Signals provided by the erythropoietin (Epo) receptor are essential for the development of red blood cells, and at least 15 distinct signaling factors are now known to assemble within activated Epo receptor complexes. Despite this intriguing complexity, recent investigations in cell lines and retrovirally transduced murine fetal liver cells suggest that most of these factors and signals may be functionally nonessential. To test this hypothesis in erythroid progenitor cells derived from adult tissues, a truncated Epo receptor chimera (EE372) was expressed in transgenic mice using a GATA-1 gene-derived vector, and its capacity to support colony-forming unit-erythroid proliferation and development was analyzed. Expression at physiological levels was confirmed in erythroid progenitor cells expanded ex vivo, and this EE372 chimera was observed to support mitogenesis and red blood cell development at wild-type efficiencies both independently and in synergy with c-Kit. In addition, the activity of this minimal chimera in supporting megakaryocyte development was tested and, remarkably, was observed to approximate that of the endogenous receptor for thrombopoietin. Thus, the box 1 and 2 cytoplasmic subdomains of the Epo receptor, together with a tyrosine 343 site (each retained within EE372), appear to provide all of the signals necessary for the development of committed progenitor cells within both the erythroid and megakaryocytic lineages.
HEMATOPOIESIS and hemostasis involve the continuous production of 6 major types of blood cells (erythrocytes, megakaryocytes, monocytes, granulocytes, B lymphocytes, and T lymphocytes) at tightly controlled rates. This is regulated in part through the differential expression and activation of lineage- and stage-specific hematopoietic growth factor (HGF) receptors of the type 1 superfamily.1 For example, lymphocyte production is known to depend on interleukin-7 (IL-7) receptor expression and signaling,2,3 whereas erythropoietin (Epo) receptor expression and activation is required for the development of erythroid progenitor cells beyond the colony-forming unit-erythroid (CFUe) stage.4 One key effect exerted by HGFs and their receptors is an inhibition of programmed cell death. This effect is well-illustrated in the IL-7 receptor system, in which transgenic expression of the survival factor Bcl-2 in IL-7 receptor α-deficient mice has been shown to rescue T-cell development.5,6 By comparison, certain HGFs appear to affect more than the survival of targeted lineage-restricted progenitor cells. Epo, the prime hormonal regulator of red blood cell production,7 provides one such example and, interestingly, neither Bcl-2 nor Bcl-xLexpression in erythroid progenitor cells is able to rescue red blood cell development in the absence of Epo signaling.8-10 Based on these considerations and on clinical significance,11significant efforts have focused on establishing the nature of key Epo receptor-induced signals that promote progenitor cell survival, growth, and/or differentiation.
One approach to dissecting Epo signaling has been to identify effectors that associate directly with activated wild-type Epo receptor complexes. For example, our laboratory has demonstrated that binding of Jak2 kinase to the Epo receptor box 1 domain and its rapid activation are generally important for signaling,12,13 whereas others have worked to establish the identity of a complex set of SH2 domain-encoding factors and associated cofactors that associate at phosphorylated tyrosine sites within the carboxyl-terminal region of the murine Epo receptor. This includes the commonly activated cytokine effectors Grb2/mSos/Raf/Ras,14 PI3 kinase,15and phospholipase C-γ (PLC-γ)16; the tyrosine phosphatases Syp17 and HCP18; the putative nucleotide exchange factors Vav19 and C3G (via the SH2 adaptor Cbl)20; the inositol phosphatase SHIP (via the adaptor Shc)21; Cis as an inhibitor of Jak2 kinase22; the latent signal transducers and activators of transcription STATs 5 a and b23,24; the Src family tyrosine kinase Lyn25; and at least 1 additional molecular adaptor, Gab1.26 However, recent studies of tyrosine point-mutated and truncated receptor forms in cell lines and fetal liver have provided provocative evidence that a membrane proximal cytoplasmic subdomain linked to a single phosphotyrosine (PY) site may be necessary and sufficient to support essentially all biological responses normally relayed via endogenous wild-type Epo receptors.27 Studies ex vivo using fetal liver cells are of particular interest. However, fetal liver is a short-term site of hematopoiesis28 and only transiently supports heightened nonhemostatic erythropoiesis.29 Thus, the extent to which fetal progenitor cells might correspond to permanent erythroid progenitor cells in adult marrow and spleen is unclear.28-33 For these reasons, and to quantitatively determine the extent to which signals derived from the above-delimited Epo receptor cytoplasmic subdomain support red blood cell development, we presently have used a GATA-1 gene-derived expression vector to express a minimal human epidermal growth factor (hEGF) receptor/murine Epo receptor chimera (EE372) in transgenic mice and have investigated the ability of this receptor construct to support proliferative signaling and red blood cell formation in adult erythroid tissues ex vivo. In addition, based on a direct structural relatedness between the receptors for Epo and thrombopoietin (Tpo)34 and upon the prediction that this EE372 transgene also should be expressed in megakaryocytic progenitor cells,35 the ability of this minimal chimera to support megakaryocyte development also has been investigated.
MATERIALS AND METHODS
Transgenic mice.
For expression in mice, a cDNA encoding a minimal hEGF receptor/murine Epo receptor chimera (EE372)8 was subcloned into a GATA-1 gene-derived vector that contains an upstream activating region, exons 1a and 1b, and the nontranslated region of exon 2.36 A 17-kb Kpn I-Cla I restriction fragment containing this linked cDNA construct was injected into pronuclei of fertilized eggs, and eggs were implanted into pseudo-pregnant BDF1 females. Transgene-positive founders were identified initially by polymerase chain reaction (PCR) using primers specific to the hEGF receptor (5′-TCC ATA CAG TGC CAC CCA GAG-3′) and murine Epo receptor (5′-AGC AGC CAC AGC TGG AAG TTA-3′). Southern blotting was performed on Bgl II digests of genomic DNA using a 770-bp Bgl II-Xba I fragment of a murine Epo receptor cDNA.37
Erythroid progenitor cell preparations and proliferation assays.
Enriched populations of erythroid progenitor cells were prepared from the spleens of mice treated subcutaneously with either thiamphenicol (TAP)38 or phenylhydrazine (PHZ)39 and from bone marrow. Briefly, TAP was administered as an implant on day 1 (14 g/kg), mice were phlebotomized on days 2 through 4, TAP was withdrawn on day 6, and splenocytes were prepared on day 9.5. PHZ was injected subcutaneously on days 1, 2, and 4 (50 mg/kg), and splenocytes were prepared on day 5. Mice were anesthetized with metofane (Schering-Plough, Union, NJ) during all procedures and prior to sacrifice. Disrupted spleen or bone marrow preparations were passed through a 70-μm cell strainer (Fisher, Pittsburgh, PA), collected, exposed for 4 minutes to a freshly combined solution of 50 mmol/L NH4Cl/phosphate-buffered saline (9:1) to lyse mature red blood cells (phosphate-buffered saline, 140 mmol/L NaCl, 0.27 mmol/L KCl, 0.43 mmol/L Na2HPO4, 0.14 mmol/L KH2PO4, pH 7.4), centrifuged through 50% fetal bovine serum in phosphate-buffered saline, washed, and adjusted to 1 × 107 cells/mL in 0.5% fetal bovine serum, OptiMEM 1 medium (GIBCO/BRL, Grand Island, NY) supplemented with penicillin (100 U/mL), streptomycin (100 ng/mL), and amphotericin B (250 ng/mL) (PSF). For assays of hEGF or Epo-stimulated proliferation, splenocytes (5 × 106 cells/mL in 10% fetal bovine serum, OptiMEM 1 medium supplemented with PSF) were exposed to cytokines and were cultured at 37°C, 7.5% CO2 for 48 hours. Rates of methyl [3H] thymidine incorporation then were determined as described.37
Flow cytometry.
Marrow cells from transgenic and normal mice were cultured ex vivo under conditions recently shown to selectively support the erythroid expansion of CD34+ cells.40 At day 3 of culture, cells were washed in phosphate-buffered saline and 0.5% bovine serum albumin and were incubated sequentially for 1 hour at 2°C with the Fc fragment of murine IgG (5 μg/mL; Pierce, Rockford, IL) and for 1.5 hours (in the presence of 0.02% NaN3) with a monoclonal antibody (EGFR.1; PharMingen, San Diego, CA) to the hEGF receptor extracellular domain (3.3 μg/mL). Cells (2 × 106 cells/sample) were then washed, incubated for 30 minutes at 2°C with a phycoerythrin-conjugated antibody specific to murine IgG F(ab′)2 (Jackson Research Labs, West Grove, PA), washed, and analyzed by flow cytometry (Coulter XL-MCL system; Coulter, Miami, FL). EE372 receptor density was estimated in a regression analysis by comparing fluorescence values from EE372-positive cells with values obtained for phycoerythrin-molecular equivalent beads (Spherotech, Libertyville, IL). Estimates accounted for a stoichiometry of 1 molecule of phycoerythrin per conjugated secondary antibody and the binding of 2 phycoerythrin antibodies per primary receptor-bound antibody.
Assays of erythroid and megakaryocyte colony formation.
In CFUe assays, splenocytes and bone marrow cells were prepared as described above and were cultured at 37°C, 7.5% CO2for 48 hours in Methocult HCC3242 media (Stem Cell Technologies, Vancouver, British Columbia, Canada) at 3 × 105 cells/mL. Cultures contained either Epo (5 U/mL) or hEGF (5 to 10 ng/mL) and, when specified, murine stem cell factor (50 ng/mL; Peprotech, Rocky Hill, NJ). Hemoglobin-positive colonies were stained with a freshly combined solution (1:1) of benzidine (3% in 90% glacial acetic acid) plus 5% H2O2. Megakaryocyte development from marrow progenitor cells was assayed using a serum-free MegaCult collagen system (Stem Cell Technologies). Cells were plated at both 1 × 105 and 3 × 105 cells/mL in the presence of murine IL-3 (50 ng/mL) and either hEGF (10 ng/mL) or Tpo (50 ng/mL). At day 7 of culture, cultures were dried, fixed, and stained for acetylcholinesterase-positive colonies.
RESULTS
Transgenic expression and proliferative activity of the minimal hEGF/Epo receptor chimera EE372 in erythroid progenitor cells.
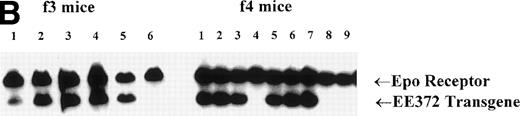
To test the ability of a minimal membrane-proximal cytoplasmic domain of the Epo receptor to support erythropoiesis in adult tissues, this domain was linked to the extracellular domain of the hEGF receptor and the resulting hEGF-activatable chimera was expressed in transgenic mice. In this construct (EE372; Fig 1A), cytoplasmic features of the murine Epo receptor include conserved box 1 and 2 motifs and a single (P)Y343 site that has been shown to bind STAT523 and to contribute significantly to growth41,42 and differentiation signaling8,27in cell line models. To provide for expression of this EE372 receptor form in erythroid progenitor cells, a GATA-1 gene-derived vector was used that recently has been shown to direct the expression of a β-galactosidase reporter gene in erythroid cells in vivo.43 Two EE372-positive founder mice were identified by PCR, and Southern blot analyses served to confirm the reliable transmission of this transgene at low copy number in mice derived from these 2 founders (Fig 1B, representative results shown for f3 and f4 generations).
The hEGF receptor (hEGF R)-murine Epo receptor (mEpo R) chimera, GATA-1 gene-derived transgenic expression vector, and integration of pG1-EE372 in transgenic mice. (A) Diagrammed are the wild-type (wt) murine Epo receptor and the minimal chimeric construct EE372. In EE372, the extracellular domain is that of the human EGF receptor, and the murine Epo receptor cytoplasmic domain is truncated to delete 7 of 8 sites of tyrosine (Y) phosphorylation. Also diagrammed is the GATA-1 gene-derived vector used to express EE372 in transgenic mice (pG1-EE372). uas, upstream activating sequence. pA, polyadenylation signal. (B) Southern blot analyses shown are for representative litters from f3 and f4 generations. Indexed areBgl II products from the endogenous Epo receptor gene and the pG1-EE372 transgene.
The hEGF receptor (hEGF R)-murine Epo receptor (mEpo R) chimera, GATA-1 gene-derived transgenic expression vector, and integration of pG1-EE372 in transgenic mice. (A) Diagrammed are the wild-type (wt) murine Epo receptor and the minimal chimeric construct EE372. In EE372, the extracellular domain is that of the human EGF receptor, and the murine Epo receptor cytoplasmic domain is truncated to delete 7 of 8 sites of tyrosine (Y) phosphorylation. Also diagrammed is the GATA-1 gene-derived vector used to express EE372 in transgenic mice (pG1-EE372). uas, upstream activating sequence. pA, polyadenylation signal. (B) Southern blot analyses shown are for representative litters from f3 and f4 generations. Indexed areBgl II products from the endogenous Epo receptor gene and the pG1-EE372 transgene.
The capacity of this minimal EE372 chimera to signal hEGF-induced proliferation in erythroid progenitor cells from adult mice was assayed in primary tests of function. To provide for an enriched population of erythroid cells, mice were treated with either TAP or PHZ under regimens shown in pilot experiments to maximize CFUe production in spleen. TAP in particular was used to generate a developmentally synchronized population of splenic CFUe while limiting the representation of megakaryocytic progenitor cells (see below). In [3H]dT incorporation assays, hEGF proved to support the proliferation of erythroid splenocytes from EE372 mice at rates that paralleled those induced by Epo via the endogenous wild-type Epo receptor (Fig 2). Data shown are for 3 transgenic mice (and nontransgenic control animals) and are representative of results obtained for 6 animals from 3 distinct generations at 5 to 20 weeks of age. These results confirmed the expression of functional EE372 receptors and indicated an essentially wild-type activation of proliferative response pathways. To directly confirm that EE372 receptors were expressed at high frequencies with low variegation (and to discount the possibility that observed proliferative response profiles might be accounted for by EE372 receptor overexpression within subpopulations), the density and distribution of EE372 transgene expression were assayed in erythroid progenitor cells expanded ex vivo. Marrow cells from transgenic and control mice were cultured under conditions recently shown to selectively support erythroid progenitor cell expansion from human CD34+ cells.40 At day 3 of culture, colony-forming assays showed that approximately 50% of expanded cells corresponded to CFUe (data not shown). For cells at this stage, an antibody to the human EGF receptor extracellular domain was used to assay EE372-positive cells (Fig 3). In cells expanded from transgenic mice, 47% of size-gated cells were positive for EE372 expression and mean densities of EE372 expression among gated cells approximated 5,000 receptors per cell (calculated as described in Materials and Methods). Thus, this EE372 transgene is expressed at high frequencies among erythroid progenitor cells at levels within the same order of magnitude as reported for endogenous Epo receptors.44
EE372 efficiently supports the ex vivo proliferation of adult splenic erythroid progenitor cells. Shown are the rates of hEGF- and Epo-stimulated [3H]dT incorporation for erythroid splenocytes from EE372 transgenic versus nontransgenic (wt, wild-type) control TAP-treated mice. In each panel, responses for 1 representative EE372 mouse and 1 nontransgenic mouse are presented. Values are means of duplicate samples expressed as a percentage of maximal [3H]dT-Incorporation induced by Epo. Standard error values are indicated by error bars for all data points. Values for maximal [3H]dT-incorporation induced by Epo for mice no. 37, 47, 50, 178, and 199 are 37.9 ± 3.5, 36.9 ± 4.6, 29.7 ± 2.0, 9.9 ± 1.44, and 41.2 ± 0.7 (×103 cpm), respectively. *Unavailable data points.
EE372 efficiently supports the ex vivo proliferation of adult splenic erythroid progenitor cells. Shown are the rates of hEGF- and Epo-stimulated [3H]dT incorporation for erythroid splenocytes from EE372 transgenic versus nontransgenic (wt, wild-type) control TAP-treated mice. In each panel, responses for 1 representative EE372 mouse and 1 nontransgenic mouse are presented. Values are means of duplicate samples expressed as a percentage of maximal [3H]dT-Incorporation induced by Epo. Standard error values are indicated by error bars for all data points. Values for maximal [3H]dT-incorporation induced by Epo for mice no. 37, 47, 50, 178, and 199 are 37.9 ± 3.5, 36.9 ± 4.6, 29.7 ± 2.0, 9.9 ± 1.44, and 41.2 ± 0.7 (×103 cpm), respectively. *Unavailable data points.
Expression of EE372 receptors on adult erythroid progenitor cells expanded ex vivo. (A) Using conditions developed by Panzenbock et al,40 erythroid progenitor cells were expanded from the marrow of transgenic (EE372) and control mice (wt, wild-type). EE372 expression was assayed using an antibody specific to the hEGF receptor extracellular domain. PE, phycoerythrin fluorescence intensity. FALS, forward angle light scatter. (B) Example estimate of EE372 receptor densities. Phycoerythrin molecular equivalent microbeads were used to generate a calibration profile (3,800, 12,000, 34,000, 124,000, and 300,000, reading left to right). This profile (and regression analyses) then were used to estimate EE372 receptor densities on marrow cells expanded ex vivo (see inset).
Expression of EE372 receptors on adult erythroid progenitor cells expanded ex vivo. (A) Using conditions developed by Panzenbock et al,40 erythroid progenitor cells were expanded from the marrow of transgenic (EE372) and control mice (wt, wild-type). EE372 expression was assayed using an antibody specific to the hEGF receptor extracellular domain. PE, phycoerythrin fluorescence intensity. FALS, forward angle light scatter. (B) Example estimate of EE372 receptor densities. Phycoerythrin molecular equivalent microbeads were used to generate a calibration profile (3,800, 12,000, 34,000, 124,000, and 300,000, reading left to right). This profile (and regression analyses) then were used to estimate EE372 receptor densities on marrow cells expanded ex vivo (see inset).
The minimal chimera EE372 promotes red blood cell development independently and in synergy with c-Kit.
Given the uniform expression of mitogenically competent chimeric receptors in EE372 mice described above, investigations next assessed the ability of the above EE372 receptor form to support red blood cell production. Specifically, the ability of hEGF to support the differentiation of CFUe within marrow and in splenocytes from TAP or PHZ treated EE372 mice was tested. For erythroid progenitor cells from each source and among all EE372 transgenic mice assayed, hEGF promoted red cell colony formation at efficiencies essentially equivalent to levels promoted by Epo at equimolar concentrations (ie, via endogenous Epo receptors; Table 1). Colony morphology (typically 16-cell hemoglobinized colonies) was indistinguishable from CFUe differentiated from control or transgenic mice in the presence of Epo (data not shown), and hEGF failed to support any detectable differentiation or outgrowth of CFUe from nontransgenic animals.
Wild-Type Activity of EE372 in Supporting CFUe Formation From Adult Erythroid Progenitor Cells
| Mouse . | CFUe Formation, % of Epo-Induced Colonies . | ||
|---|---|---|---|
| No Epo, No EGF . | Epo . | EGF . | |
| Nontransgenic no. 1 | 0 | 100 | 0 |
| Nontransgenic no. 2 | 0 | 100 | 0 |
| EE372 no. 45 | 0 | 100 | 100 |
| EE372 no. 161 | 0 | 100 | 103 |
| EE372 no. 178 | 0 | 100 | 111 |
| EE372 no. 194* | 0 | 100 | 89 |
| EE372 no. 181† | 10 | 100 | 80 |
| Mouse . | CFUe Formation, % of Epo-Induced Colonies . | ||
|---|---|---|---|
| No Epo, No EGF . | Epo . | EGF . | |
| Nontransgenic no. 1 | 0 | 100 | 0 |
| Nontransgenic no. 2 | 0 | 100 | 0 |
| EE372 no. 45 | 0 | 100 | 100 |
| EE372 no. 161 | 0 | 100 | 103 |
| EE372 no. 178 | 0 | 100 | 111 |
| EE372 no. 194* | 0 | 100 | 89 |
| EE372 no. 181† | 10 | 100 | 80 |
The ability of splenic erythroid progenitor cells from EE372 and nontransgenic control mice to form hemoglobinized colonies was assayed in the presence of either Epo (5 U/mL), hEGF (20 ng/mL), or no cytokines. Benzidine-positive colonies (CFUe) were scored at 48 hours of culture. Values are the means of duplicate samples expressed as the percentage of colonies induced in the Epo treatment groups. The mean values (± standard error) of Epo-induced colonies for mice no. 1, 2, 45, 161, 178, 194, and 181 are 10.5 ± 3.5, 34 ± 3.0, 13 ± 2, 19.5 ± 0.5, 28 ± 6.0, 8.7 ± 1.7, and 25 ± 13.7, respectively (n = 2 for all samples except no. 194, where n = 3).
Bone marrow preparation of erythroid progenitor cells.
hEGF included at 4 ng/mL.
The development of CFUe at high efficiencies is known to depend on coexposure to stem cell factor (SCF),27 and some evidence has been generated to suggest that SCF signaling via its receptor tyrosine kinase c-Kit may even depend on lateral signaling through Epo receptor complexes.45 Based on these considerations, whether the truncated chimera EE372 might act in synergy with SCF/c-Kit to support CFUe development also was tested. In control experiments, red cell formation due to Epo was enhanced markedly in the presence of SCF (Fig 4). Interestingly, this effect also was exerted at full efficiency for erythroid splenocytes from EE372 transgenic mice after their exposure to hEGF plus SCF (Fig 4). Thus, the membrane- proximal cytoplasmic subdomain of the Epo receptor efficiently mediates the synergistic effects of SCF/c-Kit on red blood cell development from adult erythroid progenitor cells ex vivo.
EE372 mediates hEGF-dependent CFUe development and synergizes with c-Kit. The ability of erythroid splenocytes from TAP-treated EE372 mice to form hemoglobinized colonies was assayed in the absence of cytokines or the presence of either Epo (5 U/mL), hEGF (20 ng/mL), SCF (50 ng/mL), Epo plus SCF, or hEGF plus SCF. Benzidine-positive colonies (CFUe) were scored at 48 hours of culture. Values are the means (± standard error) of 3 assays and are representative of 2 independent experiments.
EE372 mediates hEGF-dependent CFUe development and synergizes with c-Kit. The ability of erythroid splenocytes from TAP-treated EE372 mice to form hemoglobinized colonies was assayed in the absence of cytokines or the presence of either Epo (5 U/mL), hEGF (20 ng/mL), SCF (50 ng/mL), Epo plus SCF, or hEGF plus SCF. Benzidine-positive colonies (CFUe) were scored at 48 hours of culture. Values are the means (± standard error) of 3 assays and are representative of 2 independent experiments.
Megakaryocyte development is supported efficiently via the minimal Epo receptor chimera EE372.
Structurally, the single transmembrane receptor for thrombopoietin (Mpl) is related closely to the Epo receptor, and each is known to signal via Jak2 and to activate STAT5.34 In addition, substantial overlap exists between lineage-restricted transcription factors known to dictate commitment to megakaryocytic and erythroid lineages.35,46 This includes GATA-1, and the GATA-1 gene-derived vector used in these studies has been shown to direct expression in megakaryocytes.35 Based on these considerations and on the prediction that EE372 mice should also express this receptor chimera in megakaryocytic cells, the ability of the EE372 receptor to support the development of megakaryocytic colonies (colony-forming unit-megakaryocyte [CFU-meg]) was assayed. Remarkably, colony-forming assays in serum-free medium showed that CFU-meg development was supported efficiently by this minimal receptor form in response to hEGF at levels approximating that supported by Tpo (Fig 5A). Also, the morphology of acetylcholinesterase-positive megakaryocytic colonies was essentially indistinguishable from those of CFU-meg colonies cultured in the presence of Tpo (Fig 5B). Thus, the box 1 and 2 domains of the Epo receptor together with tyrosine 343 appear to provide all signals necessary to support both erythroid and megakaryocytic development.
EE372 mediates hEGF-dependent megakaryocyte colony formation. Bone marrow cells from adult nontransgenic control (wt, wild-type) and EE372 transgenic mice were used to test the ability of hEGF and EE372 to support CFU-meg colony formation. (A) Cultures were exposed to either hEGF (EGF, solid histograms, 20 ng/mL) or Tpo (open histograms, 50 ng/mL) in the presence of IL-3 (10 mg/mL). At day 7, acetylcholinesterase-positive CFU-meg colonies were scored (mean values ± standard error for n = 4 replicate assays). In this experiment, numbers of Tpo-induced colonies appear to be greater for the EE372 mouse. However, this effect was not observed in repeated independent analyses. (B) Shown are the similar morphologies of acetylcholinesterase-positive (brown) CFU-meg colonies propagated from EE372 mice in the presence of Tpo (top panel) or hEGF (lower panel).
EE372 mediates hEGF-dependent megakaryocyte colony formation. Bone marrow cells from adult nontransgenic control (wt, wild-type) and EE372 transgenic mice were used to test the ability of hEGF and EE372 to support CFU-meg colony formation. (A) Cultures were exposed to either hEGF (EGF, solid histograms, 20 ng/mL) or Tpo (open histograms, 50 ng/mL) in the presence of IL-3 (10 mg/mL). At day 7, acetylcholinesterase-positive CFU-meg colonies were scored (mean values ± standard error for n = 4 replicate assays). In this experiment, numbers of Tpo-induced colonies appear to be greater for the EE372 mouse. However, this effect was not observed in repeated independent analyses. (B) Shown are the similar morphologies of acetylcholinesterase-positive (brown) CFU-meg colonies propagated from EE372 mice in the presence of Tpo (top panel) or hEGF (lower panel).
DISCUSSION
Novel features of the present investigation that merit discussion include the following: (1) the nature of signals provided by the receptor form EE372 that support erythroid and megakaryocytic cell development from adult hematopoietic tissues and (2) advantages of this unique transgenic model for investigations of receptor-derived signals that promote CFUe and CFU-meg survival, proliferation, and terminal differentiation. With regards first to erythropoiesis, previous studies of the activities of carboxyl terminal-truncated and (P)Y-mutated Epo receptor forms in cell lines and fetal liver have provided compelling evidence that the minimal cytoplasmic subdomain contained in EE372 efficiently supports proliferation and differentiation signaling in these models.8,27,41,42 However, whether this receptor subdomain and derived signals might also support red blood cell development from CFUe in adult tissues has not been studied to date. This is an important issue, because fetal and adult erythropoiesis are known to differ in several notable ways. Direct evidence that Epo receptor activation mechanisms in fetal liver differ from those in adult tissues recently has been provided based on the ability of the gp55 protein of the anemia-inducing strain of Friend virus to promote Epo receptor-dependent erythropoiesis from fetal liver but not from marrow.30 In addition, progenitor cells in fetal liver are known to reconstitute hematopoiesis in irradiated mice at efficiencies higher than progenitor cells from marrow,31 and Epo produced by fetal liver per se may target stromal cells and affect their activity in supporting progenitor cell development.32In at least certain ways, programming of an erythroid fate also appears to differ in that ES cells nullizygous for the transcription factor PU.1 contribute to fetal liver but not adult marrow erythropoiesis in chimeric mice.33 Despite these differences, the present studies clearly show that, when expressed at levels comparable with the endogenous Epo receptor, the minimal Epo receptor cytoplasmic subdomain containing only the box 1 and box 2 domains and 1 Y343 site exhibits wild-type activity in promoting proliferation and hemoglobinization of erythroid progenitor cells derived from adult spleen and marrow.
The observed ability of the minimal Epo receptor chimera EE372 to also support megakaryopoiesis raises interesting questions concerning the nature of key signals that this and the endogenous receptors for Tpo and Epo transduce. In each, membrane proximal box 1 and 2 cytoplasmic domains are conserved37 and signaling depends critically on Jak2.47 However, in the Tpo receptor, tyrosine phosphorylation is limited to only 2 carboxyl terminal sites (ie, Y599, Y604 and Y626,Y631 in the murine and human receptors, respectively).47 In murine Mpl, Y599 and Y604 each appear to contribute to the activation of STAT5a/b and possibly STAT3.47 The Epo receptor and EE372 also selectively activates STAT5,23 and this signal transducer and activator of transcription therefore comprises 1 shared effector. Moreover, in the Tpo receptor, Epo receptor, and EE372, the selective uncoupling of STAT5 activation has been demonstrated to blunt proliferative and/or differentiation signaling in at least certain cell line models.8,27,41,42,48 However, in mice nullizygous for STAT5 a and b, erythropoiesis and megakaryopoiesis are essentially unperturbed.49 Thus, this latter result either is the consequence of compensatory mechanisms or no essential roles exist for STAT5a or b in these lineages. In the Tpo receptor system, Shc recently has emerged as an apparently important transducer and is recruited to PY599 of murine Mpl.50 Mutation of this site inactivates differentiation signaling in megakaryocytic F36P cells48 as well as in myeloid WEHI3B-D, M1, and 32D cells.51,52 Beyond this, Shc is known to be linked to Grb2/mSos/Raf/Ras and SHIP signaling,53 and Tpo-induced polyploidy and gpIIb/IIIa expression in F36P cells is inhibited by a dominant-negative form of c-H-Ras (S17N) and is stimulated by a dominant-active form (G12V).48 Thus, the intensity of Mpl and Shc-mediated activation of Ras has been suggested to regulate megakaryocyte development. In EE372, candidate PY sites for Shc recruitment are deleted. However, He et al54 previously have described an Epo receptor and Jak2-dependent pathway to Shc activation that efficiently proceeds in the absence of receptor tyrosine phosphorylation. Thus, Shc is proposed to comprise an attractive candidate effector of EE372, Epo, and Tpo receptor-mediated erythropoiesis and megakaryopoiesis. In addition, it is considered likely that a number of as yet undiscovered targets likely lie downstream of Jak2 per se, and it is proposed that certain of these also play important roles in Epo and Tpo response pathways. The observed ability of the minimal Epo receptor domain to support megakaryocyte formation in the absence of Tpo is inconsistent with an instructive model of Tpo signaling and is in keeping with studies by Stoffel et al55 in which the intact cytoplasmic domain of the granulocyte colony-stimulating factor receptor was shown to support megakaryopoiesis and platelet formation in Tpo receptor-deficient mice.
ACKNOWLEDGMENT
The authors thank Dr S.H. Orkin (Children’s Hospital, Boston, MA) for generously providing pA2GATA as a transgene expression vector, Dr T.L. Blankenship-Paris for expert veterinary advice, and Dr E. Kunze for directing flow cytometric analyses.
Supported by National Institutes of Health Grant No. DK40242 (D.M.W.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Don M. Wojchowski, PhD, 115 Henning Bldg, The Pennsylvania State University, University Park, PA 16802; e-mail:dmw1@psu.edu.


![Fig. 2. EE372 efficiently supports the ex vivo proliferation of adult splenic erythroid progenitor cells. Shown are the rates of hEGF- and Epo-stimulated [3H]dT incorporation for erythroid splenocytes from EE372 transgenic versus nontransgenic (wt, wild-type) control TAP-treated mice. In each panel, responses for 1 representative EE372 mouse and 1 nontransgenic mouse are presented. Values are means of duplicate samples expressed as a percentage of maximal [3H]dT-Incorporation induced by Epo. Standard error values are indicated by error bars for all data points. Values for maximal [3H]dT-incorporation induced by Epo for mice no. 37, 47, 50, 178, and 199 are 37.9 ± 3.5, 36.9 ± 4.6, 29.7 ± 2.0, 9.9 ± 1.44, and 41.2 ± 0.7 (×103 cpm), respectively. *Unavailable data points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3381.422k25_3381_3387/6/m_blod42225002x.jpeg?Expires=1763467141&Signature=Ald7Mbn02NOMDgChXDa9M64lH13SvnW2Qns8kIoP4zYovKclQebCL~2YhmfdEnAp7lgxTMrW52PlK34BumuxZwZoMguomAVgiTEMDj7qtAV6Zlm4~-7bCClUew7idjDXfbaT9829TOZT-xcOO~L7mVUJ0C8uPT1c87RnanJ7Hrl44ZRgP8~rRS7mVDCMDD01RJpj0SP0Px-KqnyYM9i3x~qZNht~wWDLzatVdyJRYgiVOI1uajF4e-V2q~ynmmxcRvb2CcOUQu4S3PCwuRyKBy02FvZNEaqejVgP3EqFmpiSWTLVSZ-pzMoPjmgMO4KO5AYR0eZnjB59ipUke5nEBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal