Abstract
Familial pseudohyperkalemia is a “leaky red blood cell” condition in which the cells show a temperature-dependent loss of potassium (K) from red blood cells when stored at room temperature, manifesting as apparent hyperkalemia. The red blood cells show a reduced lifespan in vivo but there is no frank hemolysis. Studies of cation content and transport show a marginal increase in permeability at 37°C and a degree of cellular dehydration, qualitatively similar to the changes seen in dehydrated hereditary stomatocytosis (hereditary xerocytosis). Physiological studies have shown that the passive leak to K has an abnormal temperature dependence, such that the leak is less sensitive to temperature than that in normal cells. We performed genetic mapping on the original family and found that the condition in this kindred maps to the same locus (16q23-ter) that we have previously identified for an Irish family with dehydrated hereditary stomatocytosis, which does not show the same temperature effects.
THE TERM FAMILIAL pseudohyperkalemia (FP) was coined to describe an asymptomatic, dominantly inherited, red blood cell (RBC) trait in which affected individuals presented with high plasma K+ concentrations.1-3 The electrocardiogram was normal and no hyperkalemic conditions such as renal failure or Addison’s disease were identified. The hematological abnormalities were minimal. It was determined that the rise in plasma K developed when the collected blood was allowed to stand at room temperature for some hours: plasma K estimations on fresh blood were normal. Following the first description, other kindreds with pseudohyperkalemia due to temperature-dependent K loss from RBCs in association with virtually normal hematology have been described.4-9
The pseudohyperkalemia in this family was attributable to a thermotropic abnormality in the so-called “passive leak” to K across the RBC membrane,10 the same flux that is augmented in the frankly hemolytic hereditary stomatocytoses. This passive leak can conveniently be assessed experimentally as the residual K flux that persists in the presence of both ouabain and bumetanide (inhibiting the NaK pump and NaK2Cl cotransport systems, respectively). In hemolytic variants of the hereditary stomatocytoses, such as dehydrated hereditary stomatocytosis (DHS), this ouabain and bumetanide resistant (OBR) K flux is increased at 37°C. In this kindred with FP, this flux was only slightly abnormal at 37°C, consistent with the minimal hemolysis, but the temperature curve showed a shallow slope abnormality in the interval 37°C to 20°C. Because of the different temperature sensitivities of the passive leak and the NaK pump, the cells lost K on cooling to room temperature. Minor abnormalities in cellular hydration and intracellular [Na] and [K], consistent with the minimal increase in ion fluxes at 37°C, led us to designate this kindred as “mild hereditary xerocytosis.”10
DHS, also known as hereditary xerocytosis, is the most common variant of hereditary stomatocytoses and has recently been shown to be heterogeneous. It was first identified by Glader et al.11DHS has a dominant inheritance pattern. Typically, it is characterized by a mild anemia, an increased mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), and an ektacytometric curve shifted toward the right.12 The most troublesome complication of DHS is a tendency to thrombosis manifest after splenectomy, which is contra-indicated.13 The gene responsible for DHS has been mapped to 16q23-qter in a large Irish family.14
Recently, frankly hemolytic DHS kindreds have been found to show similar temperature-related K effects to those first found in FP.15-17 A survey shows that in about a third of DHS kindreds, pseudohyperkalemia was present (Grootenboer S, Delaunay J, unpublished data, January 1999). We have also identified that, irrespective of the presence or the absence of accompanying pseudohyperkalemia, perinatal edema (clearly distinguished from hydrops fetalis) occurred in about half of the French kindreds (Grootenboer et al, unpublished data). Perinatal edema was preeminent in the perinatal period and could be fatal; it spontaneously resolved a few weeks or months after birth, and never relapsed. We hypothesized that DHS, FP, and perinatal edema, in whatever combination, were caused by different mutations within the same gene.17
This hypothesis prompted us to map the gene responsible for FP. We did so in the original Edinburgh family in which FP was first identified.3 We found that the gene for FP mapped to the same arm of chromosome 16 as DHS, confirming that FP and DHS are most likely facets of the same genetically leaky RBC disease, almost certainly representing distinct mutations within the same gene.
MATERIALS AND METHODS
Clinical case.
A four-generation Scottish kindred consisting of 32 subjects, of which 11 were affected, was studied. The clinical presentation has previously been described.3 Patients were typed according to a simple test in which aliquots of heparinized blood were stored on the laboratory bench (22°C to 25°C) for 0, 2, 4 and 6 hours, at which times samples were spun and the plasma separated for K estimation. Methods used for assessing the hematological status and measuring the cation fluxes have previously been reported.3 10 Briefly, intracellular Na and K were measured on washed cells by flame photometry. For flux studies, cells were thrice washed in MOPS-buffered saline then incubated in (mmol/L): Na, 145; K(86Rb), 5; Cl, 150, MOPS, 15 (pH 7.4 at room temperature [RT]); glucose, 5 and ouabain and/or bumetanide, 0.1. After 30 to 180 minutes incubation (depending on the temperature), cells were washed free of extracellular isotope in ice-cold 106 mmol/L MgCl2, 10 mmol/L tris Cl (pH 7.4 at RT), packed, lysed, and the trapped beta activity was counted by the Cerenkov effect. The studies reported in Table 1 were performed at 37°C.
Molecular studies and linkage calculation.
Linkage analysis was performed using nine microsatellite markers from the DHS locus on chromosome 16 (cen- D16S402 -10 cmol/L- D16S511 1 cmol/L- D16S3037 −4 cmol/L- D16S520 −1 cmol/L- D16S498 −3 cmol/L- D16S413 −3 cmol/L- D16S3026 −2 cmol/L- D16S3121- tel).18 Polymerase chain reactions (PCR) using fluorescently labeled primers were run according standard protocols. An aliquot of PCR was run in an ABI PRISM 373 or 377 DNA sequencer (Applied Biosystems, Foster City, CA) and results were processed by GENESCAN software. Allele assignation was performed using the Genotyper software. All living individuals of the family were genotyped and thus contributed to the following linkage calculations. Statistical analysis was performed on the basis of an autosomal disease with complete penetrance. The disease-gene frequency was set to 0.012, and all marker alleles were considered to be equally frequent. Two point linkage analysis was performed using the MLINK program version 5.1 from the LINKAGE computer package.19 Loops of consanguinity were accommodated as suggested by Ott.19Values for maximum LOD score were calculated with the ILINK program from the same computer package. The approximate 95% confidence limits for the maximum recombination fraction (qmax) at the maximum LOD score (Zmax) were calculated by the 1-LOD-down method.19 Alleles were downcoded without loss of informativity to reduce computing time.
RESULTS
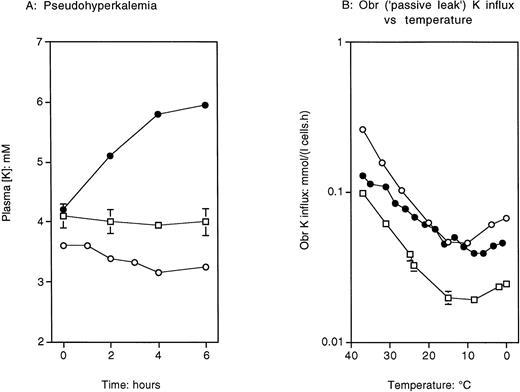
To compare temperature effects in the present family with the previously mapped DHS kindred, we examined the changes in plasma K on storage of whole heparinized blood at room temperature in FP cells, normals, and in the previously mapped DHS family (Fig1A). In the FP cells (•), there was a marked increase in plasma K+ in blood stored at 20°C, reflecting previous results,3 while in normal subjects and the DHS family (○, □), plasma K+ showed no significant change with time. The OBR K influx was studied as a function of temperature in the proposita (•), three normal subjects, and an affected member of the previously mapped DHS kindred (Fig 1B). The FP cells show a slightly increased flux at 37°C, but in the interval 37° to 20°C, the slope of the plot is significantly more shallow than that in the previously mapped DHS subject and the normal (confirming previous studies on this) family.10 Table1 illustrates the minimal ion flux and content abnormality in these FP cells compared with normal and the DHS kindred. Hemoglobin levels and MCV were within the normal range. Reticulocyte counts were on average slightly high (mean, 2.5%). We have previously shown a reduced RBC lifespan, slightly abnormal MCHC and blood film, with anisopoikilocytosis, polychromaphiliia, and few target cells and stomatocytes.3 The cells showed minimal dehydration.10
Temperature effects in FP and DHS. (A) Test for pseudohyperkalemia. From the original FP proposita and a representative affected individual of the previously mapped DHS family, whole heparinized blood was stored at room temperature for up to 6 hours before centrifugation, separation of the plasma, and estimation of K. Key, to both panels: •, FP; ○, DHS; □, normals (error bars, A, denote mean ± 2 SEM, n = 22). Only in FP does the K rise with time at this temperature. (B) OBR K influx versus temperature in the same three cases. In FP (•) the OBR flux is marginally greater than normal at 37°C, but shows the shallow slope in the interval 37° to 20°C characteristic of this family, while in DHS, the flux at 37°C is significantly greater than normal (consistent with the frank hemolysis) and shows a temperature profile which is parallel to normal.
Temperature effects in FP and DHS. (A) Test for pseudohyperkalemia. From the original FP proposita and a representative affected individual of the previously mapped DHS family, whole heparinized blood was stored at room temperature for up to 6 hours before centrifugation, separation of the plasma, and estimation of K. Key, to both panels: •, FP; ○, DHS; □, normals (error bars, A, denote mean ± 2 SEM, n = 22). Only in FP does the K rise with time at this temperature. (B) OBR K influx versus temperature in the same three cases. In FP (•) the OBR flux is marginally greater than normal at 37°C, but shows the shallow slope in the interval 37° to 20°C characteristic of this family, while in DHS, the flux at 37°C is significantly greater than normal (consistent with the frank hemolysis) and shows a temperature profile which is parallel to normal.
Comparative Diagnostic Data on Present Kindred With FP and Previously Mapped DHS Family
| Condition . | Hematology . | Intracellular Electrolytes . | Tracer Flux Rates . | ||||
|---|---|---|---|---|---|---|---|
| Hb . | Retic . | [Na]i . | [K]i . | Pump . | Cotransp . | Leak . | |
| (g/dL) . | (%) . | (mmol/L cells−1) . | (mmol/L cells−1 h−1) . | ||||
| FP (Edinburgh) | 14 to 18 | 2 to 4 | 12 | 85 | 2.6 | 1.2 | 0.12 |
| DHS (Irish family) | 11 to 12 | 6 to 12 | 12 to 14 | 75 to 86 | 4.6 to 5.9 | 0.9 to 1.2 | 0.2 |
| Normal | 14 to 18 | <2 | 5 to 11 | 88 to 105 | 0.8 to 2.0 | 0 to 1.2 | 0.075 to 0.090 |
| Condition . | Hematology . | Intracellular Electrolytes . | Tracer Flux Rates . | ||||
|---|---|---|---|---|---|---|---|
| Hb . | Retic . | [Na]i . | [K]i . | Pump . | Cotransp . | Leak . | |
| (g/dL) . | (%) . | (mmol/L cells−1) . | (mmol/L cells−1 h−1) . | ||||
| FP (Edinburgh) | 14 to 18 | 2 to 4 | 12 | 85 | 2.6 | 1.2 | 0.12 |
| DHS (Irish family) | 11 to 12 | 6 to 12 | 12 to 14 | 75 to 86 | 4.6 to 5.9 | 0.9 to 1.2 | 0.2 |
| Normal | 14 to 18 | <2 | 5 to 11 | 88 to 105 | 0.8 to 2.0 | 0 to 1.2 | 0.075 to 0.090 |
Intracellular Na and K were measured by flame photometry on fresh, washed RBCs. K influx was measured at 37°C using 86Rb as a tracer in MOPS with inhibitors at 0.1 mmol/L if required (see text). “Pump” denotes the ouabain-sensitive NaK pump fraction: “Cotransp,” the bumetanide-sensitive NaK2Cl cotransport; and “Leak,” the ouabain + bumetanide-resistant, residual passive leak fraction. Hematological values denote typical range on all 11 affected family members; isotopic flux results denote typical value on proposita.15
The four-generation kindred was analyzed using nine different microsatellite markers of DHS locus. A summary of the results is reported in Table 2. Significant LOD scores have been obtained with most of the markers used (D16S511, D16S3037, D16S520, D16S498, and D16S3026). The highest LOD score (4.14) was obtained with marker D16S3037 at theta (θ) of recombination frequency. Additional positive LOD scores were also detected with the remaining four markers. These findings clearly showed that FP disease locus in this family maps at the same position as DHS on the long arm of chromosome 16 (16q23-qter).
Pairwise LOD scores between FP Family and Chromosome 16 Markers
| Recombination Frequencies . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | Zmax | qmax | |
| D16S402 | 2.08 | 2.04 | 1.84 | 1.60 | 1.12 | 0.67 | 0.29 | 2.08 | 0.00 |
| D16S511 | 3.51 | 3.44 | 3.16 | 2.80 | 2.05 | 1.27 | 0.54 | 3.51 | 0.00 |
| D16S3037 | 4.14 | 4.07 | 3.76 | 3.35 | 2.48 | 1.57 | 0.69 | 4.14 | 0.00 |
| D16S520 | 3.08 | 3.02 | 2.78 | 2.47 | 1.81 | 1.13 | 0.48 | 3.08 | 0.00 |
| D16S498 | 3.82 | 3.75 | 3.46 | 3.07 | 2.27 | 1.43 | 0.63 | 3.82 | 0.00 |
| D16S3074 | 1.26 | 1.22 | 1.06 | 0.87 | 0.52 | 0.24 | 0.06 | 1.26 | 0.00 |
| D16S413 | 2.71 | 2.65 | 2.41 | 2.10 | 1.45 | 0.81 | 0.29 | 2.71 | 0.00 |
| D16S3026 | 3.02 | 2.95 | 2.70 | 2.38 | 1.73 | 1.09 | 0.49 | 3.02 | 0.00 |
| D16S3121 | 1.96 | 1.93 | 1.77 | 1.58 | 1.28 | 0.78 | 0.38 | 1.96 | 0.00 |
| Recombination Frequencies . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | Zmax | qmax | |
| D16S402 | 2.08 | 2.04 | 1.84 | 1.60 | 1.12 | 0.67 | 0.29 | 2.08 | 0.00 |
| D16S511 | 3.51 | 3.44 | 3.16 | 2.80 | 2.05 | 1.27 | 0.54 | 3.51 | 0.00 |
| D16S3037 | 4.14 | 4.07 | 3.76 | 3.35 | 2.48 | 1.57 | 0.69 | 4.14 | 0.00 |
| D16S520 | 3.08 | 3.02 | 2.78 | 2.47 | 1.81 | 1.13 | 0.48 | 3.08 | 0.00 |
| D16S498 | 3.82 | 3.75 | 3.46 | 3.07 | 2.27 | 1.43 | 0.63 | 3.82 | 0.00 |
| D16S3074 | 1.26 | 1.22 | 1.06 | 0.87 | 0.52 | 0.24 | 0.06 | 1.26 | 0.00 |
| D16S413 | 2.71 | 2.65 | 2.41 | 2.10 | 1.45 | 0.81 | 0.29 | 2.71 | 0.00 |
| D16S3026 | 3.02 | 2.95 | 2.70 | 2.38 | 1.73 | 1.09 | 0.49 | 3.02 | 0.00 |
| D16S3121 | 1.96 | 1.93 | 1.77 | 1.58 | 1.28 | 0.78 | 0.38 | 1.96 | 0.00 |
DISCUSSION
Positive LOD scores have been obtained in this family with FP with a series of microsatellite markers from the DHS locus. In particular, significant LOD scores higher than three have been obtained for the following markers: D16S402, D16S511, and D16S3037. These results allowed us to colocalize FP and DHS loci at the same chromosomal position. While in DHS the marker showing the highest LOD score was D16S520, here we obtained the highest score with marker D16S3037. This finding can be most likely explained by the different informativity of the marker itself in the present family as compared with the DHS family.14 We conclude that FP and DHS are almost certainly caused by alterations of the same gene. The possibility of two adjacent alleles cannot formally be dismissed, but seems unlikely. In five families showing this combination, we have never observed any recombination (Grootenboer S, Delaunay J, unpublished data, January 1999).
The incidence of FP is unknown. Its occurrence must certainly be underestimated, because it is asymptomatic and its discovery is always accidental. On functional grounds, the slight hematological and ion flux abnormalities at 37°C allow it to be classified as a mild variant of the hemolytic conditions gathered under the generic label hereditary stomatocytosis syndromes. It is the temperature effects that define this syndrome, and in particular, the shallow slope profile of the OBR fluxes. The present mapping work confirms this relationship, and the comparison of temperature effects shows that different thermotropic variants can be due to mutations of what is almost certainly the same gene.
It should be noted that the temperature profiles of the OBR K fluxes in other kindreds with FP may be different from the shallow slope variant seen here. In particular, the ouabain-plus-furosemide K efflux in the family described by Meenaghan et al6 showed a U-shaped curve, similar to that seen in normal RBCs suspended in media in which either Cl− is replaced by salicylate or thiocyanate,20 or in which Na+ is replaced by an organic cation.21 The French family described by Dagher et al8 was different again. Given these heterogeneities, it may not be appropriate to extrapolate the present mapping results to other families with the FP combination of almost-normal hematology and temperature-dependent pseudohyperkalemia.
Biochemical data and laboratory findings suggest that the function of a candidate gene should be related to monovalent cation movements across the membrane. Unfortunately, there are no known genes resembling a Na+K+ transporter or channel located within the FP/DHS locus. However, several gene fragments (expressed sequence tags) whose function remains unknown have already been mapped within this locus. Work is in progress to define the possible function of each of them and to see if one could be involved in determining FP/DHS. Recombinants with more centromeric markers (D16S503, D16S515, D16S516, and D16S3091) spanning the region of LCAT and KCC genes exclude these two genes as candidates and reduce the region for pseudohyperkalemia to 20 cM. This region has a minimum of overlapping of approximately 1.5 cM with that for xerocytosis, and most likely support the hypothesis of allelism (one gene with different mutations).
In conclusion, this work shows that a variant of that group of genetically leaky RBC conditions maps to a locus on chromosome 16 to which we have already mapped DHS. As previously suggested by hematological studies,17 FP and DHS may be variants of the same genetically leaky RBC disease. Much work will be necessary to indentify the assumed “FP/DHS gene,” and to account for the simple or compound phenotypes arising from the various mutations within this gene.
ACKNOWLEDGMENT
We are grateful to the patients for their cooperation.
Supported in part by Telethon to MC (E783). We also thank the Wellcome Trust and Action Research for support.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A. Iolascon, MD, PhD, Dipartimento di Biomedicina dell’Età Evolutiva, Piazza Guilio Cesare 11-70124, Bari, Italy; e-mail: a.iolascon@bioetaev.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal