 . | |
| B220 | 82 (7) | 81 (9) |
| CD19 | 49 (13) | 67 (11) |
| CD24 | 99 (0) | 94 (4) |
| CD43 | 89 (8) | 78 (9) |
| cIgM | 0 (0) | 0 (0) |
| c-kit | 89 (4) | 93 (3) |
| flt3 | 0 (0) | 0 (0) |
| Gr1/Mac1 | 0 (0) | 4 (2) |
| Antigen . | % Expression . | |
|---|---|---|
| FL + IL-7 . | KL + FL + IL-7 . | |
| B220 | 82 (7) | 81 (9) |
| CD19 | 49 (13) | 67 (11) |
| CD24 | 99 (0) | 94 (4) |
| CD43 | 89 (8) | 78 (9) |
| cIgM | 0 (0) | 0 (0) |
| c-kit | 89 (4) | 93 (3) |
| flt3 | 0 (0) | 0 (0) |
| Gr1/Mac1 | 0 (0) | 4 (2) |
Lin−/loSca1+c-kit+ cells were cultured in serum-depleted medium supplemented with FL + IL-7 or KL + FL + IL-7 for 17 days, at which time cells were analyzed for the expression of the indicated antigens. Data represent the mean (±SEM) from 3 individual experiments.
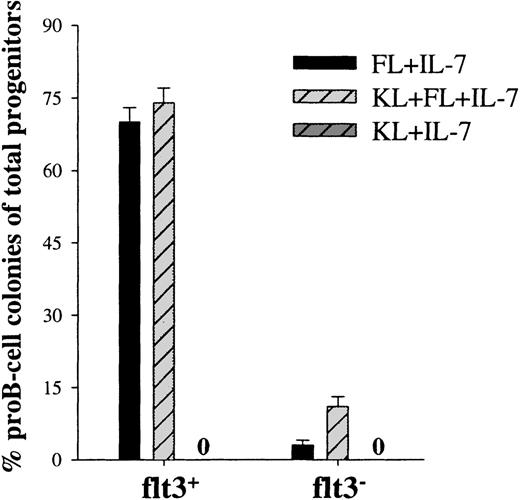
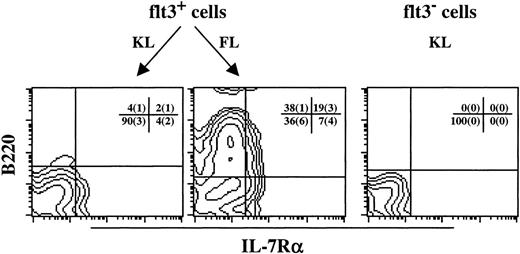
The potent ability of KL to enhance FL + IL-7–stimulated proB-cell formation (Fig 2) could either be the result of enhanced proliferation of cells already committed to the B-lymphoid lineage in response to FL + IL-7, and/or it could reflect recruitment of an increased number of uncommitted Lin−/loSca1+c-kit+ cells toward B-lymphoid development. Single-cell cloning experiments with Lin−/loSca1+c-kit+ cells showed that KL enhanced FL + IL-7–stimulated formation of proB-cell clones by 33% (O.J.B. and S.E.W.J., unpublished observations). We next addressed whether this rather limited increase in proB-cell development was a result of enhanced recruitment from the Lin−/loSca1+c-kit+flt3+and/or Lin−/loSca1+c-kit+flt3−subpopulations. Such a distinction is of considerable importance, as only the flt3− subpopulation of Lin−/loSca1+ cells has been demonstrated to be highly enriched in LTRC.16 In agreement with this, Lin−/loSca1+c-kit+flt3+cells isolated in the present studies provided efficient short-term (4 weeks), but little long-term reconstitution (6 months) in lethally irradiated recipients, whereas Lin−/loSca1+c-kit+flt3−cells were highly enriched in LTRC (O.J.B. and S.E.W.J., unpublished observation). In support of the studies on the whole Lin−/lo Sca1+c-kit+population, neither flt3+ nor flt3− cells showed any proB-cell formation in response to KL + IL-7 (Fig 3). As expected, virtually all of the FL + IL-7 responders were located in the flt3+ population, resulting in as much as 70% of the flt3+ progenitors forming proB-cell clones (Fig 3). This response could not be further enhanced by KL. The successful isolation of a flt3−population was supported by as little as 3% of these progenitors forming proB-cell clones in response to FL + IL-7 (Fig 3). Interestingly, KL enhanced FL + IL-7–stimulated proB-cell colony formation almost 4-fold in the flt3− subpopulation (Fig 3; P < .01). Thus, importantly, whereas FL + IL-7 efficiently promote B-lymphoid development from a flt3+-population of short-term reconstituting stem cells, KL appears important for early B-lymphoid development from the Lin−/loSca1+c-kit+flt3−population containing most if not all LTRC.
KL, as well as FL, are required for optimal proB-cell development from flt3− candidate murine stem cells. Lin−/loSca1+c-kit+ cells were sorted into flt3+ and flt3− populations and cultured in the presence of the indicated cytokines at a density of 1 cell per well. Fifteen to 25 days after initiation of culture, individual colonies were picked and analyzed by flow cytometry to verify the presence of proB-cells, as defined by combined B220 and CD19 expression. No proB-colonies were found in response to KL + IL-7. Results represent the mean (+SEM) of 4 individual experiments. Cells stimulated with an optimal combination of cytokines (KL + FL + MGDF + IL-3 + G-CSF + GM-CSF + Epo) served as a control for total number of in vitro clonogenic progenitors. Three independent single-cell experiments with flt3+ and flt3− cells showed a high cloning frequency, 98 (1)% and 97 (1)%, respectively. Thus, the data are presented as percent B-lymphoid colonies of the total number of clonogenic progenitors.
KL, as well as FL, are required for optimal proB-cell development from flt3− candidate murine stem cells. Lin−/loSca1+c-kit+ cells were sorted into flt3+ and flt3− populations and cultured in the presence of the indicated cytokines at a density of 1 cell per well. Fifteen to 25 days after initiation of culture, individual colonies were picked and analyzed by flow cytometry to verify the presence of proB-cells, as defined by combined B220 and CD19 expression. No proB-colonies were found in response to KL + IL-7. Results represent the mean (+SEM) of 4 individual experiments. Cells stimulated with an optimal combination of cytokines (KL + FL + MGDF + IL-3 + G-CSF + GM-CSF + Epo) served as a control for total number of in vitro clonogenic progenitors. Three independent single-cell experiments with flt3+ and flt3− cells showed a high cloning frequency, 98 (1)% and 97 (1)%, respectively. Thus, the data are presented as percent B-lymphoid colonies of the total number of clonogenic progenitors.
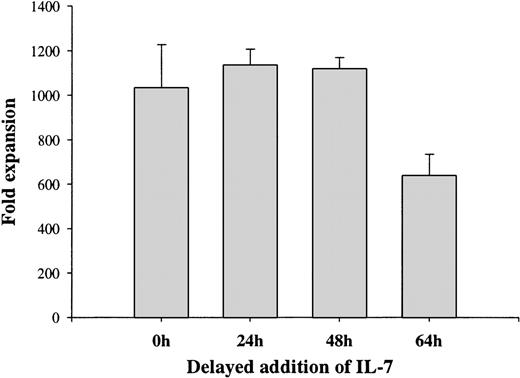
Delayed addition studies were next performed to determine to what degree FL, KL, and IL-7 were required to promote B-lymphoid development from Lin−/loSca1+c-kit+ cells at an early stage or whether they might act in a sequential manner. Delaying addition of IL-7 to FL-stimulated cultures for as little as 64 hours resulted in reduced formation of proB-cells by as much as 38%, whereas a 24- or 48-hour delay had no effect (Fig 4). Thus, FL-stimulated Lin−/loSca1+c-kit+progenitors become IL-7 responsive at an early stage of development. Interestingly, other studies demonstrated early (by 40 hours) requirements for KL as well as FL activation for optimal proB-cell formation from the Lin−/loSca1+c-kit+flt3−population (Table 4), suggesting that KL might rapidly induce FL responsiveness in the Lin−/loSca1+c-kit+flt3−stem cell population.
Requirement for IL-7 during early lymphoid development from Lin−/loSca1+c-kit+progenitors. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of FL from initiation of culture. IL-7 was added to the cultures at the indicated time points, whereas KL was added to all groups 64 hours after initiation of culture. Total cell expansion was evaluated 12 days after initiation of culture. Each data point represents the mean (+SEM) of 3 individual experiments with duplicate wells in each experiment.
Requirement for IL-7 during early lymphoid development from Lin−/loSca1+c-kit+progenitors. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of FL from initiation of culture. IL-7 was added to the cultures at the indicated time points, whereas KL was added to all groups 64 hours after initiation of culture. Total cell expansion was evaluated 12 days after initiation of culture. Each data point represents the mean (+SEM) of 3 individual experiments with duplicate wells in each experiment.
Requirement for Early KL and FL Activation for Optimal ProB-Cell Formation From Lin−/loSca1+c-kit+flt3−Cells
| Cytokines . | No. of B220+ Cells Produced per Lin−/loSca1+c-kit+flt3−Cell . | |
|---|---|---|
| 0 to 40 Hours . | 40 Hours to 12 Days . | |
| Medium | KL + FL + IL-7 | 3 (2) |
| KL | KL + FL + IL-7 | 56 (30) |
| FL | KL + FL + IL-7 | 23 (12) |
| KL + FL | KL + FL + IL-7 | 289 (40) |
| Cytokines . | No. of B220+ Cells Produced per Lin−/loSca1+c-kit+flt3−Cell . | |
|---|---|---|
| 0 to 40 Hours . | 40 Hours to 12 Days . | |
| Medium | KL + FL + IL-7 | 3 (2) |
| KL | KL + FL + IL-7 | 56 (30) |
| FL | KL + FL + IL-7 | 23 (12) |
| KL + FL | KL + FL + IL-7 | 289 (40) |
Lin−/loSca1+c-kit+flt3−cells were preincubated in serum-depleted medium alone or in the presence of KL, FL, or KL + FL for 40 hours before being supplemented with KL + FL + IL-7 for the rest of the 12-day culture period. After a total of 12 days cells were harvested, counted, and analyzed for the expression of B220. The data represent the mean (±SEM) from 4 individual experiments.
KL enhances FL + IL-7–stimulated proliferation of lymphoid-committed progenitor cells derived from Lin−/loSca1+c-kit+ progenitor cells.
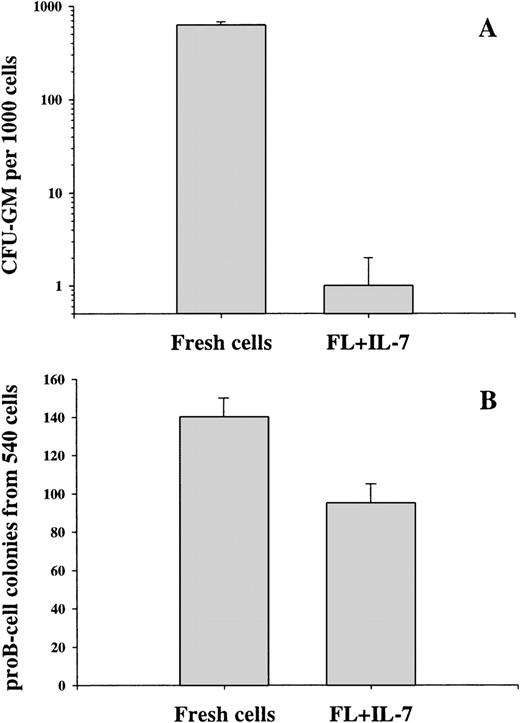
Whereas 63% of fresh Lin−/loSca1+c-kit+ cells formed myeloid colonies in methylcellulose, only 0.1% of the cells generated after 12 days in FL + IL-7 maintained such an ability (Fig 5A). In comparison, the frequency of FL + IL-7–derived cells capable of forming proB-cell colonies was 260-fold higher than those with a myeloid potential and almost as high as in the starting Lin−/loSca1+c-kit+ population (Fig 5B).
In vitro myeloid and lymphoid potential of progeny derived from FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of FL + IL-7 for 12 days, at which time cells were washed and either transferred to myeloid MC cultures (duplicates) supplemented with G-CSF + GM-CSF + IL-3 + KL (A) or plated at a density of 1 cell per well in serum-depleted medium supplemented with FL + IL-7 (B). The CFU-GM content was analyzed 7 to 10 days after transfer and the proB-cell potential was evaluated (as described in Fig 3) after 12 to 20 days of culture. The results represent the mean (+SEM) of 3 individual experiments.
In vitro myeloid and lymphoid potential of progeny derived from FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of FL + IL-7 for 12 days, at which time cells were washed and either transferred to myeloid MC cultures (duplicates) supplemented with G-CSF + GM-CSF + IL-3 + KL (A) or plated at a density of 1 cell per well in serum-depleted medium supplemented with FL + IL-7 (B). The CFU-GM content was analyzed 7 to 10 days after transfer and the proB-cell potential was evaluated (as described in Fig 3) after 12 to 20 days of culture. The results represent the mean (+SEM) of 3 individual experiments.
Because these studies clearly showed an almost exclusive development of lymphoid-restricted progenitors in response to FL + IL-7, we next investigated the cytokine responsiveness of these progenitors. Cells derived from Lin−/loSca1+c-kit+ cells in response to FL + IL-7 were washed and replated in medium with or without fresh cytokines after 7, 12, and 17 days incubation. Whereas fresh Lin−/loSca1+c-kit+cells showed absolutely no responsiveness to IL-7 alone (O.J.B. and S.E.W.J., unpublished observations), day 7 cells showed a significant IL-7 responsivess (Fig 6). However, KL (2.3-fold) and in particular FL (6.9-fold) synergistically enhanced the IL-7 responsiveness, and KL further enhanced FL + IL-7–stimulated growth. This suggested that KL enhances FL + IL-7–stimulated proB-cell formation at 2 levels; by promoting recruitment of multipotent Lin−/loSca1+c-kit+flt3−progenitors and by enhancing growth of lymphoid-restricted progenitors. Regardless of whether day 7 cells were stimulated with IL-7, FL + IL-7, or KL + IL-7, production of cells with a proB-cell phenotype dominated the cultures, and no IgM+ (surface or cytoplasmic) cells were observed (O.J.B. and S.E.W.J., unpublished observation).
Cytokine responsiveness of progeny derived from FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells. Lin−/loSca1+c-kit+ cells were cultured in the presence of FL + IL-7. After 7 or 12 days, cells were harvested, washed, and stimulated with the indicated cytokines for an additional 3 days before 3H-thymidine incorporation was determined (Materials and Methods). The data represent the mean (+SEM) of 3 experiments, in which KL + FL + IL-7–induced3H-thymidine uptake was used as a control (100%) in each experiment.
Cytokine responsiveness of progeny derived from FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells. Lin−/loSca1+c-kit+ cells were cultured in the presence of FL + IL-7. After 7 or 12 days, cells were harvested, washed, and stimulated with the indicated cytokines for an additional 3 days before 3H-thymidine incorporation was determined (Materials and Methods). The data represent the mean (+SEM) of 3 experiments, in which KL + FL + IL-7–induced3H-thymidine uptake was used as a control (100%) in each experiment.
Cells obtained after 12 days (Fig 6) or 17 days (O.J.B. and S.E.W.J., unpublished observation, March 1998) of culture in FL + IL-7 showed a dramatically different pattern of cytokine responsiveness than the starting and day 7 populations. Most importantly, cells now responded strongly and almost optimally to IL-7 alone (Fig 6). Flt3-expression is suggested to decrease with increased B-lymphoid differentiation,19 41-44 and in agreement with this, the day 17 cells had no detectable flt3-expression (Table 3) and FL had no ability to enhance IL-7–induced proliferation, whereas KL synergized slightly with IL-7 (Fig 6).
FL induces B220 and IL-7Rα expression on Lin−/loSca1+c-kit+flt3+progenitor cells.
As previously demonstrated by others,2 45 freshly isolated Lin−/loSca1+c-kit+ cells did not express detectable levels of IL-7Rα (O.J.B. and S.E.W.J., unpublished observation). However, as our delayed addition studies suggested that FL induced IL-7 responsiveness within a few days of incubation, the ability of FL to induce expression of B220 and IL-7Rα on Lin−/loSca1+c-kit+flt3+cells was investigated (Fig 7). After 7 to 8 days of stimulation with FL, 26% of the cells expressed detectable levels of IL-7Rα, and most of these coexpressed B220. In addition, 38% of FL-stimulated cells expressed B220, but lacked detectable IL-7Rα expression. Noteworthy, KL, inefficient at supporting B-lymphoid development from Lin−/loSca1+c-kit+flt3+progenitors, induced expression of IL-7Rα on only 6% of the cells, and most of these did not coexpress B220. Furthermore, because FL-stimulated cultures contained 8-fold more cells, the formation of IL-7Rα+ cells was more than 30-fold higher in response to FL than KL. In contrast to what was observed on flt3+cells, KL was incapable of inducing IL-7Rα expression on Lin−/loSca1+c-kit+flt3−cells, suggesting that the ability of KL to enhance FL + IL-7–stimulated proB-cell formation from Lin−/loSca1+c-kit+flt3−cells is not mediated through induction of IL-7Rα, but more likely results from acquisition of FL responsiveness as indicated by the delayed addition studies (Table 4).
FL, but not KL, efficiently promotes development of B220+IL-7R+ cells from Lin−/loSca1+c-kit+flt3+progenitors. A total of 2 to 10,000 Lin−/loSca1+c-kit+flt3+(flt3+) and Lin−/loSca1+c-kit+flt3−(flt3−) cells was cultured in serum-depleted medium supplemented with either KL or FL, as depicted in the figure. After 7 to 8 days in culture, cells were counted and evaluated for B220 and IL-7R expression. The figure shows 1 representative experiment of 3 individual experiments, whereas the mean (±SEM) is shown for each quadrant. Flt3− cells cultured in FL did not survive and thus could not be investigated.
FL, but not KL, efficiently promotes development of B220+IL-7R+ cells from Lin−/loSca1+c-kit+flt3+progenitors. A total of 2 to 10,000 Lin−/loSca1+c-kit+flt3+(flt3+) and Lin−/loSca1+c-kit+flt3−(flt3−) cells was cultured in serum-depleted medium supplemented with either KL or FL, as depicted in the figure. After 7 to 8 days in culture, cells were counted and evaluated for B220 and IL-7R expression. The figure shows 1 representative experiment of 3 individual experiments, whereas the mean (±SEM) is shown for each quadrant. Flt3− cells cultured in FL did not survive and thus could not be investigated.
FL + IL-7–derived progeny of multipotent Lin−/loSca1+c-kit+progenitors have ability to short-term reconstitute lymphoid, but not myeloid, cell lineages in vivo.
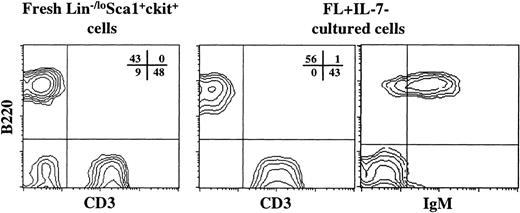
In vivo experiments were next initiated to further characterize the progeny generated from Lin−/loSca1+c-kit+ cells in response to FL + IL-7. The objectives of these studies were multiple. First, we wanted to determine to what degree these cells maintained an ability to reconstitute in vivo. Next, it was important to show that in vitro FL + IL-7–derived cells, which were blocked at the proB-cell stage under appropriate conditions, could develop into normal mature circulating B cells. Furthermore, although our in vitro studies had suggested that these cells would lack myeloid reconstituting ability, we wanted to confirm this through in vivo reconstitution experiments. Finally, and most importantly, we examined whether FL + IL-7–derived cells also had an ability to regenerate T cells. This possibility was supported by the persistence of a minor (1% to 2%) portion of B220−CD19− as well as B220+CD19− cells lacking myeloid potential as well as expression of B-lymphoid–specific markers after as much as 12 days of incubation (Fig 1). Thus, the day 12 FL + IL-7–derived progeny from 1,000 Lin−/loSca1+c-kit+ cells (Ly5.1) were transplanted into lethally irradiated C57Bl/6 mice (Ly5.2). The level of reconstitution in the peripheral blood was assayed after 10 weeks. A high level of donor-derived Ly5.1 (mean of 12%) reconstitution was observed in the peripheral blood of all transplanted mice. In addition to donor-derived B cells, CD3+ T cells were observed in all transplanted mice (Fig 8). These CD3+ cells included single positive CD4 and CD8 cells (O.J.B. and S.E.W.J., unpublished observations, February 1999). Importantly, myeloid cells (Gr1/Mac1+CD3−B220−) could not be detected in any of the mice analyzed, whereas myeloid cells could easily be detected in mice transplanted with freshly isolated Lin−/loSca1+c-kit+ cells (Fig8). Thus, FL + IL-7 specifically promote development of lymphoid-restricted progenitors from multipotent Lin−/loSca1+c-kit+ BM cells.
FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells support lymphoid-restricted reconstitution in vivo. A total of 1,000 Lin−/loSca1+c-kit+ cells from B6SJL mice (Ly5.1, donor) was either transplanted directly or cultured in the presence of FL + IL-7 for 14 days before transplantation into lethally irradiated C57Bl/6 mice (Ly5.2, recipient), together with 150,000 (Ly5.2) fresh BM cells. Peripheral blood was analyzed for the presence of donor and recipient-derived cells and lineage distribution 6 and 10 weeks after transplantation. Similar results were obtained at both time points. The CD3 and B220 reconstitution (only from Ly5.1 donor cells) from 1 typical mouse is shown for fresh, as well as FL + IL-7–cultured cells. In addition, for FL + IL-7–cultured cells, the coexpression of B220 and IgM on circulating B cells is shown. The percentage in each quadrant shows the mean from 2 or 3 mice (in 1 of 2 representative experiments) in the fresh Lin−/loSca1+c-kit+ and FL + IL-7–culture group, respectively. B220−CD3− cells detected when transplanting fresh Lin−/loSca1+c-kit+cells were practically all Gr1/Mac1+ myeloid cells (J.A. and S.E.W.J., unpublished observations, February 1999).
FL + IL-7–stimulated Lin−/loSca1+c-kit+ cells support lymphoid-restricted reconstitution in vivo. A total of 1,000 Lin−/loSca1+c-kit+ cells from B6SJL mice (Ly5.1, donor) was either transplanted directly or cultured in the presence of FL + IL-7 for 14 days before transplantation into lethally irradiated C57Bl/6 mice (Ly5.2, recipient), together with 150,000 (Ly5.2) fresh BM cells. Peripheral blood was analyzed for the presence of donor and recipient-derived cells and lineage distribution 6 and 10 weeks after transplantation. Similar results were obtained at both time points. The CD3 and B220 reconstitution (only from Ly5.1 donor cells) from 1 typical mouse is shown for fresh, as well as FL + IL-7–cultured cells. In addition, for FL + IL-7–cultured cells, the coexpression of B220 and IgM on circulating B cells is shown. The percentage in each quadrant shows the mean from 2 or 3 mice (in 1 of 2 representative experiments) in the fresh Lin−/loSca1+c-kit+ and FL + IL-7–culture group, respectively. B220−CD3− cells detected when transplanting fresh Lin−/loSca1+c-kit+cells were practically all Gr1/Mac1+ myeloid cells (J.A. and S.E.W.J., unpublished observations, February 1999).
DISCUSSION
The hematologic actions of KL and FL are predominantly restricted to the early progenitor/stem cell compartment, and both ligands and their corresponding tyrosine kinase receptors have been demonstrated to play key roles in early hematopoiesis.19 Thus, physiologically KL appears to be involved in the maintenance and expansion of long-term repopulating stem cells and regulation of myeloerythropoiesis,14,15,19,46 whereas FL appears to primarily play a role in early lymphoid, and in particular, early B-lymphoid development.17 19 Despite these rather clear distinctions in nonredundant hematopoietic functions, there are several unresolved and key questions relating to the effects mediated through c-kit and flt3, in particular to their effects on progenitor/stem cells with a combined myeloid and lymphoid differentiation potential. Thus, in the present studies, we specifically addressed: (1) to what degree such multipotent progenitor/stem cells express c-kit and flt3 and (2) whether KL and FL can promote the transition from multipotent adult BM progenitor/stem cells to lymphoid-restricted progenitor cells.
A number of studies have clearly demonstrated that both KL and FL can promote the growth of lymphoid-restricted progenitor cells.19-23,25-29 What remains unclear and somewhat disputed is at which level of development these two cytokines can promote growth and development of lymphoid progenitor cells. Thus, the present studies focused primarily on the potential ability of these two cytokines to promote the transition from multipotent progenitor cells (with a combined myeloid and lymphoid differentiation potential) to lymphoid-restricted progenitors. The challenge (and, thus, often weakness) of such studies is that firm conclusions regarding the transition from a multipotent progenitor to a lineage-restricted progenitor require ultimate proof that the initial targets/responders had such a mixed lineage potential and that the resulting progeny are more restricted in their lineage potential. Thus, the main limitations with such studies are usually the heterogeneity of the multipotent progenitor cell population, the inefficiency of the specific progenitor/lineage assays, and the burden of proof for demonstrating absence of a lineage potential. These are also likely explanations as for why limited and partially conflicting data have been available with regard to the abilities of KL and FL (as well as other cytokines) to promote the transition from multipotent to lymphoid-restricted progenitor cells. Although our previous studies had already suggested that FL + IL-7 could promote such a transition,30 36 the conclusions were somewhat limited by a low fraction of FL + IL-7 responders, an even lower fraction of responders that could be analyzed, and by potential limitations of the assays used to demonstrate presence and absence of lineage potentials. In the present studies, we have overcome these challenges allowing us not only to reach more definite conclusions, but also provide new and important information regarding the distinctions between FL and KL in promoting lymphoid commitment and development.
By using a purified population of Lin−/loSca1+c-kit+flt3+BM cells, we demonstrate at the single-cell level that virtually all (97% to 98%) starting cells have a myeloid differentiation potential and that as much as 70% of these undergo lymphoid-restricted development in response to FL + IL-7. This unequivocally demonstrates that the Lin−/loSca1+c-kit+flt3+cells undergoing lymphoid-restricted development in response to FL + IL-7 originally had a mixed myeloid-lymphoid differentiation potential. In contrast, no Lin−/loSca1+c-kit+flt3+progenitors underwent lymphoid-restricted development in response to KL + IL-7. Importantly, these observations were confirmed in single-cell serum-depleted cultures, excluding indirect effects of accessory cells. In addition to pointing to a unique difference between c-kit and flt3 in promoting lymphoid commitment from multipotent BM progenitors, these observations are particularly intriguing in light of the fact that the starting cells all expressed high levels of c-kit. Thus, the inability of KL to promote lymphoid development from Lin−/loSca1+c-kit+flt3+cells could not be explained by lack of c-kit receptor expression. Although the mechanism for lack of KL responsiveness was not addressed in these studies, it was not due to a suboptimal KL preparation, as this efficiently promoted myeloid growth from Lin−/loSca1+c-kit+ cells (O.J.B. and S.E.W.J., unpublished observations). Neither was it due to a blocking effect of the anti–c-kit antibody used for isolation of Lin−/loSca1+c-kit+flt3+cells, because the Lin−/loSca1+c-kit+flt3−cells (isolated simultaneously) responded optimally to KL and because Lin−Sca1+ cells that (were not further purified based on c-kit expression) showed the same lack of lymphoid development in response to KL+IL-7.30
The conclusion that multipotent Lin−/loSca1+c-kit+flt3+progenitors undergo lymphoid-restricted development in response to FL + IL-7 was based on demonstration both in vitro (morphology and phenotyping) and in vivo (phenotyping) that their progeny lacked myeloid differentiation potential. In addition, it was demonstrated that FL + IL-7–stimulated Lin−/loSca1+c-kit+flt3+cells efficiently produced B220+CD19+proB-cells in vitro and that these promoted in vivo reconstitution of surface IgM+ B-cells for as much as 10 weeks. Because a fraction of the B220+ cells generated in vitro lacked CD19 expression, it remained possible that there might be cells generated in response to FL + IL-7, which were not B-lymphoid committed. However, as virtually all B220+CD19− cells generated in vitro in response to FL + IL-7 appeared to coexpress λ5, thought to be expressed in a B-lymphoid–specific manner,4,7,35 it appeared that most if not all cells generated from Lin−/loSca1+c-kit+flt3+cells in response to FL + IL-7 were committed to the B-lymphoid lineage. Thus, it was surprising that all mice transplanted with FL + IL-7–stimulated Lin−/loSca1+c-kit+flt3+cells also reconstituted with high levels of circulating mature T cells. Although not the scope of these studies, we are currently addressing the identity of the lymphoid progenitors reconstituting T cells. The very few B220−CD19−and/or B220+CD19−λ5−cells remaining in the cultures could be the source of the observed T-cell reconstitution, however, it is tempting to also speculate that λ5 might not necessarily be B-lymphoid–specific and could potentially also be expressed in a bipotent B-/T-cell progenitor. In this regard, it is noteworthy that our kinetic studies clearly demonstrated that λ5 expression on B220+ cells always preceded CD19 expression. Also other lineage-“specific” genes have recently been shown to be expressed before lineage commitment.47 48
Lin−Sca1+c-kit+ BM cells have been shown to be highly enriched in long-term reconstituting activity when compared with Lin−Sca1+c-kit−cells.11-13 Previous studies have proposed that although most Lin−Sca1+ BM stem cells lack flt3 expression, there were also Lin−Sca1+flt3+LTRC.16 In contrast, recent and more detailed studies from our laboratory suggest that long-term multilineage reconstitution is exclusively derived from Lin−/loSca1+c-kit+flt3−and not Lin−/loSca1+c-kit+flt3+BM cells (O.J.B. and S.E.W.J., unpublished observations). However, we cannot exclude that there are flt3low LTRC with flt3 expression below our level of detection, although it was evident that the sorted flt3− cells unlike the flt3+ cells failed to respond to FL. In that regard, it was noteworthy that KL was found to be crucial for optimal B-lymphoid development from the Lin−/loSca1+c-kit+flt3−candidate stem cell population. Interestingly, although showing little or no response to FL + IL-7 alone, FL was absolutely required (together with KL and IL-7) for lymphoid-restricted development from the Lin−/loSca1+c-kit+flt3−cells. Although not specifically investigated, it seems likely that KL is acting by inducing flt3 expression and/or responsiveness of Lin−/loSca1+c-kit+flt3−cells, as FL had no effect on the level of recruitment of Lin−/loSca1+c-kit+flt3−progenitors seen in response to KL and as KL showed no ability to induce IL-7Rα expression on Lin−/loSca1+c-kit+flt3−cells. In addition to its ability to enhance lymphoid development from the Lin−/loSca1+c-kit+flt3−population, KL also enhanced the growth of proB-cell progenitors generated in response to FL + IL-7. These in vitro–derived proB-cells showed a normal growth and development pattern in that they remained IL-7–dependent, but gradually lost their FL and subsequently KL-dependence.19
The finding that FL and not KL can promote lymphoid commitment from a multipotent progenitor cell population is intriguing and deserves further mechanistic studies. We show that coexpression of B220 and IL-7Rα, both associated with early lymphoid development, are efficiently induced on Lin−/loSca1+c-kit+flt3+cells in response to FL, but not KL, and that KL has no ability to induce B220 or IL-7Rα expression on Lin−/loSca1+c-kit+flt3−cells. These findings further point to an important role of FL and not KL in promoting the earliest stages of lymphoid development, although expression of IL-7Rα and probably also B220 follow rather than precede lymphoid commitment. Future studies will seek to establish whether flt3/FL might play a permissive and/or instructive role in the lymphoid commitment process.
ACKNOWLEDGMENT
We thank Per Anders Bertilsson and Sverker Segren for expert assistance in cell sorting and Ingbritt Åstrand-Grundström, Eva Gynnstam, Irene Persson, and Lilian Wittman for technical assistance. We are also grateful to Dr Koichi Akashi for important technical suggestions regarding IL-7 staining and Drs Ian K. McNiece, Graham Molineux, and Stewart D. Lyman for generously supplying cytokines. We thank David Bryder, Dr Veslemøy Ramsfjell, Dr Stewart D. Lyman, and Dr Stefan Karlsson for critically reviewing the manuscript.
Supported by grants from the A-G. Crafoord Foundation, the Crafoord Foundation, the Medical Faculty, University of Lund, the Swedish Medical Research Council (MFR) and the Swedish Foundation for Strategic Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sten E.W. Jacobsen, MD, Stem Cell Laboratory, Institute for Laboratory Medicine, Department of Internal Medicine, University of Lund, S-221 85 Lund, Sweden; e-mail:sten.jacobsen@molmed.lu.se.

![Fig. 2. KL enhances FL + IL-7–stimulated proB-cell formation from Lin−/loSca1+c-kit+progenitors. A total of 500 Lin−/loSca1+c-kit+ cells/mL was cultured in the presence of the indicated cytokines. After 12 days of culture, total cells were counted and analyzed for expression of B220 and CD19. The B220+ and CD19+ bars indicate the fraction of total cells represented by B220+and CD19+ cells, respectively. Data presented are the mean (+standard error of mean [SEM]) of 4 individual experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/11/10.1182_blood.v94.11.3781/4/m_blod42304002x.jpeg?Expires=1769188639&Signature=4csFKzHiCwx23gM9C1ON6Zt1JyOtVI7CStELzaItQ1OYGKgMVfXAUU~mUxnqYRdg~zEMr2sD4r7xFc7laYpotMDOk14hpcSkLuDRC~DGJdXx1XSNlIu-zFz-SUBhmpoQ60wXLNUT6N0p-Z~ySHh~W9xGhHvjXHwDbMOj-ZNr1xZSSyNjUHjjDxywzYuMrgckL4CVn8iT9cBbqACot-b3xl3GssuKFGV8CdN0QG7L-2VRviUMHPcHhPFR1K7S3d4aCAn6XFERs9LZclkotJTTilGo8P9MD9tw-DeUrZJlV20U~HECrLDbCBjFCFPenbPOIVuoPzbcOOOXC4lemYOJZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal