Abstract
Mice deficient for the transcription factor, interferon consensus sequence binding protein (ICSBP), are immunodeficient and develop disease symptoms similar to human chronic myeloid leukemia (CML). To elucidate the hematopoietic disorder of ICSBP−/− mice, we investigated the growth, differentiation, and leukemogenic potential of ICSBP−/−myeloid progenitor cells in vitro, as well as by cell-transfers in vivo. We report that adult bone marrow, as well as fetal liver of ICSBP-deficient mice harbor increased numbers of progenitor cells, which are hyperresponsive to both granulocyte macrophage colony-stimulating factor (GM-CSF) and G-CSF in vitro. In contrast, their response to M-CSF is strongly reduced and, surprisingly, ICSBP−/− colonies formed in the presence of M-CSF are mostly of granulocytic morphology. This disproportional differentiation toward cells of the granulocytic lineage in vitro parallels the expansion of granulocytes in ICSBP−/− mice and correlates with a 4-fold reduction of M-CSF receptor expressing cells in bone marrow. Cell transfer studies showed an intrinsic leukemogenic potential and long-term reconstitution capability of ICSBP−/− progenitors. Further experiments demonstrated strongly reduced adhesion of colony-forming cells from ICSBP−/− bone marrow to fibronectin. In summary, ICSBP−/− myeloid progenitor cells share several abnormal features with CML progenitors, suggesting that the distal parts of signaling pathways of these two disorders are overlapping.
INTERFERON CONSENSUS sequence binding protein (ICSBP)1 is a member of the interferon regulatory factor family (IRF) of transcription factors.2,3 IRFs were first described as regulators of the expression of interferons (IFNs) and IFN-regulated genes. However, recent studies also indicated additional growth regulatory functions. IRF-1 has been shown to have some tumor suppressor activity and IRF-2 an oncogenic function.4 All 7 members of this family identified to date, IRF-1,5 IRF-2,6 IRF-3,7IRF-4/Pip,8,9 IRF-7,10 ISGF3γ,11and ICSBP1 possess a conserved N-terminal DNA binding region and a more variable C-terminal part, which mediates protein interactions and transcriptional activity.
In vitro experimental data initially suggested that ICSBP is a negative regulator of γ-IFN and α/β-IFN–induced genes.12 To gain more insight into its in vivo function, we have generated ICSBP-deficient mice (ICSBP−/−) by gene targeting. We reported that these mice are immunodeficient and develop a myeloproliferative syndrome that is in some respects similar to human chronic myeloid leukemia (CML).13 14 It manifests with high counts of granulocytic and B-lymphoid cells, as well as an increased number of immature cells in peripheral blood and in hematopoietic organs. The composition and the cellularity of hematopoietic organs in ICSBP−/− mice changes progressively with their age.
The primary genetic lesions of the hematopoietic disorder in CML and in ICSBP−/− mice are clearly different. The molecular hallmark of CML is the t9;22 chromosomal translocation, resulting in bcr-abl fusion and activation of c-abl tyrosine kinase.15,16 Previously, we have not been able to detect any altered abl mRNA expression and the genomic organization of c-abl in ICSBP−/− mice.13 Nevertheless, although the role of ICSBP in human CML has not yet been studied in detail, its lack of or strongly reduced expression was found in 79% of CML and 66% of acute myeloid leukemia (AML) patients.17
Myeloid progenitor cells from CML patients exhibit several cellular features in vitro potentially relevant for the development and/or progression of clinical disease. These include exaggerated growth responses to cytokines18,19 and an altered adhesion to components of the extracellular matrix.20-22 Therefore, we investigated the response of ICSBP−/−hematopoietic progenitor cells to interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, and M-CSF in vitro, as well as their adhesion to fibronectin and laminin. We also studied their proliferative and differentiation potential in vivo with a series of cell transfer experiments in lethally irradiated mice. Our results show several features shared by myeloid progenitor cells from CML patients and ICSBP-deficient mice and also demonstrate that ICSBP−/− precursors harbor an intrinsic, cell-autonomous leukemogenic potential. Thus, while the primary genetic lesion underlying both syndromes differs, the cellular signaling pathways driving a leukemic phenotype characterized by hyperproliferation of myeloid cells may be partially overlapping.
MATERIALS AND METHODS
Mice.
ICSBP-deficient mice were generated as described.13 All experiments reported in the present report were performed with ICSBP−/− mice bred on a mixed C57/BL6 × 129/Ola background.
Cell preparation.
Single-cell suspensions from bone marrow were prepared by flushing femora with Iscove’s modified Dulbecco’s medium (IMDM) containing 2% fetal calf serum (FCS). Single-cell suspensions from spleen and liver were obtained by pressing the organs through a stainless steel sieve. Fetal liver single-cell suspension were made by passing the homogenized organ through a 22-G and subsequently a 27-G needle in the same medium. Peripheral blood leukocytes were obtained by centrifugation after erythrocyte lysis in 0.83% ammonium chloride solution and washing in phosphate-buffered saline (PBS).
Quantification of c-fms expression in bone marrow.
Bone marrow cells were flushed out of femora with fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% newborn calf serum, 2 mmol/L EDTA, 0.1% sodium azide), subjected to red blood cell (RBC) lysis, and stained with a 1:100 dilution of monoclonal antibody (MoAb) 604B5 2E11,23followed by staining with phycoerythrin (PE)-conjugated anti-rat F(ab)2 (mouse-Ig cross-adsorbed; Jackson Immunoresearch, West Grove, PA). After removal of unbound antibody, cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-F 4/80 (Serotec, Oxford, UK). Flow cytometry was performed on a FACS-calibur (Becton Dickinson, Heidelberg, Germany); cells were gated for viability by propidium iodide exclusion.
Enrichment of c-kit+-progenitor cells from bone marrow.
Bone marrow single-cell suspensions, prepared as detailed above, were depleted with a cocktail of the following FITC-conjugated antibodies: Gr-1, CD19, CD4, CD8, DX5, Thy-1.2, CD11b, F4/80, and Ter 119 (all from Pharmingen, Hamburg, Germany) followed by magnet-activated cell sorting (MACS)-depletion on an AS-depletion column (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the instructions of the manufacturer. Absence of lineage markers and c-kit expression in depleted fractions was documented by FACS analysis (see Results).
In vitro colony-forming assays.
The assays were performed essentially as described by Heyworth and Spooncer.24 Single-cell suspensions of bone marrow (5 × 104/mL), spleen (105/mL), fetal or adult liver (5 × 104/mL or 105/mL), and blood (105/mL), were plated in triplicate in 1 mL IMDM (ICN, Eschwege, Germany) supplemented with 20% FCS (Biochrom, Berlin, Germany), 1% bovine serum albumin (BSA) (Boehringer, Mannheim, Germany), glutamine, 2-mercaptoethanol (5 × 10−5 mol/L), and 0.3% bacterial grade soft agar (DIFCO, Augsburg, Germany) in 35-mm petri dishes and incubated at 37°C in an humidified incubator gassed with 5% CO2 and 5% O2. For assays of committed myeloid progenitors, purified recombinant murine (rm) IL-3, rmGM-CSF, rmG-CSF, rm-stem cell factor (SCF) (TEBU GMBH, Frankfurt, Germany), rmM-CSF (R & D, Wiesbaden, Germany) were used at the concentrations indicated in the Results. Individual colonies (defined by >50 cells) were scored at 7 days postplating. For the determination of colony morphology, agar cultures were fixed, dried onto glass slides, and stained with Giemsa solution. In all cases, triplicate determinations were performed on each sample. Statistical significance compared with the ICSBP+/+ populations was determined using Student’st-test.
Colony-forming unit-spleen (CFU-S) assay and determination of self-renewal capacity.
CFU-S assays were performed as described by Lord.25 Bone marrow cells from ICSBP−/− and ICSBP+/+ mice (1 × 105 cells/mouse) were injected intravenously (IV) into lethally irradiated (C57/BL6 × 129/Ola) F2 mice (for the irradiation protocol, see below). After 12 days, the recipient mice were killed, the spleens were removed, and the number of colonies per spleen (CFU-S12) was counted (s). The seeding efficiency (f) was measured by a double transplantation technique.25 The total number of spleen colony-forming cells (CFC-S) is given by CFU-S12 divided by f.
Determination of self-renewal.
A known fraction (1/k) of each primary recipient spleen was injected into lethally irradiated secondary recipient mice. The colonies per spleen in each secondary recipient (m) were counted after 12 days as detailed above for the CFU-S assay. Self-renewal capacity of the CFU-S colonies in the primary recipient mice was defined as average CFU-S per primary colony as follows: (k × m/s).
Long-term reconstitution assay.
Adult recipient mice (>8 weeks old) were irradiated with a dose of 12 Gy total body irradiation using a 18 MeV photon beam from a linear electron accelerator with a dose rate 0.18 Gy/min. These mice were reconstituted IV with 5 × 106 or 1 × 106 freshly isolated bone marrow or fetal liver cells in 0.2 mL PBS within 24 hours of irradiation. Reconstituted mice were tail-bled every 2 weeks beginning 4 weeks after transplant and every 4 weeks begining at 18 weeks posttransplant. Differential white blood cells counts were performed on May-Grünwald-Giemsa–stained blood smears.
Cell adhesion assay.
c-kit+–enriched bone marrow fractions were prepared as described above. Forty-eight–well plates (Falcon, Heidelberg, Germany) were coated with 50 μg/mL of mouse fibronectin (Chemicon, Hofheim, Germany), 50 μg/mL laminin (Boehringer Mannheim), or with 1% BSA (Sigma, Deisenhofen, Germany) in PBS overnight at 4°C. Nonspecific binding was blocked with PBS supplemented with heat-denaturated 1% BSA for 2 hours at room temperature. Cells were resuspended to 2 × 106cells/well in serum-free IMDM and plates were incubated for 60 minutes at 37°C in a 5% CO2 incubator in the presence or in the absence of the cytokines indicated in Results. Nonadherent cells were removed by shaking the plate and washing twice with warm IMDM. Adherent cells were harvested using trypsin/EDTA solution (GIBCO) followed by 3 additional washes with warm IMDM. Nonadherent cells were treated with trypsin/EDTA solution and IMDM medium as well. Both adherent and nonadherent cell fractions were then washed in IMDM, counted, and cultured in soft agar for CFC in the presence of rmIL-3 (10 ng/mL), as described above. Percentage of adherent CFC was calculated as the number of CFC in the adherent fraction divided by the number of CFC in both fractions × 100%.
FACS analysis of integrins on c-kit+ bone marrow cells.
Bone marrow cells of ICSBP+/+ and ICSBP−/− mice were prepared as detailed above including RBC lysis. Staining was performed by incubation of single-cell suspension with MoAbs against β1 (9EG7), α4 (R1-2), or α5 integrins (MFR5) at a dilution of 1:100 simultaneously with biotinylated anti–c-kit and R-PE–labeled anti-GR–1 (all from Pharmingen) for 30 minutes at 4°C followed by staining with anti-rat FITC-conjugated F(ab) (cross-adsorbed to mouse; Jackson ImmunoResearch) and streptavidin-allophycocyanin (APC) conjugate (Pharmingen). Control samples were only stained with secondary reagents. Flow cytometry was performed on a FACSCalibur (Beckton Dickinson); viability gates were set by propidium iodide exclusion.
Functional determination of α4β1 and α5β1 integrins on committed myeloid progenitor cells.
MoAbs against β1 (9EG7), α4 (R1-2), or α5 integrins (MFR5) (Pharmingen) were coated on 48-well plates in PBS, pH 7.4, at a concentration of 2 μg/mL overnight at 4°C. IGg2a and IGg2b (Pharmingen) were used as isotype-matched controls. Nonspecific binding was blocked with 0.2% BSA. Cells were added to the coated plates at 106 cells in 200 mL per well in IMDM and incubated for 1 hour at 37°C. Nonadherent and adherent cells were processed exactly as detailed above and subjected to CFC assay in the presence of rmIL-3 as described above.
RESULTS
Hypersensitivity of hematopoietic progenitors from ICSBP−/− mice to IL-3, GM-CSF, and G-CSF.
To dissect the impact of the ICSBP mutation on hematopoietic activity, we analyzed colony-forming ability of progenitor cells in soft agar cultures containing cytokines important in myeloid development. Hematopoietic organs and peripheral blood of ICSBP−/− mice contain higher numbers of cells that generate in vitro colonies in the presence of either IL-3, GM-CSF, or G-CSF in comparison to ICSBP+/+ mice (Fig 1A and B). In addition, our results show the postnatal persistence of myeloid progenitors in peripheral blood, as well as in adult liver and spleen of ICSBP−/− mice. A significant increase of CFC is seen in 17-day-old ICSBP−/− mice, progressing with age. The increase of myeloid progenitor cells was observed in fetal livers as early as 12.5 days postcoitum (dpc) (Fig 1C), demonstrating a critical role of ICSBP in the early stages of hematopoiesis.
Increased number of myeloid progenitor cells generated in ICSBP−/− mice in response to IL-3 and GM-CSF in vitro. Single-cell suspensions from hematopoietic organs and peripheral blood from either ICSBP−/− or from wild-type mice were plated in soft agar in the presence of the rmIL-3 (10 ng/mL) and GM-CSF (10 ng/mL). Colonies of more than 50 cells were scored on day 7 postplating. Data shown represent the mean ± standard error of mean (SEM) of colonies counted in 2 independent experiments performed in triplicate with 4 mice per group in each experiment. (A) Bone marrow, spleen, and liver cells at 17 days and 6 weeks of age. (B) Peripheral blood leukocytes of 17-day-old and 6-week-old mice (G-CSF, 100 ng/mL). (C) Fetal liver cells (12.5 dpc).
Increased number of myeloid progenitor cells generated in ICSBP−/− mice in response to IL-3 and GM-CSF in vitro. Single-cell suspensions from hematopoietic organs and peripheral blood from either ICSBP−/− or from wild-type mice were plated in soft agar in the presence of the rmIL-3 (10 ng/mL) and GM-CSF (10 ng/mL). Colonies of more than 50 cells were scored on day 7 postplating. Data shown represent the mean ± standard error of mean (SEM) of colonies counted in 2 independent experiments performed in triplicate with 4 mice per group in each experiment. (A) Bone marrow, spleen, and liver cells at 17 days and 6 weeks of age. (B) Peripheral blood leukocytes of 17-day-old and 6-week-old mice (G-CSF, 100 ng/mL). (C) Fetal liver cells (12.5 dpc).
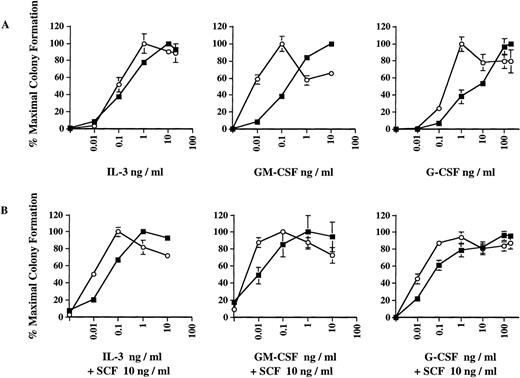
Dose-response curves obtained in the presence of IL-3, GM-CSF, and G-CSF, respectively (Fig 2A), indicate that the increased number of ICSBP−/− colonies observed in Fig 1 is correlated with a significant hyperresponsiveness of ICSBP−/− bone marrow progenitor cells to GM-CSF and, to a lesser extent, to G-CSF. As much as 10-fold lower concentrations of GM-CSF or G-CSF supported maximal growth of ICSBP−/− progenitor cells, as compared with wild-type cells. We also noted that the ICSBP−/− colonies generated in response to G-CSF and, to some extent, in response to GM-CSF, were much larger than wild-type colonies, indicating increased proliferative potential of the CFCs (data not shown). The hyperresponsiveness of ICSBP−/− cells to GM-CSF parallels the behavior of progenitor cells in patients with juvenile CML.18
Dose-response curves of colony formation in the presence of cytokines in vitro. Colony formation was performed exactly as described in the legend to Fig 1. Data shown represent mean ± SEM of 1 of 4 independent experiments with 3 mice per group in each experiment. (A) Bone marrow cells from 6-week-old ICSBP−/− (-○-) or ICSBP+/+ (-▪-) mice were plated in the presence of rmIL-3, rmGM-CSF, or rmG-CSF at the indicated concentrations. (B) As in (A), with the addition of 10 ng/mL of rmSCF to the medium.
Dose-response curves of colony formation in the presence of cytokines in vitro. Colony formation was performed exactly as described in the legend to Fig 1. Data shown represent mean ± SEM of 1 of 4 independent experiments with 3 mice per group in each experiment. (A) Bone marrow cells from 6-week-old ICSBP−/− (-○-) or ICSBP+/+ (-▪-) mice were plated in the presence of rmIL-3, rmGM-CSF, or rmG-CSF at the indicated concentrations. (B) As in (A), with the addition of 10 ng/mL of rmSCF to the medium.
Because SCF has been found to synergize with GM-CSF in promoting proliferation of CML progenitors,19 we tested the effect of SCF in combination with either IL-3, GM-CSF, or G-CSF. The addition of a low concentration of murine SCF (at 10 ng/mL) did not significantly increase the maximal number of CFCs generated (data not shown). However, it did potentiate the hypersensitive pattern of colony growth with all 3 cytokines tested, as is evident from the left shifted dose response (Fig 2B, lower panels). SCF alone did not support colony growth at this concentration (data not shown). We also examined whether the enhanced colony formation of ICSBP−/−progenitors in response to SCF could be due to an autocrine production of other growth factors. However, conditioned media harvested after 24 hours of incubation with ICSBP−/− bone marrow cells with SCF (100 ng/mL) did not support colony formation of ICSBP+/+ bone marrow cells (data not shown). Therefore, the growth potentiation elicited by SCF is not due to a costimulatory effect of other extracellular factors released from the ICSBP−/− cells.
These data suggest that the increased sensitivity of ICSBP−/− progenitor cells to the growth factors is mediated by intracellular signaling events and may contribute to the dramatic expansion of myeloid progenitors observed in vivo.
Quantitatively reduced and qualitatively altered response to M-CSF.
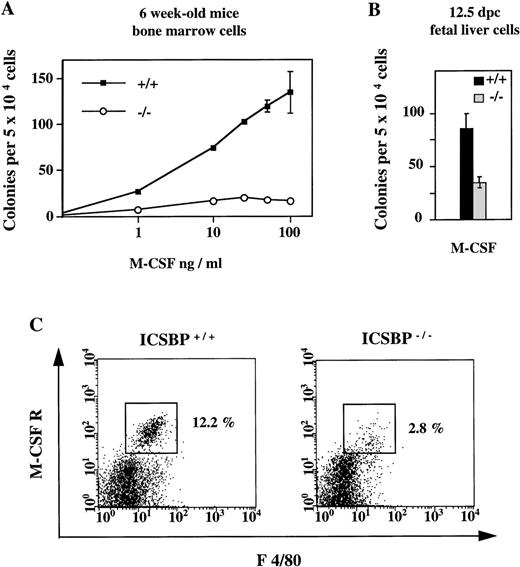
In contrast to the elevated number of CFCs in response to IL-3, GM-CSF, and G-CSF described above, the number of CFCs generated in the presence of M-CSF was significantly reduced in ICSBP−/−mice (Fig 3A). The reduced response to M-CSF is detectable as early as 12.5 dpc (Fig 3B).
Reduced response of hematopoietic progenitor cells from ICSBP−/− mice to M-CSF. (A) Dose-response curves of bone marrow cells from 6-week-old ICSBP+/+ (-▪-) and ICSBP−/− (-○-) mice cultured in the presence of rmM-CSF. Data shown represent mean ± SEM of 1 of 6 independent experiments performed in triplicate with 2 mice per group. (B) Fetal liver cells isolated at day 12.5 postcoitum were cultured in the presence of M-CSF at 10 ng/mL. (C) Reduced number of M-CSFR–expressing cells in ICSBP−/− bone marrow. Freshly prepared cells from wild-type (left) or ICSBP−/− (right) bone marrow were stained with antibodies recognizing the murine M-CSF receptor (c-fms) and the macrophage marker F 4/80 and analyzed by flow cytometry as described in Materials and Methods.
Reduced response of hematopoietic progenitor cells from ICSBP−/− mice to M-CSF. (A) Dose-response curves of bone marrow cells from 6-week-old ICSBP+/+ (-▪-) and ICSBP−/− (-○-) mice cultured in the presence of rmM-CSF. Data shown represent mean ± SEM of 1 of 6 independent experiments performed in triplicate with 2 mice per group. (B) Fetal liver cells isolated at day 12.5 postcoitum were cultured in the presence of M-CSF at 10 ng/mL. (C) Reduced number of M-CSFR–expressing cells in ICSBP−/− bone marrow. Freshly prepared cells from wild-type (left) or ICSBP−/− (right) bone marrow were stained with antibodies recognizing the murine M-CSF receptor (c-fms) and the macrophage marker F 4/80 and analyzed by flow cytometry as described in Materials and Methods.
The response of ICSBP−/− cells to M-CSF is not only quantitatively reduced, but also qualitatively altered (Table 1). A severely altered differentiation profile of ICSBP−/− progenitors is seen in the presence of M-CSF, where 69% of colonies are granulocytic and 22% monocytic as compared with 5% granulocytic and 89% monocytic colonies in control animals. A less pronounced reduction of monocytic colonies is also seen for GM-CSF, whereas the differentiation profile of colonies made in response to IL-3 is unchanged.
Morphology of Colonies From Bone Marrow Cells of 6-Week-Old Mice Stimulated With Purified CSFs
| Factor . | ICSBP Genotype . | Total Colonies per Culture* . | Morphology of Colonies (%)† . | ||
|---|---|---|---|---|---|
| GM . | M . | G . | |||
| IL-3 10 ng/mL | +/+ | 84 ± 4.0 | 10 ± 3 | 6 ± 2 | 84 ± 6 |
| −/− | 109 ± 8.6 | 6 ± 1 | 4 ± 2 | 90 ± 4 | |
| GM-CSF 10 ng/mL | +/+ | 50 ± 2.5 | 13 ± 5 | 78 ± 7 | 9 ± 4 |
| −/− | 89 ± 19 | 28 ± 4 | 58 ± 5 | 13 ± 1 | |
| M-CSF 10 ng/mL | +/+ | 69 ± 6.0 | 6 ± 3 | 89 ± 3 | 5 ± 2 |
| −/− | 38 ± 8.4 | 9 ± 2 | 22 ± 2 | 69 ± 4 | |
| Factor . | ICSBP Genotype . | Total Colonies per Culture* . | Morphology of Colonies (%)† . | ||
|---|---|---|---|---|---|
| GM . | M . | G . | |||
| IL-3 10 ng/mL | +/+ | 84 ± 4.0 | 10 ± 3 | 6 ± 2 | 84 ± 6 |
| −/− | 109 ± 8.6 | 6 ± 1 | 4 ± 2 | 90 ± 4 | |
| GM-CSF 10 ng/mL | +/+ | 50 ± 2.5 | 13 ± 5 | 78 ± 7 | 9 ± 4 |
| −/− | 89 ± 19 | 28 ± 4 | 58 ± 5 | 13 ± 1 | |
| M-CSF 10 ng/mL | +/+ | 69 ± 6.0 | 6 ± 3 | 89 ± 3 | 5 ± 2 |
| −/− | 38 ± 8.4 | 9 ± 2 | 22 ± 2 | 69 ± 4 | |
Colony number was determined using an inverted microscope. Subsequently, the agar cultures were dried onto glass slides and stained with Giemsa solution for the determination of colony morphology.
Abbreviations: M, macrophage; GM, granulocyte-macrophage; G, neutrophilic granulocyte.
Values represent mean ± SEM of 3 independent experiments with 2 to 5 mice per group.
Values represent mean ± SEM of 2 independent experiments with 4 mice per group.
Reduced number of M-CSF receptor-expressing cells in ICSBP−/− mice.
The reduced response of ICSBP−/− progenitors to M-CSF prompted us to analyze the expression of the M-CSF receptor (c-fms), which is known to be specifically expressed on myeloid cells and is upregulated during monocytic differentiation.26 By FACS analysis, we found that the percentage of M-CSF receptor-positive cells was reduced 4-fold in ICSBP−/− mice (Fig3C). Thus, survival and/or proliferation of cells responsive to M-CSF may also be reduced in vivo. This is probably not due to a direct transcriptional regulation of c-fms by ICSBP, as expression of c-fms on mature peritoneal macrophages was normal (data not shown).
High self-renewal capacity of multipotential progenitors.
The increased number of committed myeloid progenitors in ICSBP−/− mice posed the question as to whether more primitive multilineage progenitors are also affected. Therefore, we performed a CFU-S assay according to Lord.25ICSBP−/− and control bone marrow cells were injected into lethally irradiated wild-type mice, and the number of spleen colonies was determined. Table 2shows that ICSBP−/− and control bone marrow cells generated equal amounts of spleen colonies both when expressed as CFU-S12 per femur and when corrected for seeding efficiency (CFC-S). We then tested self-renewal capacity of the primitive multilineage progenitors by injecting fractions of CFU-S–containing spleens into secondary lethally irradiated recipients. This experiment showed a drastic difference in that ICSBP−/−cells generated 3-fold higher number of secondary CFU-S as compared with controls (Table 2). This result shows that not only committed precursors, but also multipotential progenitor cells, are affected by the lack of ICSBP.
CFU-S and Self-Renewal Capacity of CFU-S
| CFC-S . | Self-Renewal of CFU-S . | |||||
|---|---|---|---|---|---|---|
| ICSBP Genotype . | CFU-S12 per Femur . | f Seeding Efficiency . | CFC-S/Femur | s(primary CFU-S12 per 105 injected cells) . | m (secondary CFU-S12 per a known fraction of spleen − 1/k) . | Average CFU-S per Primary Spleen |
| +/+ | 1350 ± 90* | 0.113 | 11,940 ± 840* | 9.6 ± 0.3 | 17.6 ± 2.4 | 10 ± 1.14† |
| (n = 15) | (n = 10) | (n = 4) | ||||
| −/− | 1023 ± 92* | 0.089 | 11,482 ± 1,015* | 4.0 ± 1.0 | 22.8 ± 1.2 | 28 ± 2.6† |
| (n = 19) | (n = 10) | (n = 4) | ||||
| CFC-S . | Self-Renewal of CFU-S . | |||||
|---|---|---|---|---|---|---|
| ICSBP Genotype . | CFU-S12 per Femur . | f Seeding Efficiency . | CFC-S/Femur | s(primary CFU-S12 per 105 injected cells) . | m (secondary CFU-S12 per a known fraction of spleen − 1/k) . | Average CFU-S per Primary Spleen |
| +/+ | 1350 ± 90* | 0.113 | 11,940 ± 840* | 9.6 ± 0.3 | 17.6 ± 2.4 | 10 ± 1.14† |
| (n = 15) | (n = 10) | (n = 4) | ||||
| −/− | 1023 ± 92* | 0.089 | 11,482 ± 1,015* | 4.0 ± 1.0 | 22.8 ± 1.2 | 28 ± 2.6† |
| (n = 19) | (n = 10) | (n = 4) | ||||
Abbreviation: n, number of recipient mice.
Not significant.
P < .00001.
Long-term reconstitution.
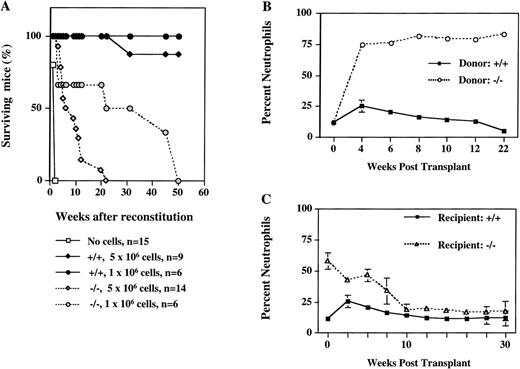
To investigate whether the microenvironment of bone marrow contributes to the altered properties of progenitors in ICSBP−/− mice and to determine their leukemogenic potential in vivo, we performed a series of cell transfer experiments. Lethally irradiated ICSBP+/+ recipient mice were reconstituted with bone marrow cells from ICSBP−/− mice and their survival was followed (Fig 4). The results show that ICSBP−/− marrow cells are capable of long-term hematopoietic reconstitution, as 50% of mice injected with 106 cells lived longer than 6 months (Fig 4A). However, mice transplanted with ICSBP−/− bone marrow had a decreased life span, depending on the number of transplanted cells. This was associated with the development of a dramatic granulocytosis in peripheral blood (Fig 4B). The leukemogenic potential was also established for ICSBP−/− fetal liver cells as early as day 12.5 dpc (data not shown). These data show that the microenvironment of adult bone marrow is not critical for the hematopoietic aberration of ICSBP−/− progenitor cells.
Long-term reconstitution of lethally irradiated mice. (A) Percent survival of lethally irradiated wild-type mice transplanted with 2 different amounts of bone marrow cells from ICSBP−/− or ICSBP+/+ mice as indicated. (B) Induction of granulocytosis in wild-type mice transplanted with ICSBP−/− bone marrow cells. The graph shows the percentage of circulating neutrophils in peripheral blood from reconstituted mice. The results shown represent mean ± SEM of wild-type mice transplanted with 5 × 106ICSBP−/− bone marrow cells (dashed line; n = 14) or with 5 × 106 ICSBP+/+ bone marrow cells (solid line; n = 9). Total leukocyte number in peripheral blood of wild-type recipient mice at 8 weeks posttransplant was 20.9 × 106/mL (ICSBP+/+) and 21.4 × 106/mL (ICSBP−/−), respectively. (C) Peripheral leukemia in ICSBP−/− mice is rescued by lethal irradiation and subsequent reconstitution with wild-type bone marrow cells. The graph shows the percentage of circulating neutrophils in peripheral blood from reconstituted mice. The results represent mean ± SEM of lethally irradiated ICSBP−/− mice (dashed line; n = 16) or wild-type mice (solid line; n = 9) transplanted with 5 × 106 wild-type bone marrow.
Long-term reconstitution of lethally irradiated mice. (A) Percent survival of lethally irradiated wild-type mice transplanted with 2 different amounts of bone marrow cells from ICSBP−/− or ICSBP+/+ mice as indicated. (B) Induction of granulocytosis in wild-type mice transplanted with ICSBP−/− bone marrow cells. The graph shows the percentage of circulating neutrophils in peripheral blood from reconstituted mice. The results shown represent mean ± SEM of wild-type mice transplanted with 5 × 106ICSBP−/− bone marrow cells (dashed line; n = 14) or with 5 × 106 ICSBP+/+ bone marrow cells (solid line; n = 9). Total leukocyte number in peripheral blood of wild-type recipient mice at 8 weeks posttransplant was 20.9 × 106/mL (ICSBP+/+) and 21.4 × 106/mL (ICSBP−/−), respectively. (C) Peripheral leukemia in ICSBP−/− mice is rescued by lethal irradiation and subsequent reconstitution with wild-type bone marrow cells. The graph shows the percentage of circulating neutrophils in peripheral blood from reconstituted mice. The results represent mean ± SEM of lethally irradiated ICSBP−/− mice (dashed line; n = 16) or wild-type mice (solid line; n = 9) transplanted with 5 × 106 wild-type bone marrow.
In a reciprocal experiment, lethally irradiated ICSBP−/− mice were reconstituted with bone marrow cells from ISCBP+/+ donors. Figure 4C shows that ICSBP−/− mice are effectively cured by bone marrow transplantation, as documented by a rapid decrease of granulocytes in the peripheral blood of transplanted animals and their long-term survival.
Taken together, these data argue against a stromal involvement in leukemogenesis and suggest that this is an intrinsic defect of ICSBP−/− hematopoietic cells.
Reduced number of fibronectin-adhering hematopoietic progenitors in ICSBP−/− mice.
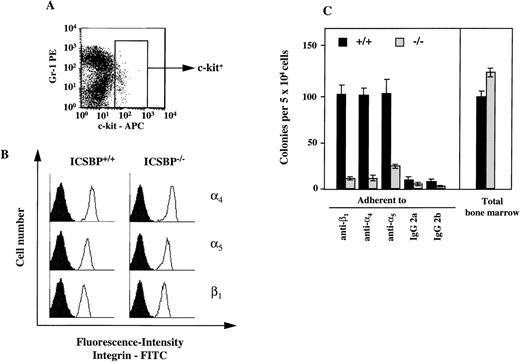
The presence of ICSBP−/− progenitor cells in peripheral blood and their persistence in adult spleen and liver suggested that a defective homing and adhesion could contribute to their altered distribution. Therefore, we measured the number of progenitors in c-kit+–enriched bone marrow cells adhering to fibronectin, a major extracellular matrix component with a known role in the adhesion of hematopoetic progenitor cells.27 As seen in Fig 5A, the percentage of myeloid CFCs adhering to fibronectin is greatly reduced in ICSBP−/− c-kit+–enriched bone marrow cells. In contrast, the percentage of ICSBP−/− CFCs adhering to laminin was increased 2-fold (Fig 5B), thus documenting that the adhesion deficiency of ICSBP-deficient precursor cells is specific for fibronectin. Both the reduced adhesion to fibronectin and increased adhesion to laminin are also characteristics of CML progenitors.21
Hematopoietic progenitors from ICSBP−/−mice display reduced adhesion to fibronectin, but not to laminin. c-kit+–enriched cell fractions were prepared as described in Materials and Methods. The percentage of c-kit+ cells in lineage-depleted fractions was as follows: 92%, 88.5% (ICSBP+/+), and 89%, 81% (ICSBP−/−) in 2 independent sorting experiments, respectively. (A) c-kit+–enriched bone marrow cells from wild-type (black bars) or ICSBP−/− mice (gray bars) were plated on 48-well plates precoated with either fibronectin or BSA in the presence or the absence of the cytokines indicated. Subsequently, adherent, as well as nonadherent fractions, were cultured in the presence of IL-3 (10 ng/mL). Percent adherence was calculated as the number of CFC in the adherent fraction divided by the number of CFC in both fractions × 100% (see Materials and Methods). Data shown represent mean ± SEM of 2 independent experiments. (B) Bone marrow cells from wild-type (black bars) or ICSBP−/− mice (gray bars) were incubated on 48-well plates precoated with laminin (see Materials and Methods). Adherent cells were subjected to CFC assay as described above. Data shown represent mean ± SEM of 2 independent experiments performed in triplicate with 2 mice per group.
Hematopoietic progenitors from ICSBP−/−mice display reduced adhesion to fibronectin, but not to laminin. c-kit+–enriched cell fractions were prepared as described in Materials and Methods. The percentage of c-kit+ cells in lineage-depleted fractions was as follows: 92%, 88.5% (ICSBP+/+), and 89%, 81% (ICSBP−/−) in 2 independent sorting experiments, respectively. (A) c-kit+–enriched bone marrow cells from wild-type (black bars) or ICSBP−/− mice (gray bars) were plated on 48-well plates precoated with either fibronectin or BSA in the presence or the absence of the cytokines indicated. Subsequently, adherent, as well as nonadherent fractions, were cultured in the presence of IL-3 (10 ng/mL). Percent adherence was calculated as the number of CFC in the adherent fraction divided by the number of CFC in both fractions × 100% (see Materials and Methods). Data shown represent mean ± SEM of 2 independent experiments. (B) Bone marrow cells from wild-type (black bars) or ICSBP−/− mice (gray bars) were incubated on 48-well plates precoated with laminin (see Materials and Methods). Adherent cells were subjected to CFC assay as described above. Data shown represent mean ± SEM of 2 independent experiments performed in triplicate with 2 mice per group.
Adhesion to fibronectin is mediated via specific receptors, α4β1-and α5β1-integrins. Therefore, we also analyzed the presence of integrin receptors on progenitor cells. By FACS analysis, we observed no difference in the surface expression of integrins on c-kit–positive bone marrow cells (Fig 6A). To assess integrin surface expression only on CFCs, we performed a CFC assay in vitro with bone marrow cells capable of binding to immobilized monoclonal integrin antibodies (Fig 6B). Similar to the adhesion studies performed with fibronectin, we observed a reduction in the number of CFCs in ICSBP−/− bone marrow binding to immobilized integrin antibodies (Fig 6B).
Expression of 4, 5, and β1-integrins on ICSBP-deficient progenitor cells. (A) Gating of bone marrow cells according to surface expression of c-kit, as measured by FACS. (B) FACS-analysis of 4- (top panel), 5- (middle panel), and β1-integrin (lower panel) expression on c-kit+–gated (see A) bone marrow cells from ICSBP+/+ (left) and ICSBP−/− (right) mice. Filled histograms represent control traces from cells stained only with FITC-labeled secondary Ig. (C) Adhesion of CFCs to immobilized antiintegrin antibodies. Bone marrow cells from wild-type (black bars) or ICSBP−/−mice (gray bars) were incubated in 48-well plates precoated with antibodies to the indicated integrins. Adherent cells were subjected to a CFC assay as described in Materials and Methods and in the legend to Fig 5A. Data shown represent mean ± SEM of 2 independent experiments performed in triplicate with 2 mice per group.
Expression of 4, 5, and β1-integrins on ICSBP-deficient progenitor cells. (A) Gating of bone marrow cells according to surface expression of c-kit, as measured by FACS. (B) FACS-analysis of 4- (top panel), 5- (middle panel), and β1-integrin (lower panel) expression on c-kit+–gated (see A) bone marrow cells from ICSBP+/+ (left) and ICSBP−/− (right) mice. Filled histograms represent control traces from cells stained only with FITC-labeled secondary Ig. (C) Adhesion of CFCs to immobilized antiintegrin antibodies. Bone marrow cells from wild-type (black bars) or ICSBP−/−mice (gray bars) were incubated in 48-well plates precoated with antibodies to the indicated integrins. Adherent cells were subjected to a CFC assay as described in Materials and Methods and in the legend to Fig 5A. Data shown represent mean ± SEM of 2 independent experiments performed in triplicate with 2 mice per group.
Because the activation state of integrins can be modulated by cytokines,28 29 we tested whether the deficient adhesion to fibronectin in ICSBP−/− progenitor cells could be upregulated by IL-3, GM-CSF, and SCF. As shown in Fig 5A, treatment of ICSBP−/− with SCF for 1 hour upregulated adhesion of ICSBP−/− CFCs, but not of ICSBP+/+ controls to fibronectin, while GM-CSF and IL-3 had no effect on cells from either genotype, demonstrating the specificity of the effect elicited by SCF. These data support the notion that the low adhesion to fibronectin of ICSBP−/− CFCs is not due to transcriptional downregulation, but rather to decreased surface presentation and/or function of integrin receptors.
DISCUSSION
Previous analysis of ICSBP-deficient mice showed altered differentiation and proliferation of several hematopoietic cell lineages, dominated by the expansion of granulocytic cells, and suggested that ICSBP is an important regulator of hematopoiesis.13 Congruently, we show here that hematopoietic organs and peripheral blood of ICSBP−/− contain significantly higher numbers of cells generating in vitro colonies in the presence of either IL-3, GM-CSF, or G-CSF in comparison to ICSBP+/+ mice. Moreover, the CFC analyses showed that ICSBP deficiency affects hematopoiesis not only in adult mice, but already in very early stages of embryonal differentiation. The observed increase of CFCs results from the enhanced sensitivity of ICSBP−/− progenitors to GM-CSF, and G-CSF in vitro, and and may contribute to the expansion of myeloid progenitors observed in vivo. It is likely that the altered responses to the growth factors are mediated by intracellular signaling events, and that ICSBP might be a part of the signaling network for several cytokines. Hypersensitivity to GM-CSF is a marker of progenitors from juvenile-CML patients18 and has also been noted in hematopoietic disturbances in mice deficient for NF-1 or SH2-containing inositol-5-phosphatase (SHIP).30,31 An increase of myeloid cells in these 2 mouse models was ascribed to elevated Ras signaling.30-32
A striking result is the quantitatively and qualitatively reduced response of ICSBP−/− cells to M-CSF. The low numbers of monocytic cells generated in response to M-CSF in vitro reflects most likely the observed strong reduction of the M-CSF receptor-bearing cells in bone marrow. Importantly, the expression of the M-CSF receptor is not affected in ICSBP-deficient peritoneal macrophages (data not shown). Thus, the lack or reduced level of M-CSFR expression in bone marrow cells is not likely due to a direct effect of ICSBP on M-CSFR transcriptional regulation. Rather, it suggests that this is due to a differentiation defect of an earlier progenitor cell in this compartment.
On the basis of our results, one can hypothesize that the observed reduction of macrophage lineage-committed progenitors, together with the altered response to M-CSF, may lead to a disproportional differentiation towards cells of the granulocytic lineage. Thus, ICSBP plays a role during an early differentiation step that influences the lineage commitment. Recently, other transcription factors such as CEBP α, ε, and, egr-1 have been identified, which influence the differentiation of myeloid progenitors along the granulocytic or macrophage lineage.33-35 Whether ICSBP also acts as lineage-switch module is actively being studied at present.
The cell transfer-experiments presented show that bone marrow cells of ICSBP−/− mice are competent in long-term reconstitution and capable of transferring the leukemic phenotype into wild-type recipients independent of their environment. We conclude that the primary defect, which leads to the myeloproliferative syndrome in ICSBP−/− mice, is due to an expansion and deregulated differentiation of early multilineage progenitors.
Aberrations in adhesive interactions affecting cell homing and migration can also affect cell proliferation and differentiation and lead to pathological disorders.20,21 The interactions of normal and malignant hematopoietic progenitors with fibronectin has been extensively studied. It has been shown that direct adhesion to bone marrow stroma via fibronectin receptors (α4β1 and α5β1integrins) inhibits proliferation of hematopoietic progenitor cells.36 The number of CFCs in ICSBP-deficient mice that bind to fibronectin is severely reduced. Our experiments indicate that this could be due to either a reduced surface presentation or function of α4β1 and α5β1 integrin-bearing progenitors. The reduced adhesion could therefore be an additional factor contributing to hematopoietic alterations of ICSBP-deficient mice.
The hematopoietic disorder caused by the ICSBP gene defect in mice has distinct similarities to CML.13 We have shown here that several characteristics of CML progenitors, hypersensitivity to GM-CSF18, synergistic proliferation in the presence of SCF,19 and the altered adhesion to fibronectin and laminin21 are also shared by the ICSBP−/− progenitors. Together, these observations suggest that despite different primary lesions, the signaling pathways leading to deregulated myeloid proliferation in CML and in ICSBP−/− mice may overlap in their distal parts. Because the signaling cascades for both disorders have not yet been elucidated, detailed knowledge of the ICSBP gene function is desired.
ACKNOWLEDGMENT
We thank Drs Peter Rosenthal and Wolfgang Hinkelbein (Department of Nuclear Medicine, Free University Berlin) for access to the irradiation facility.
Supported by the Deutsche Forschungsgemeinschaft (SFB 506) and by the Wilhelm-Sander-Stiftung.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ivan Horak, MD, Department of Molecular Genetics, FMP, Krahmerstr. 6, 12207 Berlin, Germany; e-mail:horak@fmp-berlin.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal