Fcγ receptors convey to phagocytic cells the ability to recognize, bind, and internalize IgG-coated cells and microorganisms. The present study demonstrates the use of adenovirus (Ad)-mediated gene transfer of human Fcγ receptor IIA cDNA to convert normally nonphagocytic cells (hepatocytes) into functional equivalents of phagocytic cells. Ad vector in vitro transfer and expression of FcγRIIA cDNA in primary rat hepatocytes was confirmed by flow cytometry anti-FcγRIIA immunodetection, and the function of the receptor was demonstrated by enhanced binding and phagocytosis of 51Cr-labeled IgG-opsonized erythrocytes. After in vivo gene transfer to rats, expression of FcγRIIA cDNA in hepatocytes was confirmed by Northern analysis and immunohistochemistry. Rats infected with the Ad vector carrying the FcγRIIA cDNA demonstrated enhanced clearance of opsonized erythrocytes, but not nonopsonized erythrocytes, from the circulation with increased sequestration within the liver. Together, these data demonstrate that Ad-mediated FcγRIIA gene transfer can convert normally IgG-nonphagocytic cells into phagocytic cells capable of recognizing, binding, and ingesting an opsonized particulate antigen, suggesting that gene transfer strategies might be used to transiently augment host defense by enhancing the clearance of immune complexes.
PHAGOCYTOSIS OF IgG-coated cells and microorganisms, an important component of host defense, is mediated by receptors for the Fc portion of IgG (Fcγ receptors) expressed on monocytes/macrophages and neutrophils.1-4 The 3 classes of Fcγ receptors (FcγRI, FcγRII, and FcγRIII) differ by size, structure, ligand binding specificity, and cellular distribution.1-3 The importance of Fcγ receptors in host defense is highlighted by disorders such as systemic lupus erythematosus and chronic renal and liver failure, in which enhanced susceptibility to infection is associated with a downregulation of Fcγ receptors.5-7 Likewise, when there is a deficiency in the number and/or function of mononuclear and/or polymononuclear phagocytes, such as occurs in association with chemotherapy and prolonged administration of corticosteroids, the adaptive immune system cannot effectively use IgG opsonization as a strategy to aid in host defense.8-11
Based on these concepts, we hypothesized that in vivo clearance of IgG-coated cells might be enhanced by inducing Fcγ receptor expression in normally nonphagocytic cells in the liver, an organ that normally plays a major role in the clearance of IgG-coated complexes.12-14 To evaluate this hypothesis, we used an adenovirus (Ad) gene transfer vector to express normal human FcγRIIA, a FcγRII allotype with affinity for IgG2,2 15 cDNA in rat liver cells in vitro and in vivo, and evaluated the ability of the modified liver cells to phagocytize opsonized red blood cells. The in vitro data demonstrate that FcγRIIA cDNA genetic modification of primary rat hepatocytes enhances the ability of these cells to bind and phagocytize IgG opsonized cells. The in vivo data demonstrate that FcγRIIA cDNA transfer to hepatocytes enhances the clearance of IgG opsonized cells by the liver from the circulation.
MATERIALS AND METHODS
Adenovirus vectors.
The replication-deficient recombinant Ad vectors AdCMVFcγRIIA and AdCMVNull are both E1a−, partial E1b−, partial E3− vectors based on adenovirus type 5 (Ad5), in which an expression cassette containing a promoter driving the expression of a recombinant gene is inserted at the site of the E1 deletion.16,17 AdCMVFcγRIIA contains an expression cassette of the cytomegalovirus (CMV) early/intermediate promoter/enhancer followed by the human FcγRIIA cDNA isoform H/R 13118 and a SV 40 stop/poly (A) signal. AdCMVNull is identical, except that it lacks the gene in the expression cassette.19 The Ad vectors were propagated, purified, and stored at −70°C, as previously described.16,17Titers of viral preparations were determined by plaque assay using 293 cells.20 All preparations were free of replication competent Ad.21
Primary hepatocyte cultures.
Primary hepatocyte cultures22 were established from 250 to 300 g female Sprague-Dawley rats (Taconic, Germantown, NY). Animals were anesthetized by intramuscular injection of ketamine (60 mg/kg; Fort Dodge Lab, Inc, Fort Dodge, IA) and xylazine (5 mg/kg; Butler Co, Columbus, OH). The portal vein was cannulated and the liver was perfused and digested with collagenase solution (GIBCO BRL, Gaithersburg, MD). Hepatocytes were grown in 1:1 mixture of Dulbecco’s Modified Eagle Medium (DMEM; Biofluids, Rockville, MD) and Waymouth Medium (Biofluids) containing dexamethasone (20 ng/mL; Sigma, St Louis, MO), insulin-transferrin-sodium-selenite media supplement (1 μL/mL; Sigma), and gentamicin (10 μg/mL; Sigma), penicillin G (50 U/mL; GIBCO BRL), and streptomycin (50 μL/mL; GIBCO BRL) on fibronectin (Sigma)-coated 6-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ).
Expression and function of FcγRIIA in primary rat hepatocytes.
To assess expression of FcγRIIA by immunodetection flow cytometry analysis (EPICS XL; Coulter Corp, Miami, FL), primary rat hepatocyte cultures were infected with AdCMVFcγRIIA at multiplicity of infection (moi) 10, using noninfected cells and cells infected with AdCMVNull as controls. The cells were incubated with phosphate-buffered saline solution, pH 7.4 (PBS; Biofluids) containing 2% goat serum on ice for 30 minutes followed by mouse anti-FcγRIIA monoclonal antibody IV.3 (20 μg/mL; Medarex, Annandale, NJ) for 30 minutes on ice. The cells were then washed in PBS, incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse [F(ab)2] fragments (Boehringer Mannheim, Indianapolis, IN) for 30 minutes, washed in PBS, and analyzed by flow cytometry. Isotype-matched mouse monoclonal antibodies (IgG2b; Sigma) were analyzed as negative controls.
The function of the FcγRIIA protein expressed by the rat hepatocytes was assessed by quantifying binding and phagocytosis of Ig-opsonized51Cr-labeled sheep red blood cells (SRBC)23 by the hepatocytes. Primary rat hepatocytes (5 × 105/well) were infected with AdCMVFcγRIIA (moi 10), using noninfected cells and cells infected with AdCMVNull as controls. After 48 hours, the hepatocytes were incubated for 60 minutes with IgG rabbit antisheep red blood cell antibody (Accurate Chemical & Scientific Corp, Westbury, NY) -coated, 51Cr-labeled (DuPont NEN, Boston, MA) SRBC (Accurate). Nonopsonized 51Cr-labeled SRBC were used as controls. To evaluate the binding of IgG-opsonized51Cr-SRBC to the FcγRIIA cDNA-modified hepatocytes, the hepatocytes were washed 3 times in PBS and then lysed by incubation in 0.5% sodium dodecyl sulphate (SDS) solution (Sigma) for 10 minutes. To evaluate phagocytosis, hepatocytes were incubated with SRBC for 60 minutes, washed 3 times in PBS, incubated for 60 seconds in hypotonic SRBC lysing buffer (31 mmol/L ammonia chloride, 2 mmol/L potassium bicarbonate, 20 μmol/L ethylenediaminetetraacetic acid; all from Sigma) to lyse bound noninternalized SRBC, washed, and then lysed in 0.5% SDS for 10 minutes. To evaluate phagocytosis at different time points, hepatocytes were incubated with SRBC for 10, 30, and 60 minutes. The hepatocyte to SRBC ratio for all incubations was 1:500. The radioactivity of lysates was measured using a gamma-counter.
Expression and function of FcγRIIA in vivo.
To evaluate the expression of FcγRIIA cDNA and the function of FcγRIIA protein in vivo, female Sprague-Dawley rats (250 to 300 g) were anesthetized by intramuscular injection of ketamine (60 mg/kg) and xylazine (5 mg/kg). Based on the knowledge that greater than 90% of an intravenously administered Ad goes to the liver,24 25 the Ad vectors (AdCMVFcγRIIA, AdCMVNull) were administered to the liver via the external jugular vein (109 plaque-forming units [pfu] in 100 μL 0.9% NaCl). Naive animals were used as controls.
Northern analysis was used to demonstrate in vivo FcγRIIA transcripts in the liver. After 48 hours, animals were killed (pentobarbital overdose intraperitoneally), total RNA was extracted, and expression of FcγRIIA mRNA was assessed by Northern analysis. Total RNA was extracted (RNA Extraction Kit; Clontech, Palo Alto, CA) and transferred (10 μg/lane) to nylon membranes after electrophoretic separation through a 1% agarose gel. The membranes were assessed using a human FcγRIIA cDNA probe labeled with 32P-deoxycytidine triphosphate (dCTP; Random Primer Labeling Kit; Stratagene, La Jolla, CA) for 2 hours using standard methods.2632P-labeled human γ-actin cDNA was used as a positive control.22 To analyze the expression and distribution of the FcγRIIA in the liver, livers were harvested 48 hours after administration of AdFcγRIIA, AdNull or from uninfected animals. The organs were fixed with 4% paraformaldehyde for 24 hours and then transferred to 70% ethanol before embedding. Immunohistochemical staining for FcγRIIA was performed on 5-μm, paraffin-embedded sections using the anti-FcγRII antibody IV.3. An isotype (IgG2b) -matched antibody was used as a control. The antibodies were incubated for 16 hours at 4°C and then washed and incubated with biotinylated rabbit antimouse [F(ab′)2] (Boehringer Mannheim) for 30 minutes, followed by streptavidin-FITC (Boehringer Mannheim) for 30 minutes. A rabbit anti-FITC alkaline phosphatase-labeled antibody (Boehringer Mannheim) was then incubated for 30 minutes, followed by detection with 4-nitro blue tetrazolium chloride (NBT)–5-bromo-4-chloro-3-indoyl-phosphate (BCIP) substrate (Boehringer Mannheim). The samples were counterstained with nuclear fast red (DIGENE, Beltsville, MD) and analyzed by light microscopy.
To determine if in vivo transfer of the FcγRIIA to the liver was associated with enhanced clearance of IgG opsonized RBC from the circulation, rats were anesthetized and Ad vectors were administered as described above. Forty-eight hours later, 51Cr-labeled rat red blood cells (RRBC) were opsonized with IgG rabbit anti-RRBC antibodies (Accurate). The IgG-coated, 51Cr-labeled RRBC were administered intravenously via the femoral vein (3.4 × 108 RRBC in 500 μL 0.9% NaCl). Nonopsonized51Cr-labeled RRBC were used as controls. At 5, 15, 30, 60, 90, and 120 minutes after injection of the 51Cr-labeled RRBC, blood samples were collected (100 μL) from the external jugular vein and radioactivity of the samples was measured using a gamma counter. Clearance of RBC from the circulation was expressed as a percentage of RBC survival in the peripheral blood.27 The value obtained at 5 minutes after injection was considered 100%. Animals were killed after 120 minutes by pentobarbital overdose.
To determine the uptake of the IgG-opsonized 51Cr-labeled RRBC in different organs, rats were infected with Ad vectors, and then opsonized and nonopsonized RRBC were administered as described above. Animals were killed 120 minutes after administration of the RRBC. Liver, lung, and spleen were removed, weighed, and homogenized in 20 mL of H2O. Total radioactivity of the organ (dpm) was determined using a gamma-counter and expressed as a percentage of the total radioactivity injected intravenously.
To further analyze the uptake of IgG coated particles in vivo, fluorescent-labeled IgG-coated microspheres were injected intravenously in rats that had been infected with AdFcγRIIA or AdNull 48 hours previously. The fluorescent microspheres were prepared by mixing Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) with human IgG (Sigma) for 10 minutes at 4°C, pH 8.0, followed by the addition of glycine (1 mg/mL) to stop the reaction and dialysis in 10 mmol/L HEPES, 140 mmol/L KCl, pH 7.2, for 2 hours to remove unbound biotin. The biotinylated IgG was then bound to 40 nm Neutr-Avidin–labeled red fluorescent microspheres (Molecular Probes, Eugene, OR). The number of microspheres injected was 6 × 1012 particles/animal. One hour after the injection of the fluorescent-coated microspheres, the animals were killed and frozen sections of the livers were prepared by immediately freezing the tissue in TBS tissue freezing medium (Electron Microscopy Sciences, Fort Washington, PA). Frozen sections were fixed in 4% paraformaldehyde for 30 minutes and evaluated by fluorescence microscopy.
Statistical analysis.
The results are expressed as the mean ± standard error of the mean. Statistical comparisons were made using the unpaired 2-tailed Student’s t-test.
RESULTS
AdFcγRIIA transfer to rat hepatocytes in vitro.
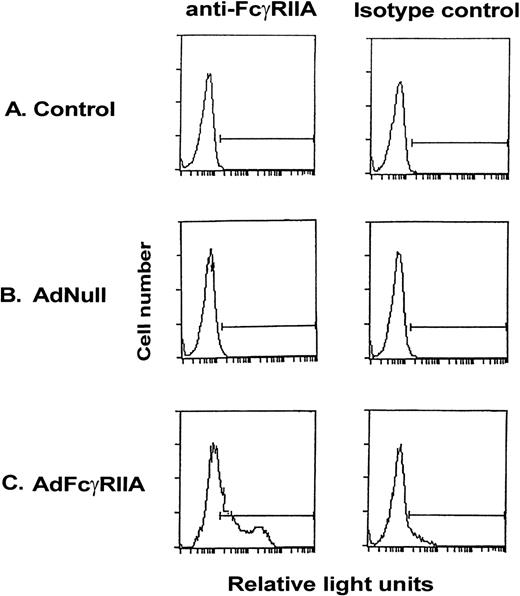
To demonstrate the ability of an Ad vector expressing the AdFcγRIIA cDNA (AdFcγRIIA) to transfer and express human FcγRIIA in vitro in rat hepatocytes, primary rat hepatocyte cultures were infected with AdFcγRIIA (moi, 10). Quantification of expression of FcγRIIA on the cell surface, determined by flow cytometry, demonstrated positive FcγRIIA staining in 46% of cells after AdFcγRIIA infection (Fig 1). No expression of FcγRIIA was demonstrated in noninfected or AdNull-infected primary rat hepatocytes. These results suggest that Ad-mediated FcγRIIA cDNA transfer results in expression of the transgene in primary rat hepatocyte tissue cultures.
Expression of FcγRIIA on the surface of primary rat hepatocyte cultures after Ad vector-mediated gene transfer of FcγRIIA cDNA. Primary hepatocytes were infected with AdFcγRIIA and AdNull at 10 moi. After 48 hours, cells were incubated with anti-FcγRIIA monoclonal antibody for 30 minutes, washed, and labeled with FITC-conjugated goat antimouse F(ab′)2 IgG for 30 minutes, washed, and fixed in 1% paraformaldehyde. Isotype-matched controls were used for all reactions. Shown is flow cytometry of (A) naive controls, (B) AdNull-infected cells, and (C) AdFcγRIIA-infected cells.
Expression of FcγRIIA on the surface of primary rat hepatocyte cultures after Ad vector-mediated gene transfer of FcγRIIA cDNA. Primary hepatocytes were infected with AdFcγRIIA and AdNull at 10 moi. After 48 hours, cells were incubated with anti-FcγRIIA monoclonal antibody for 30 minutes, washed, and labeled with FITC-conjugated goat antimouse F(ab′)2 IgG for 30 minutes, washed, and fixed in 1% paraformaldehyde. Isotype-matched controls were used for all reactions. Shown is flow cytometry of (A) naive controls, (B) AdNull-infected cells, and (C) AdFcγRIIA-infected cells.
Function of FcγRIIA transferred in vitro to rat hepatocytes.
Function of the FcγRIIA protein expressed on the primary rat hepatocytes after in vitro Ad-mediated gene transfer was assessed using a phagocytic assay using radiolabeled SRBC. Cells infected with AdFcγRIIA (moi, 10) showed increased binding of IgG-coated SRBC (Fig 2A; P < .001, for 30 and 60 minutes compared with values for noninfected and AdNull-infected cells) and phagocytosis of IgG-coated SRBC (Fig 2B; P < .006, for 30 and 60 minutes compared with values for noninfected and AdNull-infected cells). Increased binding and phagocytosis by hepatocytes expressing FcγRIIA was observed after 10 minutes of incubation with opsonized erythrocytes with significant increase and plateau after 30 and 60 minutes. Noninfected and AdNull-infected rat hepatocytes showed a minimal amount of nonspecific binding and internalization of both nonopsonized and IgG-opsonized SRBC. These observations provide evidence that Ad-mediated transfer of FcγRIIA cDNA results in expression of a functional phagocytic receptor on primary hepatocytes, enabling these cells to recognize, bind, and internalize opsonized particles.
Binding and phagocytosis of opsonized SRBC by primary rat hepatocyte cultures at different time points after Ad-mediated FcγRIIA cDNA gene transfer. Primary hepatocytes were infected with AdNull and AdFcγRIIA at 10 moi. After 48 hours, cells were incubated with IgG-coated 51Cr-labeled SRBC for 10, 30, and 60 minutes. As a control, the cells were incubated with nonopsonized SRBC for 60 minutes. To evaluate binding of IgG-coated SRBC to primary hepatocytes, cells were washed with PBS and then lysed by incubation with 0.5% SDS for 10 minutes. To evaluate SRBC phagocytosis by the primary hepatocytes, cells were washed with PBS and the SRBC bound to the cell surface were lysed by incubation with hypotonic lysis buffer for 1 minute. The cells were lysed by incubation with 0.5% SDS for 10 minutes and the radioactivity of lysate was quantified. (A) Binding of opsonized SRBC at 10, 30, and 60 minutes to hepatocytes that were not infected (control; •), infected with AdNull (▪), or infected with AdFcγRIIA (▴). Also indicated as controls are parallel cultures of cells incubated with nonopsonized SRBC for 60 minutes with uninfected hepatocytes (○), AdNull-infected hepatocytes (□), and AdFcγRIIA-infected hepatocytes (▵). (B) Phagocytosis of opsonized RBC at 10, 30, and 60 minutes. The symbols are identical to that in (A). For all data, shown are the means of activity (dpm)/well from 3 measurements ± standard error.
Binding and phagocytosis of opsonized SRBC by primary rat hepatocyte cultures at different time points after Ad-mediated FcγRIIA cDNA gene transfer. Primary hepatocytes were infected with AdNull and AdFcγRIIA at 10 moi. After 48 hours, cells were incubated with IgG-coated 51Cr-labeled SRBC for 10, 30, and 60 minutes. As a control, the cells were incubated with nonopsonized SRBC for 60 minutes. To evaluate binding of IgG-coated SRBC to primary hepatocytes, cells were washed with PBS and then lysed by incubation with 0.5% SDS for 10 minutes. To evaluate SRBC phagocytosis by the primary hepatocytes, cells were washed with PBS and the SRBC bound to the cell surface were lysed by incubation with hypotonic lysis buffer for 1 minute. The cells were lysed by incubation with 0.5% SDS for 10 minutes and the radioactivity of lysate was quantified. (A) Binding of opsonized SRBC at 10, 30, and 60 minutes to hepatocytes that were not infected (control; •), infected with AdNull (▪), or infected with AdFcγRIIA (▴). Also indicated as controls are parallel cultures of cells incubated with nonopsonized SRBC for 60 minutes with uninfected hepatocytes (○), AdNull-infected hepatocytes (□), and AdFcγRIIA-infected hepatocytes (▵). (B) Phagocytosis of opsonized RBC at 10, 30, and 60 minutes. The symbols are identical to that in (A). For all data, shown are the means of activity (dpm)/well from 3 measurements ± standard error.
AdFcγRIIA transfer to rat hepatocytes in vivo.
To analyze if FcγRIIA can be expressed in vivo in rat hepatocytes after intravenous administration of the AdFcγRIIA vector (109 pfu), total RNA was extracted from rat liver and assessed by Northern analysis. This experiment demonstrated that intravenous administration of AdFcγRIIA resulted in expression of FcγRIIA mRNA transcripts in the liver of the experimental animals. No FcγRIIA mRNA transcripts were observed in the liver of noninfected animals or animals infected with AdNull, although control γ-actin mRNA transcripts were similar in all samples (not shown). Analysis of liver sections of animals infected with Ad FcγRIIA or AdNull by immunohistochemistry showed positive staining for FcγRIIA in liver parenchymal cells (hepatocytes) of the animals infected with Ad FcγRIIA (Fig 3C and E), but not in control naive (Fig 3A and D) or AdNull-infected animals (Fig 3B and E). These observations confirm that in vivo Ad-mediated transfer of FcγRIIA results in expression of the FcγRIIA transgene in the liver.
Expression of FcγRIIA in rat liver in vivo after Ad-mediated FcγRIIA cDNA gene transfer. Sprague Dawley rats received Ad FcγRIIA or AdNull intravenously (109 pfu). After 48 hours, the livers were removed and fixed, and paraffin-embedded sections were analyzed for FcγRIIA expression by immunohistochemistry using anti-FcγRIIA antibody IV.3. (A and D) Naive control; (B and E) AdNull; (C and F) Ad FcγRIIA-infected. (A, B, and C, bar = 50 μm; E, D, and F [high power representative of A, B, and C], bar = 50 μm.)
Expression of FcγRIIA in rat liver in vivo after Ad-mediated FcγRIIA cDNA gene transfer. Sprague Dawley rats received Ad FcγRIIA or AdNull intravenously (109 pfu). After 48 hours, the livers were removed and fixed, and paraffin-embedded sections were analyzed for FcγRIIA expression by immunohistochemistry using anti-FcγRIIA antibody IV.3. (A and D) Naive control; (B and E) AdNull; (C and F) Ad FcγRIIA-infected. (A, B, and C, bar = 50 μm; E, D, and F [high power representative of A, B, and C], bar = 50 μm.)
AdFcγRIIA induced enhanced clearance of opsonized RBCin vivo.
The function of FcγRIIA expressed after intravenous administration of AdFcγRIIA (109 pfu) was assessed by the clearance of radiolabeled IgG-opsonized and nonopsonized RRBC from the peripheral blood of experimental animals. Nonopsonized RRBC were not effectively cleared in noninfected animals or in AdNull and AdFcγRIIA-infected animals (Fig 4A; P > .2, all comparisons, all time points). In contrast, IgG-coated (opsonized) RRBC were rapidly cleared from the blood in the animals receiving AdFcγRIIA. One hour after administration of RRBC in noninfected animals, 54% ± 3% RRBC remained in the blood compared with 33% ± 4% in AdNull and 9% ± 1% in FcγRIIA-infected animals (Fig 4B; P < .001, comparing values for FcγRIIA-infected animals with AdNull and noninfected animals at all time points after intravenous injection of radiolabeled RRBC). Interestingly, infection with AdNull also increased clearance of IgG-coated RRBC compared with noninfected animals, but much less than that observed with AdFcγRIIA (P < .01, all comparisons).
Clearance of 51Cr-labeled SRBC from the circulation after in vivo Ad-mediated FcγRIIA cDNA gene transfer. Sprague-Dawley rats were administered AdFcγRIIA or AdNull intravenously (109 pfu). After 48 hours,51Cr-labeled opsonized (IgG-coated) RRBC or nonopsonized RRBC as controls were injected intravenously. At 5 to 120 minutes, blood samples were collected and 51Cr-radioactivity measured using a gamma scintillation counter. The level of51Cr-RRBC in the blood was expressed as a percentage of the value obtained at 5 minutes (100%) after injection. (A) Clearance of nonopsonized RRBC. Control, no vector administered (○); AdNull (□); AdFcγRIIA (▵). Each point represents the mean and standard error from 3 animals in each group. (B) Clearance of opsonized RRBC. The symbols are identical to those used in (A). Each point represents mean and standard error from 14 animals in control group, 12 animals in group infected with AdNull, and 17 animals infected with AdFcγRIIA.
Clearance of 51Cr-labeled SRBC from the circulation after in vivo Ad-mediated FcγRIIA cDNA gene transfer. Sprague-Dawley rats were administered AdFcγRIIA or AdNull intravenously (109 pfu). After 48 hours,51Cr-labeled opsonized (IgG-coated) RRBC or nonopsonized RRBC as controls were injected intravenously. At 5 to 120 minutes, blood samples were collected and 51Cr-radioactivity measured using a gamma scintillation counter. The level of51Cr-RRBC in the blood was expressed as a percentage of the value obtained at 5 minutes (100%) after injection. (A) Clearance of nonopsonized RRBC. Control, no vector administered (○); AdNull (□); AdFcγRIIA (▵). Each point represents the mean and standard error from 3 animals in each group. (B) Clearance of opsonized RRBC. The symbols are identical to those used in (A). Each point represents mean and standard error from 14 animals in control group, 12 animals in group infected with AdNull, and 17 animals infected with AdFcγRIIA.
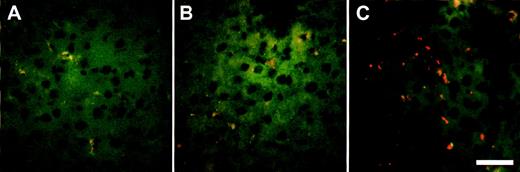
To determine the uptake of RRBC in different organs (liver, lung, and spleen), animals were killed 120 minutes after administration of radiolabeled RRBC. No significant difference was observed in uptake of nonopsonized RRBC in noninfected and AdNull and AdFcγRIIA-infected animals (Fig 5A; P > .3, all comparisons). In contrast, AdFcγRIIA-infected animals showed significant increase in the liver uptake of IgG-coated RRBC (69% ± 6%) compared with AdNull infected (41% ± 4%) and noninfected animals (27% ± 2%; Fig 5B; P < .04, AdFcγRIIA-infected animals with AdNull and noninfected animals). An increased number of IgG-coated fluorescent microspheres was present in the liver sections of the animals infected with Ad FcγRIIA (Fig 6C) compared with naive controls (Fig 6A) or animals infected with AdNull (Fig 5B). The presence of fluorescent microspheres in the control and Ad Null group most likely reflects the uptake by resident liver macrophages. Taken together, these observations provide evidence that Ad-mediated transfer of FcγRIIA cDNA leads to the expression of a functional phagocytic receptor in hepatocytes and transforms these normally nonphagocytic cells into cells that are able to internalize opsonized particles and enhance their clearance from the peripheral blood.
Organ sequestration of radiolabeled RRBC after in vivo Ad-mediated FcγRIIA cDNA gene transfer. Sprague-Dawley rats were administered AdFcγRIIA or AdNull intravenously (109 pfu). After 48 hours, 51Cr-labeled opsonized (IgG-coated) RRBC or nonopsonized RRBC as controls were injected intravenously. After 120 minutes, the liver, lung, and spleen were removed and homogenized. Total radioactivity of the organs, measured in a gamma scintillation counter, is presented as a percentage of the total dose of injected radioactivity (dpm). (A) Organ sequestration after administration of nonopsonized RRBC. (B) Organ sequestration after administration of opsonized RRBC. Shown are mean percentages with standard errors from 4 animals in the control group, 6 animals in the AdNull-infected group, and 4 animals in the AdFcγRIIA-infected group.
Organ sequestration of radiolabeled RRBC after in vivo Ad-mediated FcγRIIA cDNA gene transfer. Sprague-Dawley rats were administered AdFcγRIIA or AdNull intravenously (109 pfu). After 48 hours, 51Cr-labeled opsonized (IgG-coated) RRBC or nonopsonized RRBC as controls were injected intravenously. After 120 minutes, the liver, lung, and spleen were removed and homogenized. Total radioactivity of the organs, measured in a gamma scintillation counter, is presented as a percentage of the total dose of injected radioactivity (dpm). (A) Organ sequestration after administration of nonopsonized RRBC. (B) Organ sequestration after administration of opsonized RRBC. Shown are mean percentages with standard errors from 4 animals in the control group, 6 animals in the AdNull-infected group, and 4 animals in the AdFcγRIIA-infected group.
Localization of IgG fluorescent microspheres in vivo after Ad-mediated FcγRIIA cDNA gene transfer. Sprague Dawley rats received Ad FcγRIIA or AdNull intravenously (109 pfu). After 48 hours, IgG fluorescent microspheres (6 × 1012particles) were injected intravenously, and 1 hour later the livers were removed and frozen section were prepared and analyzed by fluorescence microscopy. (A) Naive control; (B) AdNull-infected; and (C) Ad FcγRIIA-infected. Bar = 50 μm.
Localization of IgG fluorescent microspheres in vivo after Ad-mediated FcγRIIA cDNA gene transfer. Sprague Dawley rats received Ad FcγRIIA or AdNull intravenously (109 pfu). After 48 hours, IgG fluorescent microspheres (6 × 1012particles) were injected intravenously, and 1 hour later the livers were removed and frozen section were prepared and analyzed by fluorescence microscopy. (A) Naive control; (B) AdNull-infected; and (C) Ad FcγRIIA-infected. Bar = 50 μm.
DISCUSSION
This study demonstrates Ad-mediated transfer of FcγRIIA receptor cDNA can convert nonphagocytic cells to phagocytic cells, capable of accelerating the clearance of IgG opsonized particulates in vivo. First, in vitro infection of primary rat hepatocytes with AdFcγRIIA resulted in expression of the FcγRIIA on the cell surface. Second, the transfected receptor was functional, enabling normally nonphagocytic cells to recognize, bind, and phagocytose opsonized RBC. Third, intravenous administration of AdFcγRIIA resulted in expression of FcγRIIA in hepatocytes, resulting in increased clearance of opsonized RRBC from the circulation of the experimental animals, with enhanced sequestration of RRBC in the liver.
FcγRIIA receptor.
Fcγ receptors are receptors on the surface of phagocytic cells that recognize and bind the Fc portion of the IgG molecule.1-4In humans, there are 3 major Fcγ receptors: FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). They all exhibit a disulfide loop structure in their extracellular domain that is characteristic for all Ig gene superfamily members. Additional heterogeneity of the Fcγ receptors results in the occurrence of different allelic forms.28,29 The FcγRIIA allotype is a 40-kD protein with wide cellular distribution and is present on the surface of monocytes, macrophages, granulocytes, platelets, Langerhans’ cells, B and T lymphocytes, and some mesangial cells.2 FcγRIIA is the only allotype of the Fcγ receptor that can directly mediate a phagocytic signal in the absence of an accessory chain and in the absence of other Fcγ receptors.2,3,15 It is the only human Fcγ receptor, which recognizes IgG2 efficiently,2,3,15 a component of the host defense system that plays an important role in defense against infection with encapsulated bacteria, such as, Streptococcus pneumoniae,Hemophilus influenzae, and Neisseria meningitis.30 31
Transfer of FcγRIIA to nonphagocytic cells.
Using transfection with recombinant plasmids, various Fcγ receptors have been expressed on nonphagocytic cell lines (COS-1, Jurkat T cells, and fibroblasts).15,18,32 These in vitro experiments resulted in the expression of the functional Fcγ receptors on the cell surface assessed by the phagocytosis of opsonized RRBC. Tyrosine phosphorylation of multiple proteins (eg, FcγRIIA, ZAP-70, p72SYK, and phospholipase Cγl subunit) and an increase in intracellular Ca2+ concentration was observed after cross-linking of FcγRIIA with anti-FcγRIIA monoclonal antibody,15 and incubation with inhibitors of tyrosine kinase reduced phagocytic function of the transfected cells.33
In the present study, expression of FcγRIIA was observed in liver parenchymal cells. Furthermore, increased clearance of IgG opsonized RBC with increased sequestration of RBC in the liver was observed in experimental animals infected with AdFcγRIIA, when compared with noninfected animals and animals infected with AdNull. Some degree of enhancement of the clearance of opsonized RRBC was also observed after infection with AdNull compared with noninfected animals. This is likely due to activation of resident phagocytic cells by administration of the Ad vector per se, because there was no expression of the human FcγRIIA visible in the AdNull- infected animals. Infection with the Ad vectors (AdFcγRIIA, AdNull) did not affect the clearance of nonopsonized RBC. The increased localization of IgG-coated fluorescent microspheres after Ad FcγRIIA transfer in vivo in the liver provide additional evidence for increased uptake of IgG opsonized particles by liver cells of these animals.
Possible clinical significance.
One application of this approach may be in in vivo enhancement of host defense in clinical situations in which phagocytosis of IgG-targeted complexes is impaired due to the downregulation of the Fcγ receptors, eg, in systemic lupus erythematosus, chronic liver disease, and chronic kidney disease.5-7 In regard to defects that alter the binding of IgG-coated targets due to genetic polymorphisms (and resulting dysfunction of FcγRIIA), an arginine (R131)/histidine change at amino acid position 131 in the second Ig-like domain of the FcγRIIA receptor is common.28,29 This mutation is clinically significant because FcγRIIA on leukocytes of individuals homozygous for R131/R131 (∼25% of Caucassians) are unable to efficiently bind IgG2-opsonized bacteria. These individuals have a higher incidence of infections with encapsulated bacteria and increased mortality.30,31 The R131/R131 genotype is also a risk factor for development of lupus nephritis in patients with systemic lupus erythematosus probably due to its lower affinity for certain immune complexes.34 Production of reactive oxygen intermediates by neutrophils in response to antineutrophil cytoplasmic antibodies is also significantly increased in individuals with Wegener’s granulomatosis who express the R131/R131 genotype.35 36
ACKNOWLEDGMENT
The authors thank H. Carpenter and B. Ferris for technical assistance and N. Mohamed for help preparing this manuscript.
Supported in part by Grants No. P01 HL51746, P01 HL59312, and AI 22193 from the National Institutes of Health; the Cystic Fibrosis Foundation (Bethesda, MD); the Will Rogers Memorial Fund (White Plains, NY); GenVec, Inc (Rockville, MD); and InKine Pharmaceutical Co, Inc (Blue Bell, PA).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ronald G. Crystal, MD, Division of Pulmonary and Critical Care Medicine, The New York Hospital-Cornell Medical Center, 520 E 70th St, ST 505, New York, NY 10021; e-mail: geneticmedicine@mail.med.cornell.edu.


![Fig. 3. Expression of FcγRIIA in rat liver in vivo after Ad-mediated FcγRIIA cDNA gene transfer. Sprague Dawley rats received Ad FcγRIIA or AdNull intravenously (109 pfu). After 48 hours, the livers were removed and fixed, and paraffin-embedded sections were analyzed for FcγRIIA expression by immunohistochemistry using anti-FcγRIIA antibody IV.3. (A and D) Naive control; (B and E) AdNull; (C and F) Ad FcγRIIA-infected. (A, B, and C, bar = 50 μm; E, D, and F [high power representative of A, B, and C], bar = 50 μm.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3448.422k02_3448_3455/6/m_blod422bezd3z.jpeg?Expires=1767837287&Signature=l6KV8a3w32IJAn8mOd~iq04qia68tB3fvop6JK~QVbYczcxNsHPLjamuyZ~cX2AOuLudUovbkq0~slLLCp3~33rlBg8B22uEtdGBYASh4wCZbI8BS-E8sBRsP2eq86p5wzHvoPUu2jbTxWhD6Kk8dWrD7LlIauy-6b~BUxGZRGwZMHMjBAZGUIkpsI67vK7x~ktI1Bqcl6lOBImJCDhRVs5NpsaxUQe6IZwND7C9z9DYWUudl9dzhNx59nwsLyxG7viDajkPgIGYLez-05FZU0QGpHi3fvPcbqzNPYEGj1NRsrISc~UDaZKiWCCRsx-4v3Eiv1zTt1k-gTkhImSmtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal