Abstract
The threshold for prophylactic platelet transfusions in patients with hypoplastic thrombopenia generally recommended in the standard literature is 20,000 platelets/μL. A more restrictive transfusion policy may be indicated in patients with chronic severe aplastic anemia (SAA) in need of long-term platelet support. We evaluated the feasibility and safety of a policy with low thresholds for prophylactic transfusions (≤5,000 platelets/μL in stable patients; 6,000 to 10,000 platelets/μL in cases with fever and/or hemorrhagic signs) combined with progressive lengthening of transfusion intervals (up to at least 7 days irrespective of the interim course of platelet counts). The study was based on a retrospective analysis of a total of 18,706 patient days with platelet counts ≤10,000/μL in patients with chronic SAA treated (for more than 3 months) on an outpatient basis. Altogether, 1,135 platelet transfusions were given, 88% at counts ≤10,000/μL and 57% at counts ≤5,000/μL. The mean transfusion interval was 10 days. During the period of observation, three major nonlethal bleeding complications occurred, which could be well controlled. We conclude that the restrictive policy with low transfusion thresholds and prolonged transfusion intervals proved feasible and safe in chronic SAA patients.
SEVERE APLASTIC ANEMIA (SAA) is a mostly chronic bone marrow (BM) failure leading to severe hypoproliferative pancytopenia. The majority of these patients depend on successful long-term platelet transfusion support because only a minority can be treated by allogeneic BM transplantation (BMT). Moreover, if subjected to immunosuppressive treatment attempts, recovery of platelet counts usually takes several months. Thus, they represent an excellent opportunity for the evaluation of long-term platelet transfusion policies. Such evaluation may be of interest because no consensus exists regarding long-lasting prophylactic platelet support.
Prophylactic transfusions to prevent hemorrhagic complications are commonly given when platelet counts fall below 20,000/μL.1 2 This approach usually calls for transfusion intervals of 2 to 3 days. However, the risk of complications such as infectious disease transmission, early alloimmunization, and impairment of quality of life because of frequent hospital visits are increased by a series of transfusions at short intervals. In addition, economical considerations and shortage of platelet resources also demand a more stringent policy.
Reduced utilization of platelets may be attained by various restrictions. Thresholds for prophylactic platelet transfusions may be lowered below 20,000 platelets/μL as was done successfully by Gmür et al3 in patients with acute leukemia undergoing induction chemotherapy. On the other hand, platelets could be given for bleeding complications rather than prophylactively. In this study, we analyzed the results of a policy using low thresholds for prophylactic platelet transfusion combined with a progressive lengthening of transfusion intervals irrespective of the interim course of platelet counts in all cases with good tolerance. In particular, the feasibility and safety of this more restrictive transfusion policy were evaluated in patients with SAA in need of extended series of platelet transfusions.
MATERIALS AND METHODS
Study design.
The analysis includes all patients with SAA treated for time periods of at least 3 months on an outpatient basis at the University Hospital of Zurich, in the years of November 1973 to December 1996. The retrospective evaluation took into account both outpatients and inpatient periods. Patients were followed until partial (transfusion independence, but abnormal blood counts) or complete remission of SAA, BMT, or death. After ensuring the diagnosis of SAA, long-term platelet support was started according to the following criteria for a same-day platelet transfusion: (1) Platelet count <5,000/μL in stable patients (body temperature ≤38°C, no coagulation disorder, no extensive minor or major bleeding); (2) platelet count between 5,000 and 10,000/μL in case of recent hemorrhage and/or fever >38°C only; (3) platelet count >10,000/μL in case of major bleeding events (World Health Organization grade >2 [gross blood loss and/or life threatening bleeding]) or before minor surgery only.
Besides this stringent threshold for prophylactic platelet transfusions, our goal was to lengthen the transfusion intervals irrespective of the interim course of the platelet count to at least 7 days when good tolerance was observed (ie, no major or no intolerable minor bleeding complication). Shorter intervals were allowed in cases of insufficient increase of posttransfusion platelet count (absolute increase <10,000/μL), in the presence of major bleeding complications, in preparation for surgical interventions, or for bridging over long weekends or vacations.
Platelet counts and transfusions.
Platelet counts were obtained from edetic acid (EDTA) anticoagulated venous blood as previously published.3 Platelets were counted before and 30 to 60 minutes after the transfusion to evaluate the posttransfusion increment. If two consecutive platelet counts were documented ≤10,000/μL, the assumption was made that for the days in between, the platelet counts were also ≤10,000/μL. An extrapolation was made by summation of these days. Platelets for transfusion were obtained from single random donors unless alloimmunization required HLA-matched platelet transfusions. Criteria to use HLA-compatible platelets included refractoriness (corrected count increment <4,000 platelets/μL in two consecutive transfusions) and anti–HLA-antibody positivity.
Statistics.
Data were collected from the patient records. Mean values, median values, ranges, and standard deviations were calculated using a Systat software package (Systat Inc, Evanston, IL). The Student’s t-test (independent samples) and Pearson chi-squared test were used for comparisons of the samples. Two-sided Plevels of 5% were taken to indicate statistical significance.
RESULTS
The data of 25 patients fulfilling the requirements were analyzed (Table 1). One (additional) case could not be included for lack of complete data. In 19 cases, the SAA was idiopathic, in the other cases it was assumed to be due to drug toxicity (5) or to viral hepatitis (1). All cases were treated by at least one course of immunosuppressive therapy (ALG, cyclosporine, ± steroids). Three patients received androgens and 3 others received BMT after a prolonged course of chronic SAA. The period of observation amounted to a total of 55,239 patient-days. On the average, the patients were followed for over 1,775 days (median) with a range from 293 to 8,414 days. The total number of patient-days with platelet counts below 10,000/μL was 18,706. The median number of days per patient with platelet counts ≤10,000 was 310 (range, 17 to 3,696 days). The total number of days without transfusion despite a documented platelet count <10,000/μL was 773. In 23 patients platelet transfusions were withheld despite a documented platelet count ≤5,000/μL at 230 events. The total of platelet transfusions given to the patients was 1,135 (range, 2 to 253 per patient). A total of 1,318 inpatient-days, mainly for primary diagnosis or for intercurrent infections, was recorded. Bleeding complications led to 28 inpatient-days.
Patients’ Characteristics
| Male/female | 7/18 |
| Median age (range), yr | 43 (15-76) |
| Male/female | 7/18 |
| Median age (range), yr | 43 (15-76) |
| . | Median . | Mean ± SD . | Range . |
|---|---|---|---|
| Follow-up time | 1,775 | 2,209 ± 8,414 | 293-8,414 |
| No. of days, per patient with Tc <10,000/μL | 310 | 748 ± 891 | 17-3,696 |
| No. of medical visits, per patient with Tc <10,000/μL without transfusion | 17 | 31 ± 31 | 1-116 |
| No. of platelet transfusions per patient | 28 | 45 ± 55 | 2-253 |
| No. of RBC units transfused per patient | 40 | 75 ± 84 | 0-130 |
| . | Median . | Mean ± SD . | Range . |
|---|---|---|---|
| Follow-up time | 1,775 | 2,209 ± 8,414 | 293-8,414 |
| No. of days, per patient with Tc <10,000/μL | 310 | 748 ± 891 | 17-3,696 |
| No. of medical visits, per patient with Tc <10,000/μL without transfusion | 17 | 31 ± 31 | 1-116 |
| No. of platelet transfusions per patient | 28 | 45 ± 55 | 2-253 |
| No. of RBC units transfused per patient | 40 | 75 ± 84 | 0-130 |
Abbreviations: Tc, thrombocyte; RBC, red blood cell.
Pretransfusion platelet counts.
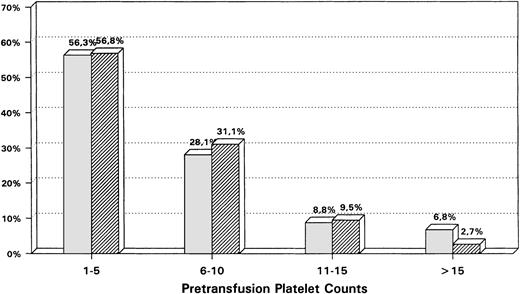
The mean pretransfusion platelet count was 6,000 ± 5,000/μL. Eighty-eight percent of all transfusion were given at platelet counts ≤10,000 platelets/μL and 57% with platelet counts ≤5,000/μL (Fig1). The pretransfusion platelet counts in outpatients and inpatients showed only small differences, which were statistically barely significant (outpatient average 5,800 platelets/μL v inpatient average 6,500 platelets/μL;P = .046). This shows that in most cases both outpatients and (severely ill) inpatients received transfusions according to the same restrictive policy.
Comparison of the relative distribution of pretransfusion platelet counts (%) between a hospital ( ) versus an outpatient setting (▨) (platelet counts in 103/μL).
) versus an outpatient setting (▨) (platelet counts in 103/μL).
Comparison of the relative distribution of pretransfusion platelet counts (%) between a hospital ( ) versus an outpatient setting (▨) (platelet counts in 103/μL).
) versus an outpatient setting (▨) (platelet counts in 103/μL).
Platelet transfusion intervals.
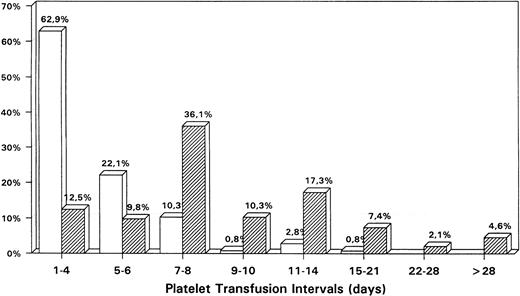
The mean interval between two consecutive platelet transfusions (Fig2) was 10 ± 18 days (median, 7 days; range, 0 to 363 days). In outpatients the goal of keeping transfusion intervals at 7 days or longer was attained in the majority (77.8%) of cases with a mean of 11.9 days (median, 7 days). In nearly half of the outpatient transfusions (47.4%) the interval was 7 to 10 days, and in an additional quartile (24.7%) the interval could be lengthened to 11 to 21 days. Inpatients were given transfusions at shorter intervals (mean and median, 4 days). Only 14.8% of transfusions given to hospitalized patients showed intervals of 7 days or longer. This difference between the two groups (outpatient v inpatient) was statistically highly significant (P < .01). This indicates to what extent the inpatients (ie, the severely ill) are monitored and transfused more frequently than outpatients (generally in a stable phase).
Comparison of the relative distribution of platelet transfusion intervals (%) between a hospital (□) versus an outpatient setting (▨).
Comparison of the relative distribution of platelet transfusion intervals (%) between a hospital (□) versus an outpatient setting (▨).
Major bleeding complications.
A total of three major, nonlethal bleeding episodes were observed (gastrointestinal hemorrhage with angiodysplasis, retinal hemorrhage with blurred vision, hemascos). These three cases were hospitalized (with a total of 19 inpatient days). Bleeding complications leading to or contributing to death were recorded in 5 cases. However, all of them were no longer transfused according to our transfusion policy. Alloimmunization had developed in 4 of them preventing effective platelet support because of lack of HLA-compatible donors. All 5 were in a terminal condition after a prolonged period of chronic SAA and refused any medical treatment including transfusions before lethal hemorrhage ensued (1 cerebral and 1 gastrointestinal bleeding, totaling 9 inpatient days; 3 suspected hemorrhages of unknown location—death at home, no autopsy).
Final outcome.
The 19 survivors showed the following outcomes: complete remissions or transfusion independent partial remissions, 14 patients; BMTs, 3; deaths not related to SAA (cardiac failure), 1; lost to follow-up, 1; still under outpatient follow-up with persistent SAA, 1.
DISCUSSION
There is no uniform consensus about the optimal platelet count threshold below which prophylactic platelet transfusion should be given for prevention of bleeding. Standard literature still recommends a threshold of 20,000 platelets/μL.1,2 In fact, a survey of 630 hospitals in the United States showed that the 20,000/μL threshold still is common practice in about 60% of the institutions.4 However, Gmür et al3reported their results of a 10-year study on prophylactic platelet transfusion in acute leukemia applying much more restrictive decision policies—the same policy that was adopted in the evaluation reported here. Later on, Heckman et al5 did not find any significant differences in morbidity and in mortality when comparing groups with thresholds of 10,000 and 20,000 platelets/μL, respectively, during induction therapy for acute leukemia. Rebulla et al6confirmed the safety of the 10,000/μL threshold. Recently Wandt et al7 also proved the safety and cost effectiveness of a 10,000/μL threshold. However, these studies3 5-7 showed effectiveness and safety of a stringent policy only for short periods of platelet transfusion dependence.
This is the first study showing that a restrictive policy for prophylactic platelet transfusions (≤5,000 platelets/μL in stable patients; ≤10,000 platelets/μL in cases with fever and/or fresh bleeding) can be safely applied in chronic SAA patients in need of prolonged platelet support. According to our policy, 57% of all transfusions were at platelet counts ≤5,000/μL. Even more importantly, the majority tolerated a progressive lengthening of transfusion intervals. It is worth noting that intervals of 7 days or longer were achieved in 78% of all outpatient transfusions, in contrast to the 2 to 3 days generally observed.
The reported deaths from hemorrhage were associated either with alloimmunization and/or patient refusal of further medical treatment including transfusions. The few other major bleeding complications were manageable. The improvement in quality of life with fewer hospital visits, the decreased risks of infection transmission, and alloimmunization are important advantages of a more restrictive transfusion practice. Cost savings and reduced utilization of platelet resources due to fewer transfusions should also be taken into account.
We conclude that our restrictive policy with low thresholds for platelet transfusions (outpatients and hospitalized patients), combined with a gradual lengthening of transfusion intervals to at least 7 days in outpatients with chronic SAA, has proven feasible, safe, and economical.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jürg Gmür, MD, Department of Internal Medicine, Division of Hematology, University Hospital Zürich, Rämistrasse 100, CH 8091 Zürich, Switzerland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal