Abstract
To determine the safety, biologic, and clinical benefits of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF; Amgen, Thousand Oaks, CA) after myelosuppressive chemotherapy in acute myeloid leukemia (AML), 108 adult patients with de novo AML were randomized to receive either PEG-rHuMGDF (2.5 μg/kg/d or 5 μg/kg/d) for up to 21 doses (group A), a single dose of 2.5 μg/kg PEG-rHuMGDF, 7 daily doses of 2.5 μg/kg PEG-rHuMGDF (group B), or placebo. The greatest biologic activity was seen in group A with a median peak platelet count of 1,084 × 109/L, occurring at a median 9 days after the last dose of study drug, compared with 517 × 109/L and 390 × 109/L in group B and placebo group, respectively. Thrombocytosis (platelets >1,000 × 109/L) was seen at rates of 52%, 8%, and 9% in groups A, B, and placebo, respectively, but were not associated with any adverse event. There was no effect on median time to transfusion independent platelet recovery (≥20 × 109/L). The median time to neutrophil recovery (≥500/μL) and red blood cell transfusion requirements were similar in all groups, and there was no apparent stimulation of leukemia. PEG-rHuMGDF was biologically active and well tolerated. Further investigation of dose and scheduling is required, specifically earlier dosing before and during chemotherapy.
ACUTE MYELOID LEUKEMIA (AML) is characterized by an uncontrolled proliferation of a clone of immature myeloid cells in the bone marrow, leading to profound myelosuppression. Anemia, neutropenia, and thrombocytopenia are present in most patients at presentation. Intensive induction chemotherapy is the first step of any curative therapy and usually consists of a combination of daunorubicin and cytarabine (Ara-C).1 2
Therapy-related deaths are mainly related to infection.3Nevertheless, hemorrhagic events are the source of significant mortality and morbidity both before treatment due to disease-related coagulation disorders and thrombocytopenia4 and as a result of chemotherapy-induced thrombocytopenia. Thrombocytopenia after chemotherapy may last for 3 weeks or more2-5 and prophylactic platelet transfusions are usually used when platelet counts fall below 20 × 109/L or 10 × 109/L.6,7 Recent randomized studies have shown that increasing intensity of induction and consolidation therapy in young patients with AML leads to increased disease-free and overall survival.2,5 Such increases in treatment intensity are likely to result in more severe, prolonged thrombocytopenia, with higher associated morbidity rates. Reducing the duration of severe thrombocytopenia could potentially reduce morbidity and mortality rates and the cost of AML therapy.8 Prophylactic platelet transfusions are routinely administered during periods of thrombocytopenia and to treat bleeding events. However, platelet transfusions are expensive and subject to the risks of acute transfusion reactions, alloimmunization, and transmissible disease.9
The recent identification and cloning of thrombopoietin by a number of groups has lead to considerable interest in the application of this agent in the setting of AML.10-16
Polyethylene glycol-conjugated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF; Amgen, Thousand Oaks, CA) is a polypeptide related to thrombopoietin, which includes the receptor-binding N-terminal domain. The polypeptide has 163 amino acids and is conjugated with polyethylene glycol on the N-terminal by reductive alkylation. In vitro, PEG-rHuMGDF is a growth and development factor for megakaryocytes.17,18Administration of PEG-rHuMGDF to normal animals increases megakaryocyte mass and platelet counts.19,20 After myelosuppressive treatment, animals treated with PEG-rHuMGDF recover their platelet counts more quickly than controls.21-23
Early human studies indicate that PEG-rHuMGDF will produce rises in the platelet counts of patients before receiving chemotherapy and will accelerate platelet recovery after myelosuppressive chemotherapy.24,25 The platelets produced in response to PEG-rHuMGDF appear to be morphologically and functionally normal.26 PEG-rHuMGDF appeared to be well tolerated at the doses used in these studies. We conducted a study to determine the safety and biologic activity of PEG-rHuMGDF in patients undergoing remission induction and consolidation treatment for de novo AML.
MATERIALS AND METHODS
Patients
The study protocol was approved by the institutional review board of the participating centers, and all patients gave written informed consent before the study. Patients aged 16 and over with de novo AML as defined by the French-American-British (FAB) classification system and an Eastern Cooperative Oncology Group (ECOG) performance score of 0, 1, 2, or 3 were eligible. Patients with FAB subtypes M3 and M7, in blast transformation of chronic myeloid leukemia, or with a history of clinically relevant coagulation disorders within the previous 6 months were excluded.
Study Design
Patients were registered for the trial at the start of the 7-day chemotherapy regimen (day 1) and were randomized on days 5, 6, or 7 to receive PEG-rHuMGDF or placebo. In the first series, patients were randomized to receive either 2.5 μg/kg/d PEG-rHuMGDF subcutaneously (sc), 5 μg/kg/d PEG-rHuMGDF sc, or placebo (1:1:1) from 24 hours after the last dose of chemotherapy until a transfusion-independent platelet count greater than or equal to 50 × 109/L. In the second series, patients were randomized to receive 2.5 μg/kg PEG-rHuMGDF sc either as a single dose administered on day 7, or for a duration of 7 days (day 8 to day 14), or placebo (1:1:1). Patients were stratified by age (<60 years and ≥60 years). The nomenclature for patient groups adopted for analysis was as follows: patients receiving PEG-rHuMGDF in the first series were referred to as group A, patients receiving PEG-rHuMGDF in the second series were referred to as group B, and all patients from both series receiving placebo were combined into a single placebo group. Investigators, study site staff, and monitors were blind to treatment assignment. The first induction chemotherapy was administered from day 1 to day 7. A further 2 courses of chemotherapy could be adminstered within the protocol, with study drug administered after each course as originally randomized. Tests, including diagnostic bone marrow, cytogenetics, full blood count, and blood chemistry were obtained at baseline. Full blood counts were measured daily from the start of study drug, and blood chemistry was obtained at weekly intervals. Remission status was assessed from a bone marrow examination after hematologic recovery. Serum was taken for measurement of antibodies to PEG-rHuMGDF at baseline and at the end of the study.
Chemotherapy
The first induction chemotherapy consisted of daunorubicin (45 mg/m2) for 3 days, Ara-C (100 mg/m2) twice a day for 7 days, and etoposide (100 mg/m2) for 5 days (DAV 3+7+5). The second course of chemotherapy was determined by age and by remission status. Patients younger than 60 years old received a second course of DAV 3+7+5, if in remission, and Ara-C (1 g/m2) for 4 days and mitoxantrone (12 mg/m2) for 3 days (MiDAC) if not in remission. All patients greater than or equal to 60 years old, regardless of remission status, received a second course consisting of daunorubicin (45 mg/m2) for 2 days, Ara-C (100 mg/m2) twice a day for 5 days, and etoposide (100 mg/m2) for 5 days (DAV 2+5+5). Patients not in complete remission (CR) after a second induction were considered to have completed the study. After CR, patients aged less than 60 years old received Ara-C (3 g/m2) for 6 days and daunorubicin (30 mg/m2) for 2 days; patients older than or equal to 60 years old received DAV 2+5+5 followed by the study drug. All chemotherapy courses were followed by the study drug as randomized.
Transfusion Policy
Prophylactic platelet transfusions were given in the absence of other clinical indications when the morning platelet count was less than 20 × 109/L. Therapeutic transfusions were administered when clinically indicated according to institutional policy. Packed red blood cell (RBC) transfusions were given when the hemoglobin level was less than 8 g/dL or when clinically indicated.
Statistical Analysis
All patients who received at least 1 dose of the study drug were included in the analyses. The time to transfusion-independent platelet recovery, neutrophil recovery, and peak platelet count were compared between the treatment groups by using the log-rank test. The days of platelet transfusion and of RBC transfusion were compared between groups by using the Kolmogorov-Smirnov test. The number and types of transfusion and reasons for transfusion were tabulated by treatment group. All statistical tests have been performed with a two-sided alternative hypothesis and the α = 0.05 (5%) level of significance. Estimates of treatment difference have also been calculated along with associated 95% confidence intervals.
RESULTS
Patients
One hundred eleven patients were registered in the trial, however, only 108 were randomized because 3 patients withdrew before randomization through ineligibility, death, and withdrawal of consent. In the first series, 24 patients were randomized to each of the PEG-rHuMGDF dose-to-target cohorts and 22 were randomized to placebo. In the second series, 12 patients were randomized to the single dose group, 14 to the 7-day dose group, and 12 to the placebo group. Patients treated with PEG-rHuMGDF in the first and second series are referred to as groups A and B, respectively, and the 2 placebo groups have been combined. There were no significant differences between the groups with respect to age or other baseline characteristics (Table 1).
Demographics and Disease Characteristics
| . | Group A . | Group B . | Placebo (%) . | ||
|---|---|---|---|---|---|
| PEG-rHuMGDF 2.5 μg/kg/d to Target (%) . | PEG-rHuMGDF 5 μg/kg/d to Target (%) . | PEG-rHuMGDF 2.5 μg/kg/d Single Dose [Day 8 only] (%) . | PEG-rHuMGDF 2.5 μg/kg/d [Days 8 to 14] (%) . | ||
| No. of patients | 24 | 24 | 12 | 14 | 34 |
| Age | |||||
| <60 yr | 17 (71) | 16 (67) | 8 (67) | 7 (50) | 23 (68) |
| ≥60 yr | 7 (29) | 8 (33) | 4 (33) | 7 (50) | 11 (32) |
| Median (yr) | 49 | 48 | 55 | 60 | 52 |
| Range | 19-71 | 20-77 | 21-78 | 17-71 | 20-75 |
| Sex | |||||
| Male | 11 (46) | 14 (58) | 5 (42) | 11 (79) | 16 (47) |
| Female | 13 (54) | 10 (42) | 7 (58) | 3 (21) | 18 (53) |
| ECOG status | |||||
| 0 | 2 (8) | 10 (42) | 3 (25) | 6 (43) | 6 (18) |
| 1 | 17 (71) | 10 (42) | 7 (58) | 7 (50) | 20 (59) |
| 2 | 4 (17) | 4 (17) | 2 (17) | 1 (7) | 6 (18) |
| 3 | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
| FAB subtype | |||||
| M0 | 1 (4) | 0 (0) | 1 (8) | 1 (7) | 0 (0) |
| M1 | 6 (25) | 4 (17) | 4 (33) | 2 (14) | 9 (26) |
| M2 | 6 (25) | 12 (50) | 2 (17) | 3 (21) | 9 (26) |
| M4 | 6 (25) | 5 (21) | 2 (17) | 1 (7) | 8 (24) |
| M5 | 3 (13) | 3 (13) | 1 (8) | 4 (29) | 6 (18) |
| M6 | 1 (4) | 0 (0) | 1 (8) | 1 (7) | 2 (6) |
| Not assessed | 1 (4) | 0 (0) | 1 (8) | 2 (14) | 0 (0) |
| Cytogenetic status | |||||
| Normal | 11 (46) | 9 (38) | 6 (50) | 5 (36) | 12 (35) |
| Abnormal* | 12 (50) | 12 (50) | 6 (50) | 7 (50) | 20 (59) |
| Good | 2 (8) | 3 (13) | 2 (17) | 1 (7) | 7 (35) |
| Intermediate | 1 (4) | 6 (25) | 3 (25) | 4 (29) | 5 (25) |
| Poor | 9 (38) | 3 (13) | 1 (8) | 2 (14) | 8 (40) |
| Not assessed | 1 (4) | 3 (13) | 0 (0) | 2 (14) | 2 (6) |
| . | Group A . | Group B . | Placebo (%) . | ||
|---|---|---|---|---|---|
| PEG-rHuMGDF 2.5 μg/kg/d to Target (%) . | PEG-rHuMGDF 5 μg/kg/d to Target (%) . | PEG-rHuMGDF 2.5 μg/kg/d Single Dose [Day 8 only] (%) . | PEG-rHuMGDF 2.5 μg/kg/d [Days 8 to 14] (%) . | ||
| No. of patients | 24 | 24 | 12 | 14 | 34 |
| Age | |||||
| <60 yr | 17 (71) | 16 (67) | 8 (67) | 7 (50) | 23 (68) |
| ≥60 yr | 7 (29) | 8 (33) | 4 (33) | 7 (50) | 11 (32) |
| Median (yr) | 49 | 48 | 55 | 60 | 52 |
| Range | 19-71 | 20-77 | 21-78 | 17-71 | 20-75 |
| Sex | |||||
| Male | 11 (46) | 14 (58) | 5 (42) | 11 (79) | 16 (47) |
| Female | 13 (54) | 10 (42) | 7 (58) | 3 (21) | 18 (53) |
| ECOG status | |||||
| 0 | 2 (8) | 10 (42) | 3 (25) | 6 (43) | 6 (18) |
| 1 | 17 (71) | 10 (42) | 7 (58) | 7 (50) | 20 (59) |
| 2 | 4 (17) | 4 (17) | 2 (17) | 1 (7) | 6 (18) |
| 3 | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
| FAB subtype | |||||
| M0 | 1 (4) | 0 (0) | 1 (8) | 1 (7) | 0 (0) |
| M1 | 6 (25) | 4 (17) | 4 (33) | 2 (14) | 9 (26) |
| M2 | 6 (25) | 12 (50) | 2 (17) | 3 (21) | 9 (26) |
| M4 | 6 (25) | 5 (21) | 2 (17) | 1 (7) | 8 (24) |
| M5 | 3 (13) | 3 (13) | 1 (8) | 4 (29) | 6 (18) |
| M6 | 1 (4) | 0 (0) | 1 (8) | 1 (7) | 2 (6) |
| Not assessed | 1 (4) | 0 (0) | 1 (8) | 2 (14) | 0 (0) |
| Cytogenetic status | |||||
| Normal | 11 (46) | 9 (38) | 6 (50) | 5 (36) | 12 (35) |
| Abnormal* | 12 (50) | 12 (50) | 6 (50) | 7 (50) | 20 (59) |
| Good | 2 (8) | 3 (13) | 2 (17) | 1 (7) | 7 (35) |
| Intermediate | 1 (4) | 6 (25) | 3 (25) | 4 (29) | 5 (25) |
| Poor | 9 (38) | 3 (13) | 1 (8) | 2 (14) | 8 (40) |
| Not assessed | 1 (4) | 3 (13) | 0 (0) | 2 (14) | 2 (6) |
Good: t(15,17), t(8,21), inv(16); Intermediate: abnormal; Poor: monosomy 5, 7, 7q-, 5q-, trisomy 8, 11q 23 abnormalities, complex abnormalities.
The number of patients starting each course of chemotherapy is shown in Table 2. There were 8 deaths during the study (3 patients in groups A/B, 5 patients in placebo); of the 8 deaths, 7 occurred during induction treatment and 1 after the second consolidation treatment. Eight patient withdrawals were due to adverse events (5 patients in groups A/B, 3 patients in placebo) and 1 was due to withdrawal of consent (group B). Five patients proceeded to alternative chemotherapy after completion of the first induction course (4 patients from groups A/B, 1 patient from the placebo group).
Disease Outcome
The remission rates for the individual patient cohorts are given in Table 3. The overall remission rate in the study population was 60% after 1 course of chemotherapy and 76% after 2 courses. There was no evidence of an effect of PEG-rHuMGDF on remission rate.
Outcome of Induction Chemotherapy
| . | Group A . | Group B . | All PEG-rHuMGDF Patients . | Placebo . | ||
|---|---|---|---|---|---|---|
| PEG-rHuMGDF 2.5 μg/kg/d to Target . | PEG-rHuMGDF 5 μg/kg/d to Target . | PEG-rHuMGDF 2.5 μg/kg/d Day 8 . | PEG-rHuMGDF 2.5 μg/kg/d Days 8 to 14 . | |||
| Induction 1 | ||||||
| No. of patients | 24 | 24 | 12 | 14 | 74 | 34 |
| Complete remission | 16 | 13 | 8 | 7 | 44 | 21 |
| Persistant disease | 8 | 10 | 3 | 6 | 27 | 9 |
| Induction 1 deaths | 0 | 1 | 1 | 0 | 2 | 4 |
| Remission rate after induction 1 | 67% | 54% | 67% | 50% | 59% | 62% |
| Induction 2 | ||||||
| No. of patients | 7 | 9 | 2 | 4 | 22 | 9 |
| Complete remission | 3 | 4 | 1 | 2 | 10 | 7 |
| Persistant disease | 4 | 4 | 0 | 2 | 10 | 2 |
| Not assessed | 0 | 1 | 1 | 0 | 2 | 0 |
| Remission rate after induction 2 | 43% | 44% | 50% | 50% | 45% | 78% |
| Overall remission rate | 79% | 71% | 75% | 64% | 73% | 82% |
| . | Group A . | Group B . | All PEG-rHuMGDF Patients . | Placebo . | ||
|---|---|---|---|---|---|---|
| PEG-rHuMGDF 2.5 μg/kg/d to Target . | PEG-rHuMGDF 5 μg/kg/d to Target . | PEG-rHuMGDF 2.5 μg/kg/d Day 8 . | PEG-rHuMGDF 2.5 μg/kg/d Days 8 to 14 . | |||
| Induction 1 | ||||||
| No. of patients | 24 | 24 | 12 | 14 | 74 | 34 |
| Complete remission | 16 | 13 | 8 | 7 | 44 | 21 |
| Persistant disease | 8 | 10 | 3 | 6 | 27 | 9 |
| Induction 1 deaths | 0 | 1 | 1 | 0 | 2 | 4 |
| Remission rate after induction 1 | 67% | 54% | 67% | 50% | 59% | 62% |
| Induction 2 | ||||||
| No. of patients | 7 | 9 | 2 | 4 | 22 | 9 |
| Complete remission | 3 | 4 | 1 | 2 | 10 | 7 |
| Persistant disease | 4 | 4 | 0 | 2 | 10 | 2 |
| Not assessed | 0 | 1 | 1 | 0 | 2 | 0 |
| Remission rate after induction 2 | 43% | 44% | 50% | 50% | 45% | 78% |
| Overall remission rate | 79% | 71% | 75% | 64% | 73% | 82% |
Remission rate odds ratio (all PEG-rHuMGDF patients vplacebo patients). Induction 1: 0.91 (0.40, 2.09); Induction 2: 0.23 (0.04, 1.35).
Effect of PEG-rHuMGDF on Platelet Recovery
Induction.
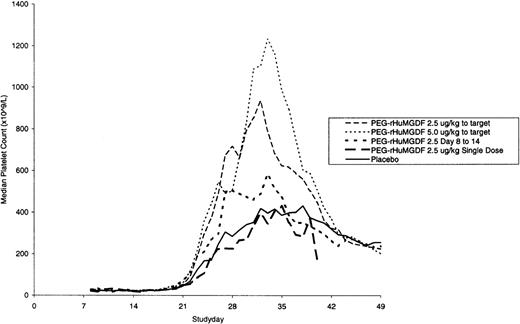
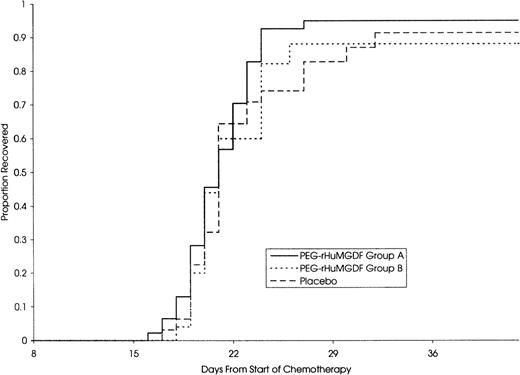
The median daily platelet count for each individual treatment schedule, at the first induction, is shown in Fig 1. On subsequent grouping of patients from the 4 MGDF treatment schedules into groups A and B, group A patients showed a dose-dependent stimulation of platelet count peaking a median of 9 days after stopping the study drug and a median of 20 days and 13 days in group B and placebo patients, respectively. Taken from the start of chemotherapy, the median time to peak platelet count was 29, 27, and 28 days in the 3 groups, respectively. Table 4 shows the platelet parameters; the median peak platelet count in group A was 1,084 × 109/L, compared with 517 × 109/L and 390 × 109/L in group B and placebo patients, respectively. In addition, platelet counts rose to greater than or equal to 1,000 × 109/L in 52%, 8%, and 9% of patients in the 3 groups. The stimulation of platelet production in group A did not translate into a significant shortening of the time to transfusion-independent platelet recovery to greater than or equal to 20 × 109/L. Figure 2 shows that there was no difference in the median time to transfusion-independent platelet recovery between all patient groups. There was no significant difference in the number of days on which a platelet transfusion was required (4, 5, and 5.5 days in groups A, B, and placebo, respectively; group A vplacebo, P = .41; group B v placebo, P = .44). Remission status was not an influencing factor as time to platelet recovery for patients with persistent disease was similar to those achieving CR. There was no effect of PEG-rHuMGDF administration on time to neutrophil recovery to greater than or equal to 500 × 109/L (24.5, 28, and 26 days in groups A, B, and placebo, respectively). Granulocyte colony-stimulating factor (G-CSF) usage was balanced between the groups.
Platelet Parameters—First Induction
| . | Group A . | Group B . | Placebo . |
|---|---|---|---|
| No. of patients | 48 | 26 | 34 |
| Median peak platelet count (×109/L) | 1,084 | 517 | 390 |
| Range | 33-3,745 | 25-1,390 | 18-1,514 |
| Median time to peak platelet count after last dose (days) | 9 | 20 | 13 |
| Range | 0-15 | 0-25 | 0-28 |
| Median time to peak platelet count after start of chemotherapy (days) | 29 | 27 | 28 |
| Range | 12-37 | 8-38 | 10-52 |
| Percentage of patients with platelet count >1,000 × 109/L | 52% | 8% | 9% |
| Median days to transfusion-independent platelet recovery >20 × 109/L | 21 | 20.5 | 21 |
| Range | 13-43 | 14-32 | 12-36 |
| . | Group A . | Group B . | Placebo . |
|---|---|---|---|
| No. of patients | 48 | 26 | 34 |
| Median peak platelet count (×109/L) | 1,084 | 517 | 390 |
| Range | 33-3,745 | 25-1,390 | 18-1,514 |
| Median time to peak platelet count after last dose (days) | 9 | 20 | 13 |
| Range | 0-15 | 0-25 | 0-28 |
| Median time to peak platelet count after start of chemotherapy (days) | 29 | 27 | 28 |
| Range | 12-37 | 8-38 | 10-52 |
| Percentage of patients with platelet count >1,000 × 109/L | 52% | 8% | 9% |
| Median days to transfusion-independent platelet recovery >20 × 109/L | 21 | 20.5 | 21 |
| Range | 13-43 | 14-32 | 12-36 |
Consolidation.
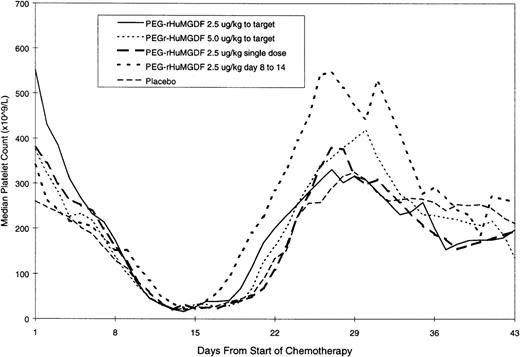
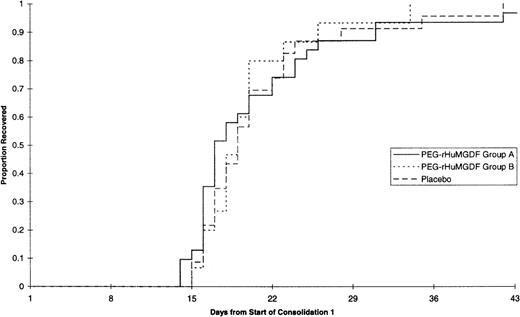
The median daily platelet count for each individual treatment schedule at the first consolidation is shown in Fig3; the platelet parameters are summarized in Table 5, and time to platelet recovery is shown in Fig 4. There was no significant difference between any of the curves presented in Figs 3 and 4. Thus, none of the patient groups undergoing consolidation and treated with PEG-rHuMGDF behaved differently from other groups nor from placebo treated subjects in terms of platelet response, which reflects the response found at induction.
Platelet Parameters—Consolidation
| . | Group A . | Group B . | Placebo . |
|---|---|---|---|
| No. of patients | 31 | 15 | 23 |
| Median peak platelet count (×109/L) | 416 | 498 | 409 |
| Range | 73-2,425 | 49-1,041 | 66-1,101 |
| Median time to peak platelet count after last dose (days) | 11 | 21 | 14 |
| Range | 7-28 | 12-42 | 7-32 |
| Median time to peak platelet count after start of chemotherapy (days) | 29 | 28 | 29 |
| Range | 9-43 | 23-40 | 22-43 |
| Percentage of patients with platelet count >1,000 × 109/L | 19% | 6% | 4% |
| Median days to transfusion-independent platelet recovery >20 × 109/L | 17 | 19 | 19 |
| Range | 14-48 | 15-34 | 15-42 |
| . | Group A . | Group B . | Placebo . |
|---|---|---|---|
| No. of patients | 31 | 15 | 23 |
| Median peak platelet count (×109/L) | 416 | 498 | 409 |
| Range | 73-2,425 | 49-1,041 | 66-1,101 |
| Median time to peak platelet count after last dose (days) | 11 | 21 | 14 |
| Range | 7-28 | 12-42 | 7-32 |
| Median time to peak platelet count after start of chemotherapy (days) | 29 | 28 | 29 |
| Range | 9-43 | 23-40 | 22-43 |
| Percentage of patients with platelet count >1,000 × 109/L | 19% | 6% | 4% |
| Median days to transfusion-independent platelet recovery >20 × 109/L | 17 | 19 | 19 |
| Range | 14-48 | 15-34 | 15-42 |
Adverse Events
The nature and incidence of reported adverse events were similar in all treatment groups for all phases of the study. In induction 1, the most frequently reported events considered related to the study drug were fever (PEG-rHuMGDF 4%, placebo 0%) and thrombocytosis (>1,000 × 109/L) (PEG-rHuMGDF 13%, placebo 0%). All other events reported as treatment related were single patient reports. In consolidation, the overall incidence of treatment-related adverse events was 8% in PEG-rHuMGDF treated patients and 4% in the placebo group, for which all were single patient reports (1 case each of colitis, rash, and thrombophlebitis in the active groups and 1 case of venous thrombosis in the placebo group). There were 4 reports of thrombotic events during the study period, 2 in PEG-rHuMGDF and 2 in placebo patients (Table 6); the platelet counts at the time of each event do not show an association with the administration of the study drug. There were no differences between the treatment groups in the nature, frequency, or severity of events. Overall, PEG-rHuMGDF was well tolerated. No neutralizing antibodies to MGDF were detected in serum samples obtained from 103 patients before and after the study drug was administered with a solid phase screening radioimmunoassay.
Thrombotic Events
| Event . | Platelet Count (×109/L) at Time of Event . | Treatment Arm . |
|---|---|---|
| Cerebral infarction | 8 × 109/L | Placebo |
| Subclavian vein thrombosis (catheter associated) | 3,174 × 109/L | PEG-rHuMGDF to target (Gp A) |
| Thrombosis in mesenteric vein (operative specimen) | 7 × 109/L | PEG-rHuMGDF to target (Gp A) |
| Brachiocephalic/subclavian vein thrombosis (catheter associated) | 546 × 109/L | Placebo |
| Event . | Platelet Count (×109/L) at Time of Event . | Treatment Arm . |
|---|---|---|
| Cerebral infarction | 8 × 109/L | Placebo |
| Subclavian vein thrombosis (catheter associated) | 3,174 × 109/L | PEG-rHuMGDF to target (Gp A) |
| Thrombosis in mesenteric vein (operative specimen) | 7 × 109/L | PEG-rHuMGDF to target (Gp A) |
| Brachiocephalic/subclavian vein thrombosis (catheter associated) | 546 × 109/L | Placebo |
DISCUSSION
This is the first reported experience of the use of PEG-rHuMGDF after chemotherapy for AML and suggests that daily sc doses of 2.5 μg/kg/d and 5 μg/kg/d administered to recovery of a target platelet count stimulate platelet production and are well tolerated. These results are consistent with observations made in other recently published studies of PEG-rHuMGDF in patients with solid tumors.24,25 27
The doses and schedule of PEG-rHuMGDF administered to the first series of patients led to marked thrombocytosis after recovery, peaking 9 days after discontinuing the cytokine.
Despite marked postrecovery thrombocytosis, PEG-rHuMGDF had no effect on the time to transfusion independent recovery of platelets to greater than or equal to 20 × 109/L. The explanation for this is not clear, but may lie in the kinetics of megakaryocyte differentiation and platelet production in response to PEG-rHuMGDF as indicated by in vitro studies.17 These suggest that there is a relatively fixed time period of 6 to 8 days from exposure of primitive progenitor cells to PEG-rHuMGDF and the appearance of megakaryocytes and a further 4-day delay in platelet release. In this study, in the placebo group, spontaneous platelet recovery occurred 14 days after the initiation of the study drug, most likely stimulated by the higher than normal levels of endogenous thrombopoietin in patients with chemotherapy-induced thrombocytopenia.28 In retrospect, it may have been unrealistic to expect PEG-rHuMGDF to substantially shorten this period of severe thrombocytopenia. As a consequence, there was little impact on the use of platelet transfusions.
Although maximum platelet counts exceeding 1,000 × 109/L were observed in approximately 50% of the patients, no excess of thrombotic events was noted in the PEG-rHuMGDF–treated patients. This may indicate that the platelets produced in response to PEG-rHuMGDF function normally and do not exhibit enhanced aggregation in vivo. This is consistent with the observation of relatively modest effects on platelet aggregation activation by PEG-rHuMGDF in vitro29-32 and the absence of activation in vivo.20 26 Interestingly, we also observed platelet counts of greater than 1,000 × 109/L in patients in the placebo group, most likely because of the effects of endogenous hematopoietic growth factors, including endogenous thrombopoietin, after intensive chemotherapy.
The most likely explanation for the observed thrombocytosis is the time-dependent generation of megakaryocytes in response to PEG-rHuMGDF, which in vitro takes a minimum of approximately 8 days. Since the maximum platelet counts occurred approximately 8 days after cessation of PEG-rHuMGDF administration, it is likely that progenitor cells, present in the bone marrow at the time of the last few doses, were stimulated to begin differentiation into megakaryocytes and completed their maturation approximately 8 days later. Another potential contributing factor may be the prolonged circulation of PEG-rHuMGDF after discontinuation of therapy. The in vivo half-life of PEG-rHuMGDF in this population has not been evaluated. However, it is estimated to be 30 to 36 hours in cancer patients after conventional chemotherapy.33
The administration of 2.5 μg/kg PEG-rHuMGDF as a single dose and 2.5 μg/kg for 7 days showed no difference from placebo with respect to platelet recovery or transfusion requirement. Accordingly, there was no evidence of significant thrombocytosis after recovery, unlike that observed in the earlier groups of patients who received the same daily doses of PEG-rHuMGDF until platelet recovery.
Effects of PEG-rHuMGDF after consolidation chemotherapy were similar to those observed after induction. Daily administration of PEG-rHuMGDF did not significantly affect the duration of severe thrombocytopenia. Alternative schedules of 7-day or single doses of PEG-rHuMGDF did not significantly change the profile of platelet recovery, although postrecovery thrombocytosis was avoided.
There was no effect of PEG-rHuMGDF on the rate of neutrophil recovery or the requirement for RBC transfusions, indicating the lack of clinically important effects on other cell lineages in this setting. The lack of effect on neutrophil recovery observed in our patients is consistent with the majority of animal studies,19,21,23,34although some studies showed an acceleration of neutrophil recovery by thrombopoietin.35,36 Phase 1 clinical studies also showed no evidence of significant stimulation of neutrophil recovery by PEG-rHuMGDF.24,25 Stimulation of erythropoiesis may be anticipated in some settings, given the known in vitro synergy between the 2 molecules37 as was observed in some animal models35,38,39 but not in phase 1 studies.24 25
The overall CR rate observed in our PEG-rHuMGDF–treated patients was 73%, not significantly different from the rate observed in the placebo arm, indicating a lack of any marked stimulation of leukemic cells by the administration of PEG-rHuMGDF. Such a stimulation is theoretically possible, despite exclusion of the rare subgroup of M7 AML from our study, because it is known that leukemic cells from 20% to 60% of patients with nonmegakaryoblastic AML express Mpl,40-43and, in some cases, show proliferative responses in vitro to PEG-rHuMGDF.41,44 The safety of myeloid growth factors after chemotherapy for AML (G-CSF45-48 and GM [granulocyte-macrophage]-CSF49-51) has been well established.
This study has shown that PEG-rHuMGDF is biologically active and well tolerated in patients undergoing induction and consolidation therapy for AML. So far, no antibodies against PEG-rHuMGDF have been detected in AML patients, including the patients in this study. Despite clear evidence of lineage-dominant activity, treatment with PEG-rHuMGDF at these doses and schedules, after chemotherapy, produced no clinical benefit. The explanation for this is not clear, but may reflect the difficulty in influencing the apparently obligatory 8 to 12 days required for progenitor cells to proliferate and mature into megakaryocytes. After the end of induction chemotherapy, platelet production in control patients recovered to 20 × 109/L platelets in approximately 14 days. Because platelets appearing in the circulation at that point are produced by megakaryocytes whose maturation began at least 8 days earlier, this suggests that there may be a very limited “window,” of perhaps only 6 days, after chemotherapy, in which PEG-rHuMGDF may realistically influence the number or differentiation of megakaryocyte progenitors. To exert such an influence, the presence of progenitors capable of responding to PEG-rHuMGDF in the bone marrow would be essential during this early period after the end of chemotherapy. In view of these considerations, alternative, earlier dosing schedules of PEG-rHuMGDF may need to be investigated. The scope for this in the context of induction therapy may be limited by the disease and by the need to initiate chemotherapy as quickly as possible. Such limitations may not apply in consolidation, in which prospective expansion of the megakaryocyte mass in the bone marrow, before chemotherapy, may offer the best opportunity of exploiting the biologic effects of PEG-rHuMGDF.
Submitted April 6, 1999; accepted July 26, 1999.
Supported by Amgen Ltd, Cambridge.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dieter Hoelzer, MD, PhD, Klinikum der JWG-University of Frankfurt, Med. Klinik III/Haematologie/Onkologie, Theodor-Stern-Kai 7, D-60596 Frankfurt, Germany.
This paper is dedicated to Prof Eric Archimbaud, Hôpital Edouard Herriot, Lyon, France, who died in March 1998.