Genetic modification of hematopoietic stem cells with genes that inhibit replication of human immunodeficiency virus-1 (HIV-1) could lead to development of T lymphocytes and monocytic cells resistant to HIV-1 infection after transplantation. We performed a clinical trial to evaluate the safety and feasibility of this procedure, using bone marrow from four HIV-1–infected pediatric subjects (ages 8 to 17 years). We obtained bone marrow, isolated CD34+ cells, performed in vitro transduction with a retroviral vector carrying arev-responsive element (RRE) decoy gene, and reinfused the cells into these subjects with no evidence of adverse effects. The levels of gene-containing leukocytes in peripheral blood samples in the 1 year after gene transfer/cell infusion have been extremely low. These observations support the potential of performing gene therapy for HIV-1 using hematopoietic cells, but emphasize the need for improved gene transfer techniques.
A POTENTIAL THERAPEUTIC approach to human immunodeficiency virus-1 (HIV-1) infection is the genetic modification of cells of a patient to make them resistant to HIV-1, termed intracellular immunization.1,2 Gene therapy for HIV-1 could target either mature peripheral blood T lymphocytes or hematopoietic stem cells. While T lymphocytes are more readily isolated and transduced, hematopoietic stem cells may be an attractive target because of their theoretical ability to generate a broad repertoire of mature T lymphocytes, as well as the monocytic cells (macrophages, dendritic cells, and microglia), which are also involved in HIV-1 pathogenesis. We have previously shown that overexpression of HIV-1rev-responsive element (RRE) sequences, as part of the transcript from the long-terminal repeat (LTR) of a retroviral vector, led to inhibition of HIV-1 replication in human T lymphocytes and in monocytic cells derived from transduced CD34+ hematopoietic progenitor cells. There was no evidence that expression of the RRE decoy adversely affected cell function.3 4
STUDY DESIGN
We have performed a pilot clinical trial to assess the safety, feasibility, and efficacy of retroviral-mediated transfer of the RRE decoy gene into CD34+ cells isolated from the bone marrow of HIV-1–infected pediatric subjects. The protocol and informed consent document were reviewed by the Committee on Clinical Investigations (CCI) and the Institutional Biosafety Committee at Childrens Hospital Los Angeles, the Recombinant DNA Advisory Committee of the National Institutes of Health, and the Center for Biologics Evaluation and Review of the Food and Drug Administration.
Subjects were recruited from the Pediatric AIDS Program at Childrens Hospital Los Angeles. No specific criteria for CD4+ cell count or HIV-1 viral loads were used, to allow enrollment of subjects with a range of disease severity. Informed consent was obtained from parents and subjects 12 years or older or assent from subjects 7 to 11 years of age, following the guidelines of the CCI. Screening studies were performed to exclude subjects with severe organ dysfunction or contraindications to general anesthesia for bone marrow harvest. Bone marrow aspirates (5 to 10 mL) were obtained to determine the frequency of CD34+ cells and to grow autologous bone marrow stromal cells for support of the CD34+ cells during transduction. All subjects met the inclusion criteria of ≥1% CD34+cells in the marrow aspirate. The anti-retroviral drug regimens that the subjects received were those previously prescribed by their primary physicians and were not influenced by participation in the study.
RESULTS AND DISCUSSION
Four HIV-1+ pediatric subjects were enrolled and underwent the gene transfer protocol. Their age, weight, absolute CD4+ T-lymphocyte count, and plasma HIV-1 viral load are shown in Table 1. Subjects no. 1, 2, and 4 were teenagers who had been HIV-1+ for 12 to 16 years, after infection via transfusion. Subject no. 3 was an 8-year-old who was infected perinatally. Two of the subjects had very low CD4+ cell counts (2 and 6 CD4/μL) and two had CD4+ cell counts between 250 and 450/μL. All had high viral loads, despite two or three anti-retroviral drug therapy.
Patients’ Characteristics and Cell Yields
| Patient No. . | Age (yr) . | Weight (kg) . | Plasma HIV (copies/mL) . | Bone Marrow . | Post-Isolex . | Posttransduction . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM vol (mL) . | %CD34+* . | %CD34+ . | % Transduced CFU-C† (G418-R/PCR+) . | |||||||||
| 1 | 14 | 42 | 259 | 24,503 | 500 | 12.0 | 6.5 | 3.2 | 70.5 | 1.0 | 1.0 | 12/25.5 |
| 2 | 16 | 38 | 6 | 30,762 | 425 | 11.0 | 5.4 | 0.65 | 75.6 | 0.76 | 0.35 | 7/13 |
| 3 | 8 | 32 | 428 | 15,585 | 430 | 13.3 | 4.8 | 1.5 | 83.7 | 1.5 | 0.75 | 26/21.5 |
| 4 | 17 | 53 | 2 | 96,528 | 226 | 4.3 | 5.1 | 1.3 | 69.7 | 0.28 | 0.05 | 22/16 |
| Patient No. . | Age (yr) . | Weight (kg) . | Plasma HIV (copies/mL) . | Bone Marrow . | Post-Isolex . | Posttransduction . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM vol (mL) . | %CD34+* . | %CD34+ . | % Transduced CFU-C† (G418-R/PCR+) . | |||||||||
| 1 | 14 | 42 | 259 | 24,503 | 500 | 12.0 | 6.5 | 3.2 | 70.5 | 1.0 | 1.0 | 12/25.5 |
| 2 | 16 | 38 | 6 | 30,762 | 425 | 11.0 | 5.4 | 0.65 | 75.6 | 0.76 | 0.35 | 7/13 |
| 3 | 8 | 32 | 428 | 15,585 | 430 | 13.3 | 4.8 | 1.5 | 83.7 | 1.5 | 0.75 | 26/21.5 |
| 4 | 17 | 53 | 2 | 96,528 | 226 | 4.3 | 5.1 | 1.3 | 69.7 | 0.28 | 0.05 | 22/16 |
Post-Ficoll.
Aliquots of the CD34+ cells after transduction were plated in a methylcellulose-based colony assay in quadruplicate plates, with and without G418.23 After 14 days of culture, the numbers of hematopoietic colonies (CFU-C) were determined under phase-contrast microscopy. Individual colonies were collected from the methylcellulose and assayed for the presence of vector provirus by DNA-PCR. The percentages of CFU-C that were G418-resistant were determined by dividing the total number of colonies grown in the presence of G418 by the total number of colonies grown in the absence of G418 × 100%. The percentage of PCR+ colonies was determined by dividing the number of PCR+ colonies by the total number of colonies evaluated. All colonies grown from each patient (from L-RRE-neo transduced, LN transduced) were summated.
Bone marrow was obtained under general anesthesia from the bilateral posterior iliac crests and collected into RPMI 1640 with 500 U/mL heparin. Subjects remained in hospital overnight on the General Clinical Research Center after marrow donation and were discharged to home the next day without complications. The bone marrow was processed by centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden), followed by CD34+ cell isolation using the Isolex 300i (Nexell Therapeutics Inc, Irvine, CA).
Table 1 shows the volumes of bone marrow collected and cell yields. The target volume of ≥10 mL/kg was reached in subjects no. 1 through 3, but not in subject no. 4. The unprocessed marrow samples contained between 1.4% and 3.0% CD34+ cells, reconfirming in each case that the subjects met the inclusion criteria for marrow CD34+ cell content of ≥1%. The cell products produced by the Isolex 300i contained between 69.7% and 83.7% CD34+cells. The two subjects with extremely low CD4+ cell counts (nos. 2 and 4) had lower cell yields than the two subjects with higher CD4+ cell counts, although the small number of subjects preclude any firm conclusions.
To determine whether T lymphocytes expressing the RRE decoy have improved survival, we used an internal comparative marking technique, as has been used by others.5,6 One half of each patient’s cells were transduced with the L-RRE-neo vector that contains a 41-bp portion of the HIV-1 RRE sequences, inserted immediately upstream of the neo gene.3 The other half of their cells were transduced with the control LN vector. Both vectors were packaged as clones in PA317 amphotropic packaging cells.7 Clinical grade supernatants were produced by the Gene Vector Laboratory at Baylor College of Medicine in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum. Titers of the supernatants were 1 to 3 × 106/mL based on G418-resistance of serial dilutions on 3T3 cells. Testing for adventitious agents and replication-competent retroviruses (RCR) were performed by the Gene Vector Laboratory at Baylor College of Medicine or by Microbiologic Associates (Rockville, MD) and were all negative.
The CD34+ cells were divided into two portions of equal cell numbers, one for transduction with L-RRE-neo and one for transduction with LN. Autologous bone marrow stromal cells (irradiated with 2,000 cGy, 1 day before use) were used as support layers during transduction of cells from subjects no. 1 through 3.8 For subject no. 4, recombinant fibronectin CH-296 (RetroNectin; Takara Shuzo Co, Ltd, Otsu, Shiga, Japan) was used instead of autologous stroma. CD34+ cells were transduced for three 24-hour transduction cycles with recombinant cytokines interleukin-3 (IL-3) (20 ng/mL; Novartis, Piscataway, NJ), IL-6 (50 ng/mL; Novartis), and Steel factor (100 ng/mL; R & D Associates, Minneapolis, MN) and protamine sulfate (4 μg/mL; Lyphomed, Deerfield, II), as described previously.4 Because subject no. 4 had a poor yield of marrow and CD34+ cells, no conclusions can be drawn about any differences in cell recovery or transduction using stroma versus fibronectin CH-296.
After the three transduction cycles, the nonadherent cells and culture medium were collected from the flasks, and then the adherent cells were collected from the flasks using enzyme-free cell dissociation buffer (GIBCO-BRL, Bethesda, MD). The nonadherent and adherent cells were combined and washed four times with Hanks’ balanced salt solution with 1 U/mL heparin and resuspended in 15 mL plasma-lyte A with 1 U/mL heparin. The two cells pools transduced separately with L-RRE-neo and LN were combined for a total final volume of 30 mL, which constituted the final cell product. Patients were monitored clinically during the cell infusion and afterward overnight.
All monitoring gram stains, bacterial, fungal, and mycoplasma cultures, endotoxin assays, and measurements for RCR in culture medium and cell pellets were negative. There was no detectable HIV-1 in the transduced CD34+ cell pellets or culture medium by DNA polymerase chain reaction (PCR) or reverse transcriptase (RT)-PCR, respectively.
Subjects received their cells by intravenous infusion without complications, based on clinical monitoring and laboratory studies. Postinfusion evaluations showed no perturbations in vital signs, complete blood count (CBC), or chemistry panels. There was no evidence that the patients were exposed to RCR, by assay for infectious RCR from their peripheral blood mononuclear cells (PBMC) (days +1, +7, and +30) or by Western blot analysis for the development of serum antibodies reacting against Moloney murine leukemia virus (MoMuLV) proteins (months +1, +2, +3). There were no detectable changes in any of the subject’s plasma HIV-1 levels from the marrow harvest or cell reinfusion. Thus, the subjects did not receive a significant inoculum of HIV-1 upon reinfusion of their transduced cells. Apparently, the transduced cells do not serve as a site of HIV-1 replication in vivo, despite their activation in vitro from culture with the recombinant cytokines used to stimulate stem cell proliferation to allow retroviral-mediated transduction.9
Transduction of clonogenic progenitor cells within the CD34+ cells was evaluated by growing CFU-C colonies and measuring the percentage that were G418-resistant and the percentage that contained vector DNA by DNA-PCR of individual colonies which were not selected in G418 (Table 1). Transduction efficiencies were between 7% and 30% by either assay and the percentages of transduced colony-forming unit-cells (CFU-C) measured by either G418-resistance or PCR were not significantly different (P = .77). The two vectors (L-RRE-neo and LN) led to similar extents of transduction (data not shown, P = .87).
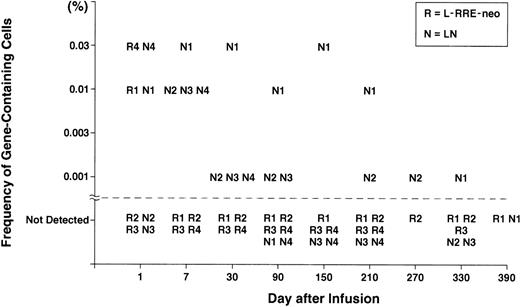
Venous blood samples were obtained at serial times after the infusion of transduced CD34+ cells to measure the percentage of PBMC containing vector sequences. Positive samples have been seen in all four subjects (Fig 1). Cells containing both the L-RRE-neo and LN vectors were detected on the day after cell infusion in two of the subjects, at frequencies of 0.03% and 0.01% (1 to 3 gene-containing cells per 10,000). With the exception of those day +1 samples, all subsequent samples were negative for the L-RRE-neo vector. Cells containing the LN vector were detected in some later samples for as long as 270 to 330 days, at frequencies of 1 cell per 100,000.
Gene frequency in PBMC after infusion of transduced CD34+ cells. Isolation of genomic DNA from patient leukocytes and PCR amplification and analysis were performed as described previously.11 To develop quantitative standards, mixtures of genomic DNA extracted from CEM cells containing one proviral copy of either the LN or the L-RRE-neo vectors were diluted in genomic DNA extracted from nontransduced cells. These standards plus multiple samples of DNA from nontransduced PBMC were analyzed with each assay of patient samples. A single pair of PCR primers was made to simultaneously detect the presence of sequences from the LN and L-RRE-neo vectors. The 5′ primer was complimentary to the MoMuLV Psi region (5′-CGAGACCTCATCACCCAGGTTAAG-3′ sense) and the 3′ primer was complimentary to the neo gene (5′-CATCAGAGCAGCCGATTGTCTG-3′ antisense). This pair of primers produces a product of 391 bp from the LN vector (N) and 451 bp from the L-RRE-neo vector (R); the increase in the L-RRE-neo product being caused by the presence of the RRE sequences immediately 5′ of the neo gene, within the span of the primer pair. An oligonucleotide (5′-TCGATCCTCCCTTTATCCAGCC-3′) complimentary to a sequence present in the resultant PCR products from each vector was labeled with -32P-dATP and used as probe.
Gene frequency in PBMC after infusion of transduced CD34+ cells. Isolation of genomic DNA from patient leukocytes and PCR amplification and analysis were performed as described previously.11 To develop quantitative standards, mixtures of genomic DNA extracted from CEM cells containing one proviral copy of either the LN or the L-RRE-neo vectors were diluted in genomic DNA extracted from nontransduced cells. These standards plus multiple samples of DNA from nontransduced PBMC were analyzed with each assay of patient samples. A single pair of PCR primers was made to simultaneously detect the presence of sequences from the LN and L-RRE-neo vectors. The 5′ primer was complimentary to the MoMuLV Psi region (5′-CGAGACCTCATCACCCAGGTTAAG-3′ sense) and the 3′ primer was complimentary to the neo gene (5′-CATCAGAGCAGCCGATTGTCTG-3′ antisense). This pair of primers produces a product of 391 bp from the LN vector (N) and 451 bp from the L-RRE-neo vector (R); the increase in the L-RRE-neo product being caused by the presence of the RRE sequences immediately 5′ of the neo gene, within the span of the primer pair. An oligonucleotide (5′-TCGATCCTCCCTTTATCCAGCC-3′) complimentary to a sequence present in the resultant PCR products from each vector was labeled with -32P-dATP and used as probe.
The failure to achieve significant transduction and engraftment of hematopoietic stem cells in this trial stands in contrast to our results using similar methods of gene transfer into CD34+cells from the umbilical cord blood of infants with adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID).10,11 In the ADA-deficient subjects, the continued presence of gene-containing cells of myeloid and lymphoid lineages for over 5 years showed that long-lived stem cells were transduced and engrafted. The difference may reflect the presence of a modest percentage of hematopoietic stem cells in cord blood which are actively undergoing cell replication, in contrast to the near-absence of replicating stem cells in bone marrow.12 The spontaneously dividing stem cells in umbilical cord blood would be susceptible to transduction by MoMuLV retroviral vector, whereas the quiescent stem cells from bone marrow would not. It is also possible that immunologic responses to either the neomycin phosphotransferase protein encoded by the vectors or to components of the cell culture medium may have led to in vivo elimination of the transduced cells.
Recent incremental improvements in retroviral-mediated gene transfer into human hematopoietic stem cells (HSC) have been achieved, using gibbon ape leukemia virus (GALV) pseudotypes, “mobilized bone marrow,” recombinant fibronectin support, new cytokines (Flt-3 ligand, thrombopoietin), and manipulation of cell-cycle kinetics.13-18 Combinations of these techniques have resulted in modest, yet significant, increases in gene marking in primate stem cell transplant models (eg, 10%, up from the previous ceiling of 0.1% to 1.0%).19,20 However, even higher levels of gene transduction of stem cells are likely to be needed for applications to many genetic diseases and acquired immunodeficiency syndrome. The development of vectors based on lentiviruses, such as HIV-1 or feline immunodeficiency virus (FIV), holds the promise of producing increased transduction of HSC, due to their ability to transduce quiescent cells such as neurons, hepatocytes, and macrophages.21 22 The safety and feasibility shown by the present study will allow future studies to evaluate these newer methods for their potential efficacy.
ACKNOWLEDGMENT
We thank the patients and families who participated in this study. Essential reagents were generously provided by Katie Harding (Novartis, Piscataway, NJ), John MacManus and Virginia Mansour (Baxter/Nexell, Irvine, CA), and Dr Ikunoshin Kato and Setsuko Yoshimura (Takara, Otsu, Shiga, Japan).
These studies were performed in the General Clinical Research Center at Childrens Hospital Los Angeles (3 MO1 RR0043-35S1) with support by the GCRC Gene Therapy Core Laboratory, a SPIRAT grant from the National Institute of Allergy and Infectious Diseases (1U19 AI36606), and research grants from the Pediatric AIDS Foundation (no. 30001-17-GT) and the T.J. Martell Foundation. D.B.K. is the recipient of an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Donald B. Kohn, MD, Mailstop #62, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail:dkohn@chla.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal