T-cell precursors that undergo productive rearrangements at the T-cell receptor (TCR) β locus are selected for proliferation and further maturation, before TCR expression, by signaling through a pre–TCR composed of the TCRβ chain paired with a pre–TCR (pT) chain. Such a critical developmental checkpoint, known as β-selection, results in progression from CD4−CD8− double negative (DN) to CD4+CD8+ double positive (DP) TCRβ−thymocytes. In contrast to mice, progression to the DP compartment occurs in humans via a CD4+ CD8−intermediate stage. Here we show that the CD4+CD8− to CD4+ CD8+ transition involves the sequential acquisition of the and β chains of CD8 at distinct maturation stages. Our results indicate that CD8, but not CD8β, is expressed in vivo in a minor subset of DP TCRβ− thymocytes, referred to as CD4+CD8+ pre-T cells, mostly composed of resting cells lacking cytoplasmic TCRβ chain (TCRβic). In contrast, expression of CD8β heterodimers was selectively found on DP TCRβ− thymocytes that express TCRβicand are enriched for cycling cells. Interestingly, CD4+CD8+ pre-T cells are shown to be functional intermediates between CD4+ CD8−TCRβic− and CD4+CD8β+ TCRβic+thymocytes. More importantly, evidence is provided that onset of CD8β and TCRβic expression are coincident developmental events associated with acquisition of CD3 and pT chain on the cell surface. Therefore, we propose that the CD4+CD8+ to CD4+CD8β+ transition marks the key control point of pre-TCR–mediated β-selection in human T-cell development.
BONE MARROW–DERIVED lymphoid progenitors seed the postnatal thymus in which they undergo the sequential rearrangement of the β and α T-cell receptor (TCR) genes to finally generate mature T lymphocytes bearing an αβ TCR.1-3Early during the intrathymic developmental process, pre-T cells that have succeeded in productive rearrangements at the TCRβ locus are rescued from apoptotic cell death and selected for further maturation, before TCRα expression, by signaling through a pre–TCR composed of the TCRβ chain paired with a pre–TCRα (pTα) chain and associated with CD3.4-7 Expression of this pre–TCR complex promotes at the same time a cell-cycle transition that results in the expansion of those thymocytes expressing a functional TCRβ chain, a process that has been termed β-selection.7-9
In both mice and humans, such a critical developmental checkpoint is associated with the acquisition of the CD4+CD8+ double-positive (DP) phenotype, which is then followed by a first round of TCRα gene rearrangement and expression.3,7-10 Differences exist, however, in the developmental timing of appearance of CD4 and CD8 in both species. Progression to the DP compartment occurs in mice through the CD44− CD25+ stage of double-negative (DN; CD4− CD8−) thymocytes via CD44− CD25− DN thymocytes.8,9 The latter subset, although currently referred to as the last DN stage, includes cells that are already transcribing and expressing low (barely detectable) levels of both CD4 and CD8, so that they spontaneously differentiate in vitro to the DP stage within 24 hours.11-13 Isolated CD44− CD25+ DN cells, in contrast, are unable to generate CD4+ CD8+ DP cells in vitro13; however, they can acquire the CD8α chain in response to certain combinations of cytokines,14 thus expressing the CD8αα homodimeric form. Interestingly, such CD8αα+ cells remain negative for CD3 and CD4 in vitro, but can differentiate into conventional CD4+CD8αβ+ DP thymocytes expressing the CD3-associated αβ TCR under the influence of the thymic microenvironment.15 In contrast to mice, developing DN thymocytes in humans acquire CD4 before CD8 and, therefore, the DN-to-DP transition occurs in humans via CD3−CD4+ CD8− intermediates.16These thymocytes, similarly to their mouse CD44−CD25+ DN counterparts, differentiate in vitro into cells expressing exclusively the CD8αα homodimer17 but generate common CD4+ CD8αβ+ DP thymocytes with the mature CD3-TCRαβ complex in vivo.16
Current data on the physiologic expression of CD8 either as an αα homodimer or as an αβ heterodimer on distinct CD8+ cell types support the notion that CD8αα+ T cells, such as intestinal intraepithelial lymphocytes (IEL), can be generated by an extrathymic maturation pathway independent of CD8β expression, whereas induction of CD8β and, hence, the generation of CD8αβ+ cells is thymus-dependent.18,19 This is further supported by functional studies showing that the CD8β polypeptide is critically involved in the maturation of CD8-lineage cells inside the thymus, so that both positive and negative selection of major histocompatibility complex (MHC) class-I–restricted T cells is impaired in CD8β−/− mice.19-22All these data concur with the fact that the CD8αα+phenotype is prominent among CD8+ T cells from athymic mice and rats, whereas normal thymocytes and CD8+ T cells from euthymic animals are virtually all CD8αβ+.15,19,23 Nonetheless, CD8αα+ cells have been reported to exist physiologically, although in a very low proportion, in the human thymus,17 raising the possibility that they constitute an important intermediate in the pathway of generation of thymus-derived T cells in humans. Supporting this notion, results from a very recent study by Blom et al24 have shown that such CD8αα+ thymocytes, which coexpress surface CD4 but still lack a mature αβ TCR,7 have extensive TCRβ gene rearrangements. However, functional studies on the developmental potential and precursor-product relationships of isolated CD4+ CD8αα+ thymocytes are still lacking, precluding a better understanding of the physiological relevance of this particular cell subset in the context of thymocyte development and β-selection in humans.
Here we show that CD4+ CD8αα+ human thymocytes are actually functional intermediates between CD4+ CD8− and CD4+CD8αβ+ thymocytes. More importantly, evidence is provided that onset of CD8β expression and β-selection are coincident events associated with the acquisition of CD3 and pTα chain on the cell surface during intrathymic development in humans. These results suggest that the pre-TCR–mediated check-point of β-selection is placed in humans at the CD4+CD8αα+ to CD4+CD8αβ+ transition.
MATERIALS AND METHODS
Isolation of thymocyte subsets.
Postnatal thymus samples were removed during corrective cardiac surgery of patients aged 1 month to 3 years. Thymocyte suspensions obtained by Ficoll-Hypaque (Nycomed, Oslo, Norway) centrifugation were fractionated on stepwise Percoll (Pharmacia, LKB, Uppsala, Sweden) density gradients as previously described.25 Thymocytes recovered from the 1.068 and 1.08 density layers, referred to as large and small thymocytes, respectively, were depleted (>99% purity) of mature T, B, NK, and myeloid cells (Lin− cells) by immunomagnetic sorting as described elsewhere.10 Thymocytes coexpressing CD4 and CD8 (DP) were then magnetically sorted from the remaining large and small Lin− cell pool with anti–CD8-coated magnetic beads (Dynabeads, Dynal Corp, Oslo, Norway), whereas CD3− CD4+ CD8−thymocytes were sorted from the CD8-depleted pool of large cells by treatment with anti–CD4-coated magnetic beads (Dynal).10
Small DP thymocytes thus isolated were all negative for TCRαβ and CD3 expression, and will be hereafter referred to as small DP CD3− thymocytes.10 In contrast, large DP thymocytes included CD3− and CD3lowpTα+ thymocytes. Both subsets were independently isolated from the whole pool of large DP TCRαβ− thymocytes as described previously.10 These cells will be referred to as large DP CD3− and large DP CD3lowthymocytes, respectively. Large DP CD3− thymocytes were fractionated into CD8αα+ and CD8αβ+ cells by cell sorting as described below.
Flow cytometry and cell sorting.
Directly labeled monoclonal antibodies (MoAb) against the following antigens were used: CD3 (Leu4-PE), CD8α (Leu2a-fluorescein isothiocyanate [FITC]), and CD5 (Leu1-FITC) from Becton Dickinson & Co, San José, CA; CD4 (CD4-PE-Cy5), CD44 (CD44-FITC), CD69 (CD69-FITC), and CD3 (CD3-PE-Cy5) from Caltag Laboratories, South San Francisco, CA; CD71 (T9-FITC) from Coulter Corp, Hialeah, FL; and CD28 (CD28-FITC) from Serotec Ltd, Oxford, UK. Unlabeled MoAb against CD8β (2ST8-5H7, kindly provided by Dr E.L. Reinherz, Dana-Farber Cancer Institute, Boston, MA)26 as well as MoAb recognizing monomorphic determinants of TCRαβ (BMA031, generously provided by Dr R. Kurrle, Behringwerke AG, Marburg, Germany),27 were used in combination with FITC- or PE-coupled goat-antimouse F(ab)′2 immunoglobulin (Ig) (Caltag). Either unlabeled or directly labeled isotype-matched irrelevant MoAb (Caltag) were used as negative controls. For detection of cytoplasmic TCRβ, cells were treated with 0.5% saponin (Sigma, St Louis, MO), incubated with the anti-TCRβ chain βF1 MoAb28 (generously provided by Dr M. Brenner, Brigham and Women’s Hospital, Boston, MA) and labeled with PE- or PE-Cy5-coupled goat antimouse IgG1(Caltag). To define background fluorescence, cells were sequentially treated with a nonreactive mouse IgG1 MoAb plus PE- or PE-Cy5-conjugated goat antimouse IgG1. Surface expression of pTα chain was determined by sequential staining with a rabbit polyclonal antibody (ED-1) previously described,10 and FITC-conjugated goat antirabbit F(ab)′2 Ig (Southern Biotechnology Associates, Inc, Birmingham, AL). Preimmune rabbit serum was used as negative control. Two- or three-color staining was performed as described elsewhere.25 Stained cells were analyzed in an EPICS XL flow cytometer (Coulter Electronics Inc, Hialeah, FL). Cell cycle analyses were performed by flow cytometry using a doublet discrimination function in cells treated with 0.05% digitonin (Sigma), washed, and stained with 50 μg/mL of propidium iodide (PI) (Sigma), as described elsewhere.10 Cell sorting of CD8αα+ and CD8αβ+ cells was performed in an EPICS Elite Cell Sorter (Coulter Electronics, Inc) on isolated large DP CD3− thymocytes after labeling with anti-CD8β plus PE-labeled goat antimouse IgG2a. An irrelevant IgG2a mouse MoAb was used as negative control. Sorted cells were greater than 98% pure as determined by post-sort analysis.
Western blot analysis.
Cells were lysed for 30 minutes at 4°C in lysis buffer containing 0.5% Deoxicholate, 1% NP40, 0.1% sodium dodecyl sulfate (SDS), 50 mmol/L NaF, 1 mmol/L Na3VO4 (Sigma Chemical Co), 150 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 8.0) (Merck, Darmstadt, Germany), 1 mmol/L PMSF, and 1 μg/mL each of leupeptin, pepstatin A, and aprotinin (Sigma). The protein concentration was determined by micro-bicinchoninic acid (BCA; Pierce, Rockford, IL) assay. Defined quantities (∼7 μg/lane) were electrophoreses in 7% SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions. Western blotting was performed as previously described.25 Blots were incubated with either the anticyclin B (Transduction Laboratories, Lexington, KY), the anti-RAG1, the anti-RAG2 (Pharmingen, San Diego, CA), or the anti–β-tubulin (Amersham International) mouse MoAb, or with a polyclonal rabbit anticyclin A antibody, or a polyclonal goat anti-Retinoblastoma (anti-Rb) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), as primary reagents. Specific signals were shown with either horseradish perioxidase (HRPO)-labeled polyclonal sheep antimouse Igs, or HRPO-labeled polyclonal goat antirabbit Igs (Amersham International) or HRPO-labeled polyclonal rabbit antigoat Igs (Jackson Immunoresearch, West Grove, PA), respectively, and an enhanced chemiluminescence (ECL)-detection kit (Amersham International).
Hybrid human/mouse fetal thymic organ cultures.
The in vitro generation of mature TCRαβ+ human T cells was analyzed using a modification of the previously described hybrid human/mouse fetal thymic organ culture (hu/mo FTOC).29Briefly, thymuses removed from 15-day-old embryos of Swiss mice were precultured for 5 to 6 days in the presence of 1.35 mmol/L dGuo (Sigma). Afterwards, the thymic lobes were washed and cocultured in hanging drops in Terasaki plates (Nunc, Inc, Roskilde, Denmark) with either CD8αα+ or CD4+ (3 × 104 to 105 cells/lobe) human thymocytes. After 2 days, lobes were transferred to Millipore filters (Millipore Corp, Bedford, MA), that were layered over gelfoam rafts and cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 2% human AB serum and 5% fetal calf serum (FCS; Gibco BRL, Paisley, Scotland). Surface staining of human cells was performed at the indicated culture periods, and flow cytometric analyses (FCA) were then performed on electronically gated CD45+ human cells.
RESULTS
Characterization of CD3− CD4+CD8α+β− DP cells in the human thymus.
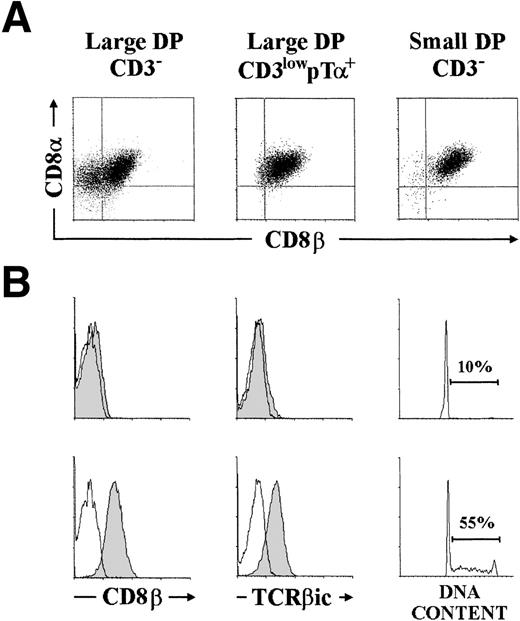
We have recently reported that surface CD3 expression and cell size define three subsets of human TCRαβ− thymocytes, namely large CD3low, large CD3−, and small CD3−, which represent distinct pre–T-cell developmental stages.10 All three pre–T-cell types expressed surface CD4 and were reactive with conventional anti-CD8 antibodies (directed against an epitope on the CD8α molecule), so that they were characterized as DP thymocytes.10 However, as shown in Fig 1A, analysis on the correlated expression of CD8α and CD8β, performed with the anti-CD8β MoAb 2ST8-5H7,26 showed that large CD3low and small CD3− pre-T cells were homogeneously CD8α+β+, whereas a significant proportion (32 ± 4%) of large CD3−pre-T cells expressed CD8α but lacked CD8β chain. Such a differential expression of the α and β chains of CD8 defines two distinct subsets of large CD3− pre-T cells in which CD8 may be expressed either as a CD8αα homodimer or as a CD8αβ heterodimer,26 so that they will hereafter be referred to as either CD8αα+ or CD8αβ+, respectively. Both cell subsets could be independently isolated by cell sorting, and were then compared for their developmental status by analyzing the intracytoplasmic expression of TCRβ chain (TCRβic). As shown in Fig 1B, a clear phenotypic pattern was obtained: TCRβic expression was not detectable in CD8αα+ thymocytes, whereas essentially all CD8αβ+ pre-T cells coexpressed TCRβic. These results confirm our previous data showing a bimodal distribution of TCRβic within the pool of large CD3− pre-T cells as a whole10 but, in addition, they suggest that expression of TCRβicparallels acquisition of the CD8αβ+ phenotype.
Differential expression of CD8 and CD8β chains on distinct subsets of human pre-T cells: Intracytoplasmic TCRβ expression and cell-cycle progression are associated with CD8β chain expression. (A) Large CD3−, large CD3lowpT+, and small CD3− pre-T cells, isolated as described in Materials and Methods, were analyzed by two-color flow cytometry for CD8 versus CD8β expression. (B) Large CD3− CD4+CD8+ (DP) pre-T cells were fractionated by cell sorting into CD8+β−(top panels) and CD8+β+ (bottom panels) cells after labeling with the 2ST8-5H7 anti-CD8β MoAb plus PE-coupled goat antimouse IgG2a. Reanalysis of surface CD8β expression postsorting is shown (shaded histograms). Sorted cells were then analyzed by flow cytometry for intracytoplasmic TCRβ chain (TCRβic) expression (shaded histograms) and DNA content. Cytoplasmic background fluorescence was determined on sorted cells stained with a nonreactive mouse IgG1 MoAb plus PE-Cy5-coupled goat antimouse IgG1 (unshaded histograms). Percentages of cycling cells (in S and G2/M phases) are indicated. Results are representative of four independent experiments.
Differential expression of CD8 and CD8β chains on distinct subsets of human pre-T cells: Intracytoplasmic TCRβ expression and cell-cycle progression are associated with CD8β chain expression. (A) Large CD3−, large CD3lowpT+, and small CD3− pre-T cells, isolated as described in Materials and Methods, were analyzed by two-color flow cytometry for CD8 versus CD8β expression. (B) Large CD3− CD4+CD8+ (DP) pre-T cells were fractionated by cell sorting into CD8+β−(top panels) and CD8+β+ (bottom panels) cells after labeling with the 2ST8-5H7 anti-CD8β MoAb plus PE-coupled goat antimouse IgG2a. Reanalysis of surface CD8β expression postsorting is shown (shaded histograms). Sorted cells were then analyzed by flow cytometry for intracytoplasmic TCRβ chain (TCRβic) expression (shaded histograms) and DNA content. Cytoplasmic background fluorescence was determined on sorted cells stained with a nonreactive mouse IgG1 MoAb plus PE-Cy5-coupled goat antimouse IgG1 (unshaded histograms). Percentages of cycling cells (in S and G2/M phases) are indicated. Results are representative of four independent experiments.
Because TCRβic expression is currently envisaged as a marker of β-selection, we concluded that the CD8αα+and the CD8αβ+ subsets could represent two distinct pre–T-cell developmental stages placed on either side of the β-selection process. This was confirmed by FCA showing that both populations differed dramatically in their cell cycle status. As expected of β-selected thymocytes, CD8αβ+ pre-T cells featured a high proportion (up to 55%) of cycling cells, whereas essentially all (>90%) CD8αα+ thymocytes were “unselected” cells arrested in the G0/G1phases of the cell cycle (Fig 1B).
The distinct developmental status of the CD8αα+ and CD8αβ+ cell subsets of large CD3−pre-T cells, prompted us to examine in more detail their phenotypic profiles, as compared with that of large cycling CD3lowpre-T cells, previously shown to represent the particular stage of β-selected thymocytes at which the CD3-associated pre–TCR is expressed in vivo.10 Regardless of CD3 expression, the two subsets (CD3− and CD3low) of β-selected CD8αβ+ TCRβic+ thymocytes were homogeneously positive for the expression of CD44, whereas CD8αα+ pre-T cells were CD44− (Fig2). In addition, all three pre–T-cell types displayed surface CD4, but levels of expression were consistently lower in the latter. Expression levels of activation markers such as CD71 (transferrin receptor) and, to a lesser extent, CD28 were also significantly lower in CD8αα+ pre-T cells, whereas CD5 and CD69 were similarly expressed in the three populations (Fig 2).
Cell-surface phenotype of individual subsets of CD8+and CD8β+ human large pre-T cells. Large CD3− DP pre-T cells shown in Fig 1 were stained with directly labeled MoAb against the indicated cell-surface molecules. FCA was performed on electronically gated CD8+β− (shaded areas) or CD8+β+ (unshaded areas, bold line) cells. Large CD3low pT+ pre-T cells, shown to be homogeneously CD8+β+ (see Fig1), were included in the study for comparison (unshaded areas, thin line). Background values (vertical lines) were determined with isotype-matched irrelevant MoAb. A representative analysis out of three independent experiments is shown.
Cell-surface phenotype of individual subsets of CD8+and CD8β+ human large pre-T cells. Large CD3− DP pre-T cells shown in Fig 1 were stained with directly labeled MoAb against the indicated cell-surface molecules. FCA was performed on electronically gated CD8+β− (shaded areas) or CD8+β+ (unshaded areas, bold line) cells. Large CD3low pT+ pre-T cells, shown to be homogeneously CD8+β+ (see Fig1), were included in the study for comparison (unshaded areas, thin line). Background values (vertical lines) were determined with isotype-matched irrelevant MoAb. A representative analysis out of three independent experiments is shown.
CD4+ CD8αα+ pre-T cells are the immediate precursors of CD4+ CD8αβ+ pre-T cells that coexpress surface pTα and CD3.
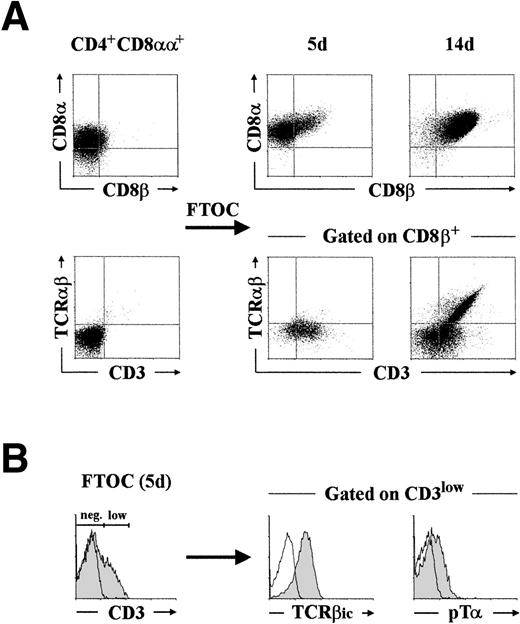
To provide direct evidence that CD4+ CD8αα+thymocytes do in fact represent a minor, but physiologically relevant, population of pre–T-cell intermediates, they were next examined for their developmental potential in a hybrid hu/mo FTOC system. Highly purified CD4+ CD8αα+ thymocytes (>98% pure after cell sorting) were consistently found to give rise to conventional DP cells coexpressing CD4 and the CD8αβ heterodimer at early periods of culture (20% by day 5 in this experiment; Fig3A, upper panel). Of notice, essentially all CD4+ CD8αβ+ progeny (>95%) generated by this time in different experiments had acquired low surface CD3 with minimal differentiation (<2%) into TCRαβ+ cells (Fig3A, lower panel). Interestingly, such CD3lowTCRαβ− CD4+ CD8αβ+pre-T cells (20% of total cells recovered by day 5) were all positive for the expression of TCRβic and, more importantly, they displayed low surface levels of the pTα chain (Fig 3B), as assessed with a polyclonal anti-pTα antibody.10
Phenotypic analysis of the cellular progeny generated after differentiation of CD4+ CD8+human pre-T cells in FTOC. (A) CD4+CD8+ pre-T cells, isolated by cell sorting as described in Materials and Methods, were cultured in a hybrid hu/mo FTOC and analyzed after 5 and 14 days for the expression of CD8, CD8β, TCRβ, and CD3. Analysis of TCRβ versus CD3 was performed by three-color flow cytometry after electronic gating on the CD8β+ progeny. (B) Intracytoplasmic TCRβ (TCRβic) and surface pT expression (shaded areas) was analyzed after gating on the CD3low progeny recovered by day 5. Background fluorescence was determined with isotype-matched irrelevant MoAb and with a rabbit preimmune serum. A representative experiment out of three is shown.
Phenotypic analysis of the cellular progeny generated after differentiation of CD4+ CD8+human pre-T cells in FTOC. (A) CD4+CD8+ pre-T cells, isolated by cell sorting as described in Materials and Methods, were cultured in a hybrid hu/mo FTOC and analyzed after 5 and 14 days for the expression of CD8, CD8β, TCRβ, and CD3. Analysis of TCRβ versus CD3 was performed by three-color flow cytometry after electronic gating on the CD8β+ progeny. (B) Intracytoplasmic TCRβ (TCRβic) and surface pT expression (shaded areas) was analyzed after gating on the CD3low progeny recovered by day 5. Background fluorescence was determined with isotype-matched irrelevant MoAb and with a rabbit preimmune serum. A representative experiment out of three is shown.
We have previously shown that CD4+ CD8αβ+pre-T cells with this particular CD3low pTα+phenotype are functional progenitors of common DP thymocytes that already express surface αβ TCR.25 Accordingly, CD4+ CD8αβ+ cells generated in the thymic lobes increased in numbers throughout culture (up to 9- to 10-fold in 6 to 7 days) to become the major cell subset (>95%) by day 14, and acquired simultaneously intermediate to high levels of both CD3 and the mature αβ TCR (50% of total cells recovered by day 14 in the experiment shown in Fig 3A). These results provide evidence that CD4+ CD8αα+ thymocytes represent the immediate precursors of the first intrathymic cells with a conventional CD4+ CD8αβ+ DP phenotype. Moreover, they indicate that the developmental onset of CD8β chain expression is closely associated with the β-selection process and parallels the acquisition of a surface pre–TCR.
CD4+ CD8αα+ pre-T cells are functional intermediates between CD4+ CD8−progenitors and CD4+ CD8αβ+ pre-T cells.
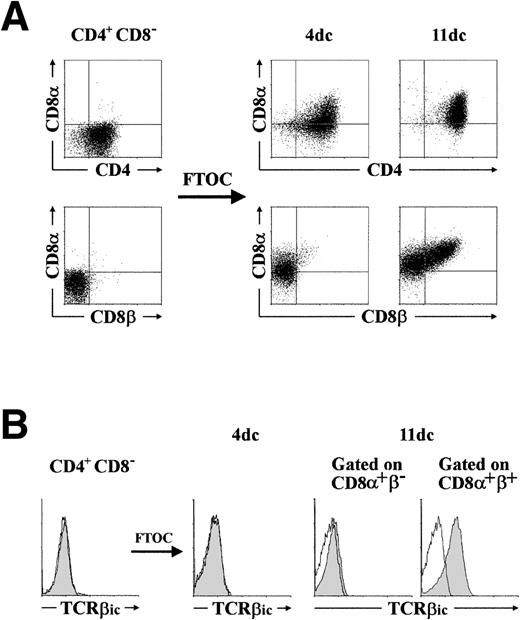
It is currently believed that CD4+ CD8−thymocytes that still lack the CD3–TCRαβ are the immediate precursors of DP thymocytes in the human thymus.16 However, we show in this study that CD4+ CD8αα+pre-T cells are efficient progenitors of CD4+CD8αβ+ DP thymocytes, suggesting that they represent very transient intermediates between CD4+CD8− and CD4+ CD8αβ+cells in vivo. To seek direct evidence that CD4+CD8αα+ thymocytes represent the normal progeny of human CD4+ CD8− thymocyte precursors in the pathway of T-cell differentiation, highly purified CD3− CD4+ CD8−thymocytes (>98% pure) were analyzed for their developmental fate in the hu/mo FTOC system. The pattern of differentiation obtained in different experiments was identical (Fig4A): CD4+ cells acquired rapidly the CD8α chain, while remaining CD8β negative (30% CD8α+β−, <2% CD8αβ+ by day 4) and, later on, CD8β chain was coexpressed with CD8α in a significant cell fraction (40% by day 11; Fig 4A) that became the major population (>90% CD8αβ+) by day 15 to 16 (data not shown). More importantly, three-color flow cytometry of the cells harvested on days 4 and 11 of culture extended our findings ex vivo, and confirmed that acquisition of the CD8αβ+ phenotype was linked to the expression of TCRβic, so that all CD8αα+progeny generated in the thymic lobes remained negative for TCRβic expression (Fig 4B). As observed when thymic lobes were reconstituted with CD4+ CD8αα+ cells, cellular proliferation lasted for 6 to 7 days in lobes injected with CD3− CD4+ CD8−progenitors. Regardless of the starting population, cellular expansion in the FTOC involved preferentially (if not exclusively) the TCRβic+ CD8αβ+ population, so that generation of CD8αα+ cells may ocurr essentially in the absence of cell proliferation. Taken together, our results provide formal proof that CD4+ CD8αα+thymocytes are the direct progeny of CD3−CD4+ CD8− thymocytes and the immediate precursors of the first DP thymocytes expressing the CD8αβ heterodimeric form. In addition, they are compatible with a model in which the CD8α chain is acquired by developing cells in the human thymus before the process of β-selection, whereas expression of CD8β is induced as a developmental consequence of the β-selection process after signaling through the pre–TCR.
CD4+ CD8+ human thymocytes are functional intermediates between CD3−CD4+ CD8− progenitors and CD4+ CD8β+ β-selected pre-T cells. (A) CD3− CD4+ CD8−thymocytes, isolated as described in Materials and Methods, were cultured in a hybrid hu/mo FTOC and analyzed after 4 and 11 days for the coexpression of CD4, CD8, and CD8β. (B) Intracytoplasmic TCRβ (TCRβic) expression (shaded areas) was analyzed by three-color flow cytometry in the cellular progeny recovered at day 4 of culture. By day 11, analysis was performed after electronic gating on the CD8+β− and the CD8+β+ progeny. Background fluorescence was determined with isotype-matched irrelevant MoAb. A representative experiment out of five is shown.
CD4+ CD8+ human thymocytes are functional intermediates between CD3−CD4+ CD8− progenitors and CD4+ CD8β+ β-selected pre-T cells. (A) CD3− CD4+ CD8−thymocytes, isolated as described in Materials and Methods, were cultured in a hybrid hu/mo FTOC and analyzed after 4 and 11 days for the coexpression of CD4, CD8, and CD8β. (B) Intracytoplasmic TCRβ (TCRβic) expression (shaded areas) was analyzed by three-color flow cytometry in the cellular progeny recovered at day 4 of culture. By day 11, analysis was performed after electronic gating on the CD8+β− and the CD8+β+ progeny. Background fluorescence was determined with isotype-matched irrelevant MoAb. A representative experiment out of five is shown.
Developmental events associated with β-selection parallel the onset of CD8β chain expression.
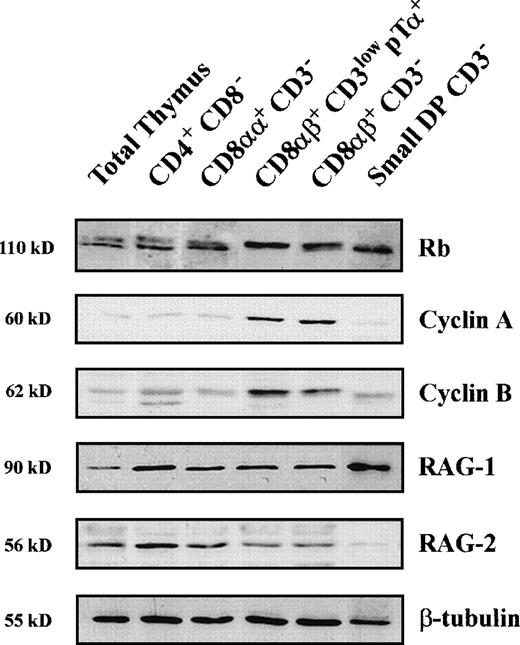
The above data allowed us to propose that CD8β expression is a cell marker of β-selection. Further support for this notion came from molecular studies aimed at analyzing individual CD8αα+and CD8αβ+ pre–T-cell stages for the expression of several proteins known to be regulated as a consequence of the β-selection process. Particularly, phosphorylation of Retinoblastoma (Rb), which is tightly associated with cell-cycle activation,30 expression of cell-cycle–associated cyclin A and cyclin B, and downregulation of RAG2 protein expression were analyzed by Western blotting, as indicators of β-selection (Fig5 and Table1).8 As shown in Fig 5, a mixture of both the hyperphosphorylated and the hypohosphorylated Rb forms was detected in CD3− CD4+CD8− as well as in CD4+CD8αα+ thymocytes. This pattern changed dramatically at the next CD3low pTα+ DP stage that showed an exclusive expression of the slow hyperphosphorylated Rb form, which was also the predominant form displayed by large CD3−CD4+ CD8αβ+ pre-T cells. Expectedly, noncycling, small CD3− DP pre-T cells10 displayed exclusively the fast hypophosphorylated Rb form. Of note, expression of the 60-kD cyclin A, which was barely detectable in CD3− CD4+CD8− and CD4+ CD8αα+thymocytes, was markedly upregulated in both the CD3lowpTα+ and the CD3− large DP pre–T-cell subsets displaying an exclusive hyperphosphorylated Rb form, whereas it dropped to basal levels in the CD3− small DP cells. An identical expression pattern was observed for the 62-kD cyclin B (Fig 5).
Analysis of Rb phosphorylation, and expression of Cyclins A and B and RAG proteins in distinct subsets of human pre-T cells. Cellular lysates from human thymocytes of the indicated phenotypes were isolated as described in Materials and Methods and analyzed by Western blotting for the expression of Rb, Cyclin A, Cyclin B, RAG1, and RAG2. Expression of β-tubulin was analyzed as an internal control. Molecular sizes are indicated on the left (kD).
Analysis of Rb phosphorylation, and expression of Cyclins A and B and RAG proteins in distinct subsets of human pre-T cells. Cellular lysates from human thymocytes of the indicated phenotypes were isolated as described in Materials and Methods and analyzed by Western blotting for the expression of Rb, Cyclin A, Cyclin B, RAG1, and RAG2. Expression of β-tubulin was analyzed as an internal control. Molecular sizes are indicated on the left (kD).
Summary of the Developmental Features of Pre- and Post-β-Selected Human Pre-T Cells
| . | Thymocyte Population . | ||||
|---|---|---|---|---|---|
| CD4+CD8− CD3− . | CD4+CD8αα+ CD3− . | CD4+CD8αβ+ CD3lowpTα+ . | CD4+CD8αβ+ CD3− large . | CD4+CD8αβ+ CD3− small . | |
| Percentage within total thymocytes ± SD | 2.1 ± 0.3 | 1.5 ± 0.3 | 5.2 ± 0.6 | 3.12 ± 0.2 | 14 ± 2.4 |
| Mean FSC ± SD | 354 ± 9.6 | 349 ± 19.2 | 396 ± 13.6 | 379 ± 15.9 | 298 ± 14.2 |
| Percentage of cycling cells ± SD | 9 ± 2.0 | 10 ± 2.1 | 50 ± 1.2 | 55 ± 3.0 | 9 ± 1.3 |
| TCRβic | − | − | + | + | + |
| CD8β | − | − | + | + | + |
| RAG-1 | ++ | + | + | + | +++ |
| RAG-2 | +++ | ++ | + | + | +/− |
| Rb phosphorylation | hypo | hypo | hyper | hyper | hypo |
| Cyclin A | +/− | +/− | +++ | +++ | +/− |
| Cyclin B | +/− | +/− | +++ | ++ | − |
| . | Thymocyte Population . | ||||
|---|---|---|---|---|---|
| CD4+CD8− CD3− . | CD4+CD8αα+ CD3− . | CD4+CD8αβ+ CD3lowpTα+ . | CD4+CD8αβ+ CD3− large . | CD4+CD8αβ+ CD3− small . | |
| Percentage within total thymocytes ± SD | 2.1 ± 0.3 | 1.5 ± 0.3 | 5.2 ± 0.6 | 3.12 ± 0.2 | 14 ± 2.4 |
| Mean FSC ± SD | 354 ± 9.6 | 349 ± 19.2 | 396 ± 13.6 | 379 ± 15.9 | 298 ± 14.2 |
| Percentage of cycling cells ± SD | 9 ± 2.0 | 10 ± 2.1 | 50 ± 1.2 | 55 ± 3.0 | 9 ± 1.3 |
| TCRβic | − | − | + | + | + |
| CD8β | − | − | + | + | + |
| RAG-1 | ++ | + | + | + | +++ |
| RAG-2 | +++ | ++ | + | + | +/− |
| Rb phosphorylation | hypo | hypo | hyper | hyper | hypo |
| Cyclin A | +/− | +/− | +++ | +++ | +/− |
| Cyclin B | +/− | +/− | +++ | ++ | − |
Pre-T cell subsets were independently isolated from human postnatal thymocytes as described in Materials and Methods. Cell size (FSC), DNA content (percent of cycling cells in S/G2 + M phases), and TCRβic and CD8β expression, was analyzed by flow cytometry, and expression of RAG-1, RAG-2, Rb, and cyclins A and B, by immunoblotting. Data presented as the mean ± SD of five independent experiments.
Analysis of RAG2 protein content in individual pre–T-cell subsets indicated that, as reported in mice,8 RAG2 downregulation may be a functional developmental consequence of β-selection also in humans. As shown in Fig 5, we found that the relative expression levels of RAG2 (as compared with those of β-tubulin) was reduced in both subsets (CD3low and CD3−) of CD8αβ+ pre-T cells, and became almost undetectable in downstream small pre-T cells. However, RAG2 protein was reexpressed at high levels at the next stage of CD3-TCRαβ–expressing DP thymocytes (data not shown) indicating that, similarly to mice, RAG2 downregulation is transient in thymocyte development in humans. Parallel analysis showed that RAG1 was not subjected to a similar developmental regulation at the protein level, because relatively high levels of RAG1 were expressed in all pre–T-cell subsets. Taken together, these results provide additional support to our proposal that β-selection occurs in humans during the transition from CD4+ CD8αα+ to CD4+CD8αβ+ DP pre-T cells and allow us to conclude that CD8β chain induction may be one of the direct consequences of the β-selection process.
DISCUSSION
The progression of thymocytes through distinct developmental stages is marked by the ordered pattern of expression of a number of cell surface molecules of which the coreceptors CD4 and CD8 are particularly relevant. In humans, the developmental expression of these two molecules marks progression of thymocytes from immature CD4− CD8− DN cells through CD4+ CD8− intermediates and finally to CD4+ CD8+ DP thymocytes, before expression of a surface αβ TCR and commitment to either CD4+CD8− or CD4− CD8+mature T cells.3,16 We have previously shown that essentially all CD4+ CD8− intermediates display a germline configuration at the TCRβ locus and lack expression of TCRβ protein, whereas both TCRβ gene rearrangements and cytoplasmic TCRβ chain expression occur in the vast majority of thymocytes at the downstream CD4+ CD8+TCRαβ− stage. The finding that the latter subset is highly enriched in large cycling cells25 allowed us to propose that the CD4+ CD8− to CD4+ CD8+ transition represents the critical developmental point at which β-selection promotes the clonal expansion and further differentiation of human pre-T cells independent of TCRα.25 Molecular support to that notion came from additional studies showing that expression of surface CD3 and pTα chain is restricted in vivo to a significant proportion of cells within the population of CD4+ CD8+TCRαβ− cycling thymocytes,10indicating that the human pre–TCR is actually expressed on pre-T cells in transit to the CD4+ CD8+ DP stage.25 However, we show in this study that the DP compartment is heterogeneous regarding the expression of CD8, so that it is mostly composed of cells with the expected CD4+CD8αβ+ phenotype, but also includes a minor fraction of cells that coexpress CD4 and CD8α without CD8β. The physiological existence of such CD4+CD8α+β− thymocytes suggests that expression of the CD8 glycoprotein either as an αα homodimer or as an αβ heterodimer26 is developmentally controlled and, therefore, the relevance of the CD4+ CD8−to CD4+ CD8+ transition as the critical check-point of β-selection needs to be revisited.
Confirming the observation by Spits et al,17 we show here that CD4+ CD8αα+ cells are very rare in the human thymus (<2% of total thymocytes), this probably precluding previous studies on their physiological role in thymic T-cell development. We have now approached this issue by taking advantage of the fact that such cells can be enriched (up to 35%) within the fraction of large-sized CD3− DP thymocytes recovered from Percoll density gradients (Fig 1). An important aspect of our study was the finding that no TCRβ chain was expressed in the cytoplasm of isolated CD4+ CD8αα+thymocytes, although they showed extensive TCRβ gene rearrangements (Blom et al,24 and our unpublished results); whereas downstream CD4+ CD8αβ+ cells were homogeneously TCRβic+. Because TCRβic expression is currently envisaged as a marker of β-selection, one might conclude that upregulation of CD8β parallels β-selection in the human thymus, whereas induction of CD8α is not coupled to that critical process. However, a very recent report by Blom et al24 has shown that TCRβic chain is already expressed in a significant proportion (up to 25%) of CD4+ CD8αα+ thymocytes that express very low surface CD3, and may thus be cells that have completed β-selection. Although it can not be ruled out that TCRβic+ cells characterized by Blom et al24 are actually conventional DP thymocytes that downregulate CD8β from the cell surface due to the ex vivo isolation procedure, it is also possible that such cells are very recent β-selected cells upregulating CD8β in vivo. Alternatively, CD4+ CD8αα+TCRβic+ cells could have escaped detection in our study. However, we consider this last possibility very unlikely because we consistently observed lack of both TCRβic and surface CD3 not only in CD4+ CD8αα+ cells isolated ex vivo, but also in those generated in vitro in FTOC from the CD4+ CD8− precursor subset. Moreover, we showed that the coincident developmental expression of TCRβic and CD8β was associated with progression through DNA synthesis and expression of cell-cycle–associated proteins both in vivo (Figs 1 and 5) and in vitro (data not shown), supporting the proposal that CD8β expression is induced simultaneously to or immediately after β-selection. A summary of the developmental events associated with progression from CD4+CD8αα+ TCRβic− cells to CD4+ CD8αβ+TCRβic+ cells in humans is illustrated in Table 1.
A second aspect of our study was the functional demonstration of a precursor-product relationship between CD4+CD8αα+ (TCRβic−) and CD4+ CD8αβ+(TCRβic+) thymocytes. This is particularly relevant because CD4+ CD8αα+ thymocytes lacking TCRβic have previously been proposed to be dead-end cells.24 Although our results do not preclude that cell death may physiologically occur within the CD4+CD8αα+ population in those cells that fail β-selection because of nonproductive TCRβ gene rearrangements, they provide direct evidence that CD4+CD8αα+TCRβic−_thymocytes represent the immediate precursors of CD4+ CD8αβ+TCRβic+ cells and the direct progeny of CD4+ CD8− intermediates. This supports again that CD8β expression is not induced before the cells have undergone a productive TCRβ gene rearrangement and express a functional TCRβ chain. Taken together, the above data allowed us to conclude that β-selection operates at the CD4+CD8αα+ to CD4+ CD8αβ+transition and, thus, upregulation of CD8β may be considered as a marker of β-selection in human thymocyte development. Our proposal has important implications regarding the existence of regulatory mechanisms that account for a differential expression of CD8α and CD8β at distinct stages of thymocyte development. In this regard, it is known that surface expression of CD8β chain is dependent on CD8α chain expression,26 so that both molecules are coordinately expressed on the vast majority of thymocytes and thymus-derived T cells.18,19 However, mechanisms might exist that allow for their discoordinate regulation as well, because other CD8+cell types such as extrathymically derived intestinal intraepithelial lymphocytes (IEL), and a subset of NK cells express exclusively the CD8αα homodimeric form.18,31,32 Evidence has been provided that CD8 lineage-specific regulatory sequences direct developmentally correct expression of the human CD8β gene on thymus-derived T cells from transgenic animals33; and recent data by Littman et al34 have shown that multiple developmental stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells in mice. These studies, however, have not addressed the question as to how lineage-specificity of CD8α and CD8β expression is achieved. As previously suggested in mice,15 it is likely that regulatory signals provided by the thymic microenvironment are involved in controlling CD8β expression also in humans. In both species, CD8αα+ thymocytes remain negative for CD8β in cytokine-supplemented cultures in vitro,15,17 but acquire CD8β when transferred into a FTOC (Hori et al17 and present study). Interestingly, we show here that progression to the CD8β+ stage is associated with acquisition of low-surface CD3 and, more importantly, of stoichiometric levels of surface pTα chain. It is thus tempting to speculate that CD8β expression in humans is coupled to signalling through the pre–TCR, which might be induced at the CD4+CD8αα+ to CD4+ CD8αβ+transition, to be rapidly downregulated from the cell surface once the cell has been brought into cycle and has attained the DP stage.25 Therefore, as reported for its murine counterpart,7-9 the human pre–TCR may participate in the transition to the conventional CD4+ CD8+ stage, at which expression of the CD8αβ heterodimer will be critically involved in the final maturation of MHC class-I–restricted CD8-lineage T cells.19-22
ACKNOWLEDGMENT
We thank Drs M. Brenner, R. Kurrle, and E.L. Reinherz for the generous gift of antibodies, Dr K. Schwarz for helpful discussions, Dr J.C. Segovia for assistance with cell sorting, and the Pediatric Cardiosurgery Units from the Centro Especial Ramón y Cajal and Ciudad Sanitaria La Paz (Madrid, Spain) for the thymus samples. We also want to express our gratitude to the people of the mouse facilities at our Institute for his continuous support.
Supported in part by grants from Glaxo Wellcome S.A.; SAF97-0161 from Comisión Interministerial de Ciencia y Tecnologı́a (CICYT); PB97-1194 from Dirección General de Enseñanza Superior e Investigación Cientı́fica (DGES); and 08.3/0013/1997 from Comunidad Autónoma de Madrid (CAM). The Centro de Biologı́a Molecular “Severo Ochoa” is partially supported by the Fundación Ramón Areces. Y.R.C., A.R.R., and V.G.Y. are fellows from the Fundación Ramón Areces, Ministerio de Educación y Ciencia, and Fondo de Investigación Sanitaria, respectively.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marı́a L. Toribio, PhD, Centro de Biologı́a Molecular “Severo Ochoa,” Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain; e-mail:mtoribio@cbm.uam.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal