Glycoprotein V (GPV), a subunit of the platelet GPIb-V-IX receptor for von Willebrand factor and thrombin, is specifically found in platelets and mature megakaryocytes. Studies of the GPV gene can therefore provide insight into the mechanisms governing megakaryocyte differentiation. The human GPV promoter was isolated, and elements important for its tissue specific transcriptional activity were localized using systematic DNase I protection and reporter deletion assays. A −1413/+25 fragment inserted into a luciferase reporter construct displayed promoter activity in Dami and HEL but not in K562, HL60, or HeLa cells. Progressive 5′ to 3′ deletion showed a putative enhancer region in the −1413/−903 segment that contained closely spaced GATA and Ets sites protected from DNase I digestion in Dami extracts. Regions similar to a GPIIb gene repressor were found at −816 and −610, with the first exhibiting repressor activity in Dami and HEL cells and the second protected from DNAse I. Deletions from −362 to −103, an area containing protected sites for Sp1, STAT, and GATA, induced a progressive decrease in activity. The −103/+1 fragment, bearing a proximal Ets footprinted site and a GATA/Ets tandem footprint, displayed 75% activity relative to the full-length promoter and retained cell specificity. In summary, this work defines several regions of the GPV gene promoter important for its activity. It contains megakaryocyte-specific signals, including erythro-megakaryocytic GATA, and Ets cis-acting elements, GPIIb-like repressor domains, and binding sites for ubiquitous factors such as Sp1, ETF, and STAT.
A CHALLENGING QUESTION in hematopoiesis is how lineage-specific patterns of gene expression can arise from a common precursor cell. The problem appears to be even more complex in the case of megakaryocyte differentiation, because megakaryocytes and erythrocytes exhibit common features at different stages along the differentiation path. Thus, several transcription factors, such as GATA-1, Tal-1, Ets-1, and NF-E2, are detected in both lineages.1-4 In this context, how can a similar subset of transcription factors differentially influence cell maturation into megakaryocytes or erythrocytes?
Strong evidence has been obtained for the implication of GATA-1 in regulation of the transcription of many megakaryocytic genes.5-9 GATA-1 is also important in vivo and in vitro to direct the differentiation of definitive hematopoietic progenitors along either the erythroid or the megakaryocytic pathway. Overexpression of GATA-1 led to megakaryocytic differentiation of the primitive myeloid cell line 416B, whereas no erythroid or mast cell differentiation was observed. Levels of GATA-2 and GATA-3 transcripts in these cells remained undetectable, which would suggest that GATA-2 and GATA-3 lie upstream of GATA-1. Moreover, enforced expression of GATA-2 or GATA-3 in 416B cells also induced megakaryocytic differentiation, suggesting that GATA-1 is a dominant regulator of maturation to this lineage.10
The original knock-out experiments did not show any direct link between GATA-1 and megakaryocytic differentiation.11,12GATA-1−/− mice present severe anemia with a total arrest of erythroid differentiation at the proerythroblastic stage, and these precursors later die by apoptosis.13,14Megakaryocyte-specific GATA-1 knock-out mice have been obtained more recently. These mice have reduced platelet numbers, in association with a deregulated proliferation of megakaryocytes and a block in their terminal cytoplasmic maturation.15 In addition, mice with disruption of GATA-1 express normal amounts of GPIIb and MPL (the thrombopoietin receptor) together with about 50-fold higher levels of GATA-2, which would suggest that GATA-2 is compensating for the loss of GATA-1 and that GATA-1 negatively regulates GATA-2 during normal erythroid maturation.16
Because the GATA-1 gene is located on the X chromosome, which is randomly inactivated in every cell, heterozygous females can bear either a wild-type or a mutant GATA-1 allele and consequently display variable anemia or thrombocytopenia. Such heterozygous mutant mice have a phenotype analogous to that of human myelodysplastic syndrome. There is marked splenomegaly, anemia, and thrombocytopenia; proerythroblasts and megakaryocytes accumulate massively in the spleen; and the animals begin to die after 5 months. These findings suggest that the hematopoietic progenitor cells start to proliferate but are unable to terminally differentiate, which leads to progenitor proliferation in the spleen and eventual death.17
Although binding sites for the transcription factor NF-E2 are present in the promoters of many erythroid genes,18,19 inactivation of the p45 NF-E2 gene has only a mild effect on erythrocytic differentiation. Conversely, this totally inhibits the terminal differentiation of megakaryocytes by blocking proplatelet formation20,21 and polyploidization,22resulting in severe thrombocytopenia. PU.1 and Fli-1, two transcription factor members of the Ets family, also play a role in erythroid and megakaryocytic differentiation. PU.1 has been identified in mature megakaryocytes and binding sites for this factor found in megakaryocytic genes like those of glycoprotein IIb (GPIIb)23 and β thromboglobulin (βTG).24 Overexpression of PU.1 in mice induced erythroleukemia through blockage of erythroid differentiation,25 and the overexpression of PU.1 on these murine erythroleukemia cells reduced their NF-E2 expression and the DNA-binding activity of GATA-1.26 Similarly, overexpression of Fli-1 in K562 cells induced a megakaryocytic phenotype characterized by markers such as GPIIb.27 Fli-1 has further been shown to transactivate the GPIbα and GPIX genes.28
In summary, GATA-1 blocks erythroid differentiation in erythroblasts and induces the hyperproliferation of megakaryocytic progenitors. Other factors, such as NF-E2, PU.1, and Fli-1, also have distinct effects on erythrocyte and megakaryocyte precursors, suggesting that the same factor could play different roles depending on its cellular context.
A traditional approach to identifying the mechanisms involved in megakaryocytopoiesis is to examine the transcriptional control of megakaryocyte-specific genes in various differentiated cell types. At the functional level, the promoters most studied to date are those of GPIIb7,29-31 and platelet factor 4 (PF4).32,33 The MPL34 and βTG24 35 promoters have also been analyzed in functional studies.
The GPIb-V-IX complex is a receptor necessary for the von Willebrand factor-dependent adhesion of platelets to the subendothelium of damaged blood vessels.36,37 Absence or defective expression of this complex leads to a severe hemorrhagic disorder known as Bernard-Soulier syndrome. GPIb-V-IX is composed of 4 subunits: GPIbα (145 kD) is disulfide-linked to GPIbβ (28 kD) to form GPIb, which is, in turn, noncovalently bound to GPIX (22 kD) and GPV (82 kD) in, respectively, 1:1 and 2:1 stoichiometry.37,38 All 4 proteins are members of the leucine-rich glycoprotein (LRG) family of adhesive receptors.39 GPIbα contains binding sites for von Willebrand factor and thrombin.40-42 The exact functions of GPIbβ and GPIX are still unknown, whereas GPV contains a cleavage site for thrombin, but its role in platelet physiology is likewise unclear.
The GPIbα,5 GPIX,8,43 and GPV genes are restricted in their expression to the platelet lineage and appear late in megakaryocytopoiesis.44 Cloning and characterization of the GPV gene45 have shown it to comprise a short intron in the 5′ untranslated region and a coding sequence contained entirely in the second exon. The 5′-flanking region contains several putative cis-acting regulatory elements, including consensus binding sites for GATA and Ets transcription factors, as in megakaryocyte-specific genes,29,46-48 and for ubiquitous factors such as Sp1. The rat and mouse genes are identical in structure and have conserved many of the putative human regulatory sequences.49
In the present study, we chose to undertake a functional characterization of the human GPV gene promoter for the following reasons. First, GPV associates in 1:2 stoichiometry with GPIb-IX and is absent or found at low levels in most Bernard-Soulier patients, suggesting a possible coordinated control of transcription. Second, GPV appears late in megakaryocyte differentiation (Lepage et al, manuscript submitted), unlike, for instance, the early GPIIb and MPL genes, which are present in CD34+CD38+hematopoietic progenitors.50 51 GPV is also absent from a majority of megakaryocytic leukemic cell lines, unlike other platelet-specific proteins such as PF4, GPIIb, or even GPIb-IX. This suggests that GPV could participate in later stages of megakaryocyte maturation and respond to late appearing growth factors or transcription factors. Characterization of the GPV promoter could thus help to identify transcriptional elements involved in the coordinated expression of GPIb-V-IX and in modulating the timing of megakaryocyte maturation.
MATERIALS AND METHODS
Cell lines.
Cell lines (American Type Culture Collection, Rockville, MD) were as follows: HeLa (epithelioid carcinoma), Raji (Burkitt’s lymphoma), K562 (chronic myelogenous leukemia), HL60 (promyelocytic leukemia), HEL (erythroleukemia), and Dami. The Dami cell line originally described as a megakaryoblastic leukemia52 has now been recognized as an HEL derivative.53 All cell lines were grown in RPMI 1640 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (Boehringer, Mannheim, Germany), 2 mmol/L glutamine, and 100 U/mL penicillin and streptomycin (GIBCO BRL).
Flow cytometry and antibodies.
Cells were washed in phosphate-buffered saline containing 1% bovine serum albumin (PBS-BSA) and resuspended in PBS-BSA at a final concentration of 5 × 105/mL. Aliquots of cells (5 × 104 per tube) were incubated for 20 minutes at 4°C with a primary antibody (20 μg/mL), washed in PBS-BSA, resuspended in the same buffer, and labeled for 20 minutes at 4°C in the dark with fluorescein isothiocyanate-conjugated goat F(ab′)2 antimouse IgG (FITC-GAM; 15 μg/mL; Jackson ImmunoResearch Laboratories Inc, West Grove, PA). After further washing in PBS-BSA, the samples resuspended in the same buffer were analyzed on a Becton Dickinson FACSort flow cytometer with CellQuest software (Becton Dickinson, Mountain View, CA).
The monoclonal antibody (MoAb) 5B12, which recognizes the αIIbβ3 integrin (GPIIb-IIIa), was obtained from DAKO (Glostrup, Denmark). Murine MoAbs against GPIbα (ALMA.12), GPIX (ALMA.16), and GPV (V.1) were produced in our laboratory,54 whereas the mouse MoAb Gi 27 recognizing GPIbβ was provided by S. Santoso (Justus-Liebig University, Giessen, Germany).
GPV mRNA analysis.
Total RNA was extracted from cell lines by the thiocyanate-guanidium method.55 Cells (107) were lysed in 1 mL Tri Pure (Boehringer) and extracted into 0.2 mL chloroform, and the extracts were centrifuged at 12,000g for 15 minutes at 4°C. RNA was precipitated from the aqueous phase with isopropanol, and the pellet was washed in 70% (vol/vol) cold ethanol.
Reverse transcription was performed on 100 ng total RNA using a specific P1 primer in the noncoding orientation (5′-TAT CAG GTC ACT GAA GGT GCC GGG GGC AA-3′ from nt 2715 to nt 2687; GPV numbering throughout the manuscript according to Lanza et al,45 EMBL/Genbank: HSGPV-Z23091). Oligonucleotides were synthesized in an Oligo 1000 synthesizer (Beckmann, Fullerton, CA). The reaction was performed in 30 μL polymerase chain reaction (PCR) buffer, pH 8.3, containing 1 U reverse transcriptase (Moloney murine leukemia virus [MMLV]-RT; GIBCO BRL), 0.1 μmol/L P1 primer, and 1.25 μmol/L of each dNTP for 30 minutes at 37°C. PCR amplification was performed in the same tube by adding 0.4 μmol/L each of P1 and a coding P2 primer (P2: 5′-AGT TAC TTT GGA GTG CAG AAC CAT TTC-3′ from nt 1433 to nt 1459) and 1 U Taq polymerase (Perkin Elmer Cetus, Norwalk, CT) and cycling in a DNA Thermal Cycler (Perkin Elmer Cetus). After denaturation for 2 minutes at 94°C, 30 cycles of 1 minute at 94°C, 2 minutes at 55°C, and 2 minutes at 72°C were followed by a final extension for 7 minutes at 72°C. The 324-bp PCR product was separated by electrophoresis on a 2% agarose gel and visualized by ethidium bromide staining and UV illumination. Contamination through the PCR amplification of genomic DNA would give a 1,282-bp fragment due to the presence of the 958 nt intron.
Nuclear extracts and DNase I protection assays.
Nuclear extracts were prepared from HeLa, Raji, K562, HL60, HEL, and Dami cells by the modified method of Dignam et al.56 All steps were performed at 4°C. The cells (0.5 to 1 × 107) were rinsed twice in PBS, pelleted by centrifugation at 1,850g for 10 minutes, resuspended in 5 times the pellet volume of hypotonic buffer (10 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.2 mmol/L phenylmethylsulfonyl fluoride [PMSF], and 0.5 mmol/L dithiothreitol [DTT]) and allowed to stand for 10 minutes. The resulting sediment was resuspended in twice the volume of hypotonic buffer and centrifuged at 1,850g. After discarding the supernatant, the nuclei pellet was resuspended in half the volume of cold low salt buffer (20 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 20 mmol/L KCl, 0.2 mmol/L EDTA, 25% [vol/vol] glycerol, 0.2 mmol/L PMSF, and 0.5 mmol/L DTT), and the same volume of cold high salt buffer (low salt buffer containing 1.2 mol/L KCl) was added slowly. This extract was gently homogenized for 30 minutes and then clarified for 30 minutes at 25,000g. After dialysis against 500 mL storage buffer (20 mmol/L HEPES, pH 7.9, 20% [vol/vol] glycerol, 100 mmol/L KCl, 0.2 mmol/L EDTA, 0.2 mmol/L PMSF, and 0.5 mmol/L DTT) for 5 hours, the dialysate was centrifuged at 25,000g for 20 minutes and the precipitate was discarded. The supernatant containing the nuclear extract was frozen in aliquots and stored at −80°C.
Fragments of GPV promoter for DNase I protection assays were obtained by PCR. PCR amplification was performed in an Expand High Fidelity PCR System (Boehringer) in a 100 μL reaction volume with 200 μmol/L of each dNTP, 0.4 μmol/L of the reverse and forward primers, 1.5 mmol/L MgCl2, 2 μL cDNA, and 2.6 U of a mix containing Taq and Pwo DNA polymerases. PCR conditions were as described above, except for the annealing temperature, which ranged from 52°C to 59°C, depending on the primer pair. The primer pairs were as follows: fragment I, FP1S (5′-GGA ACT GAA AGA TCT CCC GCG ATA C-3′, nt 6-30) and FP1AS (5′-TGA TGA ACT CGA GCA CCT TTT CAC AT-3′, nt 408-383); fragment II, FP2S (5′-ATA TCT TCT GAG ATC TAG CCT TTG TCA-3′, nt 163-189) and FP2AS (5′-GCC GCT CAC ATC TCG AGT TTA ACT GGG G-3′, nt 600-573); fragment III, FP3S (5′-TTT TGC CTA TAG ATC TAT GGG CAA AAG-3′, nt 314-340) and FP3AS (5′-TCA AGC AAT TCT CGA GCC TCG GCC TC-3′, nt 800-775); fragment IV, FP4S (5′-AGA AGC ACA GGA GAT CTC ACA AAT GGC A-3′, nt 498-524) and FP4AS (5′-TCT CAA ATC CTC GAG TTT TTT CTT CC-3′, nt 992-967); fragment V, FP5S (5′-GGT AAA ACC CCA GAT CTA CTA AAA TAC AA-3′, nt 698-726) and FP5AS (5′-GTG ATA CAG CTC GAG CAC TGC AGT AG-3′, nt 1174-1149); fragment VI, FP6S (5′-CAA GTT CAA AGA TCT TCT TAG CCT TA-3′, nt 929-954) and FP6AS (5′-AGG TCC CTA TCT CGA GCC CTT GTT CT-3′, nt 1332-1306); and fragment VII, FP7S (5′-ATG AAA AGG AAG ATC TAG GGG AAG TG-3′, nt 1203-1128) and FP7AS (5′-GTT CTG CAC TCG AGA GTA ACT GAA A-3′, nt 1453-1429). The fragments were cloned in the pCR2.1 vector using a TA Cloning Kit (InVitrogen, Leek, The Netherlands). After digestion withEcoRI, the fragments were dephosphorylated, purified, labeled with [γ32P] ATP and T4 polynucleotide kinase (Boehringer), digested on the 3′ side, and repurified. Footprinting studies were performed essentially as described by Ohlsson and Edlung.57 Briefly, nuclear extracts (50 μg) were incubated for 20 minutes at room temperature with 2 μg poly(dIdC).poly(dIdC) in a final volume of 50 μL HEPES buffer (25 mmol/L HEPES, pH 7.8, 50 mmol/L KCl, 0.05 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, and 5% [vol/vol] glycerol). A 5′-end-labeled DNA fragment (2 ng, 30,000 cpm) was added, and the mixture was incubated for 40 minutes at room temperature and then digested for 1 minute on ice with 1 to 3 U of DNase I (RQ1; Promega, Madison, WI) in the presence of 5 mmol/L MgCl2. The enzyme was inactived by incubation for 20 minutes at 37°C with 100 μL Tris buffer (100 mmol/L Tris-HCl, pH 7.8, 100 mmol/L NaCl, 1% sodium dodecyl sulfate [SDS], and 10 mmol/L EDTA) containing 100 μg/mL proteinase K and 25 μg/mL tRNA. After phenol/chloroform extraction and ethanol precipitation, the one-end-labeled DNA fragments were separated on denaturing 8% polyacrylamide gels. The gels were dried and autoradiographed under an intensifying screen.
Plasmid constructs, cell transfection, and gene reporter assays.
An approximately 1.5-kb length of the GPV gene flanking region was amplified by PCR with the primers C1 (5′-GAAGAT CTT CCC GCG ATA CCT GGC AGA GGC AGT GGC-3′, nt 20-48) and C2 (5′-GAA GAT CTT CTG CAC TCC AAA GTA ACT GAA AGA CT-3′, nt 1451-1425), which carry two Bgl II sites (underlined). This segment was subcloned into the Bgl II site of the pGL3-basic plasmid (Promega), which contains the firefly luciferase gene and the polyadenylation signal from SV40, to generate the plasmid pGL3/−1413. A series of pGL3/−1413-deleted constructs was obtained by unidirectional 5′ to 3′ progressive digestion of pGL3/−1413 with exonuclease III/ mungbean (Promega) and religation with T4 DNA ligase (Promega).
Activity of the −103/+25 region was studied further by mutating the GATA-71/Ets-66 domain. PCR amplification using the pGL3/−1413 plasmid as a template was used to generate 4 constructs with mutation of GATA-71 (GATA-71 mut), deletion of GATA-71 (GATA-71 Δ), mutation of Ets-66 (Ets-66 mut), and double mutation of GATA-71 and Ets-66 (tandem mut). The different constructs were obtained using antisense primer M1 (5′-GCT TAC TTA GAT CGC AGA TCT AAT GGT TCT GC-3′, nt 1457-1447 of GPV promoter attached to nt 36-56 of pGL3-basic) and 1 of the following sense primers. The “TTATCC” GATA-71 site was mutated to “CCGTCC” using primer M2 (5′-GGG GTA CCT ACT CTG GTA AAG TCT CCG TCC TCA GGA TGC AAG G-3′, nt 1339-1376). The first 3 bases of the “TTATCC” GATA-71 site were deleted using primer M3 (5′-GGG GTA CCT ACT CTG GTA AAG TCT TCC TCA GGA TGC AAG G-3′, nt 1339-1376). The “CAGGATGC” Ets-66 site was mutated to “CAAAGTGC” using primer M4 (5′-GGG GTA CCA AAG TCT TTA TCC TCA AAG TGC AAG G-3′, nt 1351-1376). The “TTATCCTCAGGATGC” GATA/Ets tandem was mutated to “CCGTCCTCAAAGTGC” using primer M5 (5′-GGG GTA CCA AAG TCT TTA TCC TCA AAG TGC AAG G-3′, nt 1351-1376). Primers M2-M5 and primer M1 carriedKpn I and Bgl II sites, respectively (underlined), to allow cloning in the pGL3-basic vector creating a −94/+25 or −82/+25 construct ahead of the luciferase gene.
All constructs were sequenced to check the exact boundaries and ensure that no errors had occurred during the procedure. The pRL-TK plasmid (Promega) expressing sea pansy luciferase under the control of the thymidine kinase promoter was used as an internal standard, and the pGL3/SV40 plasmid containing the firefly luciferase gene driven by the SV40 promoter was used as a control of transcription efficiency.
Cells were transfected by electroporation using a CellJect apparatus (Eurogentec, Seraing, Belgium) at 340 V, ∞ Ω, and 1,500 μF. Assays were performed with 20 μg GPV promoter construct, 5 μg pRL-TK, and 5 μg herring sperm DNA per 8 × 106cells in a total volume of 800 μL. The cells were harvested 48 hours after transfection and cell extracts were obtained by addition of LucLite from the FireLite kit (Packard, Meriden, CT). Firefly luciferase activity was measured in a BCL Book luminometer (Promega) and initially expressed in arbitrary units. Sea pansy luciferase activity was then measured in the same luminometer after the addition of RenLite substrate. The firefly luciferase activity was normalized against the sea pansy luciferase activity to correct for variable transfection efficiencies among different samples.
Primer extension.
Total RNA was extracted from Dami cells and platelets as described for mRNA analyses and poly(A)+ mRNA was purified with a commercial kit (Pharmacia, Uppsala, Sweden). The PE1 oligonucleotide (5′-AGC ACC GCG CAC AGT AGA GTC-3′, nt 2453-2433) was labeled with [γ32P] using a 5′-end-labeling kit (Amersham, Uppsala, Sweden). This 5′-end-labeled synthetic oligonucleotide was annealed to total or poly(A)+ RNA and extended with MMLV reverse transcriptase according to the manufacturer’s instructions (Superscript II plus kit; InVitrogen). The reaction products were analyzed by electrophoresis on denaturing 6% polyacrylamide gels.
Reverse transcriptase-PCR (RT-PCR) mapping.
RT-PCR assays were performed in a Titan RT-PCR System (Boehringer) using total RNA from Dami cells and poly(A)+ mRNA from platelets. Briefly, 100 ng RNA was incubated in 50 μL RT-PCR buffer containing 0.2 mmol/L of each dNTP; 0.3 μmol/L of reverse primer PM4; 0.3 μmol/L of direct primer PM1, PM2, or PM3; 5 mmol/L DTT; 5 U RNase inhibitor (Boehringer); 1.5 mmol/L MgCl2; 1 U avian myeloblastosis virus (AMV)-reverse transcriptase; and 1 U Expand High Fidelity enzyme blend (Taq and Pwo DNA polymerases) at 50°C for 30 minutes. The primers were PM1 (5′-AGT TAC TTT GGA GTG CAG-3′, nt 1433-1450), PM2 (5′-CAT GCA GAG CTC TAA GTC-3′, nt 1411-1428), PM3 (5′-GAT ACC ACC CTC TTC CTG-3′, nt 1376-1393), and PM4 (5′-GTG CGT GAG GTT GGT GGG-3′, nt 2583-2566). PCR amplification was performed in the same vial on a DNA Thermal Cycler as described above, with annealing at 52°C for PM1+PM4 and PM2+PM4 or at 54°C for PM3+PM4.
RESULTS
Dami cells are GPV-positive megakaryocytic cells.
To study GPV promoter activity, cell lines with constitutive (Dami, HEL, Meg-01, and CHRF-288) or inducible (K562) megakaryocytic features or negative cells (HL60) were screened for GPV expression by FACS analysis or for GPV mRNA expression by RT-PCR.
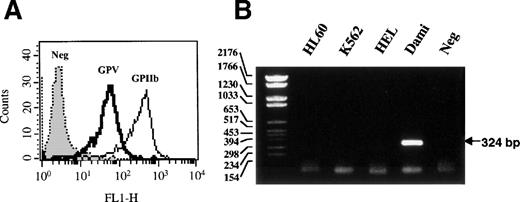
GPV was detected only on the surface of Dami cells (Fig 1A), together with the GPIb-IX subunits (data not shown). GPV was absent from the surface of HEL, Meg-01, K562, and HL60 cells (data not shown), although the first 2 cell lines are positive for the αIIbβ3integrin.58 59 Similarly, GPV mRNA was absent from HEL, K562, and HL60 cells when these cells were analyzed by RT-PCR and was only shown in Dami cells (Fig 1B). Dami was therefore used as the reference megakaryocytic cell line expressing GPV in further studies of GPV promoter function.
Expression of GPV in Dami cells. (A) Dami cells were analyzed by flow cytometry using MoAbs against GPV (V.1) or the GPIIb-IIIa complex (ALMA.17) and FITC-conjugated goat antimouse IgG. Histograms are representative of 5,000 cells. Neg corresponds to isotype-matched negative control. (B) RT-PCR analyses were performed on total RNA from HL60, K562, HEL, and Dami cells. The GPV PCR products were identified by 2% agarose gel electrophoresis and ethidium bromide staining and neg corresponds to RT-PCR without reverse transcriptase.
Expression of GPV in Dami cells. (A) Dami cells were analyzed by flow cytometry using MoAbs against GPV (V.1) or the GPIIb-IIIa complex (ALMA.17) and FITC-conjugated goat antimouse IgG. Histograms are representative of 5,000 cells. Neg corresponds to isotype-matched negative control. (B) RT-PCR analyses were performed on total RNA from HL60, K562, HEL, and Dami cells. The GPV PCR products were identified by 2% agarose gel electrophoresis and ethidium bromide staining and neg corresponds to RT-PCR without reverse transcriptase.
Identification of the GPV transcription start site.
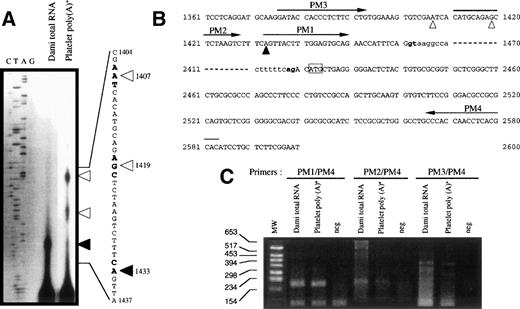
To determine the exact 5′-end of GPV mRNA, we isolated poly(A)+ RNA from platelets and Dami cells and performed primer extension analyses (Fig 2A). In both cell types, comparison with the reported genomic sequence led us to assign a major transcription start site to nt 1433, located 29 nt upstream of the intron donor splice site. Additional longer extension products matching positions 1419 and 1407 were found in platelets, consistent with the frequent observation of multiple start sites in TATA-less genes.5 42
Identification of the transcription start site of the human GPV gene. (A) Primer extension mapping of the 5′-ends of GPV transcripts from Dami cells and platelets (numbering according to Lanza et al45). (Bold arrowhead) start site common to platelet and Dami RNA; (open arrowheads) additional upstream sites in platelet RNA. (B) Positions of the primers used for PCR mapping are indicated by arrows: PM1 (nt 1433 to 1450), PM2 (nt 1411 to 1428), PM3 (nt 1376 to 1393), and PM4 (nt 2583 to 2566). The intron sequence is in lowercase characters and the GPV intron donor and acceptor splice sites are in bold characters. (C) RT-PCR mapping of the 5′-ends of GPV transcripts. The primer pairs defined in (B) were tested on Dami total RNA and platelet poly(A)+ RNA and neg corresponds to RT-PCR without reverse transcriptase. PCR products were identified by 2% agarose gel electrophoresis and ethidium bromide staining.
Identification of the transcription start site of the human GPV gene. (A) Primer extension mapping of the 5′-ends of GPV transcripts from Dami cells and platelets (numbering according to Lanza et al45). (Bold arrowhead) start site common to platelet and Dami RNA; (open arrowheads) additional upstream sites in platelet RNA. (B) Positions of the primers used for PCR mapping are indicated by arrows: PM1 (nt 1433 to 1450), PM2 (nt 1411 to 1428), PM3 (nt 1376 to 1393), and PM4 (nt 2583 to 2566). The intron sequence is in lowercase characters and the GPV intron donor and acceptor splice sites are in bold characters. (C) RT-PCR mapping of the 5′-ends of GPV transcripts. The primer pairs defined in (B) were tested on Dami total RNA and platelet poly(A)+ RNA and neg corresponds to RT-PCR without reverse transcriptase. PCR products were identified by 2% agarose gel electrophoresis and ethidium bromide staining.
The start site at position 1433 was confirmed by RT-PCR mapping of this region (Fig 2B) using the direct primers PM1 (nt 1433-1450), PM2 (nt 1411-1428), and PM3 (nt 1376-1393) and the reverse primer PM4 (nt 2583-2566). PM1/PM4 amplified a 193-bp fragment in accordance with a transcription start site at position 1433 (Fig 2C) from Dami cells and platelets. PM2/PM4 amplified the expected 215-bp band with less efficiency and specificity, whereas no band was detected in most PM3/PM4 assays. These results strongly suggest that platelet GPV mRNA has 31 nucleotides of 5′-untranslated sequence and that the first exon is 29-bp long. The GPV promoter region was numbered accordingly (Fig 3B).
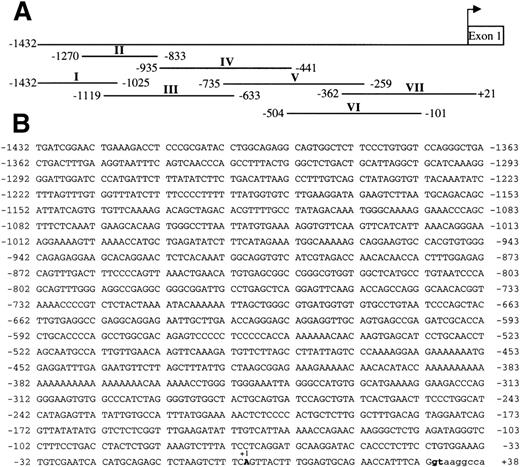
Sequence of the GPV gene promoter and DNA fragments of the 5′-flanking sequence used for DNase I protection assays. The GPV promoter was numbered from an arbitrary start site common to Dami cells and platelets, which conforms to the consensus start of platelet TATA-less gene (Table 2). (A) Alignment of the fragments I to VII with the −1432/+21 GPV 5′-flanking segment. The fragments were checked by sequencing before 5′-end-labeling and use in DNase I protection assays. Footprinting analyses of III, IV, VI, and VII are reported in Figs 4 through 7. (B) Sequence of the GPV promoter. The transcription start site is denoted +1, the intron sequence is in lowercase characters, and the intron donor splice site is in bold characters.
Sequence of the GPV gene promoter and DNA fragments of the 5′-flanking sequence used for DNase I protection assays. The GPV promoter was numbered from an arbitrary start site common to Dami cells and platelets, which conforms to the consensus start of platelet TATA-less gene (Table 2). (A) Alignment of the fragments I to VII with the −1432/+21 GPV 5′-flanking segment. The fragments were checked by sequencing before 5′-end-labeling and use in DNase I protection assays. Footprinting analyses of III, IV, VI, and VII are reported in Figs 4 through 7. (B) Sequence of the GPV promoter. The transcription start site is denoted +1, the intron sequence is in lowercase characters, and the intron donor splice site is in bold characters.
This major transcription initiation region is 100% conserved between human, mouse, and rat GPV49(Table 1), matches the consensus sequence for initiation found in the mouse terminal transferase gene, and is highly homologous to sites present in other megakaryocyte genes, such as the αIIb29 and α260 integrin, human PF4 and PF4alt,32 rat PF4,61βTG,35 and GPIX42 genes. These regions correspond to the consensus initiator site for promoters lacking TATA and CAAT boxes.62
Comparison of the Transcription Start Sites of Platelet-Specific Genes With the Human GPV Transcription Start Site Common to Dami Cells and Platelets
| Py | Py | Py | Py | −1 | +1 | Py | Py | Py | Py | |
| Tdt | C | C | C | T | C | A | T | T | C | T |
| αIIb integrin | G | G | C | C | C | A | T | T | C | C |
| α2 integrin | C | T | C | T | C | A | C | C | G | G |
| GPIX | A | T | C | C | C | A | T | A | G | A |
| PF4 human | G | A | G | T | C | A | T | T | G | G |
| PF4alt human | G | A | G | T | C | A | C | T | G | C |
| PF4 rat | G | A | G | C | C | A | C | T | G | T |
| C | T | T | T | C | A | G | T | T | A | |
| GPV rat | C | T | T | T | C | A | G | T | C | A |
| GPV mouse | C | T | T | T | C | A | G | T | C | A |
| βTG | A | A | G | C | C | A | C | T | T | A |
| Py | Py | Py | Py | −1 | +1 | Py | Py | Py | Py | |
| Tdt | C | C | C | T | C | A | T | T | C | T |
| αIIb integrin | G | G | C | C | C | A | T | T | C | C |
| α2 integrin | C | T | C | T | C | A | C | C | G | G |
| GPIX | A | T | C | C | C | A | T | A | G | A |
| PF4 human | G | A | G | T | C | A | T | T | G | G |
| PF4alt human | G | A | G | T | C | A | C | T | G | C |
| PF4 rat | G | A | G | C | C | A | C | T | G | T |
| C | T | T | T | C | A | G | T | T | A | |
| GPV rat | C | T | T | T | C | A | G | T | C | A |
| GPV mouse | C | T | T | T | C | A | G | T | C | A |
| βTG | A | A | G | C | C | A | C | T | T | A |
Bold characters represent nucleotides corresponding to the consensus sequence PyPyPyPyCAPyPyPyPy of the TdT gene.
Identification of multiple binding sites for nuclear factors on a 1,500-bp promoter fragment by DNase I footprinting.
Seven overlapping fragments (I to VII; Fig 3A) covering the −1432 to +21 5′-flanking region were generated by PCR amplification and blunt-end cloned in the pCR2.1 vector. The fragments were excised withEcoRI, 5′-end labeled with [32P], and cut with selected restriction enzymes to generate one-strand-labeled fragments. These DNA segments were analyzed on both strands for DNase I protection using nuclear extracts from megakaryocytic (Dami and HEL) and nonmegakaryocytic (K562, HeLa, Raji, and HL60) cell lines.
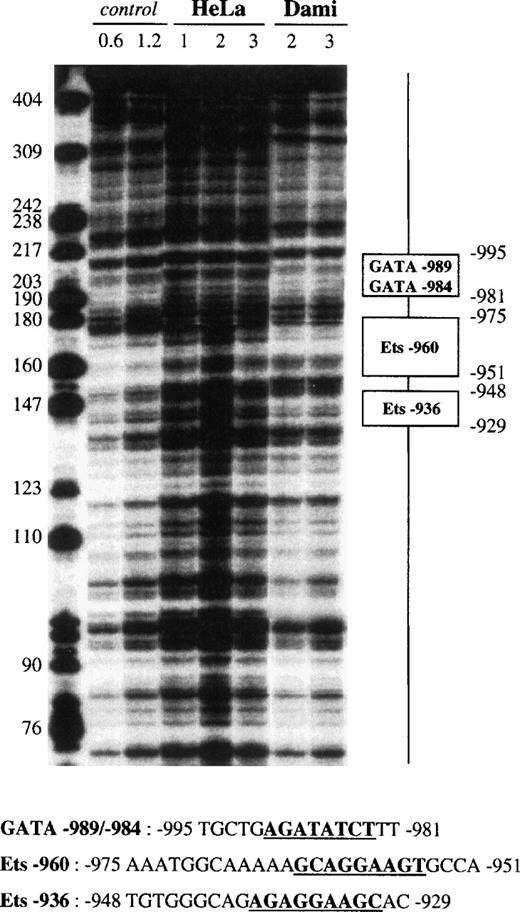
No protected region was detected on fragment I (−1432 to −1025). Fragment II (−1270 to −833) contained 3 protected sites (data not shown) that were confirmed on fragment III (−1119 to −633; Fig 4). One 15-bp footprint centered at −989 was specifically protected from nucleolytic attack when tested with Dami cell extracts. This domain contained 2 inverted sequences, named GATA-989 and GATA-984, that resemble the consensus GATA box63: 5′-WGATAR-3′ (W = A or T; R = A or G). A second 25-bp footprint, centered at −962, contained the 5′-CAGGAAGT-3′ motif, whereas a third 20-bp domain was centered at −938 and contained the 5′-AGAGGAAGC-3′ motif. These 2 regions, named Ets-960 and Ets-936, match the consensus sequence for an Ets box64: 5′-RSMGGAWRYY-3′ (R = A or G; S = C or G; M = A or C; W = A or T; Y = C or T) and were protected by Dami (Fig 4), Raji, HL60, K562, and HEL extracts (data not shown), but not by HeLa extracts.
DNase I footprint analysis of the GPV promoter fragment III using Dami and HeLa cell extracts. The 5′-end-labeled fragment III (nt −1119 to −633) was incubated with 50 μg HeLa or Dami nuclear extract in the presence of 2 μg poly(dIdC).poly(dIdC) and digested with 1, 2, or 3 U DNase I. Control corresponds to digestion of III with 0.6 or 1.2 U DNase I in the absence of nuclear extract. Digestion products were separated on an 8% acrylamide sequencing gel in the presence of 8 mol/L urea, and bands were compared with those of a DNA molecular weight marker (Hpa II digest of pBR-322). The protected regions are indicated on the right and named according to their homology with known transcription factor binding sites (“GATA” and “Ets” for putative binding sites for GATA and Ets transcription factors). The corresponding protected nucleotide sequences are indicated in the lower part of the figure and the consensus binding sites are underlined and in bold characters.
DNase I footprint analysis of the GPV promoter fragment III using Dami and HeLa cell extracts. The 5′-end-labeled fragment III (nt −1119 to −633) was incubated with 50 μg HeLa or Dami nuclear extract in the presence of 2 μg poly(dIdC).poly(dIdC) and digested with 1, 2, or 3 U DNase I. Control corresponds to digestion of III with 0.6 or 1.2 U DNase I in the absence of nuclear extract. Digestion products were separated on an 8% acrylamide sequencing gel in the presence of 8 mol/L urea, and bands were compared with those of a DNA molecular weight marker (Hpa II digest of pBR-322). The protected regions are indicated on the right and named according to their homology with known transcription factor binding sites (“GATA” and “Ets” for putative binding sites for GATA and Ets transcription factors). The corresponding protected nucleotide sequences are indicated in the lower part of the figure and the consensus binding sites are underlined and in bold characters.
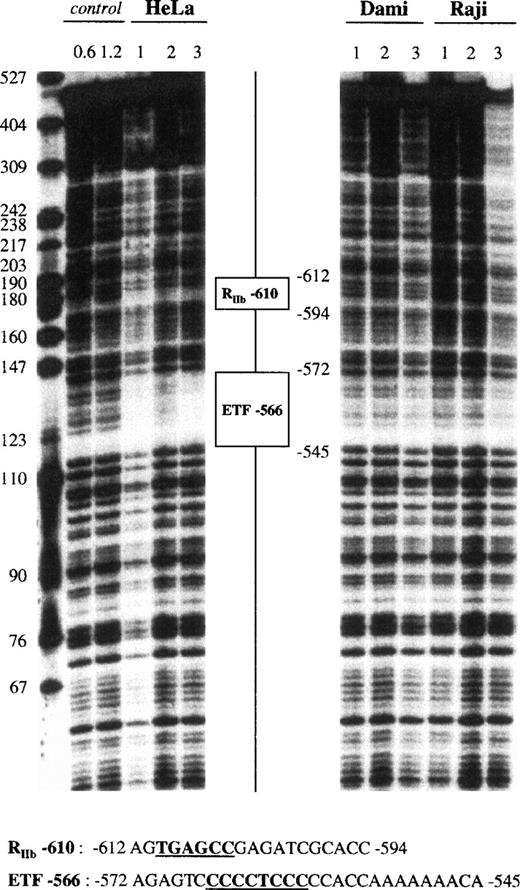
Fragment IV (−935 to −441) showed 2 footprinted areas (Fig 5). The first, a 19-bp sequence centered at −604, was protected only by HeLa extracts. It contained the 5′-TGAGCC-3′ motif, which has been named RIIb-610 due to its resemblance to the 5′-TGAGTCC-3′ motif in the 5′ sequence of the GPIIb gene (−120), which is known to exhibit repressor activity.31 A second 28-bp domain was centered at −560 and displayed no cell specificity, because it was protected by all of the nuclear extracts tested. This site, named ETF-566, contained a polypyrimidine 5′-CCCCTCCC-3′ motif matching the consensus binding site for ETF,65 a member of the Sp1 family. Fragment V (−735 to −259) contained 2 footprints already identified in fragment IV as RIIb-610 and ETF-566 (data not shown).
DNase I footprint analysis of the GPV promoter fragment IV using HeLa, Dami, and Raji cell extracts. The 5′-end-labeled fragment IV (nt −935 to −441) was analyzed as described in the legend to Fig 4, using HeLa, Dami, and Raji nuclear extracts. “RIIb” is a putative repressor binding site with homology to a repressor site in the GPIIb promoter,31whereas “ETF” is a putative binding site for ETF, a member of the Sp1 family.65
DNase I footprint analysis of the GPV promoter fragment IV using HeLa, Dami, and Raji cell extracts. The 5′-end-labeled fragment IV (nt −935 to −441) was analyzed as described in the legend to Fig 4, using HeLa, Dami, and Raji nuclear extracts. “RIIb” is a putative repressor binding site with homology to a repressor site in the GPIIb promoter,31whereas “ETF” is a putative binding site for ETF, a member of the Sp1 family.65
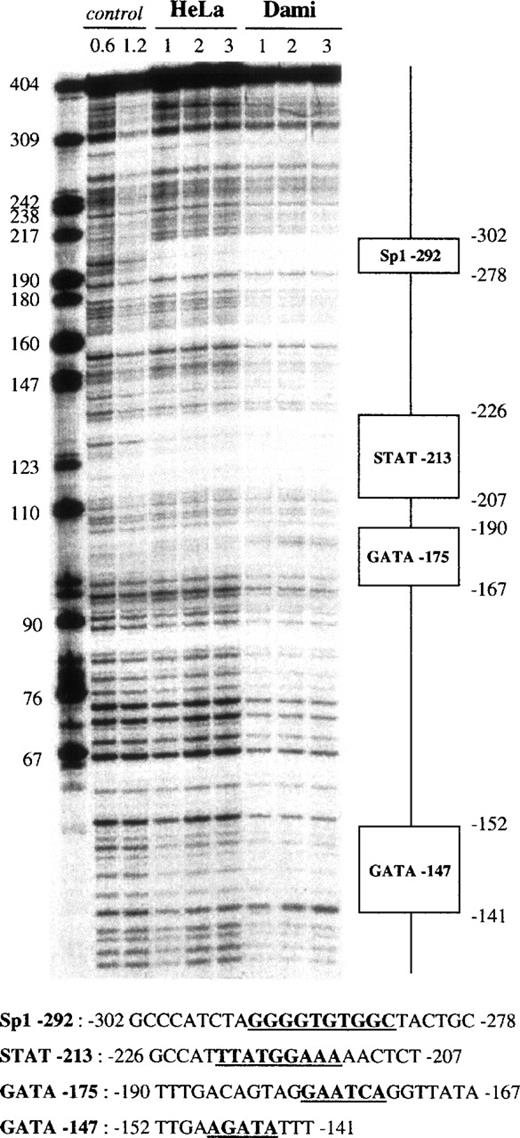
Fragment VI (−504 to −101) displayed 4 protected regions (Fig 6). The first 25-bp footprint, which was centered at −290, was protected by all of our nuclear extracts. It contained a 5′-GGGGTGTGGC-3′ sequence resembling an Sp1 binding site and was named Sp1-292. A second 20-bp domain, which was centered at −216 and named STAT-213, was protected by all nuclear extracts and contained a STAT-like 5′-TTATGGAAA-3′ motif (STAT consensus motif66: 5′-TTNNNGNAA-3′). The third 25-bp region was centered at −177 and contained the 5′-AATCA-3′ motif that corresponds to an inverted GATA site. It was named GATA-175, presented an apparent DNAse hypersensitive site shown in Fig 6, and was protected only by Dami extracts (Fig 7). A fourth 10-bp domain, named GATA-147, also matched a GATA binding site but was less cell specific, because it was protected by HeLa, Dami, Raji, and HL60 extracts.
DNase I footprint analysis of the GPV promoter fragment VI using Dami and HeLa cell extracts. The 5′-end-labeled fragment VI (nt −504 to −101) was analyzed as described in the legend to Fig4, using HeLa and Dami nuclear extracts. “STAT” is a putative binding site for a transcription factor of the STAT family.66
DNase I footprint analysis of the GPV promoter fragment VI using Dami and HeLa cell extracts. The 5′-end-labeled fragment VI (nt −504 to −101) was analyzed as described in the legend to Fig4, using HeLa and Dami nuclear extracts. “STAT” is a putative binding site for a transcription factor of the STAT family.66
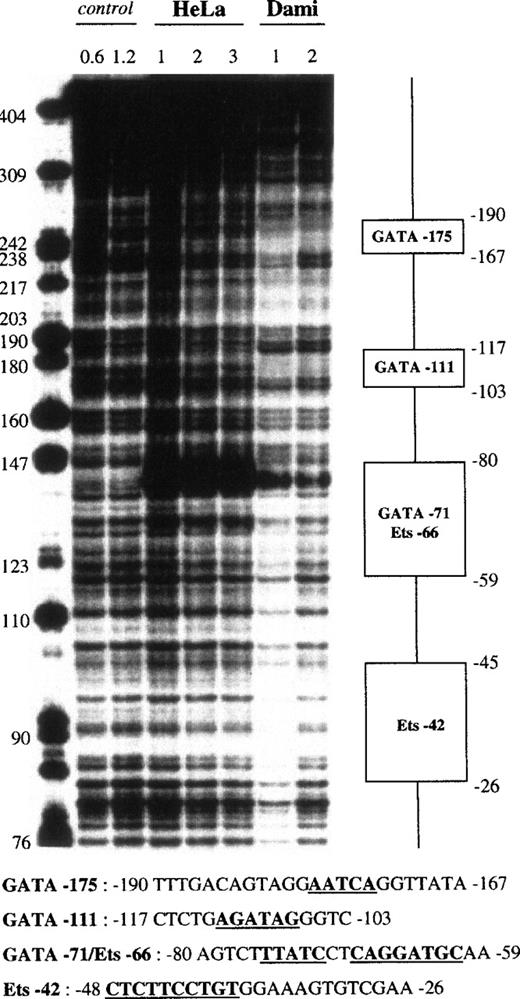
DNase I footprint analysis of the GPV promoter fragment VII using Dami and HeLa cell extracts. The 5′-end-labeled fragment VII (nt −362 to +28) was analyzed as described in the legend to Fig 4, using HeLa and Dami nuclear extracts.
DNase I footprint analysis of the GPV promoter fragment VII using Dami and HeLa cell extracts. The 5′-end-labeled fragment VII (nt −362 to +28) was analyzed as described in the legend to Fig 4, using HeLa and Dami nuclear extracts.
The proximal fragment VII (−362 to +28) also contained 4 protected areas (Fig 7), with the first corresponding to GATA-175. A second 15-bp domain, which was centered at −111 and named GATA-111, contained the 5′-AGATAG-3′ motif and was protected only by Dami extracts. The third region differed depending on the strand analyzed. In the coding strand, a 15-bp sequence centered at −68 was protected by Dami and HeLa extracts, as shown by a strong DNAse hypersensitive site in Dami and HeLa nuclear extracts, whereas the 27-bp footprint centered at −68 in the noncoding strand was protected by all of our nuclear extracts. This domain combined 2 motifs, an inverted GATA sequence (5′-TTATC-3′) and an Ets site (5′-CAGGATGC-3′), which were named GATA-71 and Ets-66, respectively. The last 20-bp region, named Ets-42, was only detected on the plus strand, where it was centered at −40, and was evident when using the lowest (1 U) DNase I concentration. It contained the 5′-CTTCCTGT-3′ motif, a putative Ets binding site.
In summary, this systematic DNase I scanning of the −1432 to +21 5′-region of the GPV promoter showed a total of 14 protected domains. Six of these corresponded to GATA and 4 corresponded to Ets binding sites, and several sites displayed putative megakaryocyte-restricted binding properties (Table 2). A number of regions proved to be highly conserved between human, mouse, and rat GPV. Regions RIIb-610 and ETF-566, present on the human Alu sequence, were, however, absent from the rat and mouse genes due to lack of the Alu repeat in these 2 species.
Cell Specificity of DNase I-Protected Regions of the Human GPV Promoter
| Dami . | Hematopoietic Cells . | HeLa . | Ubiquitous . |
|---|---|---|---|
| GATA-989/-984 | Ets-960 | RIIb-610 | ETF-566 |
| GATA-175 | Ets-936 | ||
| (+strand) | |||
| GATA-147 | |||
| Dami . | Hematopoietic Cells . | HeLa . | Ubiquitous . |
|---|---|---|---|
| GATA-989/-984 | Ets-960 | RIIb-610 | ETF-566 |
| GATA-175 | Ets-936 | ||
| (+strand) | |||
| GATA-147 | |||
The DNase I-protected regions were classified as sites protected only by Dami extracts, by extracts from a range of hematopoietic cells (megakaryocytic, erythrocytic, myeloid), or by HeLa extracts or ubiquitous footprints. Sites conserved between human, mouse, and rat GPV are underlined.
Localization of positive and negative regulatory regions using reporter gene deletion constructs.
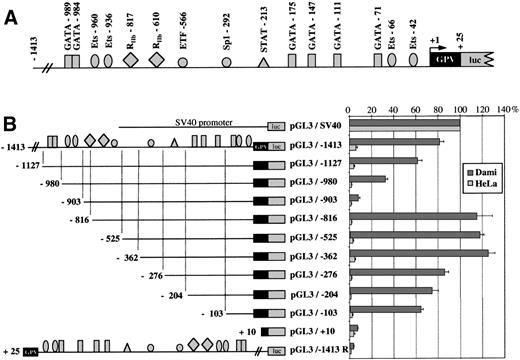
To determine its promoter strength, the −1413 to +25 region of the human GPV gene was inserted into the pGL3-basic vector upstream of the luciferase reporter gene. This construct (pGL3/−1413; Fig 8A) was transfected into the GPV-positive megakaryocytic Dami and the GPV-negative HeLa cell lines. pGL3/−1413 displayed significant promoter activity amounting to 81% ± 4% of the control SV40 activity in Dami cells but was inactive in HeLa cells (Fig 8B). The same region inserted in the inverse orientation gave a completely inactive construct (pGL3/−1413R).
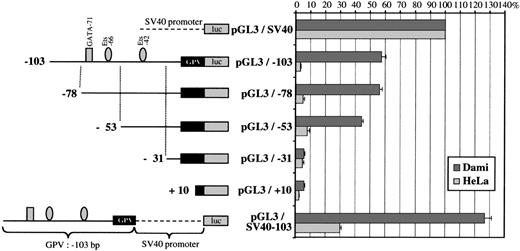
Analysis of GPV gene reporter/Exonuclease III deletion constructs after transient transfection in Dami and HeLa cells. (A) Schematic representation of the −1413 to +25 5′-flanking region of the human GPV gene linked to the luciferase reporter gene (luc) in the pGL3 vector. Binding sites for transcription factors, functionally identified by DNase I footprinting or detected by sequence analysis in the case of RIIb-817, are indicated by the symbols: (▪) GATA, () Ets, (•) Sp1 family, (▴) STAT, and (⧫) sites homologous to the GPIIb repressor. (B) On the left is a schematic diagram of the different constructs: (upper) a control SV40 promoter construct (pGL3/SV40) used to set the 100% standard activity; (middle) progressive 5′ to 3′ deletions of the −1413/+25 construct; and (lower) an inverted construct (pGL3/−1413R) of the −1413/+25 segment. On the right, the luciferase activities of the constructs transfected into Dami (shaded histograms) or HeLa cells (open histograms) are given as percentages of the control (pGL3/SV40) activity. Values were corrected for transfection efficiency by cotransfection with a sea pansy/luciferase construct under the control of the thymidine kinase promoter. Points are the mean ± SEM of at least 3 experiments performed in triplicate.
Analysis of GPV gene reporter/Exonuclease III deletion constructs after transient transfection in Dami and HeLa cells. (A) Schematic representation of the −1413 to +25 5′-flanking region of the human GPV gene linked to the luciferase reporter gene (luc) in the pGL3 vector. Binding sites for transcription factors, functionally identified by DNase I footprinting or detected by sequence analysis in the case of RIIb-817, are indicated by the symbols: (▪) GATA, () Ets, (•) Sp1 family, (▴) STAT, and (⧫) sites homologous to the GPIIb repressor. (B) On the left is a schematic diagram of the different constructs: (upper) a control SV40 promoter construct (pGL3/SV40) used to set the 100% standard activity; (middle) progressive 5′ to 3′ deletions of the −1413/+25 construct; and (lower) an inverted construct (pGL3/−1413R) of the −1413/+25 segment. On the right, the luciferase activities of the constructs transfected into Dami (shaded histograms) or HeLa cells (open histograms) are given as percentages of the control (pGL3/SV40) activity. Values were corrected for transfection efficiency by cotransfection with a sea pansy/luciferase construct under the control of the thymidine kinase promoter. Points are the mean ± SEM of at least 3 experiments performed in triplicate.
The regulatory regions were mapped by creating a series of 5′-deletions. Deletions from position −1413 to position −903 resulted in a steep 73% decrease of promoter activity in Dami cells, suggesting the removal of strong positive elements. This region contains the GATA-989, GATA-984, Ets-960, and Ets-936 sites identified by DNase I footprinting. A further deletion from position −903 to position −816 induced a 107% increase in promoter activity relative to the −903/+25 construct (pGL3/−903), which is compatible with the presence of a negative regulatory element. Sequence analysis showed a 5′-CTCATG-3′ motif, homologous in its reverse orientation to a repressor domain in the GPIIb promoter,31 that was named RIIb-817. Interestingly, the RIIb domain RIIb-610 localized by DNase I footprinting is located downstream and, hence, may be excluded from this putative negative regulatory region.
The −103 to +1 region is sufficient for efficient transcription of GPV.
Sequential deletions from −816 to −362 bp caused little change in promoter activity. In contrast, removal of the −362 to −276 bp region was responsible for a 40% decrease in activity, indicating the presence of a positive element, most likely the DNase I-protected Sp1-292 motif. Deletion from −276 to −103 bp produced hardly any further change in activity, despite the presence of 3 protected GATA sites. The −103/+25 region still supported approximately 64% of full-length promoter activity and must therefore play an important role in basal transcription of the GPV gene. Subsequent deletions from position −103 to position +10 progressively suppressed promoter activity, indicating that the elements required for minimal transcription of GPV are located in this area. This domain includes the protected GATA-71, Ets-66, and Ets-42 motifs, and the −103/+25 region is also extremely well conserved between the human, mouse, and rat genes.49
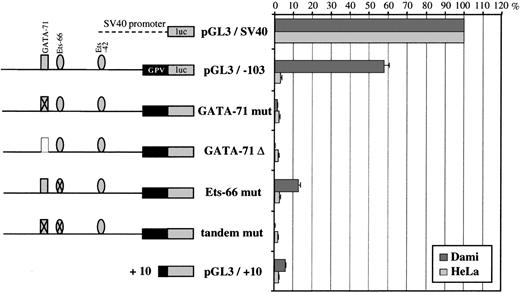
Further analysis of the −103/+25 domain (Fig 9) showed that deletion of the GATA-71/Ets-66 tandem left a construct (pGL3/−53) still having 43% of full promoter activity. On the contrary, deletion of the Ets-42 site completely abolished this activity, suggesting a critical role of Ets-42 in basal GPV promoter activity.
Reporter deletion studies of the GPV −103/+25 region. On the left is a schematic diagram of the GPV promoter/luciferase constructs. Sequential deletions of pGL3/−103 progressively removed the GATA-71/Ets-66 and Ets-42 domains and the luciferase activity was tested after transfection into Dami and HeLa cells. The pGL3/−103 construct containing the 103-bp flanking region upstream of the firefly luciferase gene is the same as in Fig 8B and had 64% activity as compared with pGL3/SV40. The lower construct (pGL3/SV40-103), which corresponds to the 103-bp flanking region of GPV linked to pGL3/SV40, was used to search for enhancer activity. On the right, the luciferase activities of the different constructs transfected into Dami and HeLa cells are given as percentages of the control (pGL3/SV40) activity. Cotransfection with a sea pansy/luciferase construct was used to normalize for transfection efficiency, and points are each the mean ± SEM of at least 3 experiments performed in triplicate.
Reporter deletion studies of the GPV −103/+25 region. On the left is a schematic diagram of the GPV promoter/luciferase constructs. Sequential deletions of pGL3/−103 progressively removed the GATA-71/Ets-66 and Ets-42 domains and the luciferase activity was tested after transfection into Dami and HeLa cells. The pGL3/−103 construct containing the 103-bp flanking region upstream of the firefly luciferase gene is the same as in Fig 8B and had 64% activity as compared with pGL3/SV40. The lower construct (pGL3/SV40-103), which corresponds to the 103-bp flanking region of GPV linked to pGL3/SV40, was used to search for enhancer activity. On the right, the luciferase activities of the different constructs transfected into Dami and HeLa cells are given as percentages of the control (pGL3/SV40) activity. Cotransfection with a sea pansy/luciferase construct was used to normalize for transfection efficiency, and points are each the mean ± SEM of at least 3 experiments performed in triplicate.
To analyze precisely the respective contribution of GATA-71 and Ets-66 on the activity of the −103/+25 region, these sites were deleted or mutated by performing G to A replacements. Reporter assays (Fig 10) showed that double mutation of GATA-71 and Ets-66 sites or that a single mutation or deletion of GATA-71 completely abolished promoter activity in Dami cells. Mutation of the Ets-66 site decreased activity by 80% as compared with the −103/+25 construct. These results suggest a critical role of both sites of the GATA/Ets tandem in GPV promoter activity, which was not shown by exonuclease III deletion analysis.
Reporter mutation analysis of the GPV GATA-71/Ets-66 tandem. On the left is a schematic diagram of the GPV promoter/luciferase constructs. Crossed-out GATA-71 or Ets-66 corresponds to mutation of these sites and is noted as “mut” (see Materials and Methods). “▵” corresponds to deletion of the GATA-71 site. On the right are the luciferase activities of the constructs after transfection into Dami and HeLa cells. Cotransfection with a sea pansy/luciferase construct was used to normalize for transfection efficiency. Each point is the mean ± SEM of at least 3 experiments performed in triplicate.
Reporter mutation analysis of the GPV GATA-71/Ets-66 tandem. On the left is a schematic diagram of the GPV promoter/luciferase constructs. Crossed-out GATA-71 or Ets-66 corresponds to mutation of these sites and is noted as “mut” (see Materials and Methods). “▵” corresponds to deletion of the GATA-71 site. On the right are the luciferase activities of the constructs after transfection into Dami and HeLa cells. Cotransfection with a sea pansy/luciferase construct was used to normalize for transfection efficiency. Each point is the mean ± SEM of at least 3 experiments performed in triplicate.
The 103-bp fragment was also tested for possible enhancer/repressor activity by placing it upstream of a weak SV40 promoter in the pGL3-SV40 vector (pGL3/SV40-103; Fig 9). A modest 25% increase in activity was observed on comparison of the effects of pGL3/SV40 and pGL3/SV40-103 in transfected Dami cells. On the contrary, the same construct yielded a 70% decrease in SV40 promoter activity in HeLa cells.
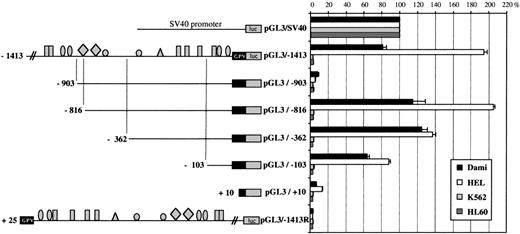
GPV promoter activity in Dami, HEL, and nonmegakaryocytic cell lines.
The −1413 promoter displayed no activity in HeLa cells (Figs 8B and 9) or in the erythroid K562 or myeloid HL60 cell lines (Fig 11). Surprisingly, 194% ± 3% of pGL3/SV40 activity was observed in transfected HEL cells, which do not express GPV, as compared with 81% ± 4% of pGL3/SV40 activity in Dami cells. Progressive deletion from −1413 to −816 bp led to a parallel decrease and then increase in promoter activity in Dami and HEL cells, with maintenance in the pGL3/−816 construct of an approximately 100% greater activity in HEL as compared with Dami cells. A different pattern of evolution appeared after removal of the region containing RIIb-610 and ETF-566. This deletion induced a significant decrease in activity in HEL cells without affecting the activity in Dami cells, whereas from −362 to +10 bp, the 2 cell lines displayed comparable promoter activities.
Comparison of the promoter activities of pGL3/−1413 and its deletion constructs in Dami, HEL, K562, and HL60 cells. On the left is a schematic diagram of the GPV promoter/luciferase constructs (see Fig 8). On the right are the luciferase activities of the constructs after transfection into Dami, HEL, K562, and HL60 cells. Cotransfection with a sea pansy/luciferase construct was used to normalize for transfection efficiency, and points are each the mean ± SEM of at least 3 experiments.
Comparison of the promoter activities of pGL3/−1413 and its deletion constructs in Dami, HEL, K562, and HL60 cells. On the left is a schematic diagram of the GPV promoter/luciferase constructs (see Fig 8). On the right are the luciferase activities of the constructs after transfection into Dami, HEL, K562, and HL60 cells. Cotransfection with a sea pansy/luciferase construct was used to normalize for transfection efficiency, and points are each the mean ± SEM of at least 3 experiments.
DISCUSSION
In this first functional study of the GPV gene promoter, we showed in gene reporter and 5′-deletion experiments that the region extending from +1 to −1413 behaves like a cell-specific promoter and identified several subdomains that act positively or negatively on its transcriptional activity. An exhaustive analysis of this region by DNase I footprinting further allowed the identification of binding sites for cell-specific and ubiquitous nuclear factors.
One feature of interest of the GPV gene is its restricted cell distribution to megakaryocytes. Previous studies of the promoters of other megakaryocyte-specific genes were often performed in leukemic cell lines displaying megakaryocytic features such as HEL or phorbol myristate acetate (PMA)-induced K562 cells. The problem of different cell lines that we have tested is that they are probably blocked at early stages of differentiation, because they express both erythroid and megakaryocytic markers. In fact, none of these cells express GPV, even at the mRNA level, whereas they express early markers such as GPIIb, with the exception of Dami cells, despite recent demonstration that present stocks of Dami cells are subclones of HEL cells. Our current Dami stock has been verified and confirmed to be a HEL derivative (H.G. Drexler, personal communication). These cells have developed a different phenotype, possibly as a result of modulation of their growth or transcription factor expression.53 The expression of GPV is accompanied by increased levels of GPIb-IX as compared with HEL cells, suggesting the existence of common regulatory pathways for the 4 subunits of the GPIb-IX-V von Willebrand factor receptor. This is consistent with our observation of the parallel appearance of these 4 subunits in the late stages of the megakaryocytic maturation of CD34+ cells from cord blood (Lepage et al, manuscript submitted). Dami was chosen for these reasons as the reference cell line for GPV expression analyses.
Our first step was to define the 5′-end of the GPV mRNA obtained from platelets and Dami cells. Because the human GPV gene bears multiple potential transcription start sites, the site common to Dami cells and platelets was numbered as +1, although some longer transcripts were isolated from platelet RNA. It corresponds to position 1433 of the GPV gene45 and is located 3 nucleotides upstream of the major site identified by Yagi et al.67 This position matches the cap site of other megakaryocytic and TATA-less genes.62
We have first shown that the −1413/+25 fragment was able to sustain luciferase activity in Dami cells but was inactive in nonmegakaryocytic leukemic cells or in nonhematopoietic HeLa cells, establishing the functionality and tissue specificity of the GPV promoter. This activity was dependent on the correct orientation of the promoter, because the construct in reverse orientation was inactive. Progressive deletion of the promoter region then allowed the identification of 5 positive/negative subdomains.
The −1413/−903 domain acts as a positive regulator and sequential deletions of this region almost abolished promoter activity (10% residual activity). Footprinting showed 4 protected areas corresponding to 2 closely linked GATA sites, GATA-989 and GATA-984, and 2 Ets binding sites, Ets-960 and Ets-936, with spacings similar to those of the GPIIb enhancer.30 The GATA-989 and GATA-984 sites contain the 5′-AGATATCT-3′ motif within the protected sequence, suggesting that these domains interact with the nuclear factor GATA-1.68 Strong evidence for the implication of GATA-1 in regulation of the transcription of megakaryocytic genes has been obtained in a number of studies.5,6,8 For example, a mutation in a GATA binding site of the GPIbβ promoter results in a Bernard-Soulier syndrome.9 In megakaryocyte-specific GATA-1 knock-out mice, the terminal megakaryocyte maturation is abnormal and results in severe thrombocytopenia in the surviving animals.15 Surprisingly, the lack of GATA-1 does not affect the expression of early megakaryocytic markers such as GIIb or MPL likely due to compensation as a result of GATA-2 overexpression, which is likewise present in megakaryocytes. However, this compensation must function only in the early stages of megakaryocytic differentiation and be ineffective at later stages, leading to the observed defects in late megakaryocytic maturation. GPV is a later marker of megakaryocyte differentiation than GPIIb or MPL and could be regulated by GATA-1 through different mechanisms.
Progressive deletion from −903 to −816 bp was accompanied by an important increase in promoter activity, indicating the presence of a potent repressor. This domain contains the motif RIIb-817 that is homologous to but behaves differently than the silencer region of the GPIIb promoter.31 69 The GPIIb repressor is responsible for GPIIb tissue-specificity. In the case of GPV, removal of the −903/−816 repressor does not induce any loss of cell specificity, because the repressor-deleted promoter is not active in K562, HL60, or HeLa cells. Hence, GPV gene transcription is strictly dependent on a megakaryocyte-specific combination of transcription factors, and this specific combination could be responsible for the late appearance of GPV during megakaryocyte maturation.
Another motif homologous to the GPIIb silencer, RIIb-610, interacted with nuclear factors in footprint experiments. However, removal of this site in the −816/−525-deleted fragment did not induce any increase in promoter activity. This could be due to the presence of a second strongly footprinted area in the −816/−525 region, ETF-566, bearing a sequence homologous to the ETF transcription factor binding site. ETF binds to typical GC box sequences and C-stretches and is a member of the Sp1 family, which is known to specifically stimulate transcription from promoters without TATA boxes.70 The −816/−525 domain thus contains a potential repressor element (RIIb-610) and a potential positive element (ETF-566), and the opposing action of these 2 elements could explain the lack of effect of the −816/−525 deletion on promoter activity. One may further hypothesize that the region including the 2 RIIb motifs might be important for regulation of the specific timing and level of GPV gene expression during megakaryocytic differentiation. Such a combination is found in the GPIbα gene in a similar environment flanking an Alu repeat region.
Sequential deletions from −362 to −103 bp induced a gradual 50% decrease in activity from 120% to 60%. This sequence contains several protected areas: Sp1-292, STAT-213, and the GATA-175, GATA-147, and GATA-111 sites. A comparison of the human, rat, and mouse GPV promoters shows that GATA-111, STAT-213, and Sp1-292 are conserved between the 3 species, although Sp1-292 is replaced by an EKLF-like recognition site in the rodent genes.49 Sp1/EKLF and GATA sites can physically interact and synergize to induce high-level transcription, as, for example, in the GPIIb71promoter. The presence of a footprinted STAT site, STAT-213, suggests that GPV transcription could be regulated by a signal transduction pathway and, if so, probably in response to thrombopoietin (TPO).23 In platelets and megakaryocytes, which coexpress STAT1, STAT3, and STAT5, thrombopoietin induces the tyrosine phosphorylation of Jak kinases and, hence, STAT3 and STAT5 activation72 73; this would, in turn, lead to STAT homodimerization or heterodimerization, translocation into the nucleus, and transcription regulation.
The proximal region from −103 to +1 bp could represent a core megakaryocytic promoter region, because it still accounts for 80% of the activity provided by the −1413/+1 region, exclusively in cells with megakaryocytic features (Dami and HEL). This promoter fragment contains 2 Ets binding sites, Ets-66 and Ets-42, and a GATA site, GATA-71. The GATA/Ets tandem of the −103/+1 domain resembles those of other megakaryocyte-specific genes such as the GPIIb, PF4, GPIbα, βTG, GPIX, and MPL genes. In fact, a proximal GATA/Ets tandem is a hallmark of megakaryocytic genes and seems to be important for their tissue-specific expression. Nevertheless, these tandems are not sufficient by themselves to support megakaryocyte-specific expression, because (1) genes such as α2 integrin and P-selectin expressed on megakaryocyte and other cell lines contain active GATA and Ets binding sites in their promoters60,74; (2) mutagenesis of these elements in the GPIIb promoter decreases its activity but does not affect its cell-specific expression7,47; and (3) the GPIIb proximal promoter still contains an active GATA/Ets tandem and is ubiquitous.31
Mutation analysis of the GATA/Ets tandem suggests that this tandem is essential for GPV transcription and that both sites are important for the activity of the GPV promoter in the megakaryocytic lineage. Progressive deletion analysis showed that the most proximal Ets site (Ets-42) is crucial for maintaining basal activity, because the −31/+25 fragment from which Ets-42 had been removed was not active. Contrary to results obtained by mutagenesis, Exonuclease III deletion of the GATA/Ets tandem did not significantly decrease activity compared with −103/+25 construct. One possible explanation is that a multiprotein complex is implicated for basal GPV transcription. In this model, the GATA/Ets tandem and Ets-42 bind their respective transcription factors and form a complex that is necessary for transcription (because pGL3/−103 presents ∼60% activity). Upon mutagenesis of the tandem, the complex cannot form and Ets-42 cannot bind its nuclear factor probably due to an unfavorable DNA context (leading to a decrease in promoter activity). When the tandem is removed by exonuclease III in the −53/+25 fragment, the Ets-42 site could be available to bind an Ets factor and provide some promoter activity.
An interesting observation was made when placing the −103 bp fragment upstream of a weak SV40 promoter that could provide some clues concerning megakaryocyte specificity of the −103 fragment. A slight increase in activity was observed in Dami cells and a remarkable 70% decrease of activity was found in HeLa cells. The inhibitory effect in HeLa cells suggests the presence of a repressor domain active in nonmegakaryocytic cells.
Mutations and deletion analysis favor an important role of Ets sites in the −103 proximal region. Different members of the Ets family have been associated with megakaryocyte differentiation. Overexpression of Fli-1 in K562 cells induces their maturation to a megakaryocytic phenotype and transfection of Fli-1 into 293 cells increases the activities of the GPIbα and GPIX28 promoters. Deletion of the 11q23-11qter, which bears the Ets-1 and Fli-1 genes, leads to severe thrombocytopenia,75 whereas Fli-1 and Ets-1 also transactivate the MPL promoter in heterologous cells.34PU.1, another member of the Ets family originally described for its role in myeloid and lymphoid cell differentiation,76 was later reported to be involved in erythroid differentiation.77 It was recently identified to play a central role in the activation of the GPIIb enhancer by TPO in UT7/MPL cells.23 An active PU.1 binding site has also been identified on the βTG promoter,24 and sequence analyses have shown potential PU.1 binding sites (5′-GAGGAA-3′)78 on the GPIX promoter.8 Sequence analysis of the GPV promoter suggests that the Ets-936 site corresponds to a PU box consensus, whereas Ets-960 (5′-CAGGAA-3′) and Ets-42 (5′-TTCCTG-3′) could also represent PU.1 binding sites. Finally, PU.1 activates the Fli-1 promoter,79 which would favor the close cooperation of Fli-1 and PU.1 during megakaryocytic differentiation. The functions of the different members of the Ets family expressed in megakaryocytes may, in fact, overlap to some extent, because PU.1−/− mice do not display any megakaryocytic abnormalities.24
In view of the differential GPV expression in Dami and HEL cells, we expected the GPV promoter to behave differently in these 2 cell lines. Surprisingly, gene reporter analyses showed a high level of promoter activity of the −1413/+25 construct in HEL cells, which do not express endogenous GPV. Deletion analyses showed very similar patterns of positive and negative regulatory regions, indicating that the same transcription factors are expressed in HEL and Dami cells. Nevertheless, whereas the −1413/+25 and −816/+25 promoter fragments were more strongly active in HEL than in Dami cells, after position −362, the strength of the promoter was roughly equivalent in the 2 cell types. This suggests that the upstream combination of the GATA/Ets-containing positive element and the RIIb repressor element had a more positive overall effect in HEL than in Dami cells. These results further suggest that slight differences (eg, in the concentrations of critical transcription factors) could influence the patterns of expression of megakaryocytic genes.
In summary, this study raises perspectives relevant to our understanding of the regulation of megakaryocyte-specific genes and, in particular, shows important differences between the GPV and GPIIb promoters. GPIIb-specific expression relies on the activity of a strong repressor, and mutation of this element leads to an ubiquitous activity of the promoter, whereas deletion of the GPV repressor does not reverse cell specificity. This study also shows that a proximal domain of the GPV promoter containing only 100 bp of the 5′ region still behaves like a generic megakaryocyte-specific promoter. Is the particular combination of a GATA and 2 Ets binding sites sufficient for the promoter specificity or does it contain another as yet unidentified megakaryocyte-specific cis-acting element? A more precise analysis of this region by linker scanning experiments should provide new insights into the regulation of gene transcription during megakaryocyte differentiation.
ACKNOWLEDGMENT
The authors thank Juliette N. Mulvihill for reviewing the English of the manuscript and Michel Prenant for his help in organizing the footprint experiments.
Supported by a GEHT-Sanofi grant and an ARC scholarship for A.L.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Corinne de la Salle, MD, INSERM U. 311, Etablissement de Transfusion Sanguine de Strasbourg, 10 rue Spielmann-B.P. 36, F-67065 Strasbourg Cedex, France; e-mail:corinne.delasalle@etss.u-strasbg.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal