Antithrombin is shown to undergo a slow spontaneous conversion to its inactive latent conformation with readily discernible amounts present in plasma on incubation at 37°C for 72 hours. More rapid conversion occurs on incubation of isolated antithrombin at 41°C or 50°C, but the appearance on electrophoresis of free latent antithrombin is preceded by the formation, in reciprocal proportions, of a new slow band. This slow component is shown to be a heterodimer of active and latent antithrombin. It can be isolated as a single stable band either by incubation of antithrombin or by mixing equimolar proportions of active and latent antithrombin under the same conditions that give overnight crystallization of the active/latent antithrombin heterodimer. Similarly, equimolar addition of latent antithrombin to plasma results electrophoretically in a quantitative shift to the slower heterodimer mobility. Clinically, the presence of latent antithrombin is potentially deleterious, because its linkage to form the heterodimer results in inactivation of the otherwise normal molecule linked to the latent antithrombin. In the case of -antithrombin, because the dimer readily dissociates, there is only a 11% additive loss of activity, but with β-antithrombin the dimer appears more stable, with the additive loss of activity from the normal β component being 21%, increasing to 33% on stabilization of the dimer with heparin. This linked and selective loss of activity of β-antithrombin provides an explanation for the unexpected severity of thrombotic episodes in heterozygotes with conformationally unstable antithrombins.
ANTITHROMBIN IS THE principal inhibitor in the plasma of the coagulation proteases thrombin and factor Xa.1 As is the case with other members of the serpin family of protease inhibitors, antithrombin is a monomer that functions by exposing its reactive site loop as a pseudosubstrate for the target protease. Cleavage at the reactive center results in entrapment of the protease, with movement of the cleaved reactive center peptide loop together with the bound protease, such that the loop forms an extra sixth strand in the middle of the A β-pleated sheet of the molecule. This movement of the cleaved loop, with its incorporation into the body of the molecule, can also be induced without cleavage of the reactive loop to give the inactive 6-stranded form of antithrombin.2This 6-stranded intact form of antithrombin has an identical crystallographic structure to that of the physiological latent conformation3 of plasminogen activator inhibitor-1 (PAI-1). For this reason, it is referred to as the latent form, even though with antithrombin this latent transition is irreversible.
The 3-dimensional structure of antithrombin was independently determined in 1994 by 2 different groups,4 5 but in each instance there was the unexpected finding that the protein had crystallized not as a monomer but as a heterodimer of antithrombin. There is now a consensus agreement between the 2 groups that this dimer is formed by the linkage of a molecule of active antithrombin to a second molecule of antithrombin in the inactive latent conformation. The puzzling implication from this finding in both laboratories was that, in each case, some of the antithrombin, which was freshly prepared for crystallization, must have spontaneously converted to the latent form. This apparent evidence that the latent conversion of antithrombin could spontaneously take place at room temperature was dismissed rather unconvincingly at the time as being a crystallographic artifact. One reason for this dismissal was the inability to demonstrate the appearance in the crystallization solutions of a component with the characteristically increased electrophoretic mobility of the latent form.
We show here that normal antithrombin does indeed undergo a slow conversion to the latent form even under in vivo conditions. However, this conversion is masked by the immediate linkage of the newly formed latent molecule to a molecule of active antithrombin to give a dimer that unexpectedly has an electrophoretic mobility close to that of the monomeric normal component. Hence, in past investigations, the presence of latent antithrombin has not been detected electrophoretically until it appears as the free species, and this does not occur until the concentration of latent antithrombin exceeds 50% of the total antithrombin. It is now apparent that, in the past, stored samples of what had been believed to be active antithrombin could actually have contained a substantial proportion of the latent form, as is demonstrated here with a commercially fractionated preparation. The presence of undetected latent antithrombin provides an explanation for puzzling inconsistencies in previous experimental results. However, we believe that the most significant implications of our findings relate to the mechanism of onset of thrombosis. The latent form not only is in itself inactive, but also its dimerization with an otherwise active molecule is shown to give an amplification of loss of inhibitory activity. The effects of this loss of activity is exacerbated by the dimerization occurring preferentially to the most active inhibitor of coagulation proteases in the plasma, the glycosaminoglycan-bound β-glycoform of antithrombin. This augmentation of the loss of inhibitory activity consequent to the latent conversion is of particular relevance to the unexpectedly severe thrombotic episodes associated with conformationally unstable variants of antithrombin.
MATERIALS AND METHODS
Antithrombin and derivatives.
Normal α-antithrombin was isolated from fresh frozen plasma using precipitation with dextran sulfate and calcium chloride, after which the supernatant was diluted with an equal volume of equilibration buffer (50 mmol/L Tris-HCl, 10 mmol/L sodium citrate, 5 mmol/L EDTA, 0.15 mol/L NaCl, pH 7.4). The mixture was then fractionated on heparin-Sepharose as previously described6,7 with residual heparin being removed by Q-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden) anion exchange chromatography. The concentration of antithrombin was determined with an extinction coefficient of 6.5.8 Latent antithrombin was prepared as previously described9 by incubating freshly prepared native antithrombin (final concentration, 1 mg/mL) with 0.25 mol/L trisodium citrate and 10 mmol/L Tris-HCl at pH 7.4 and 60°C for 16 hours. The sample was exchanged into 20 mmol/L Tris, 10 mmol/L sodium citrate, pH 7.4, by ultrafiltration on an Amicon (Beverly, MA) concentrator using a YM30 membrane, after which it was purified on heparin-Sepharose and ion-exchange chromatography as described above. The latent-antithrombin was eluted at approximately 0.4 mol/L NaCl on heparin-Sepharose chromatography. Confirmation of the latent-state was assessed by transverse urea gradient (TUG) gel electrophoresis and thermal stability measurement as previously described.10Cleaved antithrombin was prepared by incubating 5 μmol/L antithrombin with 50 nmol/L porcine pancreatic elastase for 4 hours at 37°C in 50 mmol/L Tris-HCl, pH 8.5, containing 0.15 mol/L NaCl and 0.1% polyethylene glycol (molecular weight [Mr] 8,000, wt/vol). The product was analyzed by N-terminal sequencing, and the reactive center loop cleavage sites were identified corresponding to residues 388-389 and 389-390, respectively.
Commercial antithrombin concentrate.
Bio-Products Laboratory (Elstree, UK) dried Antithrombin III in a 30 g container, prepared and heat-treated in 1992, was kindly supplied by Dr Elaine Gray (UK National Institute for Biological Standards and Control, South Mimms, UK). The concentrate had been stored in the dark at refrigerated temperature. A second sample, currently (1999) available from the manufacturers for therapeutic use, was also tested.
Incubation and thermal stress.
Normal α-antithrombin was incubated, with the addition of 20% vol/vol glycerol, at 0.5 mg/mL in buffer A (50 mmol/L Tris, 50 mmol/L NaCl, pH 7.5) at 50°C, and aliquots taken at different time intervals were analyzed by native polyacrylamide gel electrophoresis (PAGE; see Fig 1A) and activity assay. Incubation of isolated antithrombin Wibble (T85M; see Fig 1B) was performed at 50°C in buffer B (50 mmol/L Tris buffer, pH 7.4, with 50 mmol/L KCl) as in Beauchamp et al10 and at a concentration of 0.2 mg/mL. Incubations of normal antithrombin at 37°C and 41°C were performed in buffer B at 0.2 mg/mL (see Fig 1E). Aliquots taken at different incubation times were snap-frozen in liquid nitrogen. To prepare the dimer, the latent form was mixed with α- or β-antithrombin in 1:1 stoichiometric proportion to a concentration of 1 mg/mL in buffer A and incubated at 37°C for 5 minutes (see Figs1D and 3D). Competitive displacement from the dimer of α-antithrombin by β-antithrombin was performed by sequentially adding β-antithrombin to the α-dimer previously prepared as described above by the addition of 3 μg each of α-antithrombin and latent antithrombin to a volume of 30 μL (see Fig 3D). Two bags of standard donor plasma (300 mL each, collected in adenosine-citrate-dextrose) were incubated at 37°C for 0 or 72 hours and fractionated on heparin-Sepharose with a 0.1 to 2 mol/L NaCl gradient. Fractions from the peaks were pooled (as shown in Fig 2B) and analyzed on native PAGE (see Fig 3AI). The protein from the native gel was then transferred to a nitro-cellulose membrane, and the bands with the same mobility as latent antithrombin were excised and applied onto a nonreducing sodium dodecyl sulfate (SDS) gel and probed by Western blotting (see Fig3AII). A parallel check on loss of activity of plasma during 72 hours of incubation at 37°C was separately performed by the incubation of 1 mL of plasma in a stoppered microfuge tube. Activity before and after incubation was determined as described.11 To check if dimer can form in human plasma, 5 μL whole plasma was incubated with 0.9 μg latent antithrombin at 37°C for 5 minutes before loading onto native PAGE with subsequent analysis by Western blotting (see Fig 3B).
PAGE.
SDS-PAGE was performed in 10% (wt/vol) polyacrylamide as previously described.10,11 All samples for SDS-PAGE were boiled for 3 minutes under nonreducing conditions before electrophoresis. Nondenaturing PAGE was performed as previously described7but modified to obtain high resolution by use of an 8% gel and with the running time extended to 90 minutes. Isoelectric focusing was performed on precast Ampholine PAGplates (Pharmacia) using ampholytes in the range of pH 4 to pH 6.5. The gels were stained with 0.25% Commassie Blue R250.
Western blot analysis.
Proteins were separated by gel electrophoresis, blotted to nitro-cellulose, and immunostained with rabbit anti-antithrombin antibodies (Dako, Ely, UK), followed by alkaline phosphatase-coupled goat antirabbit IgG (Dako), with detection by incubating the membrane in a phosphate substrate solution consisting of 0.15 mg/mL 5-bromo-4-chloro-3-indolyl phosphate (Sigma, St Louis, MO) and 0.3 mg/mL nitroblue tetrazolium (Sigma) in 0.1 mol/L Tris, pH 9.5, containing 100 mmol/L NaCl and 5 mmol/L MgCl2.
Determination of loss of inhibitory activity.
Second order rate constants of inhibition of human thrombin (Sigma) and bovine factor Xa (Boehringer Mannheim, Ltd, East Sussex, UK) were determined using discontinuous chromogenic assays described by Olson et al1 using S-2238 and S-2222, respectively. Antithrombin isoforms α or β were mixed with latent antithrombin derived from the α-isoform to 1 μmol/L each, so that the final total antithrombin concentration was similar to that in plasma and the proteinase concentration was 100 nmol/L. Stoichiometries of inhibition were determined to be equivalent for α and β and were unaffected by the addition of latent antithrombin. Reactions were performed at room temperature in the presence of either 0.1 mg/mL polybrene or unfractionated heparin (Sigma). Values given in the presence of heparin were derived from the slope of kobs versus molar heparin concentration based on the average molecular weight of 14,000 Daltons and an occurrence of the high-afinity pentasaccharide sequence in one third of the chains. Table 1shows results obtained with factor Xa; similar results were obtained with thrombin (not shown). The reported percentage loss in inhibitory activity is the percentage decrease in rate of inhibition for the active monomer consequent to the addition of inactive latent monomer.
Change in Second-Order Rate Constants of fXa Inhibition
| Active Form . | α-Antithrombin . | β-Antithrombin . | ||||
|---|---|---|---|---|---|---|
| − Heparin (mmol/L−1s−1) . | + Heparin (mmol/L−1s−1) . | − Heparin (mmol/L−1s−1) . | + Heparin (mmol/L−1s−1) . | L:β, 10:1 (mmol/L−1s−1) . | 10:1, + Heparin (mmol/L−1s−1) . | |
| (as) Monomer | 3.75 ± 0.01 | 2,170 ± 190 | 7.16 ± 0.15 | 2,410 ± 180 | 7.16 ± 0.15 | 2,410 ± 180 |
| (in) Dimer | 3.34 ± 0.08 | 1,720 ± 160 | 5.65 ± 0.05 | 1,630 ± 130 | 3.79 ± 0.15 | 843 ± 33 |
| Activity loss | 11% | 21% | 21% | 33% | 47% | 65% |
| Active Form . | α-Antithrombin . | β-Antithrombin . | ||||
|---|---|---|---|---|---|---|
| − Heparin (mmol/L−1s−1) . | + Heparin (mmol/L−1s−1) . | − Heparin (mmol/L−1s−1) . | + Heparin (mmol/L−1s−1) . | L:β, 10:1 (mmol/L−1s−1) . | 10:1, + Heparin (mmol/L−1s−1) . | |
| (as) Monomer | 3.75 ± 0.01 | 2,170 ± 190 | 7.16 ± 0.15 | 2,410 ± 180 | 7.16 ± 0.15 | 2,410 ± 180 |
| (in) Dimer | 3.34 ± 0.08 | 1,720 ± 160 | 5.65 ± 0.05 | 1,630 ± 130 | 3.79 ± 0.15 | 843 ± 33 |
| Activity loss | 11% | 21% | 21% | 33% | 47% | 65% |
RESULTS
Dimer in vitro.
The recognition of the ready formation of latent-active heterodimers came during studies of the changes in normal (α-) antithrombin on prolonged incubation at 50°C. This incubation results in almost full conversion to the latent form by 96 hours (Fig 1A), but it was also observed that the appearance of the latent band was preceded by the initial formation of a previously unidentified slower electrophoretic band that diminished as the latent band increased. This reciprocity in appearance from an initial slow electrophoretic component to the faster band of latent antithrombin was also evident (Fig 1B) on incubation of the conformationally unstable variant,10 antithrombin Wibble (Thr85Met). Incubation of this variant at pH 8.0 for 24 hours gave almost complete conversion to the electrophoretically faster latent form, but in retrospect, careful alignment shows that this is preceded by the appearance of the new slow component.
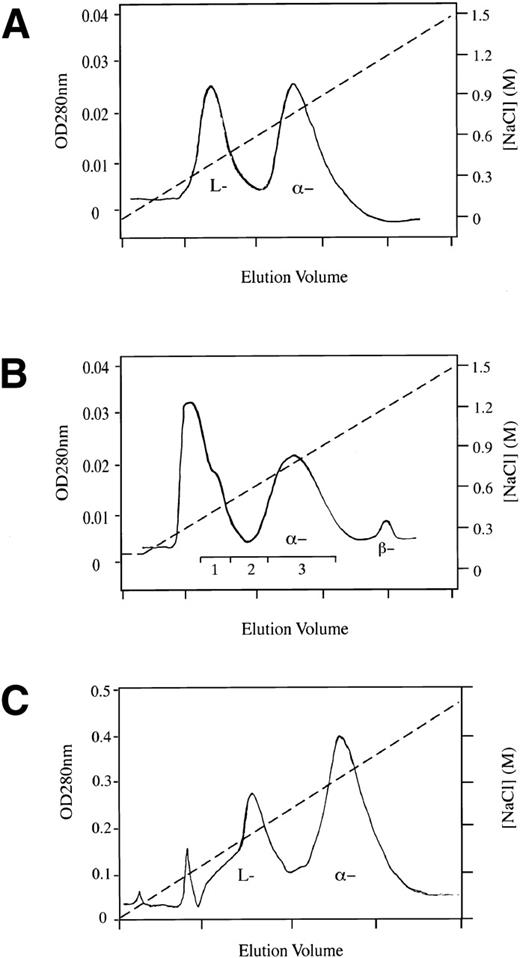
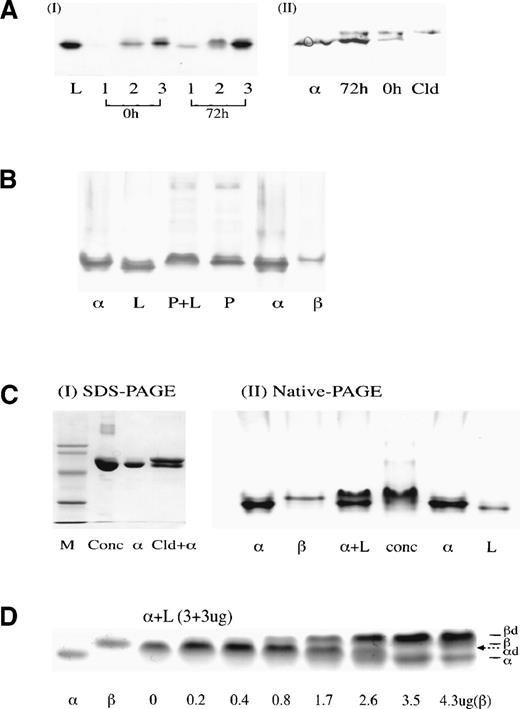
Nondenaturing PAGE electrophoreses. (A) Normal () antithrombin incubated at 50°C at pH7.4 (buffer A) and in 20% glycerol showing initial appearance at 12 hours of slow dimer band with complete conversion to latent form by 96 hours. A similar result but with more rapid change occurred under the same conditions in the absence of glycerol. L, latent control; + L, 1:1 mixture of and latent antithrombin. (B) A time sequence of incubation of the unstable antithrombin Wibble variant (50°C, pH 7.4, buffer B).10 Careful alignment shows how the transition to the latent form is preceded, in reciprocal proportions, by the formation of the slow dimeric component. Trace polymer bands are also present with the position of the loop-sheet dimer and trimer arrowed. (C) Isoelectric focusing of the stable slow electrophoretic component (lane 3), prepared as in (A), confirms its heterodimeric composition, with separation into equal bands of -antithrombin and latent antithrombin. Lanes 1 and 4, -antithrombin; lane 2, latent standard. (D) Mixing of antithrombins (left) and β (right) 1:1 with latent antithrombin immediately gives the single slow dimeric component that retains its electrophoretic integrity even after 7 days at room temperature. (E) Incubation of normal -antithrombin in buffer B (pH 7.4, 50 mmol/L KCl) showing conversion over 54 hours at 37°C to the slow dimeric form, with acceleration at 41°C to give the appearance of the free latent band at 48 hours.
Nondenaturing PAGE electrophoreses. (A) Normal () antithrombin incubated at 50°C at pH7.4 (buffer A) and in 20% glycerol showing initial appearance at 12 hours of slow dimer band with complete conversion to latent form by 96 hours. A similar result but with more rapid change occurred under the same conditions in the absence of glycerol. L, latent control; + L, 1:1 mixture of and latent antithrombin. (B) A time sequence of incubation of the unstable antithrombin Wibble variant (50°C, pH 7.4, buffer B).10 Careful alignment shows how the transition to the latent form is preceded, in reciprocal proportions, by the formation of the slow dimeric component. Trace polymer bands are also present with the position of the loop-sheet dimer and trimer arrowed. (C) Isoelectric focusing of the stable slow electrophoretic component (lane 3), prepared as in (A), confirms its heterodimeric composition, with separation into equal bands of -antithrombin and latent antithrombin. Lanes 1 and 4, -antithrombin; lane 2, latent standard. (D) Mixing of antithrombins (left) and β (right) 1:1 with latent antithrombin immediately gives the single slow dimeric component that retains its electrophoretic integrity even after 7 days at room temperature. (E) Incubation of normal -antithrombin in buffer B (pH 7.4, 50 mmol/L KCl) showing conversion over 54 hours at 37°C to the slow dimeric form, with acceleration at 41°C to give the appearance of the free latent band at 48 hours.
The unidentified slow band was prepared as a single component (Fig 1A) by incubation of α-antithrombin at 50°C for 24 hours in the presence of 20% glycerol to minimize polymerization. Even after storage at room temperature, the incubated antithrombin maintained its characteristic single slow electrophoretic mobility. However, on isoelectric focusing electrophoresis (Fig 1C) and also on heparin-Sepharose affinity chromatography (Fig 2A), the single band split into 2 equal components with the characteristic mobilities of normal α-antithrombin and of latent antithrombin. The identity of each was confirmed by native PAGE electrophoresis and activity measurements. The deduction from these results that the slow band is a heterodimer of latent and α-antithrombin was confirmed by the mixing of equimolar proportions of latent and active α-antithrombin, as shown in Fig 1A. The rapidity of formation and stability of the heterodimeric band is shown in Fig 1D, with immediate formation of the dimer on mixing of latent with either α- or β-antithrombin and its subsequent stability as a single electrophoretic band, even after 7 days of standing at room temperature.
Heparin-Sepharose affinity chromatography. Three results are aligned but that in (C; adapted and reprinted with permission from Chang and Harper12) was performed using a different affinity heparin and consequently has noncomparable elution concentrations. (A) Chromatography of the slow component (lane 4, Fig1A) gives a result identical to that obtained with a crystal of the heterodimer,16 with separation into equivalent peaks of -antithrombin and latent antithrombin. (B) Elution profile of antithrombin from plasma incubated for 72 hours at 37°C. The first peak, including fraction 1, had no thrombin-inhibitory activity. (Reprinted with permission.12) (C) Previous larger scale heparin-Sepharose chromatography of a pasteurized commercial concentrate of antithrombin.12 The inactive L (latent) forms make up 40% of the total antithrombin, but the presence of additional peaks reinforces other unpublished evidence that the latent transition may also involve minor stable intermediate forms. All of these forms apparently dimerize, as shown by the single band with dimer mobility on electrophoresis of the concentrate (Fig 3CII).
Heparin-Sepharose affinity chromatography. Three results are aligned but that in (C; adapted and reprinted with permission from Chang and Harper12) was performed using a different affinity heparin and consequently has noncomparable elution concentrations. (A) Chromatography of the slow component (lane 4, Fig1A) gives a result identical to that obtained with a crystal of the heterodimer,16 with separation into equivalent peaks of -antithrombin and latent antithrombin. (B) Elution profile of antithrombin from plasma incubated for 72 hours at 37°C. The first peak, including fraction 1, had no thrombin-inhibitory activity. (Reprinted with permission.12) (C) Previous larger scale heparin-Sepharose chromatography of a pasteurized commercial concentrate of antithrombin.12 The inactive L (latent) forms make up 40% of the total antithrombin, but the presence of additional peaks reinforces other unpublished evidence that the latent transition may also involve minor stable intermediate forms. All of these forms apparently dimerize, as shown by the single band with dimer mobility on electrophoresis of the concentrate (Fig 3CII).
To demonstrate that significant conversion to the latent form could occur at physiological pH and temperatures, active α-antithrombin samples in pH 7.4 (50 mmol/L KCl) buffer were incubated at 37°C and 41°C for 54 hours (Fig 1E). The result at 37°C shows no presence of free latent antithrombin even after 54 hours, but clearly there has been a substantial transition to the slower dimeric form. At 41°C, the transition is more rapid, with complete conversion to the dimer by 24 hours and appearance of the free latent band at 48 hours.
Formation of dimer in plasma.
In a previous study,10 we had shown the presence of trace amounts of latent antithrombin in normal donor plasma. To demonstrate that the latent transition can occur in vivo, a donor plasma bag was incubated at 37°C for 72 hours. Measurement before and after incubation of a separate tube of plasma showed a 15% loss of thrombin inhibitory activity over the 72 hours. Antithrombin from the incubated bag, together with that from a matching unincubated control bag, was then isolated by standard heparin-Sepharose chromatography (Fig 2B). The first elution peak (fraction 1 in Fig 2B), which showed no inhibitory activity to thrombin and was known to cover the elution of both latent and reactive-loop cleaved antithrombin, was collected in each of the 72-hour and 0-hour plasma incubations. Native PAGE electrophoresis (Fig 3AI) showed a faint band with the mobility of latent or cleaved antithrombin in the unincubated sample, but a much denser band in the same position in the 72-hour incubated sample. Furthermore, in fraction 2, representing the overlap with the main α-antithrombin peak, the unincubated sample shows the presence of a band of α-antithrombin, whereas the 72-hour incubated sample shows a clear band in the position of the dimer. To determine the proportions of latent versus cleaved antithrombin in fraction 1, the fast-running band was eluted from each gel (Fig 3AI) and subjected to SDS-PAGE electrophoresis (Fig 3AII). This showed in each the presence of similar trace amounts of cleaved antithrombin, but a much denser band is present in the 72-hour sample in the position of intact antithrombin. The combination of these findings of an inactive component with increased mobility on native electrophoresis but normal mobility on SDS-PAGE electrophoresis confirms its identity as latent antithrombin.
(A) (I) Native-PAGE of aliquots of fractions 1, 2, and 3 (Fig 2B) from 0- and 72-hour incubations of plasma at 37°C. Bands with the characteristic fast mobility of both the latent and cleaved forms of antithrombin are seen faintly in the 0-hour sample and strongly in the 72-hour sample. (II) Western blot of SDS-PAGE of the eluted band 1 and an intact normal control () shows the predominant presence of intact antithrombin in the 72-hour sample with the presence of some cleaved (Cld) antithrombin at 0 and 72 hours. The combined results (I) and (II), together with the absence of inhibitory activity, confirm the identity of the latent component. (B) Western immunostaining of a native PAGE of plasma shows that normal plasma (P) has a major band that aligns with -antithrombin standards (). The addition of latent antithrombin (L) in near equimolar proportions to the control plasma (P + L) fails to show the presence of the free latent band, but results in a quantitative shift of the major band from the position to the characteristic slower mobility of the /latent heterodimer. (C) Commercial antithrombin concentrate. (I) SDS-PAGE confirms that the concentrate (conc) described in Chang and Harper12 has a single major component with the molecular weight of intact antithrombin. (II) Native PAGE illustrates how the electrophoretic mobility of the concentrate could be readily mistaken for that of active -antithrombin; alignment with an + L standard (with excess ) confirms that the concentrate is predominantly /latent dimer. (D) Native PAGE showing addition of increasing amounts of β-antithrombin (to 4.3 μg) to preformed -dimer (3 μg latent:3 μg , with incubation for 5 minutes) to give complete displacement of the -antithrombin with formation of the β-dimer (βd). The position of the -dimer (d) is indicated by the dotted arrow.
(A) (I) Native-PAGE of aliquots of fractions 1, 2, and 3 (Fig 2B) from 0- and 72-hour incubations of plasma at 37°C. Bands with the characteristic fast mobility of both the latent and cleaved forms of antithrombin are seen faintly in the 0-hour sample and strongly in the 72-hour sample. (II) Western blot of SDS-PAGE of the eluted band 1 and an intact normal control () shows the predominant presence of intact antithrombin in the 72-hour sample with the presence of some cleaved (Cld) antithrombin at 0 and 72 hours. The combined results (I) and (II), together with the absence of inhibitory activity, confirm the identity of the latent component. (B) Western immunostaining of a native PAGE of plasma shows that normal plasma (P) has a major band that aligns with -antithrombin standards (). The addition of latent antithrombin (L) in near equimolar proportions to the control plasma (P + L) fails to show the presence of the free latent band, but results in a quantitative shift of the major band from the position to the characteristic slower mobility of the /latent heterodimer. (C) Commercial antithrombin concentrate. (I) SDS-PAGE confirms that the concentrate (conc) described in Chang and Harper12 has a single major component with the molecular weight of intact antithrombin. (II) Native PAGE illustrates how the electrophoretic mobility of the concentrate could be readily mistaken for that of active -antithrombin; alignment with an + L standard (with excess ) confirms that the concentrate is predominantly /latent dimer. (D) Native PAGE showing addition of increasing amounts of β-antithrombin (to 4.3 μg) to preformed -dimer (3 μg latent:3 μg , with incubation for 5 minutes) to give complete displacement of the -antithrombin with formation of the β-dimer (βd). The position of the -dimer (d) is indicated by the dotted arrow.
To assess whether latent antithrombin would combine with active antithrombin in vivo, previously prepared latent antithrombin was added to a donor plasma sample at a concentration calculated to slightly exceed the concentration of the normal component. Native PAGE electrophoresis with Western blotting showed (Fig 3B) that this addition of latent antithrombin shifted the position of the major band from that of α-antithrombin to the slower mobility characteristic of the dimer.
Dimer in antithrombin concentrates.
Earlier studies had shown12 that an antithrombin concentrate commercially prepared in 1992 contained as much as 40% in the latent form (Fig 2C). To demonstrate how this concentration of latent antithrombin could have been masked by formation of the dimer, a sample of the concentrate was electrophoresed in SDS-PAGE (Fig 3CI) that showed a single band compatible with the presence of a sole component of intact antithrombin. Electrophoresis in native PAGE of the plasma concentrate (Fig 3CII) also showed just a single major band; however, the inclusion on the gel of a control of mixed α-antithrombin plus latent antithrombin shows that the concentrate moves on electrophoresis not as α-antithrombin but as its dimer with latent antithrombin. A check of the current concentrate from the same manufacturer showed on native PAGE a main band of α-antithrombin, but, in addition, a band, scanning at 36% of the total, in the position of the dimer. This implies a presence of 18% latent antithrombin.
Dissociability, β-antithrombin, heparin, and loss of activity.
Although the mixing of latent and normal antithrombin shows that they form an electrophoretically stable dimer, the separability of the component molecules on heparin-Sepharose chromatography and by isoelectric focusing show that the dimer is readily dissociable. To assess the degree to which the transition to the latent conformation of an abnormal antithrombin in a heterozygote may affect the inhibitory activity of normal antithrombin, the second order rate constants were determined for inhibition of thrombin and factor Xa before and after the addition of previously prepared latent antithrombin. The results for factor Xa are shown in Table 1, which also tabulates the percentage loss of activity of the active inhibitory component. On addition of equimolar proportions of latent antithrombin to active α-antithrombin, there is an 11% loss of activity, compatible with the ready dissociability of the dimer. An assessment was also made of the loss of activity of active β-antithrombin both on addition of equimolar proportions of latent antithrombin and at the 10:1 molar proportion representative of that in the plasma. As Table1 shows, there is a 21% loss of activity on the addition of latent antithrombin to an equimolar solution of β-antithrombin and a 47% loss when the proportion of latent to β-antithrombin is increased to 10:1. In each case, the loss of activity is considerably further increased in the presence of long-chain (unfractionated) heparin. In keeping with this increased affinity of association with β-antithrombin and hence decreased dissociation, Fig 3D confirms that latent antithrombin preferentially dimerizes with β-antithrombin, with the sequential addition of β-antithrombin giving a displacement of α-antithrombin from the preformed heterodimer.
DISCUSSION
The results shown here are an end-point in a 15-year study of the conformational changes that result in the dysfunction of antithrombin. When the study began,13 it was known that antithrombin, as with the serpins in general, had 2 conformational forms: 1 being the active inhibitor with an intact reactive center and the other being the inactive cleaved form.14 At that time, many of the studies were performed using antithrombin samples that had been stored for long periods or purchased from biochemical suppliers or derived from commercially fractionated plasma concentrates. Careful measurement of such samples almost invariably showed deficits in the range of 10% to 40% between the antigenic concentration of antithrombin and its activity. Yet, only a small proportion of the loss of activity could be shown to be due to cleavage of the antithrombin, so clearly some other inactive form was present. We believed that a contribution to this deficit in activity could come from a conformational transition of antithrombin to give an incorporation of its reactive center into the body of the molecule, analogous to the changes that occur in the cleaved inhibitor.15 The structural confirmation for this shift of an intact serpin from a 5- to a 6-stranded form was subsequently provided3 by latent PAI-1. That the same change could occur in antithrombin was further confirmed by later structural studies.2 5 These showed that antithrombin spontaneously crystallized as a dimer, with 1 molecule being in the 6-stranded latent form.
Subsequently, it was demonstrated12 that the large deficit in activity in some commercial concentrates of antithrombin was due to the presence of up to 40% of the antithrombin in the latent conformation (Fig 2C). This transition to the inactive latent form took place during the pasteurization of the plasma concentrates, and it was shown that similar conditions, with incubation at 60°C in the presence of citrate, could be used for the quantitative preparation of latent antithrombin.9 This latent form was found to have a clearly identifiable increase in electrophoretic mobility, as in lane 1 of Fig 1A. However, the perplexing feature was that this characteristically fast band could not be seen in solutions in which there was good reason to believe latent antithrombin would be present (eg, the mother liquor from which the dimer crystallized).16 Moreover, the plasma concentrates of antithrombin, as the manufacturers pointed out, showed on electrophoresis only a single band in a position compatible with that of normal α-antithrombin and no evidence of a band in the position of latent antithrombin (Fig 3C). The possibility that these and other similar anomalies were due to the formation of the active/latent heterodimer was considered. However, it was excluded because of the assumption that such a dimer would have the same slow-moving mobility as observed with the loop-sheet linked polymers17 that are known to form under the same conditions that produce the latent transition (see arrows in Fig 1B).
The failure to identify the dimer and hence the difficulty in demonstrating the presence of latent antithrombin in plasma except as a late in vitro occurrence led to the conclusion that the latent transition of antithrombin was of biochemical rather than biological significance. This conclusion, and the results on which it is based, must be revised in view of the findings here that latent antithrombin as soon as it is formed can dimerize with active antithrombin and will only become apparent as a free component when its concentration exceeds 50% of the total antithrombin. In particular, the demonstration that the dimerization can occur in vivo has direct implications for the mechanism of onset of thrombosis, particularly for heterozygous carriers of conformationally unstable variants.7,10,18 For our own part, the presence of undetected latent antithrombin in the samples used as starting material in the experiments of 10 years ago explains the otherwise puzzling discrepancies between results obtained then15 and those in subsequent experiments years later9 19 using freshly prepared material. Even with such freshly prepared or short-storage samples, evidence can now be seen of the appearance of latent antithrombin in its dimeric form (see starting sample in Fig 1E).
Recognition and characterization of dimer.
The formation of the dimer as a precursor to the appearance of free latent antithrombin is apparent on the prolonged incubation of antithrombin not only at raised temperatures (Fig 1A and B), but also at 37°C (Fig 1E). The isolated dimeric band can readily be dissociated into equivalent proportions of α and latent forms on isoelectric focussing (Fig 1C), and heparin-Sepharose chromatography (Fig 2A) gives a separation identical to that previously obtained on heparin-Sepharose chromatography of the dimeric crystals of antithrombin.16 The final and convincing evidence of the constitution of the dimer comes from the independent demonstration by our colleague Lei Jin2 that the mixing of equimolar proportions of previously prepared α-antithrombin and latent antithrombin leads to overnight crystallization of the dimer, with confirmation of the structure (as in Fig 4) by x-ray diffraction.
Ribbon depictions of current crystallographic structures of antithrombin showing the sequential conformational changes in antithrombin leading to the exacerbation of loss of inhibitory activity by dimerization. (A) Initiation by irreversible transition from 5-stranded (β-sheet A) active antithrombin (left), with insertion of the reactive site loop (red), to give the inactive 6-stranded latent form (right). The transition is accelerated at increased body temperature particularly in the presence of conformationally destabilizing mutations. Apposition of the 2 forms, as shown, results in the induction of β-strand conformation in the reactive site loop of the active inhibitory molecule with immediate linkage to the vacated strand in the C-sheet of the latent molecule. (B) The absence of a carbohydrate sidechain at Asn135 in β-antithrombin (β - - -) at the interface of the 2 molecules explains the preferential linkage by the latent molecule to the β-isoform. The radius of the negatively charged carbohydrate is indicated in red on the latent molecule. (C) The crystallographic (charge-contour) structure of the dimer complexed with the core pentasaccharide of heparin (green) demonstrates how full-length heparin (modelled yellow) can link the 2 molecules through a continuous cationic (blue) site to stabilize the dimer. Crystal structures shown are of dimers of latent and -antithrombin (PDB 1ant, 2ant) at 2.6Å and complexed with heparin pentasaccharide at 2.9Å (PDB 1aZx) plus the completed (but awaiting publication) structure of dimeric β-antithrombin at 2.6Å. (Figures were prepared by T.R. Dafforn, A.M. Lesk, and L. Jin, using MOLSCRIPT34 and GRASP.35 Figures are adapted and reprinted from Current Opinion in Structural Biology, Volume 8, R. W. Carrell and B. Gooptu, Conformational changes and disease—Including Serpins, Prions, and Alzheimer’s, page 799, Copyright 1998, with permission from Elsevier Science.27)
Ribbon depictions of current crystallographic structures of antithrombin showing the sequential conformational changes in antithrombin leading to the exacerbation of loss of inhibitory activity by dimerization. (A) Initiation by irreversible transition from 5-stranded (β-sheet A) active antithrombin (left), with insertion of the reactive site loop (red), to give the inactive 6-stranded latent form (right). The transition is accelerated at increased body temperature particularly in the presence of conformationally destabilizing mutations. Apposition of the 2 forms, as shown, results in the induction of β-strand conformation in the reactive site loop of the active inhibitory molecule with immediate linkage to the vacated strand in the C-sheet of the latent molecule. (B) The absence of a carbohydrate sidechain at Asn135 in β-antithrombin (β - - -) at the interface of the 2 molecules explains the preferential linkage by the latent molecule to the β-isoform. The radius of the negatively charged carbohydrate is indicated in red on the latent molecule. (C) The crystallographic (charge-contour) structure of the dimer complexed with the core pentasaccharide of heparin (green) demonstrates how full-length heparin (modelled yellow) can link the 2 molecules through a continuous cationic (blue) site to stabilize the dimer. Crystal structures shown are of dimers of latent and -antithrombin (PDB 1ant, 2ant) at 2.6Å and complexed with heparin pentasaccharide at 2.9Å (PDB 1aZx) plus the completed (but awaiting publication) structure of dimeric β-antithrombin at 2.6Å. (Figures were prepared by T.R. Dafforn, A.M. Lesk, and L. Jin, using MOLSCRIPT34 and GRASP.35 Figures are adapted and reprinted from Current Opinion in Structural Biology, Volume 8, R. W. Carrell and B. Gooptu, Conformational changes and disease—Including Serpins, Prions, and Alzheimer’s, page 799, Copyright 1998, with permission from Elsevier Science.27)
Latent transition and the dimer in vivo.
An indication that the latent transition could occur in vivo came from the studies of a conformationally unstable antithrombin variant (T85M). Bruce et al7 had previously performed incubations at 37°C and 41°C of both normal α-antithrombin and of another unstable variant (N187D) and demonstrated the appearance of free latent antithrombin as a late in vitro finding with the unstable variant but found no apparent latent antithrombin in the normal control. In retrospect, it can be seen that incubation at physiologic pH and at 37°C as well as 41°C results in a steady conversion of normal α-antithrombin to the dimeric band. This is shown here in a repeat incubation of normal α-antithrombin (Fig 1E) that closely parallels the results obtained by Bruce et al in 1994.7
To show that the transition to latent antithrombin could occur in vivo, antithrombin from donor plasma was isolated by heparin-Sepharose chromatography before and after incubation at 37°C for 72 hours. Incubation over this period resulted in a 15% loss of antithrombin activity with the appearance on heparin-Sepharose chromatography of a substantially increased inactive antithrombin component (fraction 1, Fig 2B) with the electrophoretic mobilities on SDS-PAGE and native PAGE of latent antithrombin (Fig 3A). The identity was further confirmed by electrophoresis of the overlapping fraction 2, which gave a predominant dimeric band (Fig 3AII). This electrophoresis also incidentally confirms the presence10 in the control unincubated plasma of a trace but significant band of latent antithrombin. All of these results taken together show that the latent conversion does occur in vivo, although with the likelihood of rapid elimination from the circulation of this intact 6-stranded molecular form, as also occurs with protease-complexed antithrombin.20
Evidence that latent antithrombin will form a heterodimer with active antithrombin in the plasma is shown by the mixing of latent antithrombin at near equimolar proportions with the active antithrombin in donor plasma. The consequence (Fig 3B) is a quantitative shift in the position of the main band of antithrombin from that of α-antithrombin to the slower mobility characteristic of the dimer. The shift in mobility is slight, and the failure to identify it has been at the cost of both experimentalists and processors of transfusion concentrates of antithrombin. The concentrate shown in Fig 3C is substantially a solution not of active antithrombin but of the heterodimer. As discussed below, even small amounts of latent antithrombin may have deleterious effects, and this unrecognized contamination, which is still present in some current concentrates, may have contributed to the inconsistent outcome of previous trials of antithrombin therapy.21-23
β-antithrombin, heparin, and the onset of thrombosis.
The realization that the spontaneous transition of antithrombin to the latent conformation could be greatly accelerated by small increases in body temperature explains the sudden onset of thrombosis, at times of infection, observed in families with conformationally unstable antithrombins. Nevertheless, there is still a discrepancy between the severity and nature of the thrombotic events associated with the conformational variants, as compared with those resulting from a simple genetic deficiency of antithrombin. Such genetic deficiency, with a 50% deficit in plasma antithrombin, results in an increased risk of thrombosis, but this is usually not life-threatening, particularly before full adult life. By contrast, the presence of a conformationally unstable antithrombin, with a much lesser deficiency of inhibitory activity, predisposes to atypically severe thromboses, as seen, during a bout of pneumonia, in a 10-year-old with ileo-femoral thrombosis from a family heterozygous for an unstable variant.10 This unusually severe pattern of thrombosis can only be partly explained by the loss of activity of the variant antithrombin. An additional contributory cause could be the formation of longer-chain polymers, but, although these are known to accompany the conformational transition of antithrombin (Fig 1B), there is no reason to believe that such polymers are in themselves thrombogenic. The finding with an unstable variant of nearly 10% of the plasma antithrombin in the latent form suggests the alternative possibility of additive inactivation, because the latent monomer links to a normal molecule to give the heterodimer. However, these dimers are readily dissociable (Figs 1C and 2A), and, as shown here, there is only an additional 11% loss of the inhibitory activity of the normal (α-antithrombin) component in the dimer (Table 1). Even with a complete transition of the variant antithrombin, the overall deficit in activity is not significantly greater than that of the common genetic deficiency. Clearly, something else is happening.
Comparisons of known structures of antithrombin suggested the likelihood that latent antithrombin will preferentially link to the minor β-isoform of antithrombin24 that lacks a carbohydrate sidechain situated close to the interface of the 2 molecules in the dimer (Fig 4B). Such preferential binding will have considerable functional significance, because, although this isoform constitutes only 5% to 10% of the total plasma antithrombin, the greater affinity of β-antithrombin for heparin25 binds it to the endothelial wall and makes it the primary inhibitor of thrombosis in the circulation.26 Confirmation that latent antithrombin does preferentially link to β-antithrombin is shown by the electrophoretic competition studies in Fig 3D. The tighter linkage of the latent form to β-antithrombin is further supported by inhibitory assays, with a doubled additive loss of activity by the dimer with β-antithrombin as compared with that with α-antithrombin (Table 1). Another recent crystallographic structure2 of the latent-active dimer shows each molecule complexed to a 5-saccharide heparin fragment. It is apparent from this structure (Fig 4C) that linkage of the 2 molecules in the dimer will be stabilized by the longer glycosaminoglycan templates present on the heparans lining the microcirculation, and this is supported by the substantially increased loss of inhibitory activity by the antithrombin dimer in the presence of long-chain heparin (Table 1). The potential physiological consequences of the combination of these effects is particularly significant with β-antithrombin, in which the likely in vivo ratio could result in a 65% loss of activity of the heparin-bound inhibitor (Table 1). This preferential loss of β-antithrombin function will significantly contribute to the severe clinical consequences of the latent transition, because it is the inhibitory activity of endothelial bound β-antithrombin, rather than that of the total circulating antithrombin, that is the critical factor in the prevention of thrombosis.26
Conclusion: Thrombosis and conformational disease.
The conversion of active antithrombin to the latent form is a slow process that in health is likely to only contribute to the natural senescence and turnover of the protein in the plasma. However, the acceleration of the latent transition at times of stress may be a contributory factor in the increased risk of the thrombosis that accompanies ill-health. The real threat is the fulminant transition of antithrombin that can occur in carriers of unstable variants, particularly during fever and ill health.7,10 Our study completes, in structural detail, the sequential changes that follow from this initial transition and that typify the conformational diseases in general.27 A feature of such conformational diseases is not just that they result from an aberrant change in folding of a protein, but even more so from the self-association that is a consequence of this misfolding. The striking finding here is the extraordinary selectivity of that association. When we added latent antithrombin to plasma, we fully expected it to form multiple associations with a variety of plasma proteins that could fill the vacant β-strand space in latent antithrombin (Fig 4A). But, as Fig 3B shows, linkage was almost wholly confined to that with active antithrombin. It is this remarkable favoring of self-association and the subsequent consequences of polymer and fibril formation that characterizes a diverse group of degenerative diseases, including Alzheimer’s dementia28 and the prion encephalopathies.29 There is good evidence that these conformational diseases are similarly initiated by the sequence of changes that parallel those shown in Fig 4: with a conformational transition and subsequent associative propagation,30 with selective involvement of glycoforms, and with stabilization of the formed complexes by heparin-like glycosaminoglycans.31-33
ACKNOWLEDGMENT
The authors thank Linda Butler for expert technical assistance and Arthur Lesk, Tim Dafforn, and Lei Jin for help in the preparation of the figures.
Supported by the Wellcome Trust, the British Heart Foundation, and the EC. J.A.H. has a National Institutes of Health training fellowship (HL 0992702).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Robin W. Carrell, PhD, FRCP, Structural Medicine, Cambridge Institute for Medical Research, Wellcome Trust/MRC Building, Hills Road, Cambridge CB2 2XY, UK.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal