Despite the known importance of the sequences surrounding ATG start codons (Kozak sequences) for efficient translation of proteins, few reports have appeared that describe the natural variations in these sequences. Here, we report a human polymorphism in the Kozak sequence of the platelet adhesion receptor, glycoprotein (GP) Ib, a component of the GP Ib-IX-V complex, which mediates the initial adhesion of platelets to the blood vessel wall following injury. The polymorphism is based on the presence of either thymine (T) or cytosine (C) at position −5 from the initiator ATG in the GP Ib gene. The less common allele, −5C, represented 8% to 17% of the alleles in four ethnic populations surveyed. This allele more closely resembles the sequence considered optimal for efficient initiation of protein translation and is associated with increased expression of the receptor on the cell membrane, both in transfected cells and in the platelets of individuals carrying the allele. In vitro transcription/translation studies indicate that the increased expression results from more efficient translation of the −5C form of the GP Ib mRNA. Other mutations made to approximate more closely the consensus sequence described by Kozak did not increase expression of the receptor. This is the first known description of Kozak sequence polymorphism as a determinant of the surface levels of a cell adhesion receptor. This polymorphism may influence an individual’s susceptibility for the development of cardiovascular disease.
THE INITIAL INTERACTION of mammalian blood platelets with the blood vessel wall following injury is mediated by the platelet glycoprotein (GP) Ib-IX-V complex, which binds a large multimeric protein in the subendothelium called von Willebrand factor (vWF).1 This association is essential for the normal function of platelets in preventing blood loss and also is required for the initial adhesion of platelets to exposed thrombogenic materials in regions of atherosclerotic plaque rupture, which sets the stage for the formation of platelet thrombi and possible tissue infarction.
The GP Ib-IX-V complex contains four polypeptides, GP Ibα, GP Ibβ, GP IX, and GP V, which arise from different genes and are present on the platelet plasma membrane in a 2:2:2:1 stoichiometry.1All of the polypeptides are required for a fully functional complex; efficient expression of GP Ibα on the plasma membrane requires coexpression of GP Ibβ and GP IX.2 GP Ibα is the largest polypeptide in the GP Ib-IX-V complex and contains within its N-terminus the region that binds vWF. This polypeptide also contains a high-affinity binding site for thrombin that is necessary for platelet activation at low thrombin concentrations,3 and a region in its cytoplasmic domain through which it anchors the entire complex to the platelet cytoskeleton and to signaling molecules.4-6
Aside from the requirement for coexpression of GP Ibβ and GP IX, little is known about the determinants of the plasma membrane levels of GP Ibα. Its transcription in megakaryocytes is controlled by transcription factors of the Ets and GATA families,7 but no examples of transcriptional regulation of protein levels have been described, except for the boost in transcription of the gene observed in cultured human endothelial cells treated with tumor necrosis factor-α.8 In this report, we describe another mechanism by which the levels of this important adhesion receptor are determined. We found that polymorphic variation in the region surrounding the translation start site, at position −5 from the initiator ATG codon (where either T or C is present), predicts the levels of the receptor expressed on the surfaces of transfected cells and platelets. The polymorphism is prevalent in several human ethnic populations and may be a determinant of platelet responsiveness.
MATERIALS AND METHODS
Site-directed mutagenesis and expression of the polymorphic variants in heterologous cells.
Mutagenesis to produce the −5C variant of GP Ibα was performed on the cDNA cloned in the expression plasmid pDX as was previously described.9 The two GP Ibα cDNA variants were then transiently expressed in cells stably expressing GP Ibβ and GP IX, using liposome-mediated transfection (LipofectAMINE; GIBCO-BRL, Grand Island, NY). One microgram of plasmid was used in each transfection, with the two plasmids transfected either alone or as an equal mixture. Expression of the GP Ib-IX complex on the cell surface was evaluated 72 hours after the transfection. The cells were detached from the culture plates using 0.54 mmol/L EDTA and incubated for 45 minutes in 5 μg/mL final concentration of the GP Ibα monoclonal antibody, WM23 (kindly provided by Dr Michael C. Berndt, Prahran, Victoria, Australia). The cells were then washed twice in phosphate-buffered saline (PBS) and incubated in 10 μg/mL of fluorescein isothiocyanate (FITC)–conjugated rabbit antimouse IgG (Zymed, South San Francisco, CA) for an additional 45 minutes. After two subsequent washes in PBS, the cells were analyzed by flow cytometry. Ten thousand cells from each transfection were analyzed for fluorescence by exciting the fluor with laser light at 480 nm using an argon ion laser and analyzing the light emitted at greater than 520 nm. The analyses were performed on a FACStar flow cytometer (Becton Dickinson, San Jose, CA).
A separate set of experiments was also performed with the T or C plasmid cotransfected with a plasmid containing a cDNA for green fluorescent protein (GFP) as an internal control for transfection efficiency. The GP Ibα plasmid (0.5 μg) was mixed with 0.5 μg of pEGFP-C1 (Clontech, Palo Alto, CA) and transfected as described earlier. The GP Ibα was detected using WM23 and a phycoerythrin-conjugated secondary antibody. GP Ibα fluorescence was detected in FL-2 and GFP fluorescence in FL-1 after appropriate compensation for spectral overlap.
Subjects.
The DNA samples from the African American, Australian Aborigine, and Southeast Asian populations have been described previously.10 The French Caucasian population represented the Centre d’Etude du Polymorphisme Humain (CEPH) reference families. The samples were generously provided by Dr Erwin Ludwig (Department of Genetics, University of Utah, Salt Lake City).
DNA amplification and restriction analysis.
Genomic DNA was amplified by the polymerase chain reaction (PCR) using primers based on the GP Ibα gene sequence (accession no. M22403), nucleotides 2761 to 3218. The sequence of the upstream primer was 5′GAGAGAAGGACGGAGTCGAG3′ and that of the downstream primer was 5′GGTTGTGTCTTTCGGCAGG3′. Each reaction contained 100 to 300 ng of genomic DNA, 250 ng of each primer, each dNTP at a final concentration of 200 μmol/L, 2.5 U Pyrococcus furiosus (Pfu) DNA polymerase, and 5 μL Pfu buffer provided by the manufacturer (Stratagene, La Jolla, CA). The final volume of the reactions was brought to 50 μL with water. The following conditions were used in the amplification: the samples were heated to 95°C for 5 minutes, then subjected to 30 cycles of 95°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes. At the end of the 30 cycles, the samples were incubated for 10 minutes at 72°C. An aliquot of the amplification product was digested with the restriction enzyme PpuMI. The allele containing T at position −5 contains a site for this enzyme not present in the C allele. Thus, digestion of the amplified product from T/T homozygotes produces three bands (125 bp, 157 bp, and 175 bp), from C/C homozygotes, two bands (125 bp and 332 bp), and from heterozygotes, four bands (125 bp, 157 bp, 175 bp, and 332 bp).
Cell-free transcription/translation.
The transcription/translation experiments were performed using the TNT quick-coupled transcription/translation system (Promega Corp, Madison, WI). The two forms of the GP Ibα cDNA were cloned into the vector pBluescript II SK (+/−) (Stratagene), in which transcription is driven from the T7 promoter. One microgram of each DNA was added to 40 μL of the “master-mix,” which contains rNTPs, rabbit reticulocyte lysate, T7 polymerase, all of the necessary amino acids except methionine, RNAse inhibitor, buffer, and 20 μCi of [35S]methionine (Amersham Life Science, Arlington Heights, IL). The volume was brought to 50 μL with nuclease-free water. The mixture was incubated at 30°C for 60 minutes. The translation products were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, the gel was exposed to a phosphorimager plate, and the latent image developed in a phosphorimager (Fuji, Tokyo, Japan; Model BAS1000). The band density was quantitated using MacBAS version 2.0 software (Fuji).
Flow cytometry of platelets.
Blood was drawn from subjects with different GP Ibα genotypes (T/T, T/C, C/C) into 1:6 vol of acid-citrate-dextrose anticoagulant. Prostaglandin E1 (5 nmol/L) was included in the buffers at each step of the platelet preparation to prevent platelet activation. The blood was centrifuged at 250g for 15 minutes to obtain platelet-rich plasma (PRP). Creatine phosphokinase and creatine phosphate were added to the PRP as a further measure against platelet activation, and the PRP was centrifuged at 1,600g to obtain a platelet pellet. The platelets were then washed twice in Tris-buffered saline (25 mmol/L Tris, 154 mmol/L NaCl) containing 2 mmol/L EDTA. The platelets were then fixed with 1% paraformaldehyde and analyzed by flow cytometry using the GP Ibα monoclonal antibody WM23. Analysis was performed as described for the transfected CHO cells, except that 100,000 platelets were included in each analysis.
Western blot analysis of platelet GP Ibα.
Platelets were prepared as described earlier and the platelet pellet was resuspended in RIPA lysis buffer (100 mmol/L Tris, 50 mmol/L NaCl, 0.5% SDS, 1% Triton X-100), which contained a protease inhibitor cocktail (1 mg/mL leupeptin, 1.6 mg/mL benzamidine, 0.1 mg/mL soybean trypsin inhibitor, 1 mmol/L phenylmethylsulfonyl fluoride), 5 mmol/L EDTA, and 100 μg/mL DNase I. The platelet lysate was mixed with an equal volume of 2 × SDS sample buffer (containing 4% SDS and 4% β-mercaptoethanol), boiled for 10 minutes, then electrophoresed on a 7.5% SDS-polyacrylamide gel. Proteins were electrophoretically transferred to a nitrocellulose membrane. To block nonspecific binding, the membrane was incubated for 1 hour at room temperature in a solution containing 5% nonfat milk and 0.1% Tween 20 in Tris-buffered saline. The membrane was then incubated for 1 hour with 4 μg/mL WM23 to detect GP Ibα. The membrane was washed twice with the same buffer (without milk) and then incubated for 45 minutes with horseradish peroxidase–conjugated antimouse antibody (1:10,000 dilution; Amersham) and washed as before. The bound antibody was then detected using a chemiluminescence detection kit (Amersham kit no. 2106). To control for sample loading, the membrane was also probed with the monoclonal antibody G 1.9, directed against GP IIb (a kind gift from Dr Perumal Thiagarajan, University of Texas, Houston). The membrane was first submerged in stripping buffer (100 mmol/L 2-mercaptoethanol, 2% SDS, 62.5 mmol/L Tris-HCl, pH 6.7) at 50°C for 30 minutes, then washed twice for 15 minutes and blocked with 5% milk and 0.1% Tween 20 in Tris-buffered saline. The membrane was then probed with G 1.9 as described earlier for WM23.
Statistical analysis.
The data were analyzed by pair-wise comparison using Student’s two-tailed t-test for paired values. Differences were considered statistically significant for P values less than .05.
RESULTS
Cytosine at position −5 of the GP Ibα mRNA increases surface expression of GP Ibα in transfected cells.
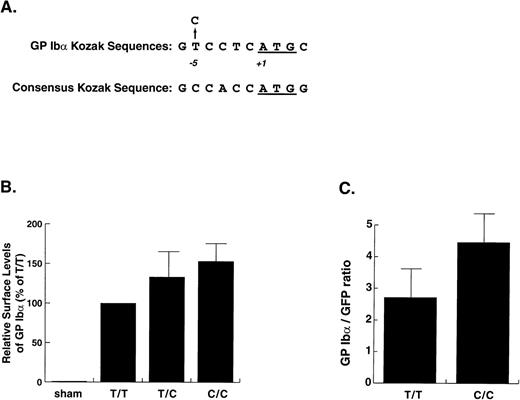
In the course of our studies on genetic variation involving the GP Ibα gene in humans,9 we found an allele with replacement of thymine (T) by cytosine (C) at position −5 from the ATG start codon (Fig 1A). This base change was recently reported as a polymorphism in a Finnish population, but no comment was made as to its effect on the platelet phenotype.11 Because the sequence surrounding the AUG initiator codon in an mRNA has been shown by Kozak to determine the efficiency with which the ribosomes and their accompanying translation machinery initiate translation,12-14 we investigated if the T→C change might influence the expression of GP Ibα.
Kozak sequence polymorphism in the GP Ib gene and in vitro expression of the polymorphic variants. (A) The GP Ib sequence surrounding the start codon and the location of the polymorphism aligned with the consensus sequence determined for these regions by Kozak.15 (B) Expression of GP Ib in CHO cells transfected with GP Ib cDNAs containing either C or T at position −5. The cells were transfected with either plasmid alone or with the same quantity of an equal mixture of the 2 plasmids. Expression of GP Ib on the cell surface was evaluated by flow cytometry after staining the cells with monoclonal antibody WM23 and a FITC-conjugated secondary antibody. Expression levels were determined by measuring the mean fluorescence of the whole cell population and are expressed as percentages of the expression obtained for the more common −5T variant. The increased surface levels of GP Ib in the cells transfected with the −5C plasmid alone or with a combination of the −5C and −5T plasmids as compared with the cells transfected only with the −5T plasmid were both statistically significant (P= .05 and P < .003, respectively, Student’s two-tail t-test, n = 5). (C) Coexpression of GP Ib variants with green fluorescent protein. Plasmids encoding the GP Ib T and C variants were cotransfected with a plasmid containing the GFP cDNA. GP Ib was detected with WM23 followed by a phycoerythrin-conjugated secondary antibody. Values are expressed as the ratio of mean fluorescence in FL-2 (PE) to the mean fluorescence in FL-1 (GFP). The expression of the C variant was again significantly higher than that of the T variant (P = .02, n = 4, Student’s two-tail t-test).
Kozak sequence polymorphism in the GP Ib gene and in vitro expression of the polymorphic variants. (A) The GP Ib sequence surrounding the start codon and the location of the polymorphism aligned with the consensus sequence determined for these regions by Kozak.15 (B) Expression of GP Ib in CHO cells transfected with GP Ib cDNAs containing either C or T at position −5. The cells were transfected with either plasmid alone or with the same quantity of an equal mixture of the 2 plasmids. Expression of GP Ib on the cell surface was evaluated by flow cytometry after staining the cells with monoclonal antibody WM23 and a FITC-conjugated secondary antibody. Expression levels were determined by measuring the mean fluorescence of the whole cell population and are expressed as percentages of the expression obtained for the more common −5T variant. The increased surface levels of GP Ib in the cells transfected with the −5C plasmid alone or with a combination of the −5C and −5T plasmids as compared with the cells transfected only with the −5T plasmid were both statistically significant (P= .05 and P < .003, respectively, Student’s two-tail t-test, n = 5). (C) Coexpression of GP Ib variants with green fluorescent protein. Plasmids encoding the GP Ib T and C variants were cotransfected with a plasmid containing the GFP cDNA. GP Ib was detected with WM23 followed by a phycoerythrin-conjugated secondary antibody. Values are expressed as the ratio of mean fluorescence in FL-2 (PE) to the mean fluorescence in FL-1 (GFP). The expression of the C variant was again significantly higher than that of the T variant (P = .02, n = 4, Student’s two-tail t-test).
Expression of GP Ibα on the plasma membrane was compared using the two forms of the GP Ibα cDNA by transient expression in Chinese hamster ovary (CHO) cells that stably express GP Ibβ and GP IX. The presence of C at −5 increased surface levels of GP Ibα in proportion to its representation in the transfection mix (C/C 153% ± 22%, T/C 132% ± 31%, T/T 100%; Fig 1B). As a further test that the observed differences were not due to differences in transfection efficiency, we repeated the transient transfections of the T and C plasmids, now cotransfecting them with a plasmid containing a cDNA for GFP as an internal control for transfection efficiency. In these experiments, expression of the C variant was 1.7 times the expression of the T variant (see Fig 3C).
Allele frequencies in different ethnic populations.
Because Kaski et al had reported that the −5C variant of GP Ibα represented a significant proportion of GP Ibα alleles in a Finnish population,11 we examined whether the same is true in other ethnic populations. Table 1 shows that the polymorphism is present in several human populations of diverse origin, with an allele frequency for the less common −5C variant ranging between 8% and 17%, and a distribution of heterozygotes close to that predicted by Hardy-Weinberg equilibrium.
Genotype and Allele Frequencies for the Kozak Sequence Polymorphism in Different Ethnic Populations
| . | CEPH . | Finnish . | African American . | Australian Aborigines . | Southeast Asian . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Genotype | ||||||||||
| T/T | 140 | 75.3 | 28 | 68.3 | 31 | 67.4 | 16 | 84.2 | 24 | 70.6 |
| T/C | 43 | 23.1 | 12 | 29.3 | 14 | 30.4 | 3 | 15.8 | 9 | 26.5 |
| C/C | 3 | 1.6 | 1 | 2.4 | 1 | 2.2 | 0 | 0 | 1 | 2.9 |
| 186 | 100 | 41 | 100 | 46 | 100 | 19 | 100 | 34 | 100 | |
| Allele | ||||||||||
| T | 323 | 86.8 | 68 | 82.9 | 76 | 82.6 | 35 | 92.1 | 57 | 83.8 |
| C | 49 | 13.2 | 14 | 17.1 | 16 | 17.4 | 3 | 7.9 | 11 | 16.2 |
| 372 | 100 | 82 | 100 | 92 | 100 | 38 | 100 | 68 | 100 | |
| . | CEPH . | Finnish . | African American . | Australian Aborigines . | Southeast Asian . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Genotype | ||||||||||
| T/T | 140 | 75.3 | 28 | 68.3 | 31 | 67.4 | 16 | 84.2 | 24 | 70.6 |
| T/C | 43 | 23.1 | 12 | 29.3 | 14 | 30.4 | 3 | 15.8 | 9 | 26.5 |
| C/C | 3 | 1.6 | 1 | 2.4 | 1 | 2.2 | 0 | 0 | 1 | 2.9 |
| 186 | 100 | 41 | 100 | 46 | 100 | 19 | 100 | 34 | 100 | |
| Allele | ||||||||||
| T | 323 | 86.8 | 68 | 82.9 | 76 | 82.6 | 35 | 92.1 | 57 | 83.8 |
| C | 49 | 13.2 | 14 | 17.1 | 16 | 17.4 | 3 | 7.9 | 11 | 16.2 |
| 372 | 100 | 82 | 100 | 92 | 100 | 38 | 100 | 68 | 100 | |
T/C polymorphism is a determinant of platelet surface levels of GP Ibα.
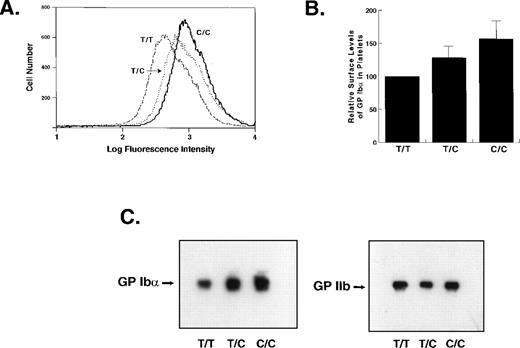
We next examined whether, as in transfected cells, the presence of C at −5 also increases GP Ib-IX-V complex surface levels in human platelets. We compared the surface levels of GP Ibα in homozygotes for either allele and in heterozygotes. As in transfected CHO cells, the amount of GP Ib-IX-V complex expressed on the platelet surface correlated directly with gene dosage. T/T homozygote platelets displayed the least GP Ibα on their surfaces, C/C homozygotes displayed the most, and heterozygotes displayed intermediate levels (T/T 100%, T/C 128% ± 16%, C/C 157% ± 26%; Fig2A and B). Both the mean and modal levels of GP Ibα were increased in the platelets of persons carrying the C allele.
Expression of GP Ib in the platelets of individuals with different GP Ib genotypes. (A) Flow cytometry histograms of platelets from three individuals with the genotypes indicated. GP Ib was detected with WM23 and a FITC-conjugated secondary antibody. The ordinate represents the relative cell number; the abscissa represents the log fluorescence intensity. (B) Average GP Ib levels from flow cytometry determinations on three individuals with each genotype. The experiments were performed six times and the mean fluorescence intensities from the individual experiments were averaged and are represented as mean ± SEM. The differences in the levels of GP Ib in the platelets of individuals with T/C and C/C genotypes as compared with T/T individuals were both statistically significant (P = .01 and P < .005, respectively, Student’s two-tailt-test, n = 6). The increased expression in the C/C individuals compared with those with the T/C genotype also showed a trend toward statistical significance (P = .075). (C) Immunoblot of whole platelet lysates from three individuals. The membrane was probed with WM23. The same blot was stripped and reprobed with antibody G 1.9 against GP IIb as a control for loading.
Expression of GP Ib in the platelets of individuals with different GP Ib genotypes. (A) Flow cytometry histograms of platelets from three individuals with the genotypes indicated. GP Ib was detected with WM23 and a FITC-conjugated secondary antibody. The ordinate represents the relative cell number; the abscissa represents the log fluorescence intensity. (B) Average GP Ib levels from flow cytometry determinations on three individuals with each genotype. The experiments were performed six times and the mean fluorescence intensities from the individual experiments were averaged and are represented as mean ± SEM. The differences in the levels of GP Ib in the platelets of individuals with T/C and C/C genotypes as compared with T/T individuals were both statistically significant (P = .01 and P < .005, respectively, Student’s two-tailt-test, n = 6). The increased expression in the C/C individuals compared with those with the T/C genotype also showed a trend toward statistical significance (P = .075). (C) Immunoblot of whole platelet lysates from three individuals. The membrane was probed with WM23. The same blot was stripped and reprobed with antibody G 1.9 against GP IIb as a control for loading.
Immunoblot analysis for GP Ibα reflected the flow cytometry results: C/C homozygotes had significantly greater quantities of GP Ibα in their platelets than did heterozygotes or T/T homozygotes (Fig 2C).
T/C polymorphism influences GP Ibα mRNA translation efficiency.
To investigate the mechanism of the increased expression, we evaluated the two allelic forms for their ability to produce protein in a cell-free transcription/translation system. Transcription in this system is driven by the T7 bacteriophage promoter and begins from the T7 transcription start site. The only difference between the two inserts was the nucleotide at position −5. In this system, significantly more protein was produced from the −5C cDNA than from the −5T form (Fig 3), which strongly suggests that the polymorphism affects the efficiency of translation.
In vitro transcription/translation analysis. Protein production from the two forms of the GP Ib cDNA was evaluated in a cell-free transcription/translation system using [35S]methionine as the radiolabel for the newly synthesized protein. The band designated GP Ib is of the molecular weight expected for the polypeptide that has not been posttranslationally modified (∼70 kD). The experiment was performed six times; a representative autoradiogram is shown. The autoradiogram was obtained by phosphorimaging; the band densities are expressed in arbitrary units. Substantially more protein was synthesized from the −5C plasmid.
In vitro transcription/translation analysis. Protein production from the two forms of the GP Ib cDNA was evaluated in a cell-free transcription/translation system using [35S]methionine as the radiolabel for the newly synthesized protein. The band designated GP Ib is of the molecular weight expected for the polypeptide that has not been posttranslationally modified (∼70 kD). The experiment was performed six times; a representative autoradiogram is shown. The autoradiogram was obtained by phosphorimaging; the band densities are expressed in arbitrary units. Substantially more protein was synthesized from the −5C plasmid.
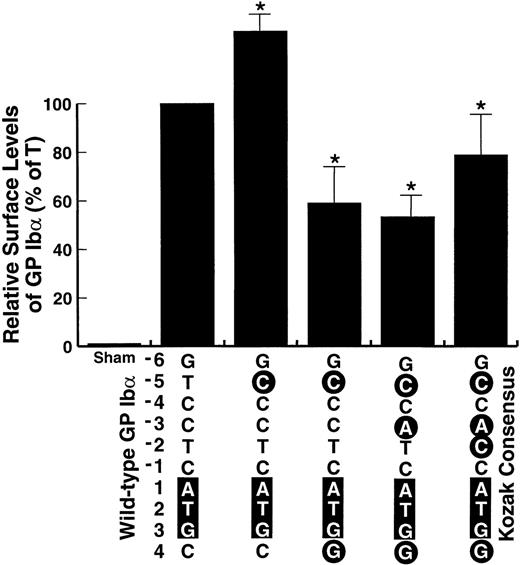
Other mutations of the GP Ibα Kozak sequence.
The consensus derived by Kozak was deduced by study of a large number of sequences and by experimental studies with a model mRNA, encoding preproinsulin.12-15 We wondered whether the same rules hold for the sequence surrounding the GP Ibα translation start site. Therefore, we studied whether mutations that further increase the similarity of the GP Ibα sequence to the consensus sequence described by Kozak12-14 would also increase the surface expression of the GP Ib-IX complex in transfected cells. The mutations progressively increase the similarity between the GP Ibα sequence and the consensus sequence, as depicted in Fig 4, by sequentially changing the nucleotides at positions +4, −3, and −2. None of the changes produced expression levels greater than produced by the wild-type sequence; only when the sequence was fully converted to the consensus Kozak sequence did expression approximate expression from the wild-type variant. In contrast, the levels produced by the −5C form were again significantly higher than those from the wild-type variant. Thus, the wild-type sequence of GP Ibα functions as well as the consensus sequence in supporting efficient translation of the polypeptide and the −5C polymorphic variant functions even better.
Effect of additional mutations of the GP Ib Kozak sequence on surface expression of the GP Ib-IX complex in transfected cells. Additional mutations surrounding the GP Ib start codon were made as indicated, with each successive mutation rendering the sequence closer to the consensus sequence derived by Kozak, shown at the right. Expression of the mutants is expressed as a percentage of the expression in cells transfected with the wild-type GP Ib cDNA. *The decrease in expression from these mutants as compared with wild-type GP Ib was statistically significant.
Effect of additional mutations of the GP Ib Kozak sequence on surface expression of the GP Ib-IX complex in transfected cells. Additional mutations surrounding the GP Ib start codon were made as indicated, with each successive mutation rendering the sequence closer to the consensus sequence derived by Kozak, shown at the right. Expression of the mutants is expressed as a percentage of the expression in cells transfected with the wild-type GP Ib cDNA. *The decrease in expression from these mutants as compared with wild-type GP Ib was statistically significant.
DISCUSSION
We describe here a polymorphism of a gene encoding a human platelet adhesion receptor that influences the levels of the receptor on the platelet plasma membrane. To our knowledge, no other similar polymorphisms have been described that affect the context of a eukaryotic translation initiation site without either adding or removing an ATG start codon. Recently, Kanaji et al showed that the level of coagulation factor XII in human plasma was profoundly influenced by a single base polymorphism within the Kozak sequence of the mRNA encoding this protein.16 The less common allele, with T replacing C at position −4, was associated with a marked decrease in the plasma levels of factor XII. The difference between that polymorphism and the one described here is that in the factor XII polymorphism T at position −4 introduces a new ATG codon upstream of the one present in the most common allele and thus may decrease expression by providing a competing out-of-frame initiation codon. In the GP Ibα polymorphism, on the other hand, only the context of the initiation site is changed. The sequence containing C instead of T at position −5 more closely approximates the consensus derived by Kozak (Fig 1A), which suggests that the mRNA with C at −5 is translated more efficiently. Consistent with this possibility, more protein was produced from the −5C form in a cell-free transcription/translation system (Fig 3). Previous work on the sequences surrounding translation initiation sites indicates that the purines at positions −3 and +4 are the most important determinants of efficient translation. The other nucleotides in the sequence were deemed of lesser importance, based on a comparison of a large number of eukaryotic mRNA sequences12 and on in vitro studies with a model mRNA.13-15 Our studies indicate that these values of relative importance may not apply generally, and certainly do not apply in the case of the GP Ibα mRNA, as the −5 position had a much more profound effect than did changes at any other position (Fig 4).
The presence of one or two copies of the −5C allele increases both the mean and modal levels of GP Ibα on the platelet plasma membrane (Fig 2A). Because the stability and plasma membrane expression of GP Ibα require that this polypeptide form a complex with GP Ibβ and GP IX, this finding suggests that the latter polypeptides are normally synthesized in excess. If this is true, then one might find that the level of the GP Ib-IX-V complex found on the platelets of heterozygous carriers of Bernard-Soulier syndrome (the deficiency disorder of the complex) would depend on which of the three genes is mutated. Those who carry an abnormal GP Ibα allele might be expected to express less of the complex on their platelets if this polypeptide is normally present in limiting quantities. As yet this hypothesis has not been thoroughly examined.
Most of the previously described differences within populations in the levels of individual proteins have been ascribed to differences in gene transcription or protein stability. It might be expected that other polymorphisms of Kozak sequences would also lead to variations in protein levels, although such polymorphisms may have escaped detection because they involve neither protein coding sequences nor promoters and consequently may have been overlooked in searches for determinants of variation in protein expression levels. Nevertheless, the large variations in translation efficiency, and thus in protein levels, that have been described following experimental alteration of these sequences in vitro,13-15 suggest that changes in translation efficiency may be a common reason for differences in protein expression between individuals.
The GP Ib-IX-V complex plays a crucial role in the adhesion of platelets to the vessel wall, both during normal hemostasis and during thrombotic events that lead to tissue infarction (notably of the myocardium in coronary artery disease). It seems possible that increasing the density of this adhesive protein on the surfaces of platelets might predispose them to attach more readily, thus increasing the likelihood of thrombosis and infarction. Increased expression of the GP Ib-IX-V complex in platelets may also provide more efficient hemostasis, and thus the −5C allele may have at some point in human evolution provided a selective advantage to individuals carrying it.
ACKNOWLEDGMENT
We gratefully acknowledge Dr Erwin Ludwig for providing DNA samples and helpful advice, Dr Jing-fei Dong for helpful discussions, and Drs Arthur Beaudet and John Belmont for carefully reading the manuscript and providing helpful suggestions.
Supported by Grants No. 96002750 and 96012670 from the American Heart Association and Grant No. HL54218 from the National Institutes of Health. J.A.L. is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to José A. López, MD, Veterans Affairs Medical Center, Hematology/Oncology (111H), 2002 Holcombe Blvd, Houston, TX 77030; e-mail: josel@bcm.tmc.edu.

![Fig. 3. In vitro transcription/translation analysis. Protein production from the two forms of the GP Ib cDNA was evaluated in a cell-free transcription/translation system using [35S]methionine as the radiolabel for the newly synthesized protein. The band designated GP Ib is of the molecular weight expected for the polypeptide that has not been posttranslationally modified (∼70 kD). The experiment was performed six times; a representative autoradiogram is shown. The autoradiogram was obtained by phosphorimaging; the band densities are expressed in arbitrary units. Substantially more protein was synthesized from the −5C plasmid.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.186.413k19_186_191/6/m_blod41319003w.jpeg?Expires=1767758490&Signature=z9XHI6zelzBCDWtFuyrkMZGwRLhcUHW7RqiPDMiQncN93ING0z8gu7FOEEn8EX4M-o6B--9wWTJ1tVW2-mqkK1WtS-sc6lO8jKgqJIXk5XNOorK99VzObC~uJAharQz5cLAdE7Db0YBJUS4yIzg2z6sFgqYUDCKBNuNE7lhY3ITn1JSHRdeA0~Zdvahx2buT2KIT6Hu~9Qajf9vNV2UL-OKc91iUF7PYw0QdEGYPxxPVlQqgN42nYrN4aBpTwSDwL0V-raHB-hK65OPT0vbVEJr9p243FPz8yQnCUHfrxQmWZjMG~GdwZx7KEHTC6DusqseAxAp7fFBu5S2M0jIWuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal