The potential for tissue factor (TF) to enhance inflammation by factor VIIa-dependent induction of proinflammatory changes in macrophages was explored. Purified recombinant human factor VIIa enhanced reactive oxygen species production by human monocyte-derived macrophages expressing TF in vitro. This effect was dose- and time-dependent, ligand- and receptor-specific, and independent of other coagulation proteins. This receptor/ligand binding induced phospholipase C-dependent intracellular calcium fluxes. Transfection studies using a human monocyte-derived cell line (U937) demonstrated that an intact intracytoplasmic domain of TF is required for factor VIIa-induced intracellular calcium fluxes. The capacity of TF to enhance proinflammatory functions of rabbit peritoneal-elicited macrophages (production of reactive oxygen species and expression of major histocompatibility complex class II and cell adhesion molecules) was demonstrated in vivo by treatment with an anti-TF antibody. These data demonstrate that, in addition to its role in activation of coagulation, TF can directly augment macrophage activation. These effects are initiated by binding factor VIIa and are independent of other coagulation proteins. These studies provide the first demonstration of a direct proinflammatory role for TF acting as a cell-signaling receptor.
TISSUE FACTOR (TF) IS A cell surface-bound glycoprotein that binds both the zymogen, factor VII, and the active serine protease factor VIIa.1 TF/factor VIIa complexes activate the extrinsic coagulation pathway and are the major in vivo initiator of coagulation. Under normal physiological conditions, TF is expressed only at extravascular sites and perivascularly in the adventitial layer of blood vessels.2Coagulation may be initiated after the breach of vascular integrity by contact of circulating factor VII and factor VIIa with constitutively expressed TF or when systemic (eg, intravascular sepsis and endotoxemia) or local inflammatory stimuli induce TF on monocyte/macrophages or endothelial cells.
Potential interactions between coagulation and inflammation have received renewed attention since the cloning of 3 inflammatory cell surface-based receptors, proteinase activated receptor-1 (PAR-1),3 effector cell protease receptor-1 (EPR-1),4 and TF,5 that interact with circulating serine proteases (thrombin, factor Xa, and factor VIIa, respectively). A proinflammatory role for EPR-1 in vivo has been suggested by the demonstration of factor Xa induced paw edema6 and the prevention of graft-versus-host disease by blocking the receptor7 in mice. Binding of thrombin to PAR-1 triggers intracellular calcium fluxes8,9 and induces proinflammatory effects in vitro.10 Proliferative and proinflammatory responses to thrombin have been demonstrated in endothelial cells11 and macrophages,12respectively. Direct proinflammatory effects of TF on cells have not previously been demonstrated.
TF has an extracellular domain of 219 amino acids5 that shares significant structural homology with the cytokine receptor superfamily and is most closely related to interleukin-10 (IL-10) and interferon-α, -β, and -γ receptors.13 The transmembrane domain consists of 23 amino acids. The intracytoplasmic tail of TF is short, comprising only 21 amino acids, with 3 serine residues that are potential phosphorylation sites.14 Unlike PAR-13 and EPR-1,4,15 TF is not constitutively expressed on leukocytes or endothelial cells. Adherence and proinflammatory stimuli such as lipopolysaccharide (LPS) and interferon-γ can induce TF expression.16
Studies using anti-TF antibodies and tissue factor pathway inhibitor (TFPI; which binds and inactivates factor Xa and TF/factor VIIa complexes) provide evidence for proinflammatory effects associated with extrinsic coagulation pathway activation. Treatment with an anti-TF antibody inhibited coagulation and fibrin formation and reduced inflammation in LPS-induced septic shock.17 In experimental glomerulonephritis (GN), treatment with anti-TF antibodies reduced indices of glomerular inflammation including proteinuria and major histocompatibility complex (MHC) class II expression in addition to reducing glomerular fibrin deposition.18 Treatment with TFPI reduced injury in spinal cord ischemia19 and experimental GN.20 These studies suggest the potential for TF to enhance inflammation by direct cellular activation in addition to its procoagulant function.
Despite evidence suggesting close links between the activation of inflammation and coagulation, no direct effects of TF on the function of inflammatory cells have yet been demonstrated. Reactive oxygen species (ROS) are inflammatory effector molecules produced by various cells, including activated monocyte/macrophages. They have important intracellular functions in cell signaling and killing pathogens and, when released extracellularly, are potent effectors of inflammatory tissue injury. In the current studies, the potential of TF/factor VIIa to act as a cell signaling receptor/ligand system that induces intracellular calcium fluxes and ROS production (as a marker of inflammatory activation) was studied in monocyte/macrophages.
MATERIALS AND METHODS
Human monocyte-derived macrophages.
Peripheral blood mononuclear cells (PBMCs) were prepared from healthy human volunteers by density centrifugation (Ficoll-Hypaque; Pharmacia Biotech, Uppsala, Sweden) of citrated venous blood. Cells were washed twice in phosphate-buffered saline (PBS), resuspended at 1 × 106/mL in serum-free Dulbecco’s Minimal Essential Medium (DMEM), and incubated for 1 to 24 hours at 37°C in a 5% CO2 atmosphere in the presence and absence of LPS. After culture, supernatants were aspirated and adhered cells were harvested by flushing with DMEM at 4°C. Cell viability, assessed by flow cytometry using propidium iodide exclusion, was greater than 97% in all experiments. LPS contamination of medium from LPS-free cultures was undetectable (<15 pg/mL) using the limulus amebocyte lysis assay.
The cellular composition of mononuclear cell preparations, assessed by flow cytometry, after ficol density separation showed 75% ± 4% macrophages, 20% ± 3% CD3+ T cells, and 5% ± 1% CD19+ B cells. After adherence for 60 minutes, macrophages were further enriched. This adherent population comprised 85% ± 2% macrophages, 12% ± 1% CD3+ T cells, and 3% ± 1% CD19+ B cells. Cell ratios did not alter significantly with further adherence up to 24 hours. Macrophage-specific parameters (below) were analyzed by selectively gating on macrophages, defined by their characteristic forward and 90° light scatter on flow cytometry.
Measurement of TF expression.
TF expression was assessed by flow cytometry as previously described.21 Cells were labeled with a monoclonal mouse antihuman TF antibody (#4504; American Diagnostica, Greenwich, CT), followed by a fluorescein isothiocyanate (FITC)-conjugated sheep antimouse Ig antibody (Silenus, Hawthorn, Victoria, Australia). An irrelevant isotype-matched mouse monoclonal antibody was used as a control for the anti-TF antibody.
Measurement of ROS.
ROS production was assessed as described previously.22After culture, PBMCs were resuspended at 5 × 105/mL in PBS containing 4% fetal calf serum (FCS). Cells were incubated at 37°C for 10 minutes with phorbol myristate acetate (PMA; 500 ng/mL; Sigma Chemicals, St Louis, MO) or normal saline (to assess spontaneous non–PMA-triggered ROS production), and then dihydro-rhodamine 123 (100 ng/mL; Molecular Probes, Eugene, OR) was added for an additional 10 minutes. Cells were placed on ice before analysis by flow cytometry. PMA-triggered ROS production was expressed as the difference in mean fluorescence between PMA-stimulated and saline-treated cells.
Factor VIIa-induced ROS augmentation.
PBMCs were incubated under the serum-free conditions described above with purified human recombinant factor VIIa (American Diagnostica) at a range of concentrations from 0 to 2.5 μg/mL in the presence or absence of 0.5 μg/mL LPS (Sigma Chemicals). ROS production was measured as described above. The ligand specificity of the response was assessed by incubation with purified native factor VII (2.5 μg/mL; American Diagnostica) and factor VIIa (2.5 μg/mL), in which the active site was irreversibly inactivated with 1,5-dansyl-Glu-Gly-Arg chloromethyl ketone (DEGRck; Calbiochem, San Diego, CA), as previously described.23
The receptor specificity of the factor VIIa-induced response was determined by incubation in the presence of a monoclonal mouse antihuman TF antibody (5 μg/mL) or an irrelevant isotype-matched monoclonal antibody. Incubations were also performed in the presence of 2 mg/mL hirudin (Ciba Pharmaceuticals, Basel, Switzerland) to exclude thrombin/PAR-1–mediated effects. This dose of hirudin abolished thrombin (1 U/mL; Armour Pharmaceutical Co, Kaneke, IL) -stimulated augmentation of macrophage ROS production. Incubations were also performed in the presence of 0.2 U/mL dalteparin (Fragmin; Pharmacia and Upjohn Pty Ltd, Rydalmere, New South Wales, Australia) to exclude potential effects of endogenous factor Xa production via EPR-1. This dose of dalteparin completely inhibited the augmentation of macrophage ROS production induced by 5 μg/mL of purified human factor Xa (American Diagnostica). The requirement for protein synthesis for PBMC augmentation of factor VIIa-induced ROS production was assessed by culture of PBMCs in the presence of 500 μmol/L cycloheximide (Molecular Probes).
Measurement of intracellular free calcium fluxes.
Intracellular Ca2+ fluxes were measured by flow cytometry in PBMCs loaded with Pluronic F and Fluo-3 (Molecular Probes), as previously described.24 PBMCs (1 × 107cells/mL) cultured with LPS (0.5 μg/mL for 24 hours, as described above) were incubated in RPMI medium (ICN Biomedicals, Aurora, OH) containing 1% FCS, Pluronic F 127 (1 μg/mL), and Fluo-3 (3 μmol/L in 0.25% dimethyl sulfoxide; Sigma Chemicals) for 45 minutes at 37°C to allow Fluo-3 loading. Cells were washed twice in RPMI at room temperature to remove extracellular Fluo-3 and were then allowed to equilibrate to 37°C for 10 minutes. At the end of this time, stable baseline fluorescence over a 20-second period was confirmed by flow cytometry. Baseline intracellular [Ca2+] was calculated from the fluorescence at the end of this 20-second period. Analysis was then interrupted to allow addition of potential agonists (<2-second delay) and fluorescence signals were reacquired after a 20-second delay. Analysis was then continued for a further 70 seconds (∼90 seconds after the addition of agonists). The fluorescence units were converted into Ca2+ concentrations by a nondisruptive calibration procedure using a nonfluorescent calcium ionophore Bromo-A23187 (10 μmol/L; Molecular Probes) followed by quenching with manganese chloride (2 mmol/L; Sigma Chemicals), as previously described.25 The following potential ligands were assessed: factor VIIa (2.5 μg/mL), native factor VII (2.5 μg/mL), and site-inactivated factor VIIa (2.5 μg/mL). Receptor specificity was assessed by measurement of factor VIIa (2.5 μg/mL) -induced Ca2+ fluxes in the presence of anti-TF antibody (5 μg/mL) or control antibody. Ca2+ fluxes induced by factor VIIa, factor VII, and factor VIIa in the presence of anti-TF antibody were also assessed on cells that were allowed to equilibrate to 37°C for 15 minutes after fluo-3 loading. To investigate the role of second messengers, factor VIIa-induced Ca2+ fluxes were studied in the presence of a tyrosine kinase inhibitor (herbimycin A; 3 μmol/L; Sigma Chemicals) or a phospholipase C inhibitor U73122 (5 μmol/L; Biomol, Plymouth Meeting, PA) or its inactive analog (U73343; 5 μmol/L; Biomol) added 3 minutes before analysis of the basal fluorescence level. Ca2+ fluxes were also measured in cells incubated with these inhibitors for 24 hours.
Transfection of TF into U937 cells.
Oligonucleotide primers were used to obtain full-length and truncated TF DNA constructs from human TF cDNA5 by the polymerase chain reaction (PCR) using Pfu polymerase. The truncated construct comprised the complete sequence for the extracellular and transmembrane regions and the sequence for only the first 5 amino acids of the intracytoplasmic domain to facilitate membrane anchoring. The sequence for the terminal 16 amino acids of the 21 in the cytoplasmic tail was deleted. These PCR products were cloned into a mammalian expression vector (pcDNA3.1) containing a cytomegalovirus promoter, and the sequences were confirmed by automated sequencing (ABI Prism; PE Biosystems, Foster City, CA). Vector DNA (containing the TF cDNA constructs) was linearized and transfected into a human monocytic cell line (U937 cells) by electroporation. Transfected cells expressing stable, high levels of TF on their cell membrane (assessed by flow cytometry as described above) were selected by repeated passage of cells in RPMI with G418 (400 μg/mL). The capacity of human factor VIIa to induce intracellular Ca2+ fluxes was investigated as described above. In contrast to PBMCs, U937 Ca2+ fluxes were measured at 20°C.
Peritoneal elicited macrophages.
New Zealand White rabbits (2.0 to 2.3 kg; Monash University, Central Animal Services, Clayton, Victoria, Australia) were injected intraperitoneally with 50 mL of 3.8% thioglycolate (Becton Dickinson, Cockeysville, MD) and treated intravenously with either functionally inhibitory sheep antirabbit TF globulin (n = 6) or normal sheep globulin (NSG; n = 6). Treatments were administered 6 hours (50 mg/kg), 30 hours (100 mg/kg), and 54 hours (50 mg/kg) after thioglycolate injection. Peritoneal exudate cells were harvested by lavage with Eagles medium (ICN Biomedicals) containing 1% FCS and 3.3% sodium citrate, 72 hours after administration of thioglycolate. Red blood cells were removed by water lysis for 30 seconds. Cells were then washed and resuspended in PBS with 4% FCS and counted using a hemocytometer. Peritoneal exudate cells were greater than 85% positive by nonspecific esterase staining26 and had a viability of greater than 97% by flow cytometry using propidium iodide exclusion. Flow cytometry was used to assess macrophage activation by their capacity to produce ROS after PMA stimulation (as described above), their expression of MHC class II using a monoclonal anti-rabbit MHC class II antibody (2CAB12),27 and their expression of β2 cell adhesion molecules, using a monoclonal antibody to the common β integrin chain (CD18; 60.3; Bristol Myers Squibb Pharmaceutical Research Institute, Seattle, WA).28
Study design and statistical methods.
Blood monocytes were collected from a group of 15 volunteers. All human monocyte-derived macrophage experiments were performed in duplicate on 2 or more separate occasions on cells from a minimum of 5 individuals for each parameter. Differences between groups were analyzed by the ANOVA with post-hoc analysis of Fisher’s protected least significant difference.
RESULTS
TF expression is upregulated during macrophage maturation.
Monocytes expressed low levels of TF (14 ± 3 mean fluorescence units [mfu]) immediately after density separation (control). TF expression increased significantly after adherence to plastic in the absence of LPS (87 ± 16 mfu at 4 hours and 225 ± 9 mfu at 24 hours, both P < .001 compared with control). In the presence of LPS (500 ng/mL), adherent monocyte-derived macrophages showed greater enhancement of TF expression at 4 hours (116 ± 13 mfu) and 24 hours (512 ± 32 mfu, P = .047) as compared with cells cultured in the absence of LPS. There was a significant correlation between TF expression and PMA-triggered ROS production of macrophages cultured under serum-free conditions (coefficient of correlation, R2 = .849; P = .0239; Table 1).
TF Expression and ROS Production by Nonadhered Human Monocyte-Derived Macrophages After Density Sedimentation (Control) and After Culture and Adherence Under Serum-Free Conditions (and the Absence of Factor VIIa)
| . | TF Expression (mfu) . | ROS Production (mfu) . |
|---|---|---|
| Control (post ficol) | 14 ± 3 | 26 ± 13 |
| Adherence, no LPS | ||
| 4 h | 87 ± 16 | 58 ± 8 |
| 24 h | 225 ± 9 | 63 ± 8 |
| Adherence and LPS | ||
| 4 h | 116 ± 13 | 64 ± 8 |
| 24 h | 512 ± 32 | 100 ± 10 |
| . | TF Expression (mfu) . | ROS Production (mfu) . |
|---|---|---|
| Control (post ficol) | 14 ± 3 | 26 ± 13 |
| Adherence, no LPS | ||
| 4 h | 87 ± 16 | 58 ± 8 |
| 24 h | 225 ± 9 | 63 ± 8 |
| Adherence and LPS | ||
| 4 h | 116 ± 13 | 64 ± 8 |
| 24 h | 512 ± 32 | 100 ± 10 |
Results are expressed as mfu.
Factor VIIa augments macrophage ROS production in a time- and dose-dependent manner.
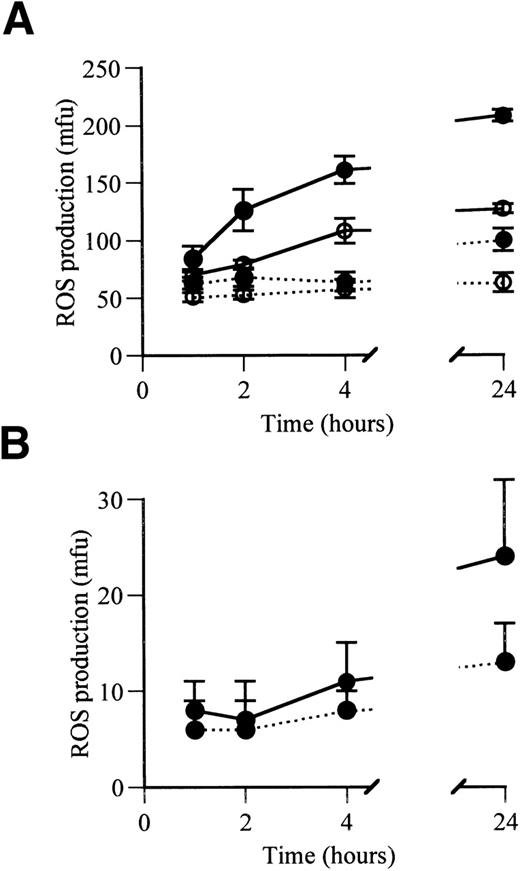
Adherence of monocyte-derived macrophages under serum-free conditions resulted in a rapid initial augmentation of their PMA-stimulated ROS production. At 1 hour in culture, ROS production was not significantly influenced by the presence of factor VIIa (2.5 μg/mL) or LPS (Fig 1A). Subsequent ROS production in the absence of factor VIIa and LPS remained stable for the following 23 hours. In the presence of LPS and absence of factor VIIa, ROS production was also stable between 1 and 4 hours, but showed a small significant increase at 24 hours (P < .008, compared with that at 1, 2, and 4 hours). Factor VIIa in the absence of LPS induced a marked increase in ROS production between 1 and 24 hours (P = .0015). This effect was accentuated in the presence of LPS, where ROS production was significantly augmented by factor VIIa at 2 hours (P = .0004), 4 hours (P < .0001), and 24 hours (P < .0001) compared with 1 hour. In the absence of PMA triggering, factor VIIa still produced a significant increase in spontaneous ROS production at 24 hours (P < .001; Fig 1B).
ROS production by adhered human monocyte-derived macrophages after 1, 2, 4, and 24 hours in serum-free culture in the presence and absence of factor VIIa. (A) PMA-triggered ROS production. (B) Spontaneous ROS production, measured without PMA triggering. (•) Values for cells cultured in the presence of LPS; (○) values for cells cultured in the absence of LPS. Continuous lines show values in the presence of factor VIIa (2.5 μg/mL); dotted lines show values in the absence of factor VIIa. ROS production is expressed as mfu.
ROS production by adhered human monocyte-derived macrophages after 1, 2, 4, and 24 hours in serum-free culture in the presence and absence of factor VIIa. (A) PMA-triggered ROS production. (B) Spontaneous ROS production, measured without PMA triggering. (•) Values for cells cultured in the presence of LPS; (○) values for cells cultured in the absence of LPS. Continuous lines show values in the presence of factor VIIa (2.5 μg/mL); dotted lines show values in the absence of factor VIIa. ROS production is expressed as mfu.
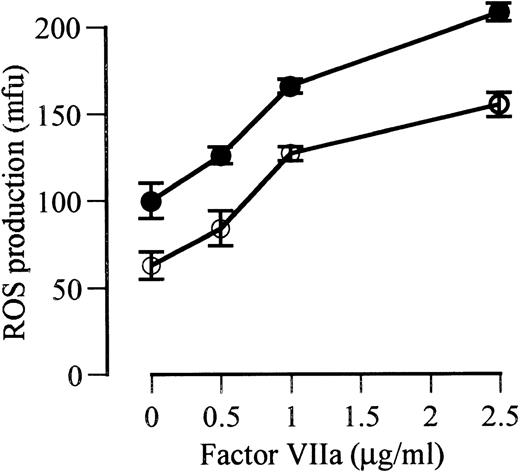
Factor VIIa-induced augmentation of ROS production is dose-dependent.
At 24 hours in the presence of LPS, there were significant increases in ROS production with each increment of factor VIIa concentration from 0 to 0.5 μg/mL (P = .025), from 0.5 to 1.0 μg/mL (P = .001), and from 1.0 to 2.5 μg/mL (P < .0001). In the absence of LPS, significant increases in ROS production also occurred with each dose increment. The relative increase in response to the maximal dose of factor VIIa (2.5 μg/mL) was similar in the presence and absence of LPS (108% and 102%, respectively, above control; Fig 2).
The effect of increasing doses of factor VIIa on PMA-triggered ROS production by monocyte-derived macrophages cultured for 24 hours in serum-free media in the presence of LPS (•) and the absence of LPS (○).
The effect of increasing doses of factor VIIa on PMA-triggered ROS production by monocyte-derived macrophages cultured for 24 hours in serum-free media in the presence of LPS (•) and the absence of LPS (○).
Factor VIIa-dependent ROS production in monocyte-derived macrophages requires protein synthesis.
Culture in the presence of cycloheximide prevented factor VIIa augmentation of ROS production at 2, 4, and 24 hours. Basal ROS production by monocyte-derived macrophages (after 1 hour of adherence) was 63 ± 5 mfu, and ROS production increased to 208 ± 5 mfu after 24 hours of culture in the presence of LPS and factor VIIa (2.5 μg/mL). This increase was prevented by the addition of cycloheximide (60 ± 8 mfu). These studies demonstrate that augmentation of ROS production in the presence of factor VIIa requires a process involving active protein synthesis by monocyte-derived macrophages. Because cycloheximide is a nonselective inhibitor of protein synthesis, the particular proteins involved in this process remain to be defined.
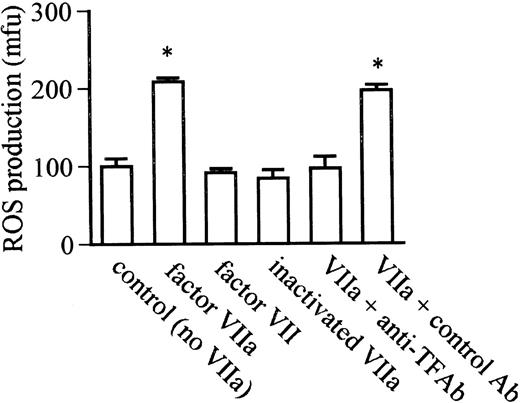
TF/factor VIIA augmentation of ROS is ligand and receptor specific.
Although factor VIIa (2.5 μg/mL) significantly augmented ROS production, no change in ROS production was observed with native factor VII or inactivated VIIa at the same concentration (Fig 3). Anti-TF antibody abolished factor VIIa-induced augmentation of ROS production, whereas no inhibition was observed with a control antibody. The ability of anti-TF antibody to abolish the response to factor VIIa and the absence of response to inactivated factor VIIa demonstrates that this response is not attributable to endotoxin.
The ligand and receptor specificity of factor VIIa/TF-stimulated monocyte-derived macrophage ROS production after 24 hours of coincubation with LPS in serum-free media. Factor VIIa induced significant augmentation of ROS production compared with the absence of factor VIIa (control-no VIIa), but augmentation was not observed with native factor VII or active site inactivated factor VIIa. Augmentation of ROS production by factor VIIa was abolished in the presence of anti-TF antibody (VIIa + anti-TF Ab) but was unaffected by a control antibody (VIIa + control Ab). *P < .0001 compared with control.
The ligand and receptor specificity of factor VIIa/TF-stimulated monocyte-derived macrophage ROS production after 24 hours of coincubation with LPS in serum-free media. Factor VIIa induced significant augmentation of ROS production compared with the absence of factor VIIa (control-no VIIa), but augmentation was not observed with native factor VII or active site inactivated factor VIIa. Augmentation of ROS production by factor VIIa was abolished in the presence of anti-TF antibody (VIIa + anti-TF Ab) but was unaffected by a control antibody (VIIa + control Ab). *P < .0001 compared with control.
Thrombin and factor Xa do not contribute to factor VIIa-induced ROS production.
Experiments were performed in the presence of hirudin or dalteparin to exclude a role for endogenous thrombin or factor Xa generation, respectively, in the augmentation of monocyte-derived macrophage ROS production by factor VIIa after 24 hours of serum-free culture with LPS. The presence of hirudin did not effect factor VIIa augmentation of ROS production (factor VIIa [2.5 μg/mL] + hirudin, 196 ± 14 mfu; factor VIIa + control, 205 ± 23 mfu). Hirudin alone in the absence of factor VIIa did not effect ROS production (hirudin alone, 110 ± 13 mfu; control, 100 ± 10 mfu), but this dose of hirudin abolished thrombin-induced augmentation of ROS production (thrombin [1 U/mL], 154 ± 11 mfu; thrombin + hirudin, 108 ± 8 mfu;P < .001). Similarly, dalteparin, a direct factor Xa inhibitor, did not inhibit augmentation of ROS production by high doses of factor VIIa (2.5 μg/mL; factor VIIa + dalteparin, 219 ± 12 mfu; factor VIIa + control, 213 ± 5 mfu) or lower doses of factor VIIa (0.5 μg/mL; factor VIIa + dalteparin, 146 ± 10 mfu; factor VIIa + control, 126 ± 5 mfu). Dalteparin alone in the absence of factor VIIa did not effect ROS production (dalteparin alone, 91 ± 7 mfu; control, 100 ± 10 mfu), but did abolish augmentation of ROS production induced by 5 μg/mL of factor Xa (factor Xa alone, 127 ± 10 mfu; factor Xa + dalteparin, 100 ± 5 mfu; P = .03) and 25 μg/mL of factor Xa (factor Xa alone, 185 ± 13 mfu; factor Xa + dalteparin, 85 ± 7 mfu; P = .001).
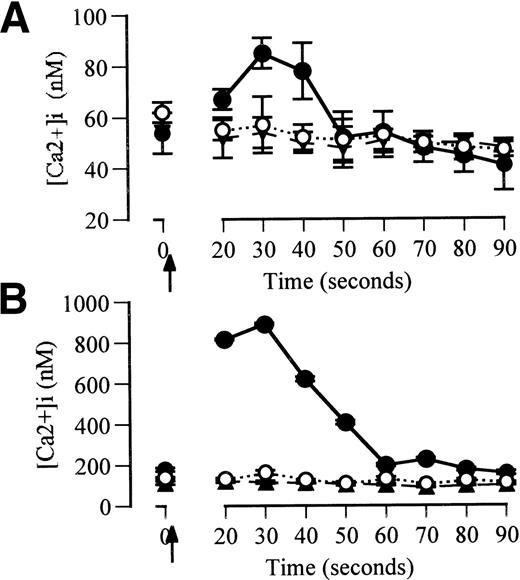
TF/factor VIIa interactions induce intracellular calcium fluxes in monocyte-derived macrophages.
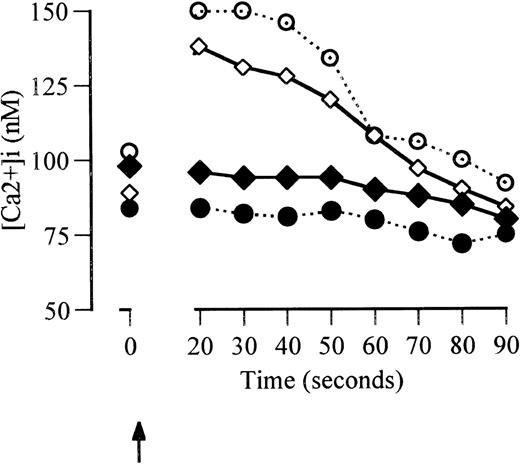
Factor VIIa (2.5 μg/mL) induced rapid Ca2+ fluxes in macrophage-derived monocytes from a baseline intracellular Ca2+ concentration of 54 ± 8 nmol/L. The percentage of responding cells was greater than 98%. Native factor VII (2.5 μg/mL) and inactivated factor VIIa (2.5 μg/mL) did not induce Ca2+ fluxes (Fig 4A). Factor VIIa-induced Ca2+ fluxes were abolished in the presence of anti-TF antibody but unaffected by control antibody (data not shown). Ca2+ fluxes were also abolished in the presence of the phospholipase-C-β inhibitor, U73122, but were unaffected by its inactive analog (U73433) or the tyrosine kinase inhibitor, herbimycin (data not shown). These Ca2+ fluxes were studied 10 minutes after the completion of fluo-3 loading to minimize leakage of Ca2+ into cytosolic compartments. The magnitude of the Ca2+ fluxes induced by factor VIIa was consistent with fluxes measured by other techniques that measure specific intracellular Ca2+ in macrophages.29
Ca2+ fluxes induced by factor VIIa in monocyte-derived macrophages. (A) Cells equilibrated for 10 minutes after fluo-3 loading. (B) Cells equilibrated for 15 minutes after Fluo-3 loading. Solid circles and continuous lines represent the response to addition of factor VIIa (2.5 μg/mL) added at 2 seconds (arrowed). Open circles and dotted lines represent the response to native factor VII (2.5 μg/mL). Triangles and broken lines represent responses to factor VIIa (2.5 μg/mL) when the cells were coincubated with anti-TF for 24 hours before the assessment of Ca2+fluxes.
Ca2+ fluxes induced by factor VIIa in monocyte-derived macrophages. (A) Cells equilibrated for 10 minutes after fluo-3 loading. (B) Cells equilibrated for 15 minutes after Fluo-3 loading. Solid circles and continuous lines represent the response to addition of factor VIIa (2.5 μg/mL) added at 2 seconds (arrowed). Open circles and dotted lines represent the response to native factor VII (2.5 μg/mL). Triangles and broken lines represent responses to factor VIIa (2.5 μg/mL) when the cells were coincubated with anti-TF for 24 hours before the assessment of Ca2+fluxes.
Increasing the delay in analysis of Ca2+ fluxes after fluo-3 loading or increasing the permeability of the cells results in higher apparent baseline Ca2+ concentrations and larger apparent Ca2+ fluxes.29 30 In the current studies, equilibration of monocyte-derived macrophages to 37°C for 15 minutes after fluo-3 loading resulted in higher baseline Ca2+ concentrations (152 ± 64 nmol/L) and greater apparent Ca2+ fluxes in response to factor VIIa (2.5 μg/mL) than cells equilibrated for 10 minutes (Fig 4B). Ca2+ fluxes in cells equilibrated for 15 minutes did not occur in response to factor VII or to factor VIIa in the presence of anti-TF antibody.
The cytoplasmic tail of TF is required for factor VIIa-induced Ca2+ fluxes.
U937 cells expressed low constitutive levels of TF (49 ± 5 mfu), but significantly enhanced their TF expression (250 ± 31 mfu;P < .001) after 24 hours of culture in the presence of LPS. U937 cells transfected with cytoplasmic truncated tail or full-length TF DNA constructs under control of a cytomegalovirus promoter expressed high constitutive levels of TF expression (212 ± 43 and 180 ± 22 mfu, respectively) that were not significantly different from the levels in LPS-stimulated U937 cells. Factor VIIa (2.5 μg/mL) induced intracellular Ca2+ fluxes in LPS-treated U937 cells and cells expressing full-length TF but not in unstimulated U937 cells or cells expressing TF in which the cytoplasmic tail had been truncated (Fig 5).
Factor VIIa induced Ca2+ fluxes in unstimulated U937 cells (•), LPS-stimulated U937 cells (○), and U937 cells transfected with full-length (◊) and truncated TF DNA sequences (⧫) under a cytomegalovirus promoter. Profiles show a representative response for each cell clone.
Factor VIIa induced Ca2+ fluxes in unstimulated U937 cells (•), LPS-stimulated U937 cells (○), and U937 cells transfected with full-length (◊) and truncated TF DNA sequences (⧫) under a cytomegalovirus promoter. Profiles show a representative response for each cell clone.
Anti-TF antibody attenuates activation of peritoneal macrophages in vivo.
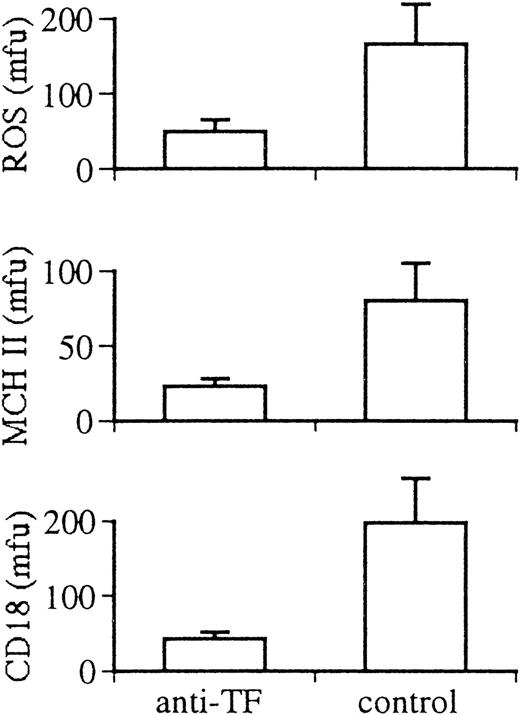
In vivo inhibition of TF with a functionally inhibitory sheep antirabbit TF antibody attenuated activation of rabbit macrophages recruited in response to intraperitoneal injection of thioglycolate. TF antibody treatment did not affect the recruitment of cells (total cell harvest at 72 hours: anti-TF–treated, 1.09 ± 0.11 × 107 cells; control, 1.06 ± 0.09 × 107cells) or cell viability (>97% in both groups). However, the mean cell size of peritoneal macrophages (measured by flow cytometry using the mean forward angle light scatter signal) in rabbits treated with anti-TF antibody was reduced by 40% (treated, 34 ± 4; control, 55 ± 6; P < .0001). Cell granularity (measured by the mean right angle light scatter signal) was also significantly reduced (treated, 23 ± 3; control, 27 ± 3; P = .04). These characteristics indicate a less activated phenotype of recruited peritoneal macrophages in rabbits after in vivo inhibition of TF. Treatment with anti-TF antibody also significantly reduced macrophage ROS production after PMA triggering (treated, 50 ± 15 mfu; control, 166 ± 15 mfu; P = .03). Expression of MHC II (treated, 23 ± 5 mfu; control, 80 ± 25 mfu; P = .04) and CD18 (treated, 43 ± 9 mfu; control, 189 ± 58 mfu; P = .02) by elicited macrophages was significantly reduced in anti-TF antibody-treated rabbits (Fig 6).
Effect of anti-TF antibody and control antibody treatment on the activation of rabbit elicited peritoneal macrophages in vivo. Panels show production of ROS, expression of MHC II antigen (MHC II) and the expression of the integrin β chain (CD18) of leukocyte adhesion molecules.
Effect of anti-TF antibody and control antibody treatment on the activation of rabbit elicited peritoneal macrophages in vivo. Panels show production of ROS, expression of MHC II antigen (MHC II) and the expression of the integrin β chain (CD18) of leukocyte adhesion molecules.
DISCUSSION
The current studies demonstrate that binding of TF to its natural ligand, factor VIIa, results in proinflammatory phenotypic changes in vitro in human monocyte-derived macrophages and in vivo in rabbit peritoneal macrophages. Circulating monocytes do not express TF.2 However, after maturation to a macrophage phenotype in vitro by adherence both in the presence and absence of endotoxin, or in vivo after recruitment across the peritoneal membrane, monocytes were induced to express TF. In these TF-expressing cells, factor VIIa augmented ROS production in vitro and anti-TF antibodies inhibited ROS production in vivo.
In addition to its recently described role in intracellular signaling,31 ROS products are important effector molecules for macrophage inflammatory functions. Macrophage ROS production is involved in the killing of intracellular pathogens,32ischemia reperfusion injury (where ROS products are associated with increased TF expression in coronary lesions),33 and macrophage-mediated tissue injury.34 35 Factor VIIa, in the absence of other coagulation proteins, augmented the capacity of macrophages to produce ROS in a time- and dose-dependent manner. Thus, direct TF/factor VIIa-mediated effects on macrophages have the potential to enhance killing of intracellular pathogens and exacerbate tissue injury in a variety of situations in which ROS are important mediators.
Previous studies have provided limited evidence for the capacity of TF to modulate cellular functions such as increased poly(A) polymerase mRNA production in fibroblasts,36 translocation of TF to the caveolae in transformed human endothelial cells,37 and enhanced metastatic potential of human melanoma cell lines.38 A potential role in embryonic angiogenesis was suggested by studies of TF gene in knock-out mice.39However, failure of hemostatic function has been suggested as alternative explanation for this observation,40 and recent studies have demonstrated angiogenesis may proceed normally in some TF-deficient mice.41 The current studies provide the first evidence for direct modification of inflammatory cell function by TF/factor VIIa-mediated signaling.
Augmentation of ROS production by factor VIIa was specific for the proteolytically active ligand. Responses did not occur with the zymogen factor VII or with factor VIIa in which the enzymatic site had been inactivated. Furthermore, the ability of a specific monoclonal antibody to human TF to inhibit the factor VIIa-induced response demonstrates its receptor specificity. Plasminogen has recently been demonstrated as an alternative ligand for TF, which binds independently of factor VIIa.42 Plasminogen is unlikely to contribute to the ROS augmentation in the serum-free conditions of the current studies.
Macrophages have the capacity to produce a prothrombinase complex,43 and in some reports their potential to produce prothrombin has been suggested.44 The ability of factor VIIa to augment ROS production in our studies was unaffected by the presence of hirudin, excluding any role for thrombin in these effects. The absence of serum and the use of recombinant factor VIIa in these studies exclude exogenous sources of factors, such as Xa, as ligands for their receptors. It is possible that monocytes may produce factor Xa in culture. However, factor VIIa-augmented ROS production was unaffected in the presence of the direct factor Xa inhibitor dalteparin,45 excluding a contribution of any endogenous factor Xa/EPR-1–mediated effects to this response.
Maximal ROS responses to factor VIIa were induced at a concentration of 2.5 μg/mL, which is the same as that required for other effects reported in fibroblasts and monocytes.36 46 Smaller responses were induced by factor VIIa at a concentration of 0.5 μg/mL, which is the concentration of factor VII in plasma. The local concentration of factor VIIa generated after activation of factor VII at the endothelial cell surface is unknown. The in vivo sensitivity of this receptor/ligand system may also vary according to the local density of TF expression and other factors. Therefore, it is difficult to directly extrapolate the in vitro dose response curve to in vivo conditions. However, the ability of anti-TF antibody to block macrophage activation in vivo points to the in vivo physiological relevance of TF/factor VIIa interactions.
Factor VIIa augmentation of ROS production was associated with rapid induction of intracellular Ca2+ fluxes after ligand/receptor binding. The short loading time for fluo-3 allows more specific assessment of intracellular Ca2+ fluxes by minimizing extracellular leakage of the fluorophore. These intracellular Ca2+ fluxes are similar in magnitude to those measured using flow cytometry after thrombin stimulation of human endothelial cells47 and are consistent with other reports of specific intracellullar Ca2+ fluxes.29Because of its ability to measure specific cell-associated fluorescence, flow cytometry using fluo-3 produces lower apparent Ca2+ fluxes than does spectrofluorimetry.48Increasing the loading time for fluo-3, which increases its extracellular leakage, results in higher baseline cytosolic Ca2+ concentrations and larger apparent Ca2+fluxes.30 48
Inhibitor studies demonstrated the requirement for phosho-inositol–specific phospholipase C, but not tyrosine kinase, suggesting that TF/factor VIIa-induced Ca2+ fluxes in macrophages are mediated by a G-protein–coupled phospho-inositol–specific phospholipase C pathway. Binding of TF with factor VIIa has also been demonstrated to induce intracellular Ca2+ fluxes (but not functional effects) in human umbilical vein endothelial cells (HUVECs) and Madin-Darby canine kidney cells.30 Ca2+ fluxes induced in monocyte-derived macrophages were ligand and receptor specific, as demonstrated by the inability of native factor VII and inactivated factor VIIa to induce Ca2+ fluxes and the ability of anti-TF antibody to prevent factor VIIa responses. Specificity for proteolytically active factor VIIa in initiating Ca2+fluxes has also demonstrated in Madin-Darby cells30 and TF-dependent metastatic behavior of Chinese hamster ovary (CHO) cells.49 Although factor VII and factor VIIa bind to the same site on TF, catalytic activation of factor VIIa by TF leads to increased binding affinity and enhanced factor X hydrolysis.50 These allosteric changes may be critical for TF signaling.
Previous studies have demonstrated that mutation of the cytoplasmic tail of TF inhibits the metastatic behavior of melanoma cell lines38 and TF-dependent metastatic behavior of CHO cells.49 In the current studies, a requirement for the cytoplasmic tail of TF for factor VIIa-induced Ca2+ fluxes in macrophages was demonstrated. Other investigators have suggested that TF does not directly cause Ca2+ fluxes but realigns factor VIIa and facilitates its interaction and signaling through other (unidentified) cell surface receptors.30 However, transfection of U937 cells demonstrated that factor VIIa cannot induce Ca2+ fluxes when the cytoplasmic tail of TF is truncated to leave only 5 amino acids in the intracellular domain. Cells expressing truncated TF fail to show Ca2+ fluxes despite levels of cell surface TF expression similar to those of cells transfected with full-length TF and LPS-stimulated U937 cells, which respond to factor VIIa. This does not exclude the possibility that TF may signal by coupling to a second intracellular receptor system, which has been suggested by studies demonstrating association of TF with the FcεRI γ chain homodimer in human monocytes.46
The in vivo capacity of TF to enhance macrophage activation was demonstrated in rabbits. Blocking TF/factor VIIa interactions using an antisheep TF antibody significantly reduced PMA-triggered ROS production by the elicited peritoneal macrophages. In addition, blocking TF reduced expression of MHC class II and β2 integrin leukocyte adhesion molecules (indicated by reduced expression of the common β chain of these heterodimers), which are additional markers of macrophage activation. Intraperitoneal administration of anti-TF antibodies did not reduce macrophage recruitment into the peritoneum or reduce macrophage viability. However, their reduced size and granularity on flow cytometry is also an indication of a less activated (more monocyte-like) phenotype. The lack of effect of TF inhibition on the transmigration of monocytes into the peritoneum is consistent with the observation that TF is not expressed on circulating monocytes.2 These studies demonstrate that factor VIIa/TF-mediated effects observed using human monocyte-derived macrophages in vitro have significant implications for macrophage activation in vivo.
In summary, this study demonstrates that the binding of cell surface expressed TF to its active serine protease ligand, factor VIIa, augments the proinflammatory functions of macrophages both in vivo and in vitro. These findings demonstrate a role for the TF/factor VIIa receptor/ligand interaction in augmentation of inflammatory processes. Modification of the TF/factor VIIa interaction by the naturally occurring inhibitor TFPI, blocking peptides, or antibodies may allow novel therapeutic approaches to ameliorate human diseases such as septic shock, crescentic glomerulonephritis, and atherosclerosis, in which the induced TF expression on the macrophage cell surface may play an important pathological role.
ACKNOWLEDGMENT
The authors thank Dr Chris Mitchell for critically reviewing the manuscript.
Supported by grants from the National Health and Medical Research Council of Australia (NH&MRC) and the Australian Kidney Foundation. M.A.C. is the recipient of a NH&MRC Medical Postgraduate Research Scholarship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal