Blood group polymorphisms have been used as tools to study the architecture of the red blood cell (RBC) membrane. Some blood group variants have reduced antigen expression at the cell surface. Understanding the underlying mechanism for this reduced expression can potentially provide structural information and help to elucidate protein trafficking pathways of membrane proteins. The Kp(a+) phenotype is a variant in the Kell blood group system that is associated with a single amino acid substitution (R281W) in the Kell glycoprotein and serologically associated with a weakened expression of other Kell system antigens by an unknown mechanism. We found by immunoblotting of RBCs that the weakening of Kell antigens in this variant is due to a reduced amount of total Kell glycoprotein at the cell surface rather than to the inaccessibility of the antigens to Kell antibodies. Using a heterologous expression system, we demonstrate that the Kpa mutation causes retention of most of the Kell glycoprotein in a pre-Golgi compartment due to differential processing, thereby suggesting aberrant transport of the Kell protein to the cell surface. Furthermore, we demonstrated that single nucleotide substitutions into the coding region of the common KEL allele, as predicted by the molecular genotyping studies, was sufficient to encode three clinically significant low incidence antigens. We found that two low incidence antigens can be expressed on a single Kell protein, thus showing that the historical failure to detect such a variant is not due to structural constraints in the Kell protein. These studies demonstrate the power of studying the molecular mechanisms of blood group variants for elucidating the intracellular transport pathways of membrane proteins and the requirements for cell surface expression.
BLOOD GROUP ANTIGENS are inherited, polymorphic amino acid or carbohydrate motifs on the surface of red blood cells (RBCs) and because they are easy to detect, a multitude of naturally occurring variants have been identified. Several examples of blood group variants that have reduced cell surface expression have been described serologically, and in some cases, the molecular basis has been elucidated and found to be associated with point mutations in the proteins encoding these variants.1 Examples include the mutations in multipass proteins, eg, the mutation in the Duffy (FY*B) gene that leads to the Fyxphenotype,2-4 and the mutations in the channel-forming integral protein (CHIP) leading to the Colton null phenotype [Co(a−b−)].5 Understanding the molecular mechanisms underlying the reduced cell surface expression can potentially help in elucidating the biosynthetic pathways of the surface membrane proteins and in obtaining useful structural information.
The Kell blood group system is one of the most polymorphic antigenic systems in human RBCs with at least 23 Kell antigens including sets of antithetical antigens K (K1) and k (K2); Kpa (K3), Kpb (K4), and Kpc (K21); Jsa (K6) and Jsb (K7).6 Kell system antigens are highly immunogenic and the resulting antibodies can cause severe reactions to transfusion of incompatible blood as well as causing fetal anemia and hemolytic disease in newborns (HDN).7 It was recently shown that anemia in the fetus/newborn is exacerbated by suppression of erythropoiesis.8 Molecular analysis of the phenotypes of the Kell blood group system has shown that for 21 antigens, the differences are due to single base mutations, each causing an amino acid substitution.6 However, with the exception of the K antigen,6 it has not been possible to determine whether single point mutations are solely responsible for each of the Kell antigens, probably due to lack of strongly reactive monoclonal antibodies (MoAbs) as detection tools. In addition, despite a deliberate search, a KEL allele that encodes two low incidence antigens has not yet been described.9
Kell antigens reside on a 93-kD type II glycoprotein, with a 665-amino acid carboxy terminal extracellular domain, a single transmembrane domain, and a 47–amino acid N-terminal cytoplasmic domain.10 The Kell protein has a striking sequence homology with neutral zinc endo peptidases,10 which activate or inactivate bioactive peptides and was recently shown to have proteolytic activity.11 There are five consensus N-glycosylation sites in the Kell sequence. In addition, Kell has 16 cysteine residues, making it highly folded and its antigens sensitive to reducing agents, thereby showing the importance of conformation for antigen expression. A weakening of all inherited, high incidence Kell antigens has been reported with RBCs of the Kp(a+) phenotype,7,12,13 but the mechanism by which this occurs is not understood. An amino acid substitution of Arg281Trp is associated with the Kpa antigen.14 Because Kell is a highly folded protein, this amino acid change may cause a conformational change throughout the protein, thereby affecting the accessibility of the antigens to Kell antibodies. Alternatively, the amino acid substitution may affect the stability of the protein or its ability to reach the cell membrane. Interestingly, the Kpcantigen, a second antithetical antigen to Kpb, is associated with amino acid substitution Arg281Gln,14 but does not result in weakening of Kell antigens. Understanding the underlying mechanism for the difference in these RBC phenotypes can potentially yield useful information on the structure of Kell and its related family members and/or help in elucidating the intracellular trafficking of Kell and its requirements for surface expression.
As a first step in studying the clinically significant Kell antigens including Kpa, we developed a transient heterologous Kell expression system and obtained sufficient surface expression levels to allow detection of antigens with human alloimmune antibodies. Using this system, we showed that a single amino acid substitution in the Kell protein gives rise to the expression of antithetical blood group antigens: k to K: T193M; Kpb to Kpa: R281W; Jsb to Jsa: L597P and demonstrated that it is possible to express two low incidence antigens (K and Jsa) on the same Kell protein. Interestingly, the weakening of Kell antigens in RBCs with the Kp(a+) phenotype can be mimicked in this heterologous system, and we used this system to understand the molecular mechanism for this reduced expression. We demonstrated that whereas other Kell variants express two forms of Kell glycoprotein, one that reaches the cell surface and one that is retained in a pre-Golgi compartment, the Kpa mutation causes the retention of most glycoprotein in the intracellular compartment. These results strongly suggest that the reduced cell surface expression of the Kell protein in the Kp(a+) phenotype is as a result of aberrant transport of the protein. These novel findings not only help in our understanding of the intracellular trafficking of Kell and other members of the endopeptidase family, but can potentially be used to study the surface expression requirements of other type II glycoproteins.
MATERIALS AND METHODS
Materials.
RBCs with known antigen types were obtained either from local blood donors, a commercial panel (Gamma Biologicals, Inc, Houston, TX) or from frozen storage. MoAb anti-K14 (6-22)15 and anti-Fy6 (NYBC-BG6, clone K6)16 were kindly supplied by Pablo Rubinstein (New York Blood Center [NYBC], New York, NY). Commercial antibodies (anti-K, anti-k, anti-Kpa, and anti-Kpb) were from Gamma Biologicals, Inc (Houston, TX) and Ortho Diagnostic Systems, Inc (Raritan, NJ). Sera containing alloantibodies with specificities identified by the Immunohematology Laboratory at the New York Blood Center were obtained from blood donors or patients. Sera used included the following Kell specificities: anti-K (6735416, 6747196), anti-k (BK, GUE), anti-Kpa (JH, FR), anti-Kpb (RAU, 303255), anti-Jsa (632443, AM) and anti-Jsb (NM, CHI).
Construction of Kell expression vectors.
The wild-type Kell cDNA (encoding the common Kell phenotype, ie, K−k+ Kp(a−b+) Js(a−b+) K14+ in pRc/CMV (InVitrogen, Inc, Carlsbad, CA)17 without the XK cDNA (hereafter referred to as pRc/CMV-wt) was used for expression studies and for site-directed mutagenesis. All Kell cDNAs with the single mutations were prepared in three steps using a DNA amplification method.18 DNA sequencing analysis using an automated system (Model 373A, version 1-2.0; Applied Biosystems, Foster City, CA) was performed on all expression constructs to confirm that only the correct mutations were obtained.
K construct.
A forward primer, 322fa (5′-AACTTCCAGAACTGTGGCCCTC-3′) and a reverse primer, NK1R (5′-ACTGACTCATCAGAAGTCTCAGCATTCGG-3′) harboring the K C698T mutation19 were used to amplify a 400-bp 5′ polymerase chain reaction (PCR) fragment using the pRc/CMV-wt as the template. The 3′ 152-bp PCR fragment with the same C698T mutation was amplified with primers NK1F (5′-GG AC TTCCTTAAACTTTAACCGAAT GCTG-3′) and RT801R (5′-CTT GA GG GG AA CA TC AA AC TC TG GC-3′). The third PCR step was performed using the 5′ and 3′ gel-purified PCR fragments with 322fa and RT801R primer pairs. This 504-bp PCR product was then digested with EcoRI (nucleotide [nt] 528) and PpuMI (nt 753) to release a 225-bp DNA fragment that was inserted at the EcoRI and PpuMI of the Kell cDNA in pBC SK.17 The resulting plasmid was digested withHindIII and PpuMI and the 652-bp fragment was placed in the HindIII and PpuMI double-digested pRc/CMV-wt to obtain pRc/CMV-K.
Kpa construct.
To obtain a 350-bp 5′ PCR fragment with the Kpa-associated C961T mutation (that replaces arginine with tryptophan at residue 281),14 primer pairs PPUF (5′-GG CC ATTTCCCTTTCTTCAGAGCCTACCT-3′) and KPAR (5′-GG GG CC TC AG AA AC TGG AA CA GC CATGAAGT-3′) were used with the linearized pRc/CMV-wt as template. A 162-bp 3′ PCR fragment with the C961T mutation was made using KPAF (5′-CT TC CT TG TC AA TC TC CA TC AC TT CATGGCTG-3′) and BSTR (5′-GA GC TT TC TG CG TG CC TC CT GG AA TTGAC-3′) as primers. Both PCR products were then gel-purified and used as templates with primer pairs PPUF and BSTR to generate a 561-bp final PCR product. This was then cut with PpuMI (nt 753) and BstEII (nt 1230) and the resulting fragment inserted atPpuMI and BstEII sites of pRc/CMV-wt Kell construct and was referred to as pRc/CMV-Kpa.
Kpc construct.
The strategy used to generate the Kpa mutation was also used to make the Kpc-associated G962A mutation14 (that encodes glutamine instead of arginine at residue 281). The exception was that the primers PPUF and KPCR (5′-GG GG CC TC AG AA ACT GG AA CA GCTGTGAAGT-3′) were used to generate the 5′ PCR fragment and primers KPCF (5′-CT TC CT TGTCAATCTCCATCACTTCACA GCTG-3′) and BSTR were used to make the 3′ PCR fragment. The final expression construct was called pRc/CMV-Kpc.
Jsa construct.
Molecular genotyping studies have shown the Jsa allele has two point mutations within the Kell gene, one at position 1910 that has been associated with T to C substitution that encodes Leu597 Pro and another occurring at position 2019, which is a silent mutation.20 Therefore, we introduced the point mutation that corresponds to T1910C. A 5′ 741 bp DNA fragment containing the 5′ terminal region with the T1910C mutation was made by PCR with forward primer, BstF (5′-CT TT CT GC AG AG CC AC AT GA TCTTAGGGC-3′) and reverse primer, JsaR (5′-TC CT GG AG GG CA TG GT TG TC AC AG GC GG GG-3′) using thePvuI linearized pRc/CMV-wt as template. A 3′ 208-bp DNA fragment also harboring the T1910C mutation was made with primers JsaF (5′-AC CA GC TC TT AC TG CC T GG GG GC TGCCCC-3′) and NheR (5′-TA AC AG CC TGTTGCTGTATGCCTGCAG-3′) using pRc/CMV-wt Kell as template. This was then followed by the third-step PCR reaction using gel purified 5′ and 3′ PCR fragments as templates and the BstF and NheR primer pairs. The resulting 904-bp PCR product was digested with BstEII (nt position 1230 of the Kell cDNA) andNheI (nt 2043) to release an 814-bp DNA fragment, which was cloned into pRc/CMV-wt at BstEII and NheI sites. This expression construct is referred to as pRc/CMV-Jsa.
Double K/Jsa mutant construct.
The strategy used to construct a Kell cDNA that encodes two low incidence antigens K and Jsa was to insert the K mutation into pRc/CMV-Jsa. pRc/CMV-K and pRc/CMV-Jsawere both digested with HindIII (5′ to the Kozak site in the polylinker of the vector) and AvrII (nt 750). Because there is an extra AvrII in the pRc/CMV vector, the pRc/CMV-Jsa was only partially digested. The gel-purified fragments (750-bp fragment encoding the K mutation and the 7 kbp pRc/CMV-Jsa) were then ligated together to make the pRc/CMV-K/Jsa double mutant.
Transfection procedure 293T cells.
The human embryonic kidney 293T cells were transfected by the calcium phosphate precipitation method as previously described21with the various (listed above) Kell cDNA expression constructs (10 μg). To control for transfection efficiency, an unrelated plasmid, pREP-FYB (3 μg), was cotransfected with each expression construct. After 24 hours, cells were washed and fed with growth medium (Minimal Essential Medium Eagle alpha [α-MEM], 10% fetal bovine serum, 1% penicillin/streptomycin) and grown for another 24 hours. Cells were then analyzed for expression by flow cytometry and immunoblotting.
Flow cytometric analyses.
Analysis of antigen expression on transfected cells was performed by flow cytometry in parallel with antigen-positive and antigen-negative RBCs to control for antibody specificity. In addition to antithetical antigen expression profile, the expression of other Kell antigens was analyzed to ensure that the Kell proteins in the transfectants were correctly folded. Thus, expression of K14 antigen was tested with each of the transfectants using MoAab anti-K14. Anti-k (n = 2) were used to detect the expression of k antigen in Kpa- and Jsa-transfected cells; anti-Kpb (n = 2) to detect the expression of Kpb antigen in K-, Jsa-, and K/Jsa-transfected cells; and anti-Jsb (n = 2) to detect the expression of Jsb antigen in K-, Kpa-transfected cells (data not shown).
Briefly, RBCs (3 μL of 0.5% suspension) or 106transfected 293T cells were washed twice with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and incubated for 1 hour at 37°C with the appropriate primary antibody diluted at a ratio of 1:2 in PBS/0.5% BSA. After three washes, the cells were incubated with fluorescein-conjugated anti-mouse or anti-human IgG (H+L) (Vector Laboratories Inc, Burlingame, CA) at 50-fold dilution for 30 minutes at 4°C. To analyze the transfected cells, 10 μg of propidium iodide (PI) was added to 0.5 mL cell suspension and PI-negative cells, representing the live cell population, were selected for analysis on the FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). For the RBC analysis, the total population was used. RBCs with the common Kell phenotype were used as the positive control for all of the high incidence antigens. In Tables 1 and 2, the median of fluorescence intensity values from several experiments were averaged and used as a measure of antigen expression. To normalize for efficiency of transfection of the different Kell constructs, expression of cotransfected Duffy plasmid vector was measured by flow cytometry using anti-Fy6. The fluorescence intensity values for Kell antigen expression in Table 2 were calculated as relative values obtained after normalization to the level of Duffy expression.
Flow Cytometric Analysis of K14 Antigen on Common Kell, Kp(a+), and Kp(c+)
| RBC . | Median of Relative Fluorescence Intensity . |
|---|---|
| Anti-K14 . | |
| K14-negative (n = 1) | 3 (2-4) |
| Kp(a−b+) (n = 2) | 84 (67-93) |
| Kp(a+b−c−) (n = 2) | 32 (27-37) |
| Kp(a−b−c+) (n = 1) | 80 (67-93) |
| RBC . | Median of Relative Fluorescence Intensity . |
|---|---|
| Anti-K14 . | |
| K14-negative (n = 1) | 3 (2-4) |
| Kp(a−b+) (n = 2) | 84 (67-93) |
| Kp(a+b−c−) (n = 2) | 32 (27-37) |
| Kp(a−b−c+) (n = 1) | 80 (67-93) |
The averaged values of relative fluorescence intensity from several experiments are shown with the range of the values indicated in parentheses. The n value refers to the number of RBC samples tested.
Comparison of K14 Antigen Expression on Wild-Type, Kpa, and Kpc Transfectants
| Kell cDNA Constructs . | Median of Relative Fluorescence Intensity . |
|---|---|
| Anti-K14 . | |
| Mock (n = 6) | 4 (3-4) |
| pRc/CMV-wt (n = 6) | 1,519 (895-2,283) |
| pRc/CMV-Kpa (n = 6) | 445 (335-621) |
| pRc/CMV-Kpc (n = 3) | 1,612 (1,071-2,025) |
| Kell cDNA Constructs . | Median of Relative Fluorescence Intensity . |
|---|---|
| Anti-K14 . | |
| Mock (n = 6) | 4 (3-4) |
| pRc/CMV-wt (n = 6) | 1,519 (895-2,283) |
| pRc/CMV-Kpa (n = 6) | 445 (335-621) |
| pRc/CMV-Kpc (n = 3) | 1,612 (1,071-2,025) |
The averaged values of relative fluorescence intensity from several experiments (n) are shown with the range of the values indicated in parentheses. The values of relative fluorescence intensity for each cell population transfected with the plasmid constructs are given after normalization for transfection efficiency.
Immunoblotting.
RBC membrane ghosts were prepared by a standard method.22Transfected 293T cells (1 × 107) were lysed in PBS/1% Triton X100 in the presence of protease inhibitors (10 μg/mL aprotonin and 1 mmol/L phenylmethylsulfonyl fluoride), centrifuged at 12,000 rpm to pellet nuclei and cell debris. Equal volumes of 2X sodium dodecyl sulfate (SDS)-loading buffer (0.5 mol/L Tris-HCl, pH 6.8, 2% SDS, 8 mol/L urea, 20 mmol/L dithiothreitol [DTT], and 0.01% bromophenol blue) was added to RBC ghost preparations and cell lysates. Samples were boiled, separated on 4-12% SDS-polyacrylamide gel electrophoresis (PAGE) gradient gels (Novex, San Diego, CA) and then transferred onto nitrocellulose paper (NCP). The NCP was stained with Ponceau-S to demonstrate approximately equal amounts of protein in each lane before blocking for 1 hour or overnight in 5% BSA. This was followed by several washes in PBS, 0.1% Tween-20 and incubation overnight at 4°C with a 1:1,000 dilution of polyclonal anti-Kell (kindly provided by David Russo, NYBC), which had been prepared from injection into rabbits of SDS-PAGE purified Kell glycoprotein immunoprecipitated from antigen positive RBCs. After several washings (4 × 15 minutes) in PBS, 0.1% Tween-20, the NCP was incubated at room temperature for 1 hour with a 1:3,000 dilution of the rabbit horseradish peroxidase (HPR)-conjugated secondary antibody in PBS, 0.5% BSA. They were then washed four times 15 minutes in PBS, 0.1% BSA, and the peroxidase activity developed by HRP color development reagent, 4-chloro-1-naphthol (Bio-Rad, Hercules, CA).
Endo H digestion.
Transfected 293T cells (1 × 105) were lysed in 300 μL of PBS/1% Triton X100 in the presence of protease inhibitors (10 μg/mL aprotonin and 1 mmol/L phenylmethylsulfonyl fluoride), centrifuged at 12,000 rpm to pellet nuclei and cell debris. A total of 100 μL of the extracts was then denatured and digested with 500 U of Endo H (New England Biolabs, Inc, Beverly, MA) at 37°C for 1 hour following manufacturer’s instructions. As control, 100 μL of extracts were also treated following manufacturer’s instructions, but in the absence of endo H. An equal volume of 2X SDS-loading buffer was then added to each sample. The proteins were separated on 4-12% SDS-PAGE gradient gels (Novex) for analysis by immunoblotting using the rabbit polyclonal anti-Kell antibody as in above.
Cell surface biotinylation.
Two days after transfection, 5 × 106 cells were washed three times with PBS. The cells were then harvested and coated for 30 minutes with 3 mL of 1 mg/mL Sulfo-NHS-LC-Biotin (Pierce Chemical Co, Rockford, IL) at 4°C. After three washes with PBS containing 20 mmol/L glycine and one wash with PBS alone, the cells were lysed by suspension in lysis buffer (PBS containing 1% Triton X-100 10 μg/mL of aprotonin and 1 mmol/L phenylmethylsulfonyl fluoride) for 10 minutes on ice. The nuclei and cell debris were pelleted by centrifugation at 12,000 rpm for 15 minutes. ImmunoPure Immobilized Streptavidin (Pierce Chemical Co) was then added at one tenth of the volume and the sample was incubated with gentle mixing at 4°C for 2 hours. The bound complexes were then washed five times with lysis buffer and then eluted with SDS-loading buffer. The proteins were separated by 4-12% SDS-PAGE gradient gels (Novex) for analysis by immunoblotting using the rabbit polyclonal anti-Kell antibody as in above. To control and ensure for comparable levels of biotinylation in the different samples, immunoblotting was performed using ImmunoPure Streptavidin horseradish peroxidase–conjugated antibody (Pierce Chemical Co) (data not shown). To control for equal amounts of protein in each lane, immunoblotting with anti-α tubulin (Sigma, St Louis, MO) was performed.
RESULTS
Expression of wild-type and variant Kell constructs.
Using a panel of alloantibodies that included patient and donor sera with specificities of anti-K, anti-Kpb, and anti-Jsb, the 293T transfected with wild-type Kell cDNA was found to express the corresponding antigens (see below), indicating that the correct conformation of the Kell protein was maintained. By site-directed mutagenesis, point mutations associated with K, Kpa, Jsa antigens were introduced into the wild-type Kell expression construct. Each construct was transfected separately into 293T cells and the antithetical antigen expression profile was compared in the mutant and wild-type Kell transfected cells (see below). Moreover, the expression of other Kell antigens was also analyzed to ensure that the correct conformation of the variant Kell proteins was maintained (see Materials and Methods).
K/k polymorphism.
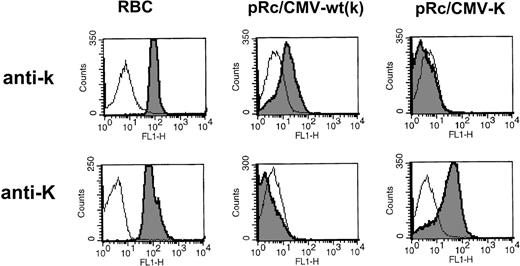
We first tested the reactivity of sera from donors containing alloanti-k (n = 2) or alloanti-K (n = 3) on cells transfected with the cDNA encoding the K allele (for representative histograms, see Fig 1). We found that, as had been demonstrated using MoAbs, the sera containing anti-K specifically detected the variant Kell protein that contained the amino acid substitution encoded by the point mutation C698T associated with the K antigen.6
Expression analysis of K/k polymorphism. Flow cytometric analysis of 293T cells transiently transfected either with the wild-type Kell cDNA construct (pRc/CMV-wt) encoding the high incidence antigen k or with the Kell cDNA encoding the C698T mutation associated with K antigen (pRc/CMV-K). Antigen-positive RBCs were tested in parallel to control for antibody specificity. Alloimmune sera used were anti-k (GUE) and anti-K (6735416). The results are depicted as overlays of open histograms to represent negative control mock/RBCs and shaded histograms, representing positive control RBCs or transfected cells. Mean fluorescence intensity (as a measure of antibody binding) in log scale is on the x-axis and the relative number of cells is represented on the y-axis.
Expression analysis of K/k polymorphism. Flow cytometric analysis of 293T cells transiently transfected either with the wild-type Kell cDNA construct (pRc/CMV-wt) encoding the high incidence antigen k or with the Kell cDNA encoding the C698T mutation associated with K antigen (pRc/CMV-K). Antigen-positive RBCs were tested in parallel to control for antibody specificity. Alloimmune sera used were anti-k (GUE) and anti-K (6735416). The results are depicted as overlays of open histograms to represent negative control mock/RBCs and shaded histograms, representing positive control RBCs or transfected cells. Mean fluorescence intensity (as a measure of antibody binding) in log scale is on the x-axis and the relative number of cells is represented on the y-axis.
Kpa/Kpb polymorphism.
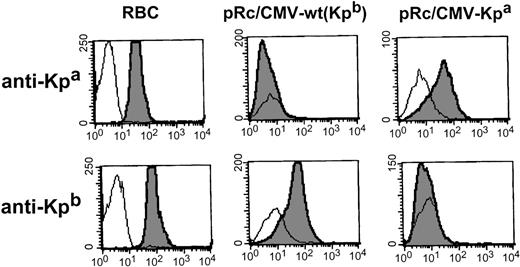
Alloanti-Kpa (n = 3) specifically detected cells transfected with cDNA encoding the amino acid substitution Arg281Trp, while alloanti-Kpb (n = 3 that had reacted with the wild-type Kell) did not detect the mutant protein (for representative histograms, see Fig 2). This confirmed that the presence of C or T at position 961 was sufficient to give rise to the expression of Kpb or Kpa antigens, respectively.
Expression analysis of Kpa/ Kpbpolymorphism. Flow cytometric analysis of 293T cells transiently transfected either with pRc/CMV-wt encoding the high incidence antigen Kpb or with the Kell cDNA encoding the C961 to T mutation associated with Kpa antigen (pRc/CMV-Kpa). Alloimmune sera used were anti-Kpb (RAU) and anti-Kpa (JH).
Expression analysis of Kpa/ Kpbpolymorphism. Flow cytometric analysis of 293T cells transiently transfected either with pRc/CMV-wt encoding the high incidence antigen Kpb or with the Kell cDNA encoding the C961 to T mutation associated with Kpa antigen (pRc/CMV-Kpa). Alloimmune sera used were anti-Kpb (RAU) and anti-Kpa (JH).
Jsa/Jsb polymorphism.
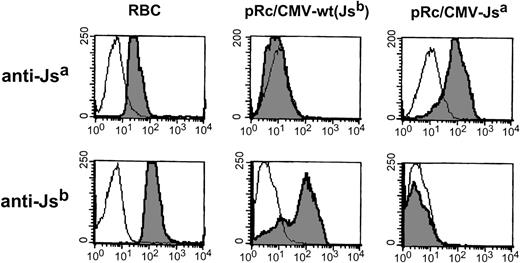
Using anti-Jsa (n = 2) and anti-Jsb (n = 2), we found that the introduction of a single point T1910C mutation into the Kell gene was sufficient to allow expression of Jsa as detected by anti-Jsa (which did not detect the wild-type Kell protein), and the expressed protein was unreactive with anti-Jsb (for representative histograms, see Fig 3).
Expression analysis of Jsa/Jsbpolymorphism. Flow cytometric analysis of 293T cells transiently transfected either with pRc/CMV-wt encoding the high incidence antigen Jsb or with the Kell cDNA encoding the T1910 to C mutation associated with Jsa antigen (pRc/CMV-Jsa). Alloimmune sera used were anti-Jsb (NM) and anti-Jsa (AM).
Expression analysis of Jsa/Jsbpolymorphism. Flow cytometric analysis of 293T cells transiently transfected either with pRc/CMV-wt encoding the high incidence antigen Jsb or with the Kell cDNA encoding the T1910 to C mutation associated with Jsa antigen (pRc/CMV-Jsa). Alloimmune sera used were anti-Jsb (NM) and anti-Jsa (AM).
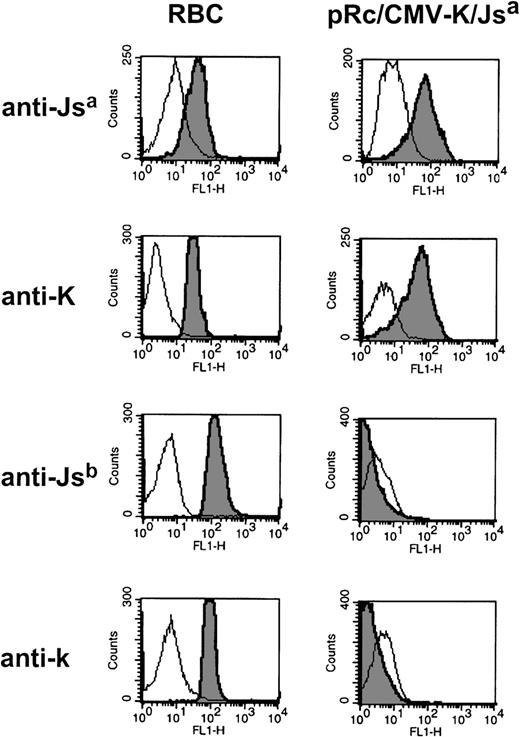
Two low incidence antigens on a single Kell protein.
To date, RBCs that carry two low incidence antigens on a single Kell protein have not been observed. To test whether this may be due to conformational constraints on the Kell protein, we created a Kell double mutant that encodes two low incidence antigens (K and Jsa) on a single Kell protein. This expression vector was introduced into 293T cells and tested for the simultaneous surface expression of K and Jsa antigens. Using flow cytometry, we found that the transfected cells were detected by anti-Jsa(n = 2) and anti-K (n = 2) and were no longer reactive with anti-Jsb (n = 2) or anti-k (n = 2) (see Fig 4, for representative histograms). This indicates that a single Kell protein can express the two low incidence antigens K and Jsa.
Surface expression of two low incidence antigens on a single Kell protein. Flow cytometric analysis of 293T cells transiently transfected with the Kell cDNA encoding mutations responsible for K and Jsa antigens (pRc/CMV-K/Jsa). Alloimmune sera used were anti-Jsa (AM), and anti-K (6735416), Jsb (NM), and anti-k (GUE).
Surface expression of two low incidence antigens on a single Kell protein. Flow cytometric analysis of 293T cells transiently transfected with the Kell cDNA encoding mutations responsible for K and Jsa antigens (pRc/CMV-K/Jsa). Alloimmune sera used were anti-Jsa (AM), and anti-K (6735416), Jsb (NM), and anti-k (GUE).
Weakening of Kell antigens in Kpa transfectants.
Whereas it is not possible to compare the reactivity of antibodies with antithetical antigens (such as Kpa/Kpb), it is possible to compare the expression levels of high incidence Kell antigens on the different RBC phenotypes. Classically, the Kpa RBC phenotype has been shown to cause weakening of high incidence Kell system antigens on the same Kell molecule as assayed by serological methods. Similarly, by flow cytometry, we found that whereas the Kpa antigen expression was readily detected by anti-Kpa (Fig 2), there was over a twofold depression of every other high incidence Kell antigen tested including K14, Jsb, and k in Kp(a+b−) RBCs as compared with wild-type or Kp(a−b−c+) RBCs (for K14 expression levels, see Table 1). Interestingly, a comparable level of depression of (high incidence) Kell antigens was also observed in 293T cells transfected with the mutant construct encoding the Kpa antigen, but not with cells transfected with wild-type (pRc/CMV wt) or Kpc(pRc/CMV Kpc) encoded plasmids (Table 2). In fact, none of the other mutant constructs (pRc/CMV-Jsb, pRc/CMV-K, or pRc/CMV-K/Jsa) showed this depression of Kell antigens (data not shown). Because we have used heterologous regulatory sequences (ie, the CMV promoter) and the cDNA to express Kpa, these results confirm that sequences outside of the coding region of the KPA gene or incorrect splicing events are not responsible for the weakening of Kell antigens in Kp(a+) RBCs. Therefore, these data strongly suggest that the reduction in the Kell antigens in the Kp(a+) RBCs is at the posttranscriptional level.
Immunoblotting.
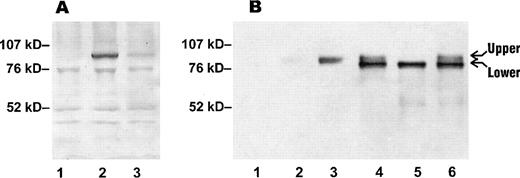
To determine the levels of Kell protein in RBC membranes of Kell variants, proteins extracted from RBCs were tested by immunoblotting with a polyclonal anti-Kell (see Materials and Methods). A specific band of apparent molecular weight of 93 kD, characteristic of Kell glycoprotein, was detected with both wild-type and Kp(a+) RBC membrane preparations, but the intensity of the band was reduced in the Kp(a+) RBC membranes (in Fig 5A, compare lanes 2 and 3). This shows that the depression of Kell antigens in Kp(a+) RBCs is due to a reduced amount of Kell glycoprotein at the cell surface and not due to conformational/folding defect of the Kell protein that leads to inaccessibility to Kell antibodies.
Immunoblotting of Kell protein in RBCs and transfectants. (A) RBC membrane preparations were separated on a 4-12% gradient SDS-PAGE under reducing conditions and immunoblotted with a polyclonal anti-Kell (see Materials and Methods). Lane 1, Kell null RBCs; lane 2, Kp(b+) (wt) RBCs; lane 3, Kp(a+) RBCs. (B) Protein extracts from 293T transfected cells and RBC membrane preparations were separated on SDS-PAGE under reducing conditions and immunoblotted with the polyclonal anti-Kell. Lane 1, mock-transfected cells; lane 2, Kp(a+) RBCs; lane 3, Kp(b+) (wt) RBCs; lane 4, cells transfected with pRc/CMV-wt Kell; lane 5, cells transfected with pRc/CMV-Kpa; lane 6, cells transfected with pRc/CMV-Kpc. The amount of protein loaded in lanes 1, 2, and 3 in (A) was threefold more than in (B) lanes 2 and 3.
Immunoblotting of Kell protein in RBCs and transfectants. (A) RBC membrane preparations were separated on a 4-12% gradient SDS-PAGE under reducing conditions and immunoblotted with a polyclonal anti-Kell (see Materials and Methods). Lane 1, Kell null RBCs; lane 2, Kp(b+) (wt) RBCs; lane 3, Kp(a+) RBCs. (B) Protein extracts from 293T transfected cells and RBC membrane preparations were separated on SDS-PAGE under reducing conditions and immunoblotted with the polyclonal anti-Kell. Lane 1, mock-transfected cells; lane 2, Kp(a+) RBCs; lane 3, Kp(b+) (wt) RBCs; lane 4, cells transfected with pRc/CMV-wt Kell; lane 5, cells transfected with pRc/CMV-Kpa; lane 6, cells transfected with pRc/CMV-Kpc. The amount of protein loaded in lanes 1, 2, and 3 in (A) was threefold more than in (B) lanes 2 and 3.
Because mature RBCs lack internal organelles, it is only possible to assess the properties of the cell surface-associated Kell protein in an RBC “ghost” preparation. To compare the characteristics of the variant Kell glycoproteins in cells with intact intracellular organelles, extracts from mock, wild-type and Kpatransfected cells were analyzed by immunoblotting (Fig 5B, lanes 1, 4, and 5). As control, cells expressing the Kpc antigen, which is associated with amino acid substitution Arg281Gln in the same codon as that of Kpa, were also analyzed (Fig 5B, lane 6). Interestingly, expression of wild-type Kell, Kpa, and Kpc yielded two species (Fig 5B, lanes 4 through 6, upper and lower). However, the amount of the upper species in protein extracts of the Kpa transfectant was barely detectable (Fig5B, lane 5). Moreover, the upper species appeared to comigrate with Kell protein extracted from RBCs (Fig 5B, compare lane 3 with lanes 4 through 6).
Endo-H treatment of transfected cells.
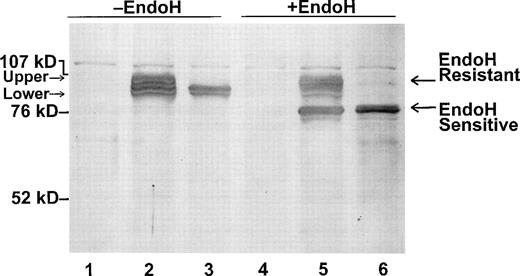
Because there are five potential N-glycosylation sites in the Kell protein, it may be that the two species in the Kell transfectants have different N-glycan moieties and/or are localized in different intracellular compartments. To address this possibility, we treated wild-type Kell and Kpa transfectants with endoglycosidase H (endo-H) (Fig 6). Endo-H cleaves “high-mannose,” but not “complex” oligosaccharide chains from proteins.23 Conversion from high-mannose to complex oligosaccharides occurs in the medial Golgi through the addition of N-acetylglucosamine to trimmed carbohydrate chains.24 After endo-H digestion, there was a shift in mobility of the lower Kell species corresponding to removal of N-linked glycans, from both wild-type and Kpa-transfected cell extracts (endo-H sensitive) (in Fig 6, compare lanes 2 and 3 with lanes 5 and 6). Thus, the N-glycans of this Kell species are not processed, suggesting that the lower species are retained in a pre-medial Golgi compartment. In contrast, the upper species were endo H-resistant (Fig 6) and by inference, they have reached the medial Golgi. Together these data strongly suggest that the Kpa mutation results in the aberrant transport of Kell protein to the cell surface.
Endo-H treatment of wild-type Kell and Kpa-transfected cell extracts. Cell extracts from mock-transfected cells (lanes 1 and 4) and cells transfected with pRC/CMV-wt Kell (lanes 2 and 5) or pRC/CMV-Kpa (lanes 3 and 6) were treated with (lanes 4 through 6) or without endo H (lanes 1 through 3), separated on SDS-PAGE and immunoblotted with the polyclonal anti-Kell.
Endo-H treatment of wild-type Kell and Kpa-transfected cell extracts. Cell extracts from mock-transfected cells (lanes 1 and 4) and cells transfected with pRC/CMV-wt Kell (lanes 2 and 5) or pRC/CMV-Kpa (lanes 3 and 6) were treated with (lanes 4 through 6) or without endo H (lanes 1 through 3), separated on SDS-PAGE and immunoblotted with the polyclonal anti-Kell.
Surface biotinylation of transfected cells.
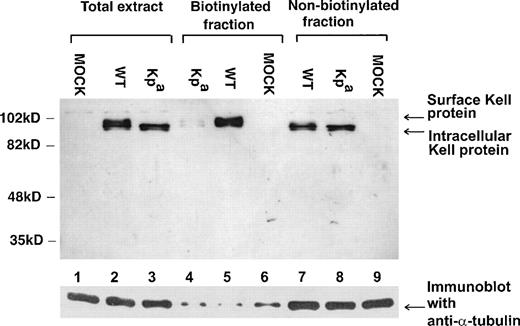
To determine whether the upper Kell species, which are endo-H resistant, are present at the cell surface, we performed surface biotinylation studies on wild-type and Kpa expressing transfected cells. As shown in Fig 7, only the upper Kell bands were detected in the biotinylated fractions, ie, the cell surface-associated fractions (in Fig 7, lanes 4 and 5). Moreover, there was considerably less of these upper species in Kpa-expressing transfected cells than in the wild-type Kell-expressing transfectants (Fig 7, compare lanes 4 and 5), as had been observed in Kp(a+) RBC membrane preparation (Fig 5). The lower, endo-H sensitive bands were only present in the nonbiotinylated fractions (Fig 7, lanes 7 and 8) and absent from the surface-labeled fraction. This is consistent with their presence in an intracellular compartment.
Surface biotinylation of wild-type Kell and Kpa transfectants. 293T cells transfected with pRc/CMV-wt (WT) (lanes 2, 5, and 7), pRc/CMV-Kpa (Kpa) (lanes 3, 4, and 8) and mock (MOCK) (lanes 1, 6, and 9) were surface biotinylated and the protein extracts immunoprecipitated using streptavidin beads, separated on SDS-PAGE, and immunoblotted with the polyclonal anti-Kell or with anti- tubulin to control for equal amounts of protein in each lane. Total extract refers to cell extracts lysed in Triton X-100 before immunoprecipitation with the streptavidin beads. Biotinylated fraction is the streptavidin immunoprecipitated fraction and therefore constitutes the cell surface-associated proteins. Nonbiotinylated fraction represents the components in the lysed cell extract that was not immunoprecipitated with the streptavidin beads.
Surface biotinylation of wild-type Kell and Kpa transfectants. 293T cells transfected with pRc/CMV-wt (WT) (lanes 2, 5, and 7), pRc/CMV-Kpa (Kpa) (lanes 3, 4, and 8) and mock (MOCK) (lanes 1, 6, and 9) were surface biotinylated and the protein extracts immunoprecipitated using streptavidin beads, separated on SDS-PAGE, and immunoblotted with the polyclonal anti-Kell or with anti- tubulin to control for equal amounts of protein in each lane. Total extract refers to cell extracts lysed in Triton X-100 before immunoprecipitation with the streptavidin beads. Biotinylated fraction is the streptavidin immunoprecipitated fraction and therefore constitutes the cell surface-associated proteins. Nonbiotinylated fraction represents the components in the lysed cell extract that was not immunoprecipitated with the streptavidin beads.
DISCUSSION
We have established a heterologous Kell expression system with similar antigenic properties as the native RBCs and were able to conclusively show that a single point mutation introduced into the wild-typeKEL gene is solely responsible for expression of the low incidence antigens: K, Kpa, and Jsa. Thus, the molecular genotyping of these antigens can be restricted to the analysis of the point mutations alone. Furthermore, because the point mutations lead to either the loss or gain of a restriction enzyme site,6 PCR-based restriction fragment length polymorphism (RFLP) assays can be used for genotyping of the three clinically significant antithetical Kell antigens: K/k, Kpa/Kpb, and Jsa/Jsb.
Despite a deliberate search, a Kell protein that carries two low incidence antigens has not been described.9 In this report, we have shown that two low incidence antigens can be expressed on the same Kell protein. This demonstrates that the failure to encode more than one low incidence antigen on a single Kell protein is not due to conformational constraints on the protein, as previously thought, but rather is a consequence of statistical improbability of two rare genetic events occurring on a single chromosome.
We found that the weakening of Kell antigens in Kp(a+) RBCs can be explained by the presence of a reduced level of the Kell protein at the cell surface, rather than inaccessibility of the antigens to Kell antibodies. In addition, because the cDNA was used to express Kpa, events that cause reduced expression due to genomic regulatory sequences (such as incorrect splicing or transcriptional regulation) can be ruled out. Because Kell is a highly folded glycoprotein, the Arg (charged, basic amino acid) to Trp (noncharged bulky aromatic amino acid) substitution that gives rise to the Kpa antigen could interfere with the biosynthetic folding of the Kell protein and, therefore, may not be transported to the cell surface. Consistent with this, we found that in Kpa-transfected cells where the weakening of Kell antigens as seen in RBCs is mimicked, most of the steady state Kell protein is retained within the cell (see below).
Kell protein extracted from wild-type transfectants migrates as two species in SDS-PAGE, whereas the RBC membrane Kell protein runs as a single band. As demonstrated by surface biotinylation studies, the upper, endo-H–resistant species of the Kell transfectants is present at the cell surface and appears to comigrate with the RBC membrane Kell protein and probably represents the form of protein that is present in the native RBC membrane. By contrast, the lower species of the Kell transfectants were absent from surface biotinylated fractions and were endo-H–sensitive. Because there is no precedent for endo- H–sensitive N-glycosylated protein species that are transported to the cell surface, it is inferred that these lower species never reach the cell surface, and pulse-chase experiments can be performed to firmly confirm this. Thus, the lower species could represent the immature form(s) of Kell glycoprotein, which are retained in an intracellular compartment, most probably in the endoplasmic reticulum (ER). In Kpatransfectants, most of the glycosylated Kell species are endo H-sensitive. Because mature Kell protein is highly folded with five potential N-linked glycans, it is conceivable that the quality control mechanisms in the ER25 would ensure that misfolded Kell species are not deployed to the cell surface. This is unlike the molecular basis of previously studied blood group variants [one type of Co(a−b−) and Fyx RBCs] where protein instability rather than defects in protein trafficking are responsible for reduced expression. Thus, in Colton null [Co(a−b−)] blood group variant, although the mutation (P38L) does not alter the initial level of the CHIP protein synthesis, it affects the glycosylation of the mutant polypeptide and the amount of protein within the cell decreases over time.5 Similarly, the missense mutation responsible for the weak Duffy phenotype (Fyx) does not result in the retention of the Duffy protein in an intracellular compartment, but rather it affects the steady state level of protein.4
Interestingly, Kpc antigen expression, which is associated with an amino acid substitution to glutamine (polar, uncharged) in the same codon as Kpa,14 does not result in weakening of other Kell antigens on Kp(c+) RBCs.26Consistent with this, we found similar levels of Kell protein and antigens in Kpc-transfected cells as in wild-type Kell transfectants. Analysis of the crystal structure of Kell will be needed to explain why Arg281Trp and not Arg281Gln has such a drastic effect on the Kell glycoprotein.
Kell is associated with Kx protein in RBC membranes.17,27,28 In McLeod phenotype RBCs where the Kx protein is absent, there is a depression of all Kell antigens.7 Based on these observations, it is possible that the Kell protein may be dependent on Kx for transport to the cell surface. However, heterologous cell surface expression of the common Kell protein in several cell lines has been shown not to require Kx,6,17,29 30 indicating that transport of Kell protein to the plasma membrane does not require heterologous expression of Kx. Alternatively, it may be that these Kell expression systems expressed endogenous Kx or Kx-related protein. In our heterologous system, all Kell cDNAs tested were expressed at levels comparable to those seen in RBCs and were correctly folded as determined by the expression of several conformational antigens. Moreover, cotransfection with an XK-expressing plasmid vector did not enhance the expression of the Kell antigens even in the context of the Kpa mutation (data not shown). However, these results do not rule out the possibility that there is endogenous Kx or Kx-related protein in the 293 cells and that the failure of these cells to transport Kpa-encoded Kell protein may be due to abnormal interaction with Kx. Coprecipitation experiments to test the interaction of Kx and Kell in Kp(a+) RBCs and transfectants are required to test this possibility.
It is interesting that anti-Kpa may sometimes be of high titer and cause HDN in infants.7 31 Thus, the amino acid substitution responsible for Kpa is immunogenic because even in the presence of reduced levels of protein, Kp(a+) RBCs can induce an immune response to this particular antigen. Further molecular studies to understand the processing of Kell protein and structure of the Kpa antigen are required to address this point.
ACKNOWLEDGMENT
We are grateful to Ruth Croson-Lowney and Jan Visser for assistance with some of the flow cytometric analyses. We also thank Colvin Redman and Christine Lomas-Francis for critically reading the manuscript and Robert Ratner for preparing the manuscript and figures.
Supported in part by Grant No. HL54459 from the National Institutes of Health Specialized Center of Research (SCOR).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Karina Yazdanbakhsh, PhD, Immunochemistry Laboratory, New York Blood Center, 310 E 67th St, New York, NY 10021; e-mail: kyazdan@nybc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal