The Fas/FasL system mediates apoptosis in several different cell types, including T lymphocytes. Fas ligand (FasL), a 40-kD type II membrane protein also expressed in activated T cells, belongs to the tumor necrosis factor ligand family. We describe a new alternative splicing of mouse FasL, named FasL short (FasLs), cloned by reverse transcriptase-polymerase chain reaction. FasLs is encoded by part of exon 1 and part of exon 4 of FasL gene. The protein encoded by FasLs mRNA has a putative initiation code at position 756 and preserves the same reading frame as FasL, resulting in a short molecule lacking the intracellular, the transmembrane, and part of the extracellular domains. RNase protection and immunoprecipitation analysis showed that FasLs is expressed in nonactivated normal spleen cells and in hybridoma T cells and that it is upregulated upon activation by anti-CD3 monoclonal antibody (MoAb). Moreover, FasLs-transfected cells expressed soluble FasLs in the supernatant and became resistant to apoptosis induced by agonist anti-Fas MoAb. Thus, FasLs, a new alternative splicing of FasL, is involved in the regulation of Fas/FasL-mediated cell death.
APOPTOSIS (PROGRAMMED cell death) plays a crucial role in the deletion of unwanted T cells in 2 different phases during the ontogeny of the immune response.1-5 In the thymus, apoptosis participates in T-cell repertoire development. The encounter of self-antigen leads to T-cell deletion characterized by apoptotic cell death (negative selection). In the periphery, chronically stimulated mature T cells undergo apoptosis upon engagement of TCR/CD3 by antigen-presenting cells (APCs) presenting antigenic peptide.6-10 This process, termed activation-induced cell death (AICD),11 appears to be mediated by the Fas/FasL system.12-18
Fas/APO-1 (CD95), a protein expressed on the surface of a variety of cells, including transformed cell lines and chronically stimulated T cells, mediates apoptosis after binding with FasL or with anti-Fas monoclonal antibody (MoAb).13,16,18 FasL, a 40-kD type II membrane protein, is normally expressed in the spleen and at low levels in the thymus. Human FasL is proteolytically released from the cell membrane in a soluble form that binds Fas and induces apoptosis.19-22
Fas/FasL-mediated apoptosis is regulated by a balance of receptor/ligand interactions.23 For example, TCR engagement induces FasL and upregulates Fas, and their interaction induces cell death.14,16,18 Moreover, Fas-mediated apoptosis is modulated by soluble proteins derived from alternative splicing of the Fas gene that inhibits anti-Fas–induced apoptosis.24-26Although it is clear that soluble forms of Fas derived from alternatively spliced transcripts exist and some have a decoy function, a similar mechanism has not been described for FasL. We investigated whether alternative spliced transcripts, coding for different forms of FasL, are produced.
We used the T-cell line hybridomas 3DO, which has been previously used in studies on apoptosis. It also undergoes apoptosis by means of TCR/CD3 cross-linking in which the Fas/FasL interaction plays a dominant role in mediating AICD.27-29 We identified and characterized a new mRNA FasL variant. It is also expressed in normal spleen cells and encodes a protein corresponding to a short form of FasL (FasLs) that inhibits Fas-mediated cell death.
MATERIALS AND METHODS
Cell line and animals.
A spontaneously dividing CD3+, CD4+, CD2+, CD44+ subline of the ova-specific hybridoma T-cell line 3DO,30 obtained by recloning the original line in our laboratory, was used for the experiments. Cells were maintained in logarithmic growth in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 10 μmol/L HEPES, and antibiotics. Spleen T cells were obtained from 4- to 6-week-old C3H/HeN mice (Charles River, Calco, Milan, Italy) and enriched by passing cells twice through nylon columns.
Antibody cross-linking and flow cytometry analysis.
Hamster antimouse CD3 (clone145-2C11; Pharmingen, San Diego, CA) MoAb at 1 μg/mL was allowed to adhere to flat-bottomed, high-binding, 96-well plates (Costar, Cambridge, MA) at 4°C in 100 μL phosphate-buffered saline (PBS). After 20 hours, plates coated with MoAb were washed, incubated at 37°C for 2 hours with PBS supplemented with 10% FCS, and washed again, and spleen and hybridoma T cells were then plated at 1 × 105 cells/well and incubated at 37°C for 15 hours. Cells were then used to perform fluorescence-activated cell sorting (FACS) analysis or to extract RNA.
For FasL membrane detection, cells were stained with hamster antimouse MoAb (clone MFL4; Pharmingen) or isotype-matched MoAb and with antihamster IgG fluorescein isothiocyanate (FITC) conjugate (Pharmingen) as a second-step reagent.
The percentage of FasL+ cells was calculated using Lysis II research software (Becton Dickinson, Mountain View, CA).
To evaluate Fas-mediated killing, cells (1 × 106) were incubated at room temperature for 30 minutes with 10 μg/mL of the anti-Fas MoAb (hamster antimouse, clone Jo2; Pharmingen) and then washed and plated in wells coated with Ab to hamster IgG (5 μg/mL; Pharmingen) for the cross-linking of the anti-Fas MoAb.27
RNA extraction and reverse transcriptase reaction.
RNA was isolated by using the TRIzol LS reagent (GIBCO-BRL, Life Technologies, Paisley, Scotland) following the manufacturer’s instructions.
The reverse transcriptase reaction was conducted in 20 μL reverse transcriptase buffer (GIBCO-BRL), 10 mmol/L dithiothreitol, 0.5 U/μL RNase inhibitor, 100 μmol/L dNTP, 1 μmol/L oligo(dT) primer (T15), and 0.1 μg DNA-free RNA. After 10 minutes of incubation at 65°C and then at 37°C, 1 μL Moloney murine leukemia virus (M-MLV) reverse transcriptase (GIBCO-BRL) was added. The reaction was performed for a further 50 minutes and the enzyme was inactivated by heating for 5 minutes at 95°C. The reaction quality was checked by polymerase chain reaction (PCR) with specific primers for β-actin mRNA amplification.
PCR.
PCR was conducted in 20 μL of PCR buffer (Perkin Elmer Corp, Norwalk, CT), 25 μmol/L dNTP, 0.5 μmol/L of each specific primer, 1 mmol/L MgCl2, and 2 μL cDNA from the reverse transcriptase reaction or 0.04 μL DNA from a previous PCR reaction (when a nested PCR was performed). After 5 minutes of incubation at 96°C (hot start) and 2 minutes at the chosen annealing temperature (68°C), 0.2 μL AmpliTaq (Perkin Elmer) was added. Denaturation was performed at 95°C for 30 seconds and extension was performed at 72°C. The DNA Thermal Cycler 480 (Perkin Elmer) was used. DNA oligonucleotide primers were synthesized in an Oligo-1000 DNA synthesizer (Beckman, Fullerton, CA).
Cloning and sequencing.
PCR products were cloned into the pCR II vector using the TA Cloning kit (Invitrogen, San Diego, CA). Sequencing was performed with the Sequenase kit (USB, Cleveland, OH).
RNase protection analysis.
Using reverse transcriptase-PCR (RT-PCR), a probe for RNase protection was constructed with primers 1 and 4 (shown in Fig 4) to clone the 207-bp probe. After cloning, the products were sequenced to exclude point mutation. Plasmide DNA was linearized with BamHI (New England Biolabs, Beverly, MA) and transcribed with T7 RNA polymerase (GIBCO-BRL) in the presence of 50 μmol/L [α-32P] UTP. After gel purification, the 2 × 105 cpm probe was hybridized to total RNA (20 μg) overnight at 60°C.
RNase digestion was performed by using an RNase A (40 μg/mL; Boehringer Mannheim, Mannheim, Germany) and RNase T1 (1.5 U/μL; GIBCO-BRL) solution at 37°C for 15 minutes. The undigested products were treated with phenol-chloroform, precipitated with ethanol, and loaded on a denaturing polyacrylamide sequencing gel. Autoradiographic exposure was performed for 2 days.
Transfection and evaluation of the transfected clones.
The FasLs sequence (406 bp) was cloned into pcDNA3 plasmid (Invitrogen) for expression in mammalian cells. 3DO cells were transfected by electroporation (300 mA, 960 μF) with 15 μg of linearized pcDNA3 vector (control clones) or 15 μg of linearized pcDNA3 vector expressing the FasLs cDNA. Thirty-six hours after transfection, cells were cultured in medium containing G418 0.8 mg/mg active-form/mL (GIBCO-BRL) and 100 μL/mL of cell suspension were plated in 96-well plates (4 for each transfection). Fifteen to 20 days later, no more than 15% of the wells contained living, growing cells. These cells were considered clones and were analyzed in RT-PCR for exogenous FasLs expression.28 As primer forward, we used the T7 eukaryotic modified (TCGAAATTAATACGACTCACTATAGGG), and as primer reverse, we used the n. 3 located on FasLs cDNA (see Fig 2) or the Sp6 eukaryotic (GCTCTAGCATTTAGGTGACACTATAG). Each RT-PCR reaction was controlled using the respective RNA without the addition of reverse transcriptase to the RT reaction.
Immunoprecipitation.
Antibodies used for immunoprecipitation were 2 different affinity-purified rabbit polyclonal antibodies raised against a peptide corresponding to amino acids 2-19 mapping at the amino terminus of rat origin FasL (FasL-NH2) and an antibody raised against a peptide corresponding to amino acids 260-279 mapping at the carboxy terminus of human FasL (FasL-COOH). Those antibodies were purchased by Santa Cruz Biotechnology (Santa Cruz, CA).
Cells (5 × 106/sample) lysed in a 1% NP-40–containing buffer (250 mmol/L NaCl, 0.5% deoxycholic acid (DOC), 0.1% sodium dodecyl sulfate [SDS], 50 mmol/L Tris, pH 8) and supernatants from transfected clones were preadsorbed with normal rabbit IgG. After removing the protein A-Sepharose by centrifugation, 10 μg of purified anti-FasL–COOH or anti-FasL–NH2 Abs or preimmune antiserum (Jackson Immuno Research Laboratories, West Grove, PA) was added to the supernatants. After incubation for 60 minutes on ice, protein A-Sepharose was added and the mixture was incubated at 4°C overnight. The immunoprecipitates were washed 3 times with cold lysis buffer, boiled for 3 minutes, and then analyzed by electrophoresis in 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel followed by transfer to nitrocellulose (Bioblot-NC; Costar) for 5 hours at 250 mA, 4°C in 25 mmol/L Tris/glycine, pH 8.3, and 20% vol/vol methanol. After immunoprecipitation, proteins were analyzed by Western blotting using the anti-FasL–COOH or anti-FasL–NH2 Abs.
To control the specificity of anti FasL-COOH Ab, Western blotting experiments were performed using FasL-GST and GST fusion proteins. In particular, 10 μg of fusion proteins was resolved by electrophoresis at room temperature on a 12% gradient polyacrylamide gel. After electrophoresis, proteins were analyzed by Western blotting using the anti-FasL–COOH Ab or the antimouse-FasL MoAb, clone MFL4.
The proteins were detected using the enhanced chemiluminescence (ECL) system (Amersham Life Science, Buckinghamshire, UK) after staining with streptavidin-conjugated horseradish peroxidase (Amersham). For metabolic labeling, cells were maintained in leucine-free minimal essential medium (Sigma, St Louis, MO) supplemented with glutamine, gentamicin, 0.02 mmol/L 2-mercaptoethanol, and 2% dialyzed fetal bovine serum (FBS). [3H] leucine (L-4,5-3H leucine; Amersham) was then added to anti-CD3–treated and untreated cells at 1 mCi per 2 × 107 cells. After 15 hours of incubation, 3H-labeled supernatants and cell lysate proteins were analyzed as described above. Blocking peptide corresponding to amino acids 260-279 mapping at the carboxy terminus of FasL of human origin was used for competition studies following the manufacturer’s instructions (Santa Cruz).
Cytotoxicity assay.
The lysis of P815 Fas+ tumor cell line was used as an indicator of FasL expression. This tumor cell line was grown in RPMI 1640 and 10% FCS and subcultured 2 to 3 times per week. Different concentrations of FasLs- or empty vector-transfected 3DO cells were cultured for 20 hours on plates coated with anti-CD3 (1 μg/well) or control medium. The 51Cr labeling and assay were as previously described.18 Spontaneous release or release in the presence of anti-CD3 with no effector cells was less than 15% of the total release. The percentage of specific lysis at various E:T ratios was calculated as follows: % cytotoxicity = (test cpm − spontaneous release cpm)/(total release cpm) × 100, where test cpm is the mean cpm released in the presence of effector cells; spontaneous release is the mean cpm released from targets cultured in medium alone; and total release cpm is the mean cpm obtained by lysing target with 0.5% Triton X-100. To demonstrate that the cytotoxic effect was dependent on FasL expression, the blocking antimouse FasL MoAb (5 μg/mL, clone MFL4) was added to some empty vector-transfected clones activated by anti-CD3 MoAb.
Apoptosis evaluation by propidium iodide (PI) solution.
Apoptosis was measured by flow cytometry as described elsewhere.31 After culturing, cells were centrifuged and the pellets gently resuspended in 1.5 mL hypotonic PI solution (50 μg/mL in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma). Test tubes were stored overnight at 4°C in the dark. The PI-fluorescence of individual nuclei was measured by flow cytometry using standard FACScan equipment (Becton Dickinson). The nuclei traversed a 488 nm argon laser light beam. A 560 nm dichroid mirror (DM 570) and a 600 nm band pass filter (band width, 35 nm) were used to collect the red fluorescence due to PI DNA staining, and the data were recorded in logarithmic scale in a Hewlett Packard (HP 9000, model 310; Hewlett Packard, Palo Alto, CA) computer. The percentage of apoptotic cell nuclei (subdiploid DNA peak in the DNA fluorescence histogram) was calculated with specific FACScan research software (Lysis II).
Preparation of FITC-labeled fusion protein.
A fusion protein containing the full FasLs amino acid sequence fused to glutathione S-transferase (GST; Pharmacia, Uppsala, Sweden) was prepared. GST-fusion protein was expressed in Escherichia coli, induced with 1 mmol/L isopropyl-β-D-thiogalactopyranoside, and purified with glutathione-agarose beads as previously described.28 The FasLs and GST fusion proteins were conjugated with biotin (Pharmacia) according to standard procedures. Serial dilution of biotinylated proteins was used to evaluate the binding to a Fas+ cell line. Streptavidin-FITC (Pharmingen) was used as a second-step reagent. To control the specificity of the staining, antimouse Fas MoAb (clone Jo2) was used to inhibit GST-FasL binding to activated 3DO cells. In particular, cells were incubated with the MoAb 30 minutes at 4°C, washed, and then incubated for a further 30 minutes with the biotinylated GST-FasL fusion protein.
Samples were analyzed on a FACScan flow cytometer.
Statistical analysis.
Each experiment was performed at least 3 times. Representative experiments are shown, unless otherwise indicated in the figure legends. Results are expressed as the mean ± SD of 3 different experiments. Because of the nonnormal distribution of the data, nonparametric tests (Kruskal-Wallis’ analysis of variance) were adopted for statistical evaluation.
RESULTS
Isolation and characterization of FasLs cDNA.
3DO cells expressed FasL on the cell membrane upon activation with cross-linked anti-CD3 MoAb (Fig 1A). To investigate whether other potential products of the FasL gene are expressed in activated 3DO cells, mRNA transcripts from both untreated cells or cells treated with cross-linked anti-CD3 MoAb were analyzed by RT-PCR. Using the primers 1 and 2 shown in Fig 2A (specific for mouse FasL cDNA), we obtained 2 products (Fig 1B). As expected, 1 of these products, which was 1,067 bp long, corresponded to the full-length FasL cDNA. Unexpectedly, a second product, which was 406 bp long, was also detected on both anti-CD3–treated (line 2) and, although less evident, resting cells (line 1, Fig 1B).
(A) FasL expression in 3DO cells. Cells were incubated for 15 hours in 96-well plates with medium alone (control) or coated with anti-CD3 MoAb (1 μg/mL). T cells were stained with an antimouse FasL MoAb and an antihamster-FITC. The number shows the percentage of positive cells calculated by Lysis II. Fluorescence intensity versus cell number. (B) RT-PCR analysis of the mouse FasL mRNA transcripts. First-strand cDNA prepared from untreated (line 1) or anti-CD3–treated (line 2) 3DO cells were subjected to PCR with the primers 1 and 2 shown in Fig 2A. A negative control, without cDNA template, was also performed (line 3). The PCR products were separated by electrophoresis on 1.5% agarose gel and stained with ethidium bromide. Marker VI was run for comparison (left line). (C) PCR products obtained using as template the PCR product (0.04 μL) of the experiment shown in (B). Line 1, forward, primer 1, reverse, primer 3; line 2, forward, primer 1, reverse, primer 2; line 3, negative control. The primers are shown in Fig 2A. Marker VI was run for comparison (left line).
(A) FasL expression in 3DO cells. Cells were incubated for 15 hours in 96-well plates with medium alone (control) or coated with anti-CD3 MoAb (1 μg/mL). T cells were stained with an antimouse FasL MoAb and an antihamster-FITC. The number shows the percentage of positive cells calculated by Lysis II. Fluorescence intensity versus cell number. (B) RT-PCR analysis of the mouse FasL mRNA transcripts. First-strand cDNA prepared from untreated (line 1) or anti-CD3–treated (line 2) 3DO cells were subjected to PCR with the primers 1 and 2 shown in Fig 2A. A negative control, without cDNA template, was also performed (line 3). The PCR products were separated by electrophoresis on 1.5% agarose gel and stained with ethidium bromide. Marker VI was run for comparison (left line). (C) PCR products obtained using as template the PCR product (0.04 μL) of the experiment shown in (B). Line 1, forward, primer 1, reverse, primer 3; line 2, forward, primer 1, reverse, primer 2; line 3, negative control. The primers are shown in Fig 2A. Marker VI was run for comparison (left line).
(A) FasL and FasLs nucleotide sequences and predicted amino acid sequence of mouse FasLs. The deleted sequence is shown in small letters. The asterisks indicate possible glycosylation sites. (B) Schematic representation of FasL and FasLs cDNA. (C) Schematic representation of FasL and FasLs protein. Arrows indicate the primers used to clone FasLs and to clone the probe used for RNase protection.
(A) FasL and FasLs nucleotide sequences and predicted amino acid sequence of mouse FasLs. The deleted sequence is shown in small letters. The asterisks indicate possible glycosylation sites. (B) Schematic representation of FasL and FasLs cDNA. (C) Schematic representation of FasL and FasLs protein. Arrows indicate the primers used to clone FasLs and to clone the probe used for RNase protection.
To investigate the shorter RT-PCR product (406 bp), a series of PCR reactions using different specific primers was performed. Figure 1C shows the results of a representative experiment in which the PCR products of the experiment reported in Fig 1B were reamplified using the same primers (line 2) or the primer 1 and the primer 3 (shown in Fig 2A) nested with respect to primer 2 (line 1). As expected, the products obtained using the nested primer were smaller than those obtained using primers 1 and 2, indicating that the short 406-bp band might be the amplification of a spliced FasL mRNA.
The short FasL cDNA, which was obtained from either anti-CD3–activated 3DO cells or from anti-CD3–treated spleen cells, was cloned and the nucleotide sequence was determined. The sequence data concurred with the size of the amplified products and with the published mouse FasL cDNA sequence, except for the deletion of part of it (Fig 2). We named this short FasL alternative splicing, FasLs. FasLs was characterized by a deletion of 661 bp starting at nucleotide position 87 and ending at position 748 of the full-length FasL cDNA. The starting codon (ATG) of full-length FasL cDNA is also deleted. However, the FasLs starting codon gives an equal reading frame to FasL, with the same termination codon. This resulted in a cDNA encoding for a putative FasL protein lacking the intracellular, the transmembrane, and part of the extracellular domains but retaining the extracellular domain of FasL with 2 glycosylation sites (Fig 2A). The molecular mass of the predicted mature FasLs protein, before further posttranslation modification, is 8,040 Daltons.
FasLs mRNA is upregulated by anti-CD3 activation.
RNase protection analysis was performed to evaluate the expression of FasLs. The probe was obtained by amplifying a smaller fragment of FasLs cDNA in RT-PCR using primers 1 and 4 shown in Fig 2A. This probe protects fragments of 207 bp (FasLs), 171 bp (FasL), and 36 bp (FasL). As shown in Fig 3, the 2 higher fragments (207 and 171 bp, respectively) were detected, whereas the smaller expected fragment of 36 bp (FasL) was not observed in the gel. The 2 observed fragments were equally evident after hybridization of the labeled probe, with RNA from anti-CD3–treated 3DO cells (Fig 3, line 4) but not with RNA from nonactivated 3DO cells that express FasLs only, although at lesser amount (Fig 3, line 3). No fragments were observed with tRNA used as negative control (Fig 3, line 2). These data indicate that FasLs but no FasL mRNA are expressed in untreated 3DO cells and that anti-CD3 activation upregulates the expression of both FasL and FasLs.
Expression of FasLs and FasL mRNA in 3DO cells. RNase protection analysis was performed as described in Materials and Methods. Probe includes a portion of exon 1 and a portion of exon 4. On the left, the fragment that the antisense probe would protect upon single-strand specific RNase digestion is shown schematically. Line 1, undigested probe; line 2, probe digested after hybridization with 30 μg tRNA; line 3, probe digested after hybridization with 30 μg RNA from 3DO cells; line 4, probe digested after hybridization with 30 μg RNA from anti-CD3–stimulated 3DO cells. The basepair length reported on the right was derived from a sequencing reaction.
Expression of FasLs and FasL mRNA in 3DO cells. RNase protection analysis was performed as described in Materials and Methods. Probe includes a portion of exon 1 and a portion of exon 4. On the left, the fragment that the antisense probe would protect upon single-strand specific RNase digestion is shown schematically. Line 1, undigested probe; line 2, probe digested after hybridization with 30 μg tRNA; line 3, probe digested after hybridization with 30 μg RNA from 3DO cells; line 4, probe digested after hybridization with 30 μg RNA from anti-CD3–stimulated 3DO cells. The basepair length reported on the right was derived from a sequencing reaction.
FasLs: RNA and protein expression in transfected cells.
It is well known that triggering of the TCR/CD3 complex results in upregulation of Fas and induction of FasL expression and that the Fas/FasL interaction mediates the TCR/CD3-induced apoptosis.17 To test the effects of FasLs expression on apoptosis mediated by TCR triggering, we transfected 3DO cells with an expression vector pcDNA3 in which FasLs cDNA is expressed under the control of the CMV promoter (clones 1 through 6). As a control, we also transfected the empty vector (clones 7 through 10). After selection with G418 antibiotic, cell clones were screened for FasLs expression by RT-PCR, using a specific primer for pcDNA3 vector (forward) and a specific primer for FasLs (reverse) or primers that bind Sp6 and T7 of pcDNA3.
Results of a representative experiment (reported in Fig 4) show that FasLs-transfected clones (clones 1 through 6) express the 517-bp mRNA band, whereas empty vector-transfected clones (clones 7 through 10) do not (Fig 4A). A bigger band was obtained with the primers binding Sp6 and T7 of pcDNA3 (Fig 4B). Each RT-PCR reaction was controlled using the respective RNA without the addition of reverse transcription to the RT reactions (Fig4B). Both FasLs-transfected (clones 1 through 6) or empty vector-transfected (clones 7 through 10) clones were tested for FasLs protein expression using an antibody recognizing the COOH terminal portion of the molecule. Figure 4C shows that a protein of molecular weight (Mr) of approximately 16,000 Daltons, which is compatible with the Mr of FasLs’ predicted protein after potential posttranslation modification, was found in FasLs-transfected, but not in empty vector-transfected clones. Moreover, the same protein was found in the supernatants obtained from FasLs-transfected clones, but not in the supernatants from empty vector-transfected clones (Fig 4D).
Expression of exogenous FasLs in transfected 3DO cells. The FasLs transfection was controlled by RT-PCR using as primer forward the T7 eukaryotic and as primer reverse the n. 3 shown in Fig 2A or using as primer forward the T7 eukaryotic and as primer reverse the SP6 eukaryotic. Each RT-PCR reaction (+) was controlled by using the respective RNA without the addition of reverse transcriptase (−) (B). The FasLs transfection was also controlled by immunoprecipitation with anti-FasL–COOH Ab of cell lysates (C) and supernatants (D). Western blotting of the GST-FasLs fusion protein. Ten micrograms of GST-FasLs (lines 1 and 3) or GST (lines 2 and 4) was resolved by electrophoresis in a 12% gradient polyacrylamide gel (E). Proteins were analyzed by Western blotting using the anti-FasL–COOH Ab (lines 1 and 2) or the anti-FasL MoAb (clone MTL4, lines 3 and 4) as described in Materials and Methods.
Expression of exogenous FasLs in transfected 3DO cells. The FasLs transfection was controlled by RT-PCR using as primer forward the T7 eukaryotic and as primer reverse the n. 3 shown in Fig 2A or using as primer forward the T7 eukaryotic and as primer reverse the SP6 eukaryotic. Each RT-PCR reaction (+) was controlled by using the respective RNA without the addition of reverse transcriptase (−) (B). The FasLs transfection was also controlled by immunoprecipitation with anti-FasL–COOH Ab of cell lysates (C) and supernatants (D). Western blotting of the GST-FasLs fusion protein. Ten micrograms of GST-FasLs (lines 1 and 3) or GST (lines 2 and 4) was resolved by electrophoresis in a 12% gradient polyacrylamide gel (E). Proteins were analyzed by Western blotting using the anti-FasL–COOH Ab (lines 1 and 2) or the anti-FasL MoAb (clone MTL4, lines 3 and 4) as described in Materials and Methods.
The Mr of the protein immunoprecipitated by the anti-FasL–(COOH) antibody (Mr ∼16,000) is larger than the Mr of FasLs as calculated from its amino acid sequence (Mr 8,040). A similar difference between predicted and actual Mr has been previously reported for FasL protein19 and is probably due to glycosylation of the glycosylation sites that are also present in the FasLs sequence (Fig2A). The specificity of anti-FasL–COOH Ab was tested by using the FasLs-GST and GST fusion proteins in Western blotting. As can be seen in Fig 4E, anti-FasL–COOH recognized the FasLs-GST fusion protein but not the GST fusion protein (Fig 4E, lines 1 and 2). In contrast, antimouse-FasL, clone MFL4, did not recognize FasLs or GST fusion proteins (Fig 4E, lines 3 and 4).
As a further control, we also used an antibody that recognizes the NH2-terminal portion of the FasL. As expected, no bands of Mr 16,000 were detected in the cell pellet or supernatants of FasLs-transfected clones (not shown).
FasLs expression protects T cells from anti-Fas– and anti-CD3–activated apoptosis.
To explore the FasLs functional activity, the cytotoxic activity of activated and resting FasLs-transfected 3DO clones was examined using the Fas+ P815 cell line as target.18,27 As shown in Table 1, upon activation with anti-CD3 MoAb, clones transfected with empty vector, as well as untransfected clones,27 induced cytolysis of P815 cell line (clones 7 through 10), whereas FasLs-transfected clones exhibited reduced or no cytotoxic activity (clones 1 through 6). The cytotoxic effect was truly FasL-dependent, because the blocking anti-FasL MoAb MFL4 reduced the cytotoxic levels of activated empty vector-transfected clones (Table 1). We also tested whether FasLs-transfected clones were susceptible to anti-Fas MoAb-induced cell death. Table 1 shows that, unlike empty vector-transfected clones, FasLs-transfected clones were resistant to apoptosis induced by anti-Fas MoAb, suggesting that FasLs can inhibit FasL-induced apoptosis. To analyze this inhibiting activity, we used the supernatant of transfected clones containing FasLs. Results indicate the supernatants from FasLs-transfected, but not those from empty vector-transfected clones, inhibited anti-Fas–induced cell death (Table 1).
Effect of FasLs Transfection on Apoptosis Induced by Anti-Fas or Anti-CD3 MoAb
| . | Control . | Anti-Fas . | Control . | Anti-Fas . | Control . | Anti-CD3 . | Control . | Anti-CD3 . |
|---|---|---|---|---|---|---|---|---|
| 1 | 11.7 | 9.3 | 6.5 | 9.0 | 0.1 | 1.3 | 2.6 | 40 |
| 2 | 5.0 | 4.5 | 6.0 | 8.1 | 0.1 | −1.1 | 2.2 | 38 |
| 3 | 6.5 | 6.0 | 8.3 | 10.0 | 0.2 | −1.2 | 3.7 | 51 |
| 4 | 4.7 | 5.2 | 9.0 | 10.0 | 0.2 | 0.6 | 1.7 | 50.1 |
| 5 | 9.4 | 5.5 | 10.7 | 12.0 | 0.1 | −1.1 | 3.1 | 52.4 |
| 6 | 3.3 | 4.1 | 7.8 | 10.1 | 0.2 | 0.7 | 3.5 | 49.1 |
| 7 | 10.7 | 39.5* | 5.8 | 30.5* | 0.1 | 14.6* | 4.2 | 39.5 |
| 8 | 10.5 | 36.8* | 8.9 | 35.3* | 0.1 | 16.6*,† | 1.4 | 46 |
| 9 | 7.4 | 31.6* | 5.0 | 27.2* | 0.3 | 18.5*,† | 2.8 | 51 |
| 10 | 8.3 | 38.9* | 6.9 | 31.0* | 0.1 | 14.9* | 4.0 | 43 |
| . | Control . | Anti-Fas . | Control . | Anti-Fas . | Control . | Anti-CD3 . | Control . | Anti-CD3 . |
|---|---|---|---|---|---|---|---|---|
| 1 | 11.7 | 9.3 | 6.5 | 9.0 | 0.1 | 1.3 | 2.6 | 40 |
| 2 | 5.0 | 4.5 | 6.0 | 8.1 | 0.1 | −1.1 | 2.2 | 38 |
| 3 | 6.5 | 6.0 | 8.3 | 10.0 | 0.2 | −1.2 | 3.7 | 51 |
| 4 | 4.7 | 5.2 | 9.0 | 10.0 | 0.2 | 0.6 | 1.7 | 50.1 |
| 5 | 9.4 | 5.5 | 10.7 | 12.0 | 0.1 | −1.1 | 3.1 | 52.4 |
| 6 | 3.3 | 4.1 | 7.8 | 10.1 | 0.2 | 0.7 | 3.5 | 49.1 |
| 7 | 10.7 | 39.5* | 5.8 | 30.5* | 0.1 | 14.6* | 4.2 | 39.5 |
| 8 | 10.5 | 36.8* | 8.9 | 35.3* | 0.1 | 16.6*,† | 1.4 | 46 |
| 9 | 7.4 | 31.6* | 5.0 | 27.2* | 0.3 | 18.5*,† | 2.8 | 51 |
| 10 | 8.3 | 38.9* | 6.9 | 31.0* | 0.1 | 14.9* | 4.0 | 43 |
FasLs expression protects T cells from anti-Fas–activated apoptosis. FasLs-transfected (clones 1 through 6) or empty vector-transfected (clones 7 through 10) clones were triggered by anti-Fas MoAb (1 μg/mL). The percentage of apoptosis (column 2, untreated control; column 3, anti-Fas–treated cells) was evaluated as described in Materials and Methods. Supernatant of FasLs-transfected clones inhibits anti-Fas–activated apoptosis. Anti-Fas–induced cell death (column 8, untreated control; column 9, anti-CD3–treated cells) was evaluated against normal untransfected 3DO cells in the presence of supernatants (100 μL supernatant added in the assay, see also Materials and Methods) from cultures of FasLs-transfected (clones 1 through 6) or empty vector-transfected (clones 7 through 10) clones. FasLs expression inhibits the cytotoxic activity of activated T cells against Fas+ P815 target cells. Cytotoxic activity of unstimulated (control, column 6) or anti-CD3–activated (column 7) clones was evaluated in a cytotoxicity assay against the Fas+ P815 cell line. FasLs-transfected (clones 1 through 6) or empty vector-transfected (clones 7 through 10) clones were triggered with anti-CD3 antibody and used as effectors in the cytotoxicity assay. The percentage of cytotoxicity, at the E:T ratio of 25:1, was calculated as described in Materials and Methods. Results are the average of 3 experiments; the standard errors, which are less than 10% of the values, are omitted for clarity. Expression of FasL in FasLs- or empty vector-transfected clones. Cells were cultured in 96-well plates coated with anti-CD3 MoAb (1 μg/mL), and FasL expression was evaluated by flow cytometry analysis after 15 hours of culture. Numbers represent the percentage of FasL+ cells of a representative experiment.
P < .01 as compared with control (respective control values are in the left column).
Blocking antimouse FasL MoAb (MFL4, 5 μg/mL) was used in 2 clones to control that the observed cytotoxic effect of anti-CD3 triggered cells was FasL-dependent. The addition of the MoAb inhibited the cytotoxicity down to 5.6% (clone 8) and 4.1% (clone 9), respectively, compared with controls (without anti-FasL blocking MoAb) giving 17.9% (clone 8) and 21.5% (clone 9).
To exclude an aspecific effect due to transfection, all clones were also tested for Fas (not shown) and FasL expression by FACS analysis. No differences were found between FasLs-transfected clones, empty vector-transfected clones, and normal 3DO cells (Table 1). Moreover, FasLs activity present in the culture supernatant of clone 6 was evaluated by different dilutions using 3DO as effector and P815 as target cells. The results, which are shown in Fig 5, indicate that FasLs-transfected clone 6 supernatant inhibited anti-CD3–activated 3DO cytotoxic activity in a dose-dependent manner.
Cytotoxic activity of 3DO cells cultured in the presence of different concentration of culture supernatant from a FasLs-transfected clone. Results are the average of 3 experiments, each performed in triplicate. The standard errors (<10%) are omitted for clarity. E:T ratio, 25:1.
Cytotoxic activity of 3DO cells cultured in the presence of different concentration of culture supernatant from a FasLs-transfected clone. Results are the average of 3 experiments, each performed in triplicate. The standard errors (<10%) are omitted for clarity. E:T ratio, 25:1.
These data indicate that FasLs prevents apoptosis induced by Fas/FasL interaction.
FasLs binds to Fas+ activated T cells.
These results indicate that FasLs expression, like FasL, is induced by T-cell activation and also indicate that FasLs, which lack the transmembrane domain, can be detected in the supernatant of activated cells. Moreover, results indicate that FasLs can prevent Fas/FasL-induced death. We performed experiments to evaluate the possible binding of FasLs to Fas using resting and activated T cells as target.18 In particular, the percentage of positive T cells after treatment with in vitro synthesized biotinylated FasLs (GST-FasLs) was evaluated by flow cytometry analysis. For that purpose resting and anti-CD3 activated T lymphocytes were treated with different concentration of biotin-GST-FasLs (Fig 6) and then with streptavidin-FITC. As shown in Fig 6, T-cell activation, which upregulates Fas expression,11 resulted in a significant increase of the percentage of fluorescent cells (a measure of fluorescent FasLs binding, Fig 6D) as compared with untreated T cells (Fig 6C). Moreover, the percentage of positive cells increased with the augmentation of biotinylated-protein concentrations used in the assay and decreased in the presence of anti-Fas MoAb whose binding to Fas receptor prevents the interaction Fas receptor/GST-FasLs fusion protein (Fig 6E). GST alone gave a percentage of FITC+ cells near to that of streptavidin-FITC alone (Fig 6A and B).
GST-FasLs fusion protein binding to untreated (A and C) and anti-CD3–treated (B and D) 3DO cells. GST fusion protein was prepared and biotinylated as described in Materials and Methods. Serial dilutions of GST (A and B) or GST-FasLs (C and D) and streptavidin-FICT 1:50 were used to stain 3DO cells. Inhibition of GST-FasLs biotin (25 μg/μL) binding with anti-Fas MoAb (5 μg/μL) (E). The numbers above the marker represent the percentage of FITC+ cells of a representative experiment as calculated by Lysis II. The doses of GST and GST-FasLs fusion proteins are shown on the top of the figure.
GST-FasLs fusion protein binding to untreated (A and C) and anti-CD3–treated (B and D) 3DO cells. GST fusion protein was prepared and biotinylated as described in Materials and Methods. Serial dilutions of GST (A and B) or GST-FasLs (C and D) and streptavidin-FICT 1:50 were used to stain 3DO cells. Inhibition of GST-FasLs biotin (25 μg/μL) binding with anti-Fas MoAb (5 μg/μL) (E). The numbers above the marker represent the percentage of FITC+ cells of a representative experiment as calculated by Lysis II. The doses of GST and GST-FasLs fusion proteins are shown on the top of the figure.
FasL and FasLs expression in normal lymphocytes.
The results given above indicate that 3DO cell activation by anti-CD3 treatment induces the expression of FasL that is not detectable in nonactivated cells and upregulates FasLs that is also detectable in nonactivated cells (Figs 1 and 3). In the attempt to further analyze the expression of FasL and FasLs in normal lymphocytes, we performed experiments using thymus and spleen cells. In particular, mRNA transcripts from both spleen cells untreated and treated with cross-linked anti-CD3 MoAb were analyzed by RT-PCR and RNase protection (Fig 7A and B). Results indicate that, in untreated lymphocytes, FasLs mRNA is expressed and that it is augmented by anti-CD3 treatment. FasL mRNA is only detectable after anti-CD3 treatment. These results suggest that FasL gene transcription is active in resting lymphocytes, although at a lesser extent in comparison to anti-CD3 treated, and also indicate that FasLs splicing is the only splicing product in nonactivated cells.
(A) PCR products obtained using as template cDNA prepared from untreated or anti-CD3–treated mouse spleen cells. Line 1, untreated spleen cells; line 2, spleen cells incubated overnight with anti-CD3 MoAb (1 μg/mL); line 3, negative control. The primers 1 (forward) and 2 (reverse) are shown in Fig 2B. RNase protection analysis of FasL and FasLs mRNA expression from nonactivated and anti-CD3–activated spleen cells. Each line was loaded with 30 μg of total RNA. Line 1, undigested probe; line 2, tRNA; line 3, nonactivated spleen cells; line 4, activated spleen cells.
(A) PCR products obtained using as template cDNA prepared from untreated or anti-CD3–treated mouse spleen cells. Line 1, untreated spleen cells; line 2, spleen cells incubated overnight with anti-CD3 MoAb (1 μg/mL); line 3, negative control. The primers 1 (forward) and 2 (reverse) are shown in Fig 2B. RNase protection analysis of FasL and FasLs mRNA expression from nonactivated and anti-CD3–activated spleen cells. Each line was loaded with 30 μg of total RNA. Line 1, undigested probe; line 2, tRNA; line 3, nonactivated spleen cells; line 4, activated spleen cells.
FasLs protein products are upregulated by anti-CD3 MoAb: detection in cell lysate and supernatant.
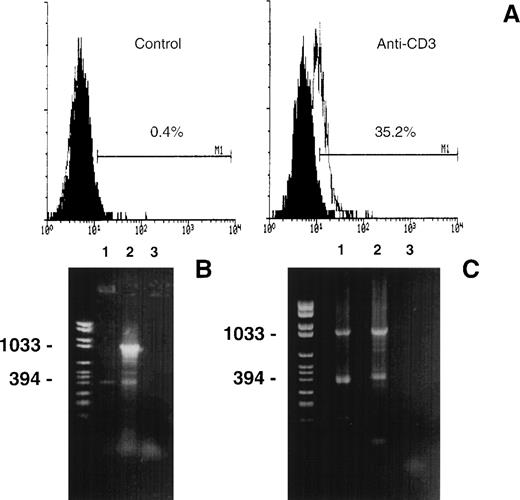
The activation of mature T cells induces the expression of membrane FasL.15,16 18 To evaluate the ratio between Fas and FasLs proteins, experiments with 3H-leucine metabolic labeling were performed. Untreated and anti-CD3-treated 3DO cells were labeled with 3H-leucine. The supernatants and the cell lysates were immunoprecipitated by an anti-FasL–(COOH) antibody. As shown in Fig 8A, a band at Mr of approximately 16 kD was detected in cell lysates and in the supernatants of activated, but not resting, 3DO cells. This band may correspond to FasLs. In fact, the detection of the band was inhibited by the addition of a specific peptide (Fig 8A, line 3). A band of Mr 40 kD corresponding to FasL was detected in the cell lysate but not in the supernatant of anti-CD3–activated cells (Fig 8A, line 2), and it was also inhibited by the specific peptide (Fig 8A, line 3).
(A) Immunoprecipitation of supernatant and cell lysate proteins from resting (left and right panels, line 1) and anti-CD3–activated (left and right panels, line 2) 3DO cells. Line 3, inhibition of cell lysate immunoprecipitation by antagonist peptide. (B) Immunoprecipitation of supernatant and cell lysate from resting (line 1) and anti-CD3–activated (line 2) spleen cells. Resting and activated (anti-CD3, 1 μg/mL) cells were labeled with [3H] leucine. The immunoprecipitation was performed with anti-FasL–COOH antibody.
(A) Immunoprecipitation of supernatant and cell lysate proteins from resting (left and right panels, line 1) and anti-CD3–activated (left and right panels, line 2) 3DO cells. Line 3, inhibition of cell lysate immunoprecipitation by antagonist peptide. (B) Immunoprecipitation of supernatant and cell lysate from resting (line 1) and anti-CD3–activated (line 2) spleen cells. Resting and activated (anti-CD3, 1 μg/mL) cells were labeled with [3H] leucine. The immunoprecipitation was performed with anti-FasL–COOH antibody.
Contrary to FasL, FasLs mRNA was slightly evident in unstimulated 3DO cells (Fig 3), and FasLs protein was also slightly detectable under our experimental conditions in resting 3DO cells (Fig 8A, line 1).
The results reported above indicate that FasLs expression, like FasL, is induced in activated T cells and also that FasLs could be expressed in resting T cells.
To further analyze this point, we compared the FasLs expression in untreated and anti-CD3–treated spleen T cells. Results in Fig 8B indicate that FasLs, but not FasL, is detectable in resting spleen cells and is upregulated in anti-CD3–activated cells when FasL is also induced. Similar results were obtained with thymus cells (not shown).
DISCUSSION
These data describe the isolation of a new short FasL alternative splicing (FasLs) that inhibits T-lymphocyte apoptosis.
Proteolytic cleavage of membrane-associated tumor necro- sis factor (TNF), CD40L, or human FasL produces soluble products with less functional activity than membrane-bound molecules, suggesting that the purpose of ligand shedding is to attenuate the signal triggered by receptor-ligand interaction.32 Furthermore, soluble forms of membrane receptors are produced either through the proteolytic cleavage of membrane bound receptors (eg, the interleukin-2 [IL-2] and the human TNF receptors), translation products of alternatively spliced mRNA (eg, the mouse IL-1, IL-4, or human Fas receptors).24-26,33-35 These soluble forms are truncated products of the membrane-bound receptors that prevent the receptor-ligand interaction.33
The Fas/Fas-L system is involved in TCR/CD3-triggered apoptosis of chronically stimulated T-cell clones or peripheral activated T cells. In these models, TCR/CD3-induced apoptosis involves expression of the FasL, which binds to the Fas receptor and triggers the death of activated T cells.12,13,15-18 A similar mechanism has been proposed for the activation induced cell death of murine T-cell hybridomas, including the 3DO cell line, responding to immobilized anti-CD3 MoAb.18,27,36 Furthermore, it has been shown that soluble forms of Fas receptor derived from alternative splicing products of Fas gene inhibit the Fas/FasL interaction and prevent anti-Fas MoAb-induced apoptosis,24-26 suggesting that the ratio between soluble and membrane-bound receptors may be critical in the regulation of cell death.
The aim of our work was to determine whether alternative splicing products of FasL might also control Fas/FasL-induced apoptosis. We isolated an alternatively spliced product of the mouse FasL gene coding for part of the extracellular domain of FasL protein. By analogy with the soluble form of Fas receptor,24-26 this short form of FasL (FasLs) may have an antagonist function, changing Fas/FasL interaction and modulating cell death.
Because of the splicing, the cDNA encoding for FasLs lacks exons 2, 3, and part of exons 1 and 4. Short conserved sequences, at the end of the introns, are found in 99% of pre-mRNA splice sites. The constant presence of these sequences defines the splicing rule: an intron starts with the GT and ends with AG dinucleotides.37,38 The junction exon/intron of FasLs is characterized at the left of the 5′ site by AG-GC, where GC is the dinucleotide localized at the end of spliced intron, and at the right 3′ site, by AG-GT, where AG is once again the dinucleotide at the end of spliced intron. FasLs splicing conforms to the GT-AG rule, except for a C instead of a T in the second position of 5′ intron end. However, apparent exceptions, proving the splicing rule, include in fact 5′ splice sites with C at the second position and an otherwise excellent match to consensus.37 Because of the splicing, the ATG of full-length FasL cDNA was deleted. Nevertheless, the possible translational initiation site of FasLs lacks the Kozak consensus sequence.
The predicted FasLs mRNA encoded protein lacks the transmembrane, the intracellular, and part of extracellular domain, resulting in a small soluble form of FasL. A Mr protein of approximately 16 kD recognized by anti-FasL–(COOH), but not anti-FasL–(NH2) antibody, was detected in the cell lysates and in the supernatants of transfected clones and anti-CD3–activated T cells (Figs 4 and 8), suggesting that TCR-triggering upregulates FasLs and results in soluble protein secretion, an effect that is not shared by FasL. Tanaka et al21 detected cytotoxic activity against Fas-expressing cells in the supernatant of COS cells transfected with human, but not murine, FasL cDNA. The active human agonist protein inducing apoptosis was a smaller (27 kD) form of FasL.21 More recently, it has been shown that a FasL soluble form can also inhibit cytotoxic activity of membrane-bound FasL.39-41
Our functional studies demonstrate that FasLs does not induce apoptosis. In fact, FasLs-transfected clones used as effectors in a cytotoxic assay against a Fas+ target exhibited reduced cytotoxicity. Furthermore, the supernatant of FasLs expressing cells inhibited anti-Fas MoAb-induced apoptosis (Table 1), suggesting that FasLs, like the soluble form of the Fas receptor, may have an inhibitory function.
The cytotoxic soluble form of human FasL exists as a trimer, and consideration of the structure of membrane-bound FasL suggests that it has the potential to form a trimeric structure, which is the prerequisite for receptor stimulation.39 In general, these trimeric structures, which are common to most ligands in the TNF ligand superfamily, induce receptor multimerization at the cell surface, which is necessary for signal transduction.39 We could hypothesize that FasLs that lacks part of the FasL molecule, including the intracellular, the transmembrane, and part of the extracellular portion, is unable to trimerize and consequently to activate the apoptosis signal when bound to the receptor. On the other hand, FasLs binding to Fas receptor (Fig 6) may cause the inhibition of anti-Fas–mediated apoptosis (Table 1). Therefore, FasLs may have an antagonistic activity and may be involved in the modulation of the Fas/FasL-activated apoptosis.
In this respect, the observation that FasLs, but not FasL, is expressed in resting T lymphocytes suggest that this short ligand is normally produced, although at lower levels as compared with activated T cells (Fig 8). It has been previously reported by many laboratories that resting T cells, although not expressing FasL, do express Fas receptor, although at a lower level as compared with activated lymphocytes.11-19 Anti-CD3–mediated activation induces FasL and upregulates Fas expression.17 Based on these observations, resting T cells can also be induced in apoptosis when FasL from different activated T cells triggers the receptor. The preferential splicing coding for the antagonist noncytotoxic FasLs could contribute to protect resting T lymphocytes from the attack by other FasL+ activated cells. When the resting lymphocyte is activated, it also expresses the mRNA coding for the cytotoxic FasL and overexpresses the pre-existing Fas. Fas/FasL interaction induces T-cell death and then limits the activated T-lymphocyte expansion and contributes to end the immune response.17
Moreover, the identification of a new FasL form with inhibiting activity opens up further potential therapeutic approaches. As an example, FasLs could be expressed in T lymphocytes to protect them against the attack from FasL+ tumor cells or in those tissues (eg, liver) that also can be destroyed via the Fas/FasL system. However, although the present results show that FasLs is expressed in normal lymphocytes, the possible in vivo role in the regulation of Fas/FasL-mediated apoptosis remains a matter of debate.
Supported by Associazione Italiana Ricerca sul Cancro (AIRC) Milan, MURST, and PF Biotecnologie, CNR, Rome, Italy.
The nucleotide FasLs sequence appears in the EMBL, GenBank, and DDBJ Nucleotide Sequence Databases under the accession no. AF119335.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Carlo Riccardi, MD, PhD, Dept. Clin. Exp. Med., University of Perugia, Via del Giochetto, 06100 Perugia, Italy; e-mail: riccardi@unipg.it.







![Fig. 8. (A) Immunoprecipitation of supernatant and cell lysate proteins from resting (left and right panels, line 1) and anti-CD3–activated (left and right panels, line 2) 3DO cells. Line 3, inhibition of cell lysate immunoprecipitation by antagonist peptide. (B) Immunoprecipitation of supernatant and cell lysate from resting (line 1) and anti-CD3–activated (line 2) spleen cells. Resting and activated (anti-CD3, 1 μg/mL) cells were labeled with [3H] leucine. The immunoprecipitation was performed with anti-FasL–COOH antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3456.422k33_3456_3467/6/m_blod42233008w.jpeg?Expires=1767697435&Signature=LNoh4SWXpWFw5ZaiTn6jDBCRelsls449SR7WyxFDPAXMUSq21jLCQClcbgOf3h~0gnD8F1hsZCznDJTq76t1RSlAKt7F986yU3~2in7HNqdZgQKL6MIiiFaCdhZPF3uyuNG~3TGo~KGnUCCHw9W7yy8WAhW1wCEGTeIPSRXSdz3foPwffCedzcC4-7w0Yd2udgNDMB5cXR-7LUgZBRFA96fJ7hOLx0DJntgkF~-7rOdzE6OTrAi3R-fAlCmBncFljRd8qUUomZn6LAped7bI0PqFJjH2XnxWSnAVKtGLAg3hvPJtB52G4c5lpnMlxhdydRYXUsO5hjG8KsXfMk~yAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal