Abstract
In this study, a mechanism is reported which determines the lifetime of polymorphonuclear neutrophils (PMN). In human PMN freshly isolated from the circulation, expression of bcl-Xl, bax-, and bak, members of the bcl-2 family of apoptosis-associated genes, was found using the reverse transcription-polymerase chain reaction technique. In contrast, no expression of bcl-2 was seen in PMN, whereas the myeloid cell line HL-60 was positive for bcl-2 mRNA. Two gene products, Bcl-Xl and Bax-, which are known to function as the regulatory machinery of programmed cell death (apoptosis), were detected at the protein level in PMN. Moreover, differential expression of these proteins was found upon induction or prevention of apoptosis by cytokines: Whereas induction of apoptosis by tumor necrosis factor- was associated with a reduction of expression of the anti-apoptotic Bcl-Xl protein, prevention of apoptosis by granulocyte-macrophage colony-stimulating factor led to a downregulation of expression of the death-promoting Bax- protein. This shift of balance of anti- and pro-apoptotic proteins was found to control caspase-3 activity which, in turn, downregulated Bcl-Xl expression in PMN undergoing apoptosis. Thus, cytokines can affect the ratio of Bax-/Bcl-Xl expression in human PMN and modulate the subsequent activity of caspase-3, which functions as executer of the programmed cell death and may promote apoptosis by a positive feed-forward mechanism that downregulates Bcl-Xl.
APOPTOSIS (programmed cell death) is thought to contribute to the homeostasis of functional leukocyte pools.1 It is an active and well-regulated process that is characterized by specific phenomena such as cell shrinkage, chromatin condensation, internucleosomal DNA fragmentation, membrane blebbing and, finally, the decay into apoptotic bodies.2-4 Mature human polymorphonuclear neutrophils (PMN) undergo apoptosis spontaneously within hours to days, and this is thought to contribute to the high turnover of these cells in the circulation.5The life span of circulating PMN is relatively short when compared with other leukocytes, but it can be further shortened or extended by accelerating or delaying apoptosis.4 Upon induction of apoptosis, the plasma membrane of the PMN remains intact, thus preventing the release of proinflammatory and histotoxic contents of these cells. Because apoptosis also leads to an impairment of PMN responsiveness6 and allows specific recognition and elimination of the cells by phagocytosis,7 8 the induction or prevention of PMN apoptosis is currently discussed as a key event in the control of inflammation.
Various inflammatory cytokines are able to affect PMN survival by modulating apoptosis. The granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to inhibit PMN apoptosis both in vitro as well as in the circulation.9,10 This effect occurs during systemic responses and is thought to strengthen the inflammatory reaction. Other cytokines such as tumor necrosis factor-α (TNF-α) serve as potent inductors of apoptosis.11 Adhesive interactions via CD11/CD18 during PMN recruitment to sites of inflammation also contribute to induction of apoptosis of emigrated PMN, which may help to subside local inflammatory reactions by safe elimination of emigrated PMN.12 13
Although PMN apoptosis seems to be critical for homeostasis as well as for the control of inflammatory processes, the molecular mechanism that underlies the control of PMN apoptosis is widely unknown. A previous report presented evidence that activation of Lyn, a member of the Src kinase family, may be involved in the GM-CSF–mediated delay of PMN apoptosis.14 Because expression of bcl-2, a member of the bcl-2 family of apoptosis-associated genes, was found to be absent in PMN, the investigators stated that apoptosis-associated genes which are critical for the control of apoptosis in many other cell systems are probably not involved in the regulation of PMN apoptosis. However, prolonged survival of PMN—caused by inhibition of apoptosis—was previously observed in bcl-2 transgenic mice,15 suggesting that the targets of the Bcl-2 protein are present in PMN.
The mammalian Bcl-2 family of apoptosis-associated proteins consists of members that inhibit apoptosis (Bcl-2, Bcl-Xl, Mcl-1, A1, etc) and others that induce apoptosis (Bax, Bak, Bad, Bcl-xs, Bik, etc), and the balance between pro-apoptotic and anti-apoptotic members determines the fate of the cells in many systems.16,17 Bcl-2 antagonizes cell death, like other members of this protein family, by forming homodimers as well as heterodimers with different homologues. The upregulation of anti-apoptotic Bcl-2 or its close homologue Bcl-Xl is known to inhibit apoptosis,18,19 whereas the downregulation of Bcl-220 or its antagonization by dimerization with, eg, Bax-α promotes programmed cell death.20 The Bcl-2 family regulates apoptosis, eg, by controlling the activity of caspases, the executioners of apoptosis, via release of cytochrome C from mitochondria.21 Although caspase-3 (CPP32) activity was identified in human PMN undergoing apoptosis,22 there is no information about the molecules that may control this protease in PMN.
The present study was undertaken to elucidate the putative role of the Bcl-2 family of apoptosis-associated proteins in the control of PMN apoptosis. Apoptosis of isolated human PMN was measured in GM-CSF– or TNF-α–treated cells as well as in untreated control cells, respectively, by flow cytometric analysis of DNA content, by DNA fragmentation assay (DNA ladder), and by measuremnt of CD16 expression on the cell surface. Expression of bcl-2, bcl-Xl, and bax-α was analyzed by reverse transcription-polymerase chain reaction (RT-PCR) as well as by the Western blotting technique at the protein level. Activation of caspase-3 was analyzed by detection of proteolytic cleavage of the pro-caspase as well as by direct measurement of caspase-3 acitivity.
MATERIALS AND METHODS
Isolation of human PMN and culture of HL-60 cells.
PMN were isolated from heparinized blood (10 I.E./mL) of healthy donors. After erythrocyte sedimentation in the presence of 40% (vol/vol) autologous plasma, the leukocyte-rich plasma was layered onto a discontinuous Percoll gradient (Sigma Chemie, Deisenhofen, Germany) as described23 and centrifuged at 600g for 20 minutes. The PMN-containing band was collected and washed in Dulbecco’s phosphate-buffered saline (PBS). Cells were resuspended in RPMI-1640 medium supplemented with 10% fetal calf serum. PMN viability was greater than 97% as assessed by the trypan blue exclusion test; purity was greater than 98% as analyzed by microscopy using Hemacolor staining (Merck, Darmstadt, Germany). The human leukemia cell line HL-60 and the B-cell line BL-41 were grown in RPMI-1640 medium supplemented with 10% fetal calf serum and antibiotics (50 U/mL penicillin, 50 μg/mL streptomycin) in 5% CO2 at 37°C.
Analysis of DNA content.
DNA content was analyzed by flow cytometry (FACScan; Becton Dickinson, San Jose, CA) using propidium iodide. Briefly, PMN (5 × 105/100 μL) were washed with PBS supplemented with 0.5 mmol/L EDTA and resuspended in 70% ethanol. PMN were permeabilized overnight at −20°C, washed with PBS supplemented with 0.5% EDTA and suspended in 250 μL PBS with 0.5 mmol/L EDTA. After addition of 20 μg/mL DNase-free RNase and 50 μg/mL propidium iodide (final concentrations), samples were incubated for 15 minutes at room temperature and kept at 4°C until flow cytometric analysis. In each sample, 104 cells were counted, gated off-line for granulocytes, and analyzed using Cell Quest software (Becton Dickinson).
Cell surface expression of CD16.
Aliquots of cells (5 × 105/100 μL) were incubated for 1 hour at 4°C in the dark with the fluorescein isothiocyanate (FITC)-conjugated anti-CD16 monoclonal antibody (MoAb; final dilution of 1:20), washed twice, and subjected to flow cytometry. In each sample, 104 cells were counted, gated off-line for granulocytes, and analyzed using Cell Quest software.
Internucleosomal DNA fragmentation assay.
PMN (107) were lysed for 10 minutes on ice in 600 μL hypotonic lysis buffer (10 mmol/L EDTA, 0.2% Triton X-100 (Sigma Chemie), 10 mmol/L Tris, pH 7.5). After centrifugation for 10 minutes at 4°C at 13,000g, DNA was isolated by phenol/chloroform extraction and subsequent precipitation with 2.5 vol ethanol containing 0.1 mol/L NaCl overnight at −20°C. After centrifugation at 13,000g, the pellets were washed in 70% ethanol, dried, and resuspended in 20 μL H2O. After treatment with DNase-free RNase in a final concentration of 0.8 mg/mL for 30 minutes at 37°C, samples were analyzed by gel electrophoresis in 1.8% agarose and ethidium bromide staining.
RT-PCR.
Total RNA was isolated using the guanidine isothiocyanate method24 using TRIZOL (Life Technologies, Eggenstein, Germany). RNA (500 ng) was transcribed into cDNA using oligo(dT) primers (Life Technologies) and 50 U reverse transcriptase Moloney murine leukemia virus (MMLV; Promega, Madison, WI). PCR amplification was performed using specific primer sets (TIP MOLBIOL, Berlin, Germany) for bcl-2 (upstream primer: 5′-GGT-GCC-ACC-TGT-GGT-CCA-CCT, downstream primer: 5′-CTT-CAC-TTG-TGG-CCC-AGA-TAG-G, 458-bp product), bcl-X (upstream primer: 5′-TTG-GAC-AAT-GGA-CTG-GTT-G, downstream primer: 5′-GTA-GAG-TGG-ATG-GTC-AGT-G, 765-bp product); bax-α (upstream primer: 5′-CTG-ACA-TGT-TTT-CTG-ACG-GC, downstream primer: 5′-TCA-GCC-CAT-CTT-CTT-CCA-GA, 289-bp product); and bak (upstream primer: 5′-TGA-AAA-ATG-GCT-TCG-GGG-CAA-GGC, downstream 5′-TCA-TGA-TTT-GAA-GAA-TCT-TCG-TAC-C, 642-bp product). For control, a specific primer set for GADPH (upstream primer: 5′-GGT CGG AGT CAA CGG ATT TGG T, downstream primer: 5′-TGT GGG CCA TGA GGT CCA CCA C) was used, which yielded a 977-bp product. PCR (30 cycles: 1 minute at 94°C, 1 minute at 55°C, 1 minute at 72°C) was performed using 1.25 U AmpliTaq DNA polymerase (Perkin Elmer, Weiterstadt, Germany). PCR products were analyzed by agarose gel electrophoresis and visualized with ethidium bromide under UV light.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
After stimulation as indicated, PMN were pelleted and lysed in 1XLaemmli-buffer (2% [wt/vol] SDS, 6% (vol/vol) 2-mercaptoethanol, 10% (vol/vol) glycerol, and a trace amount of bromphenol blue in 200 mmol/L Tris-HCl, pH 7.5). The samples were immediately heated for 5 minutes at 100°C. Total cell lysates (106 cells/sample) were subjected to SDS-PAGE on gels containing 12% (wt/vol) acrylamide under reducing conditions.25 Separated proteins were transferred to nitrocellulose filters using semidry technique at 180 mA for 1 hour. Filters were blocked by treatment with 3% BSA in TBS for 1 hour, and subsequently incubated with the primary monoclonal or polyclonal antibodies (Abs) in a final concentration of 1 μg/mL or at a final dilution of 1:1,000, respectively, for 1 hour in TBS supplemented with 0.1% BSA. After three washes in TBS containing 0.1% Tween-20, filters were incubated with peroxidase-conjugated secondary Abs (final dilution, 1:1,000) in TBS supplemented with 0.1% BSA for 1 hour and subsequently washed as described above. Detection was performed by chemiluminescence using ECL-kit (Enhanced ChemiLuminescence; Amersham Life Science, Braunschweig, Germany) and subsequent autoluminography by exposure to x-ray films (XOMAT-AR, Kodak, Stuttgart, Germany). Densitometry was performed using One-Dscan software (Scanalytics, Billerica, MA).
Measurement of caspase-3 activity.
PMN (5 × 106) were washed with PBS and lysed in 100 μL of 10 mmol/L potassium phosphate, 1 mmol/L EDTA, 0.5% Triton X-100, 2 mmol/L phenlymethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL pepstatin, and 10 mmol/L dithiothreitol. After incubation for 10 minutes on ice, samples were centrifuged at 18,000g for 20 minutes at 4°C. The protein concentration of the supernatant was determined by colorimetric measurement using the BCA protein assay reagent (Pierce, Rockford, IL) according to supplier’s instructions. An aliquot of each sample (40 μg) was diluted to a final volume of 200 μL in assay buffer consisting of 50 mmol/L HEPES, 10% sucrose, 0.1% CHAPS, and 10 mmol/L dithiothreitol supplemented with 10 μmol/L of the fluorogenic caspase-3 substrate T-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin (Z-DEVD-AFC). Samples were incubated for 90 minutes at 30°C and measured at an exitation wavelength of 400 nm and an emission wavelength of 505 nm using a luminescence spectrometer (LS 50B; Perkin Elmer). Blanks were measured in the absence of cell lysate to determine background fluorescence.
Antibodies.
The polyclonal rabbit anti-human Bcl-X (Ab-1) and the monoclonal mouse anti-human Bak Ab (clone TC100) were obtained from Calbiochem (Bad Soden, Germany). The polyclonal rabbit anti-human Bax (N-19) was purchased from Santa Cruz Biotechnology (Heidelberg, Germany). The peroxidase-conjugated goat anti-mouse IgG and the peroxidase-conjugated goat anti-rabbit IgG antibodies were obtained from Sigma (Deisenhofen, Germany). The FITC-conjugated anti-human CD16 (Fcγ receptor type III) MoAb (clone DJ130c) was purchased from Dako Diagnostica (Hamburg, Germany). The polyclonal rabbit anti-human caspase-3 Ab was obtained from PharMingen (San Diego, CA).
Reagents.
BSA, Chaps, dithiothreitol, leupeptin, ovalbumin, penicillin, pepstatin, Percoll, phenlymethylsulfonyl fluoride, Ponceau S, propidium iodide, streptomycin, sucrose, TNF-α, Triton X-100, and Tween-20 were obtained from Sigma. ECL Western blotting kit (RPN 2106) and electrophoresis calibration standards for molecular mass determination were purchased from Pharmacia (Freiburg, Germany). GM-CSF was obtained from Boehringer (Mannheim, Germany). The fluorogenic caspase-3 substrate Z-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin (Z-DEVD-AFC) and the caspase-3 inhibitor Z-Asp-Glu-Val-Asp-7-fluoromethylketone (Z-DEVD-FMK) were purchased from Calbiochem. Buffers, cell culture media, and fetal calf serum were obtained from Biochrom (Berlin, Germany).
Statistical analysis.
Data shown represent mean ± SD where applicable. Statistical significance was determined using Student’s t-test; P< .05 was considered statistically significant.
RESULTS
Regulation of apoptosis by cytokines.
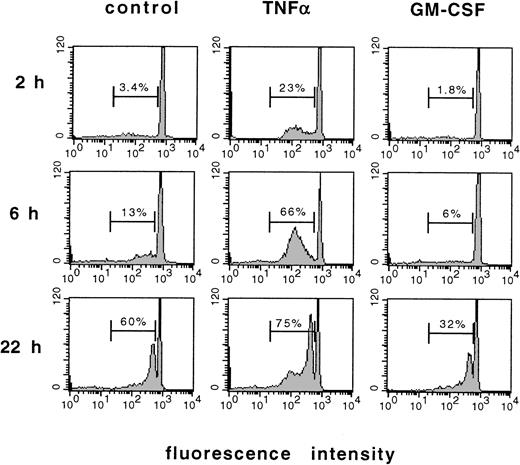
To characterize apoptosis of mature human PMN, cells were freshly isolated from the circulation and cultured in the presence of TNF-α, GM-CSF, or left untreated for control. The loss of DNA content, a well-known marker of apoptosis,2 was measured by flow cytometry using propidium iodide staining. Figure 1 shows the original fluorescence histograms obtained from these experiments. Unstimulated freshly isolated PMN showed only fluorescence peak after up to 2 hours of culture, which resulted from a uniform diploid DNA content of these cells. Only a few more cells showed a loss of DNA content when aged for 6 hours in culture, but a substantial amount of PMN showed a diminished DNA content, ie, underwent apoptosis spontaneously after 22 hours of culture. This effect was accelerated by stimulation with TNF-α, which induced a substantial loss of DNA content as early as 2 hours and 6 hours after the onset of stimulation. In contrast, GM-CSF inhibited the loss of DNA content associated with apoptosis when compared with unstimulated control cells.
Loss of DNA content in PMN undergoing apoptosis as measured by flow cytometry of prodium iodide–stained PMN. Original recordings of fluorescence histograms of PMN freshly isolated from the circulation, aged for 2, 6, or 22 hours in culture without further stimulation (control), or treated with 300 U/mL TNF- or 300 U/mL GM-CSF, respectively. Numbers indicate apoptotic PMN in percent of total cell number. Results are representative of 12 independent experiments.
Loss of DNA content in PMN undergoing apoptosis as measured by flow cytometry of prodium iodide–stained PMN. Original recordings of fluorescence histograms of PMN freshly isolated from the circulation, aged for 2, 6, or 22 hours in culture without further stimulation (control), or treated with 300 U/mL TNF- or 300 U/mL GM-CSF, respectively. Numbers indicate apoptotic PMN in percent of total cell number. Results are representative of 12 independent experiments.
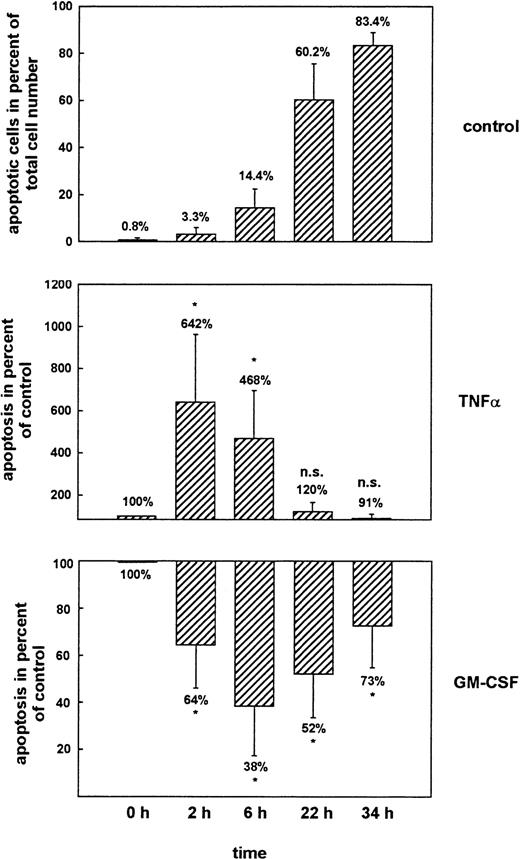
Figure 2 shows the mean values of 12 independent experiments. The prolonged time-course revealed that more than 80% of unstimulated PMN underwent apoptosis spontaneously within 34 hours of culture. TNF-α was able to increase apoptosis significantly within 2 hours and 6 hours of culture to 642% and 468% of the values seen at the same time-points in the untreated controls (100%). The relative ineffectiveness of TNF-α to induce apoptosis at later time-points (22 hours and 34 hours) was due to the fact that the majority of unstimulated cells already underwent apoptosis spontaneously within this time period. GM-CSF induced its maximal effect at 6 hours after the onset of stimulation and reduced apoptosis to 38% of control, but significant inhibition of apoptosis was also observed at earlier (2 hours) and later (22 hours and 34 hours) time-points. The effects of TNF-α and GM-CSF were dose dependent (data not shown).
Regulation of apoptosis by cytokines as measured by flow cytometry of propidium iodide–stained PMN. PMN were aged for 2, 6, 22, or 34 hours in culture without further stimulation (control), or treated with 300 U/mL TNF- or 300 U/mL GM-CSF, respectively. Data represent apoptotic PMN in percent of total cell number (top panel) or apoptotic PMN in percent of the values seen in the untreated samples at the same time points (middle and bottom panels). Mean ± SD of 12 independent experiments. *P < .05 versus unstimulated control. The effects of TNF- and GM-CSF were dose-dependent (data not shown).
Regulation of apoptosis by cytokines as measured by flow cytometry of propidium iodide–stained PMN. PMN were aged for 2, 6, 22, or 34 hours in culture without further stimulation (control), or treated with 300 U/mL TNF- or 300 U/mL GM-CSF, respectively. Data represent apoptotic PMN in percent of total cell number (top panel) or apoptotic PMN in percent of the values seen in the untreated samples at the same time points (middle and bottom panels). Mean ± SD of 12 independent experiments. *P < .05 versus unstimulated control. The effects of TNF- and GM-CSF were dose-dependent (data not shown).
Regulation of apoptosis was also apparent by loss of Fc receptor expression and internucleosomal DNA degradation.
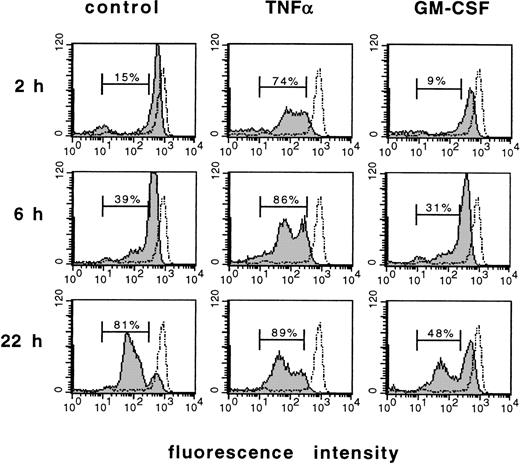
Figure 3 shows a flow cytometric analysis of CD16 (Fc receptor type III) expression on the cell surface of PMN. This surface molecule has previously been shown to be downregulated in PMN undergoing apoptosis.26 PMN freshly isolated from the circulation showed a high surface expression of CD16. Within 2 hours and 6 hours of culture, the population with low CD16 expression increased gradually when PMN were aged in culture, and reached 81% of total cell number within 22 hours. This effect was accelerated by TNF-α, which decreased CD16 expression substantially as early as 2 hours after stimulation and led to about 74% of PMN with low CD16 expression within this time period. In contrast, GM-CSF inhibited the loss of high CD16 surface expression during culture and diminished the population with low CD16 expression markedly when compared with control cells.
Induction or prevention of apoptosis by cytokines as measured by analysis of CD16 expression. Flow cytometric analysis of DNA content using the FITC-labeled anti-CD16 MoAb. PMN freshly isolated from the circulation (dotted line) were stimulated for 2, 6, or 22 hours with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Numbers indicate the cells with low CD16 expression in percent of total cell number. Results are representative of three independent experiments.
Induction or prevention of apoptosis by cytokines as measured by analysis of CD16 expression. Flow cytometric analysis of DNA content using the FITC-labeled anti-CD16 MoAb. PMN freshly isolated from the circulation (dotted line) were stimulated for 2, 6, or 22 hours with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Numbers indicate the cells with low CD16 expression in percent of total cell number. Results are representative of three independent experiments.
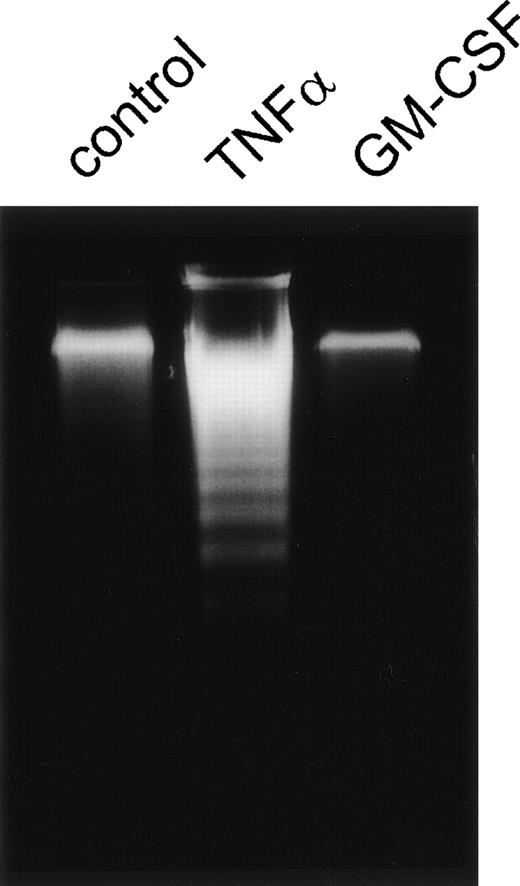
As shown in Fig 4, similar results were obtained by analysis of internucleosomal DNA degradation, a ubiquitious marker for apoptosis.3 PMN were stimulated for 6 hours by TNF-α, GM-CSF, or left untreated, respectively. Unstimulated PMN showed only weak DNA degradation within this time period whereas TNF-α–stimulated cells revealed strong DNA degradation. Almost no DNA laddering was detectable in GM-CSF–treated cells. Thus, three independent methods showed that the experimental conditions used were suitable to detect spontaneous apoptosis as well as induction or prevention of apoptosis by the cytokines TNF-α and GM-CSF, respectively.
Apoptosis of PMN measured by internucleosomal DNA degradation. Agarose gel of low-molecular-weight DNA of PMN stimulated for 6 hours with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Results are representative of three independent experiments.
Apoptosis of PMN measured by internucleosomal DNA degradation. Agarose gel of low-molecular-weight DNA of PMN stimulated for 6 hours with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Results are representative of three independent experiments.
Gene expression of members of the bcl-2 family.
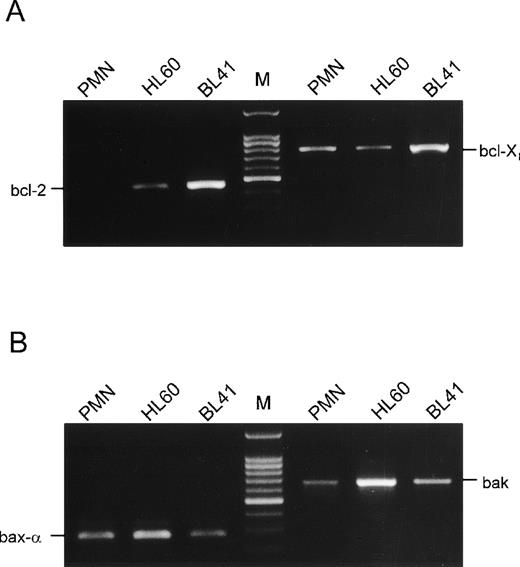
Expression of members of the bcl-2 family of apoptosis-associated genes was studied by RT-PCR of RNA derived from PMN freshly isolated from the circulation. Using specific primers, bcl-2 was found to be absent in PMN (Fig 5A). This was also true for unstimulated, GM-CSF– or TNF-α–stimulated PMN after 2, 6, 22, or 34 hours of culture (data not shown). In contrast, bcl-2 was detectable in the pro-myeloid cell line HL-60 as well as in the B-cell line BL-41, which was used for positive control.27 However, bcl-Xl, another anti-apoptotic member of this gene family, was detectable in PMN as well as in HL-60 cells and BL-41 cells, respectively. Analysis of PMN for expression of pro-apoptotic genes, which are known to heterodimerize with the bcl-Xl gene product, gave positive results (Fig 5B): RT-PCR products for both bax-α and bak were found in PMN. Both genes were also expressed in HL-60 cells as well as in BL-41 cells, respectively. Because bcl-Xl as well as its pro-apoptotic counterparts bax-α and bak have the capability to regulate apoptosis, we studied their expression by Western blotting technique.
Analysis of mRNA expression of members of the bcl-2 family. Total RNA of PMN freshly isolated from the circulation, HL-60, or BL41 cells for positive control, respectively, was subjected to RT-PCR using primers specific for bcl-2 or bcl-X (A), as well as for bax- or bak (B). PCR products on a 1.5% agarose gel are shown. Results are representative of three independent experiments.
Analysis of mRNA expression of members of the bcl-2 family. Total RNA of PMN freshly isolated from the circulation, HL-60, or BL41 cells for positive control, respectively, was subjected to RT-PCR using primers specific for bcl-2 or bcl-X (A), as well as for bax- or bak (B). PCR products on a 1.5% agarose gel are shown. Results are representative of three independent experiments.
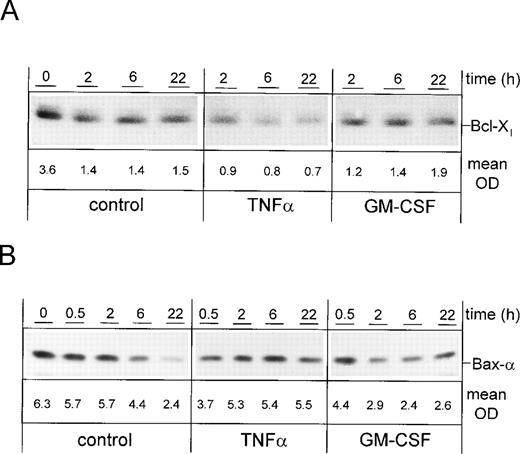
Effect of cytokines on differential protein expression of members of the Bcl-2 family.
PMN were cultured for 0, 2, 6, or 22 hours in the presence of TNF-α or GM-CSF, or were left untreated. Whole-cell lysates were subjected to SDS-PAGE and immunoblotted with an anti–Bcl-X antibody (Fig 6A). Whereas unstimulated freshly isolated PMN showed high expression of Bcl-Xl, the protein level decreased with time of culture, suggesting that the loss of the protective effect of Bcl-Xl may play a role in spontaneous apoptosis. The reduction of Bcl-Xl expression in the presence of TNF-α was much stronger when compared with control cells. This suggests that the mechanism which allows TNF-α to enhance apoptosis may be associated with an accelerated downregulation of Bcl-Xl. In contrast, the level of Bcl-Xl in GM-CSF–stimulated PMN was not altered when compared with unstimulated cells, indicating that the inhibition of apoptosis by this cytokine is not mediated by (up-)regulation of Bcl-Xl expression.
Differential expression of Bcl-Xl and Bax- upon induction or prevention of apoptosis by cytokines. PMN were stimulated for indicated times with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Whole-cell lysates were subjected to SDS-PAGE and Western blot was performed using the anti–Bcl-X (A) or anti–Bax- Abs and a peroxidase-conjugated secondary antibody (B). Numbers indicate mean OD of each lane obtained from three representative and independent experiments. Bak was present in BL41 cells but not detectable in PMN at any time point (data not shown).
Differential expression of Bcl-Xl and Bax- upon induction or prevention of apoptosis by cytokines. PMN were stimulated for indicated times with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Whole-cell lysates were subjected to SDS-PAGE and Western blot was performed using the anti–Bcl-X (A) or anti–Bax- Abs and a peroxidase-conjugated secondary antibody (B). Numbers indicate mean OD of each lane obtained from three representative and independent experiments. Bak was present in BL41 cells but not detectable in PMN at any time point (data not shown).
Next, protein expression of Bax-α was studied (Fig 6B). Whereas unstimulated PMN showed a gradual decrease of Bax-α expression with time of culture, protein expression remained almost constant upon stimulation with TNF-α. In contrast, GM-CSF induced a downregulation of Bax-α when compared with control cells, suggesting that the downregulation of this death-promoting protein is involved in PMN survival mediated by GM-CSF. Although present in BL41 cells, Bak was not detectable in PMN, which may reflect a low expression of this protein (data not shown). Thus, rather differential expression of Bcl-Xl and Bax-α, which occurred during induction, or prevention of apoptosis by cytokines may be critical for control of apoptosis: Whereas TNF-α seems to alter the Bax-α/Bcl-Xl ratio by downregulating Bcl-Xland stabilizing Bax-α expression, and thereby may promote apoptosis, GM-CSF seems to promote survival by modulating the Bax-α/Bcl-Xl ratio via downregulation of Bax-α. Data were confirmed by analysis of protein expression using densitometry.
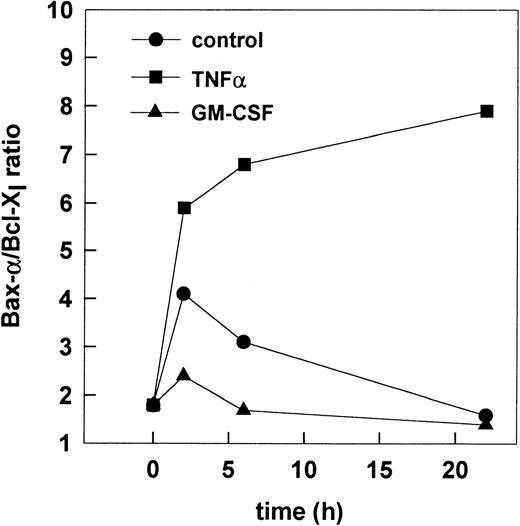
To further characterize the balance of Bax-α and Bcl-Xlin PMN undergoing apoptosis, the Bax-α/Bcl-Xl ratio was calculated using the mean optical density (OD) of Bax-α and Bcl-Xl obtained from three independent experiments (Fig 7). During spontaneous apoptosis a transient increase of the Bax-α/Bcl-Xl ratio occurred within 2 hours of culture, which may account for induction of spontaneous apoptosis. When compared with control cells, TNF-α led to a sustained increase of the Bax-α/Bcl-Xl ratio within 2 hours when compared with control cells. In contrast, a decrease of the Bax-α/Bcl-Xl ratio was observed in the presence of GM-CSF within 2 hours and 6 hours of culture when compared with the ratio obtained from unstimulated control cells at the same time-points. Thus, the Bax-α/Bcl-Xl ratio may be responsible for the induction or prevention of apoptosis. To test the hypothesis that the altered Bax-α/Bcl-Xl ratio may represent the functional relevant check-point for the regulation apoptosis, the activation of caspase-3 was studied, which finally executes the apoptotic program in PMN.28
Alteration of the Bax-/Bcl-Xl ratio upon induction or prevention of apoptosis. The Bax-/Bcl-Xlratio was calculated from mean OD of Bax- and Bcl-Xlobtained in the three independent cell experiments described in Fig6.
Alteration of the Bax-/Bcl-Xl ratio upon induction or prevention of apoptosis. The Bax-/Bcl-Xlratio was calculated from mean OD of Bax- and Bcl-Xlobtained in the three independent cell experiments described in Fig6.
Activation of caspase-3 in PMN undergoing apoptosis.
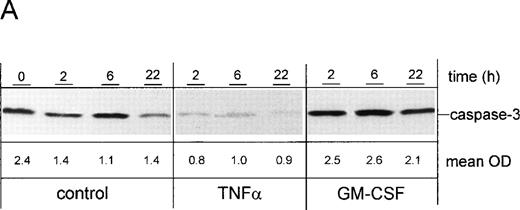
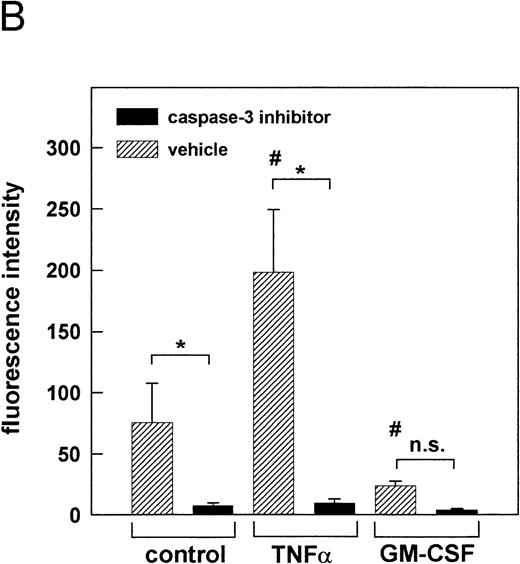
PMN were cultured for 2, 6, or 22 hours in the presence of TNF-α or GM-CSF, or were left untreated. Whole-cell lysates were subjected to SDS-PAGE and immunoblotted with a caspase-3 antibody. Results were confirmed by densitometry (Fig 8A). The expression of the proteolytically inactive pro-caspase of 32 kD was substantially decreased in the presence of TNF-α when compared with unstimulated control cells at the same time points (2, 6, and 22 hours), suggesting an enhanced cleavage into the active form. In contrast, an increased pro-caspase level was observed in the presence of GM-CSF when compared to control cells, indicating that GM-CSF may exert its protective effect by inhibiting proteolytic cleavage of the pro-caspase-3 into the active form. To confirm this result, the enzymatic activity of caspase-3 was measured in the cell lysates obtained from unstimulated control cells as well as from TNF-α– and GM-CSF–stimulated cells, respectively (Fig 8B). Using a fluorogenic substrate bearing the caspase-3–specific cleavage site DEVD, caspase-3 activity was detected as early as 2 hours after the onset of culture in unstimulated control cells. Activity of caspase-3 was markedly enhanced upon stimulation by TNF-α. As expected, GM-CSF induced a downregulation of caspase-3 activity when compared with unstimulated control cells. In all samples, caspase-3 activity was almost completely inhibited after pretreatment of the cells with 200 μmol/L of the specific caspase-3 inhibitor Z-DEVD-FMK, demonstrating the specificity of the employed assay. This shows that the modulatory role of the cytokines on the processing of the pro-caspase corresponded to the enzymatic activity of caspase-3 in the cell lysates. Thus, two independent methods showed that the cytokine-mediated alteration of the Bax-α/Bcl-Xl ratio resulted in the modulation of caspase-3 activity.
Effect of cytokines on caspase-3 activation. PMN were stimulated for indicated times with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. (A) After stimulation for indicated times, whole-cell lysates were subjected to SDS-PAGE and Western blot was performed using the anti-caspase-3 Ab and a peroxidase-conjugated secondary antibody. Numbers indicate mean OD of each lane obtained from three representative and independent experiments. (B) Caspase-3 activity was measured in whole-cell lysates obtained from PMN, which were treated for 1 hour at 37°C with 200 μmol/L of the caspase-3 inhibitor Z-DEVD-FMK or left untreated (vehicle) before stimulation for 2 hours as indicated. Caspase-3 activity is shown as mean fluorescence intensity ± SD obtained from three independent experiments. *P < .05; #P < .05 versus unstimulated control; n.s., not significant.
Effect of cytokines on caspase-3 activation. PMN were stimulated for indicated times with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. (A) After stimulation for indicated times, whole-cell lysates were subjected to SDS-PAGE and Western blot was performed using the anti-caspase-3 Ab and a peroxidase-conjugated secondary antibody. Numbers indicate mean OD of each lane obtained from three representative and independent experiments. (B) Caspase-3 activity was measured in whole-cell lysates obtained from PMN, which were treated for 1 hour at 37°C with 200 μmol/L of the caspase-3 inhibitor Z-DEVD-FMK or left untreated (vehicle) before stimulation for 2 hours as indicated. Caspase-3 activity is shown as mean fluorescence intensity ± SD obtained from three independent experiments. *P < .05; #P < .05 versus unstimulated control; n.s., not significant.
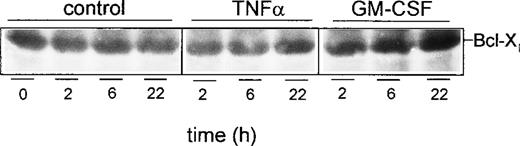
Next, additional experiments were performed to elucidate the molecular mechanism that underlies the alteration of the Bax-α/Bcl-Xl ratio, ie, the downregulation of Bcl-Xl upon TNF-α stimulation. Because caspase-3 has recently been shown to convert the anti-apoptotic Bcl-2 protein, a homologue of Bcl-Xl, into a death-promoting factor,29 the effect of caspase-3 inhibition was studied on the expression of Bcl-Xl (Fig9). PMN were treated with 200 μmol/L of the cell-permeable caspase-3 inhibitor Z-DEVD-FMK for 1 hour at 37°C and were stimulated with TNF-α or GM-CSF, or left untreated. Subsequent Western blot analysis of Bcl-Xl expression showed that spontaneous as well as TNF-α–induced downregulation of Bcl-Xl was almost completely abolished in the presence of the caspase-3 inhibitor (compare with Fig 6A). This suggests that the observed downregualtion of Bcl-Xl is due to an enhanced protein turnover, probably via direct proteolytic cleavage of Bcl-Xl by caspase-3. Thus, caspase-3 seems to play a pivotal role in mediating apoptosis of human PMN.
Effect of caspase-3 inhibition on expression of Bcl-Xl. PMN were treated for 1 hour at 37°C with 200 μmol/L of the caspase-3 inhibitor Z-DEVD-FMK before stimulation for indicated times with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Whole-cell lysates were subjected to SDS-PAGE and Western blot was performed using the anti–Bcl-X Ab and a peroxidase-conjugated secondary antibody. Results are representative of three independent experiments.
Effect of caspase-3 inhibition on expression of Bcl-Xl. PMN were treated for 1 hour at 37°C with 200 μmol/L of the caspase-3 inhibitor Z-DEVD-FMK before stimulation for indicated times with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Whole-cell lysates were subjected to SDS-PAGE and Western blot was performed using the anti–Bcl-X Ab and a peroxidase-conjugated secondary antibody. Results are representative of three independent experiments.
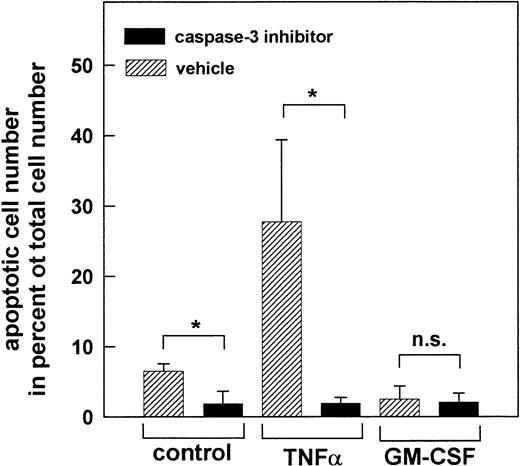
To prove the biological significance of caspase-3 activation, apoptosis of PMN was measured in the presence of the caspase-3 inhibitor Z-DEVD-FMK (Fig 10). After treatment of the PMN with 200 μmol/L the caspase-3 inhibitor for 1 hour at 37°C, apoptosis was almost reduced to the level observed in freshly isolated PMN. This was true for both, spontaneous as well as TNF-α–induced apoptosis of PMN, and shows that activation of caspase-3 is critical for the induction of apoptosis. As expected, no further reduction of apoptosis was observed when PMN were stimulated with GM-CSF after inhibition of caspase-3, suggesting that inhibition of apoptosis by this cytokine may be due to prevention of caspase-3 activation. Thus, alteration of the Bax-α/Bcl-Xl ratio seems to represent the key event in the regulation of apoptosis of human PMN by controlling the execution of the apoptotic program via caspase-3.
Prevention of apoptosis by inhibition of caspase-3. PMN were treated for 1 hour at 37°C with 200 μmol/L of the caspase-3 inhibitor Z-DEVD-FMK or left untreated (vehicle) before stimulation for 2 hours with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Data represent apoptotic PMN in percent of total cell number. Mean ± SD of three independent experiments. *P < .05; n.s., not significant.
Prevention of apoptosis by inhibition of caspase-3. PMN were treated for 1 hour at 37°C with 200 μmol/L of the caspase-3 inhibitor Z-DEVD-FMK or left untreated (vehicle) before stimulation for 2 hours with 300 U/mL TNF-, 300 U/mL GM-CSF, or left untreated (control), respectively. Data represent apoptotic PMN in percent of total cell number. Mean ± SD of three independent experiments. *P < .05; n.s., not significant.
DISCUSSION
In the present study, a mechanism that can determine the lifetime of PMN is reported. Gene expression of bcl-Xl, bax-α, and bak, members of the bcl-2 family that is known to control the apoptotic machinery, were identified in mature human PMN isolated from the circulation. The anti-apoptotic bcl-Xl gene as well as the death-promoting bax-α gene were also found in significant amounts at the protein level. Although present in control cells, expression of bak was not detectable in PMN at the protein level, suggesting that its expression may be rather low when compared with Bcl-Xl and Bax-α. The myeloid cell line HL-60 was not only positive for expression of the three above-mentioned genes, but also for bcl-2 mRNA, which was absent in mature PMN. This is in agreement with a previous finding showing that bcl-2 is downregulated in HL-60 cells upon granulocytic differentiation.30 The presence of bcl-2 expression in pro-myeloid cells and the absence in mature PMN is consistent with the observation that bcl-2 is downregulated, eg, in lymphocytes under negative selection but highly expressed in pro B cells and mature B cells that are selected to survive.31Thus, the absence of bcl-2 expression can be interpreted as determination to undergo apoptosis. However, the expression of other members of the bcl-2 family of apoptosis-associated genes implies that mature PMN do not simply die due to the absence of bcl-2 expression, but that the apoptotic program—at least its time-course—is subject to regulation. Bcl-Xl shows some functional redundancy with Bcl-2 in suppressing cell death, but only Bcl-Xl, and not Bcl-2, was observed to inhibit apoptosis in WEHI-231.7 cells in response to cross-linking of IgM and other stimuli.32Similarly, Bcl-Xl may fulfill a special anti-apoptotic function in mature human PMN. However, both Bcl-2 and Bcl-Xl are known to act as anti-apoptotic counterparts of Bax-α,33 and this may explain the observation that expression of bcl-2 in PMN of transgenic mice prevents apoptosis, although it is absent in normal PMN.20
Both the anti-apoptotic Bcl-Xl, the death-antagonizing splice variant of the bcl-X gene, and the pro-apoptic Bax-α, the death-promoting splice variant of the bax gene, are known to form homodimers and heterodimers, respectively.33 The shift of balance of the Bax-α/Bcl-Xl ratio—which can be achieved by upregulation or downregulation of both interacting partners—has previously been shown to determine survival and apoptosis in other cell systems.34 In our system, the survival factor GM-CSF induced a downregulation of pro-apoptotic Bax-α, and TNF-α led to a decrease of survival-promoting Bcl-Xl. Thus, a downregulation of the death or survival promoting proteins seems to modulate the Bax-α/Bcl-Xl ratio in PMN. When compared with other leukocytes, the life span of PMN is extremely short, and its regulation was found to occur within hours upon cytokine treatment. Downregulation may provide an appropriate mechanism that is fast enough to meet this requirement.
The fact that downregulation of Bcl-Xl was almost completely abolished upon inhibition of caspase-3 suggests that caspase-3 is critically involved in the regulation of Bcl-Xl expression. Thus, rather an enhanced turnover of Bcl-Xl than its transcriptional regulation seems to control the level of Bcl-Xl expression. This is consistent with previous findings showing that the death-preventing protein Bcl-2—a close homologue of Bcl-Xl—serves as a substrate for caspase-3 in cells undergoing apoptosis. Moreover, the cleavage product of Bcl-2 was found to exert a death-promoting function, which further strengthened the apoptotic process.29 Thus, the enhanced downregulation of Bcl-Xl expression, which promoted the shift of the Bax-α/Bclxl ratio and induced apoptosis upon TNF-α stimulation, presumably underlies direct regulation by caspase-3. This observation not only elucidates the molecular mechanism that may underlie the regulation of Bcl-Xl downregulation, but also may explain the acceleration of apoptotic cell death upon TNF-α stimulation by providing a powerful feed-forward mechanism.
Similarly, this may be relevant for spontaneous apoptosis, which was also found to be associated with a downregulation Bcl-Xl. However, Bax-α expression was also downregulated upon spontaneous apoptosis, suggesting the existence of a multi-step control mechanism. The fact that PMN underwent apoptosis spontaneously suggests an excess of death-promoting factors when PMN age in culture. The observed decrease of Bcl-Xl may enhance the overall susceptibility of PMN to undergo apoptosis, and the increase of the Bax-α/Bcl-Xl ratio may induce spontaneous apoptosis. Also, a possible role of other yet unidentified members of the Bcl-2 family has to be considered to contribute to the regulation of apoptosis of PMN besides Bax-α and Bcl-Xl.
In general, apoptosis is divided into three different steps. The initiation phase, which allows the intracellular signaling, precedes the effector phase, which includes regulation of apoptosis by the Bcl-2 family. Subsequently, irreversible cell death occurs upon activation of caspases, which function as the executers of apoptosis.35 The present study gives evidence that caspase-3 may be involved in the final degradation process in apoptosis of PMN, which is in agreement with previous findings.22Caspase-3 activation is generally thought to require—besides two further apoptosis protease-activating factors (Apafs-1 and -3)—the release of cytochrome c (Apaf-2) from mitochondria into the cytosol. This is thought to be regulated by the proteins of the Bcl-2 family associated with the mitochondrial membrane.36,37But there exists some evidence that caspase-3 may be also activated via Bcl-Xl independently of cytochrome c.38
Activation of caspase-3 has recently been shown to be required for DNA fragmentation in cells undergoing apoptosis in response to TNF-α,39 which is consistent with our finding that TNF-α promotes DNA laddering in human PMN. The observed downregulation of the 32-kD pro-enzyme is well known to reflect the activation of the enzyme, which is due to proteolytic cleavage in two fragments with molecular masses of 20 kD and 11 kD, respectively.40 41 The fact that processing of the pro-caspase was promoted in the presence of TNF-α and inhibited in the presence of GM-CSF suggests that induction or prevention of apoptosis by these cytokines depends on activation of caspase-3. This was confirmed by direct measurement of caspase-3 activity, which showed that TNF-α and GM-CSF inversely regulate its proteolytic activity toward a specific DEVD-containing substrate. Thus, this study not only gives evidence that caspase-3 activation is critical for the final degradation process of apoptosis as well as for the downregulation of Bcl-Xl expression, but it also shows that the functional endpoint of GM-CSF–mediated inhibition of apoptosis involves the Bax-α/Bcl-Xl–mediated prevention of caspase-3 activation. Thus, the critical checkpoint for both, induction as well as the prevention of apoptosis, seems to represent the control of caspase-3. The biological relevance of this pathway was confirmed by the finding that inhibition of caspase-3 activity almost completely prevented apoptosis of human PMN.
In summary, induction of apoptosis by TNF-α was found to be associated with a reduction of expression of the anti-apoptotic Bcl-Xl protein whereas prevention of apoptosis by GM-CSF led to a downregulation of expression of the pro-apoptotic Bax-α protein. This shift of balance of the Bax-α/Bcl-Xl ratio led to an induction of caspase-3 activation by TNF-α and a prevention of caspase-3 activation in the presence of GM-CSF. Thus, cytokines affected the ratio of Bax-α/Bcl-Xl expression and modulated the subsequent activity of caspase-3, which function as executers of the programmed cell death. The regulation of the Bcl-Xl/Bax-α ratio critically involves activation of caspase-3, which downregulated Bcl-Xl, probably by direct proteolytic cleavage, and thereby provides a positive feedback mechanism that may be responsible for the TNF-α–mediated acceleration of apoptosis. The reported Bax-α/Bcl-Xl–mediated control of apoptosis via caspase-3 seems to represent an important checkpoint with strong biological relevance, because apoptosis was almost completely absent upon inhibition of this pathway.
ACKNOWLEDGMENT
The expert technical assistance of S. Sölter is acknowledged.
Supported by Deutsche Forschungsgemeinschaft (SFB 366/C3).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Barbara Walzog, PhD, Freie Universität Berlin, Department of Physiology, Arnimallee 22, D-14195 Berlin, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal