Abstract
The trace element Zinc (Zn2+) has been implicated as a mediator in host defense, yet the molecular basis for its extracellular functions remains obscure. Here, we demonstrate that Zn2+can induce the adhesion of myelomonocytic cells to the endothelium, as well as to the provisional matrix proteins vitronectin (VN) and fibrinogen (FBG), which are pivotal steps for the recruitment of leukocytes into inflamed/injured tissue. Physiologic concentrations of Zn2+ increased the urokinase receptor (uPAR)-mediated adhesion of myelomonocytic cells to VN, whereas other divalent cations had smaller effects. Zn2+-induced cell adhesion to VN was abolished by cation chelators such as 1-10-phenanthroline, as well as by plasminogen activator inhibitor-1 (PAI-1) and a monoclonal antibody (MoAb) against uPAR. These characteristics could be recapitulated with a uPAR-transfected cell line emphasizing the specificity of this receptor system for Zn2+-dependent cell adhesion. Like urokinase (uPA), Zn2+ increased the binding of radiolabeled VN to uPAR-expressing cells, as well as the interaction of VN with immobilized uPAR in an isolated system. Moreover, Zn2+ enhanced leukocytic cell adhesion to FBG and endothelial cell monolayers by activating β2-integrins. Instead of the direct β2-integrin activation through the divalent cation binding site, Zn2+-induced integrin activation was mediated via uPAR, a crucial regulator of this system. The present study uncovers for the first time Zn2+-mediated cell adhesion mechanisms that may play a crucial role in modulating leukocyte adhesion to vessel wall components.
WHEN LEUKOCYTES EMIGRATE from the bloodstream into sites of inflammation or injury, they undergo a complex sequence of adhesion and locomotion steps. These highly coordinated processes require the expression and upregulation of various adhesion receptors on the surface of leukocytes and vascular cells.1,2 During their transmigrational phase, leukocytes adhere to provisional matrix substrates such as fibrinogen (FBG) and vitronectin (VN), which become deposited at these sites upon increases in vascular permeability or damage.3 Both adhesion proteins are recognized by different, temporally activated receptors: αMβ2-integrin (Mac-1, CD11b/CD18) mediates interaction with FBG, which is profoundly affected by extracellular divalent cations,4,5 whereas VN serves as a ligand for the urokinase receptor (uPAR) in a divalent cation-independent manner.6,7The VN-uPAR interaction mediates cell adhesion and is augmented by uPA, but blocked by plasminogen activator inhibitor-1 (PAI-1).6uPAR thereby serves a dual role in cellular interactions, (1) being critical for pericellular proteolysis and (2) contributing to cell adhesion in a proteolysis-independent fashion.8,9Consequently, the expression level of uPAR on various cell types correlates with their migratory and invasive potential.10Moreover, independently of its enzymatic activity, uPA stimulates monocytic cell chemotaxis11,12 and regulates adhesion of neutrophils and monocytes.13,14 For some of these functions, uPAR is suggested to use integrins as adapters, since it is able to form complexes with β2-integrins15-17or interferes with β1-integrin ligation.18
During our investigations of monocyte cell–matrix interactions, we noted a profound effect of zinc (Zn2+) on cell adhesion. The trace element Zn2+ is an important cofactor of several proteins and enzymes, such as transcription factors,19,20focal adhesion molecules,21 or matrix metalloproteinases.22 Recently, Zn2+ has been shown to promote the interaction of uPAR and kininogen and this is inhibited by VN, which suggests a role for Zn2+ in regulating cell adhesion.23 Zn2+ has also been reported to enhance interleukin-1β and tumor necrosis factor-α levels in peripheral blood mononuclear cells,24,25 to influence ICAM-1 expression,26 or to increase neutrophil adhesion.27
The observations that Zn2+ deficiency is associated with skin lesions, impaired wound healing, and cell-mediated immune disorders28-30 prompted us to investigate the effect of Zn2+ on adhesion of leukocytic cells in more detail. The results indicate that the adhesion of these cells to provisional matrix proteins VN and FBG and to the endothelium is stimulated by Zn2+ in a uPAR-dependent manner.
MATERIALS AND METHODS
Reagents.
Recombinant Gly158scuPA (noncleavable mutant of high molecular weight uPA)31 was kindly obtained from Dr H.R. Lijnen (University of Leuven, Belgium) and uPA was from Medac (Hamburg, Germany). VN was purified from human plasma and converted to the multimeric form as previously described.32,33 FBG was purchased from Kabivitrum (Munich, Germany), and recombinant uPAR was kindly provided by Dr N. Behrendt (Finsen Laboratory, Copenhagen, Denmark). MoAb 13H1 against human VN33 was provided by Dr P. Declerck (University of Leuven, Belgium), and anti-uPAR MoAb R3 was given by Dr G. Hoyer-Hansen (Finsen Laboratory, Copenhagen, Denmark). The blocking anti-β2-integrin MoAb 60.3 was obtained from Dr J. Harlan (University of Washington, Seattle, WA); MoAb 24, which recognizes an activation-dependent epitope of β2-integrin subunit, was kindly provided by Dr N. Hogg (University of London, UK), MoAb 2LPM19c was from Dako (Hamburg, Germany), and the blocking MoAb, Game 46, against mouse β2-integrins was from Pharmingen (Hamburg, Germany). Bovine serum albumin (BSA), ZnCl2, MnCl2, FeCl2, NiCl2, CuCl2, and CoCl2 were from Sigma (Munich, Germany), 1-10-phenanthroline was from Fluka (Neu-Ulm, Germany), vitamin D3 was from Biomol (Hamburg, Germany), transforming growth factor-β was from R&D Systems (Boston, MA), and interleukin-3 was from PBH (Hannover, Germany).
Cell culture.
Human myelomonocytic cells U937 and HL60 were from American Type Culture Collection (ATCC; Rockville, MD) and cultured as described by the supplier in RPMI-1640 medium containing 10% (vol/vol) fetal calf serum. BAF-3 cells were from ATCC and cultured in RPMI-1640 medium containing 10% (vol/vol) fetal calf serum and 2 ng/mL interleukin-3. All culture media were from GIBCO (Eggenstein, Germany).
Isolation of peripheral blood monocytes.
Buffy coats of healthy donors obtained from the blood bank were diluted 1:4 in phosphate-buffered saline and loaded on Histopaque 1177 (Sigma). After centrifugation at 700g for 30 minutes at room temperature, the monocytic fraction was recovered and washed twice by centrifugation, counted in a cell counter (Schärfe System, Reutlingen, Germany) before suspension in the adhesion buffer (RPMI 1640 containing 0.3% [wt/vol] BSA). Microscopic analysis was performed to check cell viability and homogeneity before the experiments.
uPAR-transfected BAF-3 cells.
BAF-3 cells (an interleukin-3–dependent mouse pre–B-cell line) were transfected by electroporation with uPAR cDNA (provided by Dr N. Behrendt, Copenhagen, Denmark) both in the sense and antisense orientation in the expression vector pCDNA3. Cells were selected in the presence of 0.8 mg/mL G418 and characterized for expression of uPAR by flow cytometry, Northern blot analysis, and uPAR enzyme-linked immunosorbent assay (ELISA) (data not shown).
Cell binding of radiolabeled VN.
Multimeric VN (100 μg) was labeled with 0.5 mCi of Na125I (Amersham, Braunschweig, Germany) using Iodogen (Pierce, Oud Beijerland, The Netherlands) according to a previously outlined procedure.6 After separation on a Sephadex G-25 column (Pharmacia, Freiburg, Germany) suspended in Tris-buffered saline containing 0.1% (wt/vol) BSA, the labeled protein was dialyzed against the same buffer. The specific activity of VN in three different preparations was 5 to 10 μCi/μg.
U937 cells were washed extensively with serum-free medium and resuspended in Dulbecco’s modified Eagle’s medium (DMEM), pH 7.4, containing 25 mmol/L HEPES, 0.3% (wt/vol) BSA. 125I-VN alone or together with excess cold ligand (at 50 μg/mL to measure nonspecific binding) or other competitors was reacted for 2 hours at 4°C in a final volume of 0.15 mL. Aliquots of the binding mixtures were layered on top of 0.2 mL DMEM containing 0.3% (wt/vol) BSA and 0.3 mol/L sucrose and centrifuged for 5 minutes at 12,000g. The bottom tip of the tube containing the radioactive ligand-associated cell pellet was counted in a γ-counter.
Binding interactions in a purified system.
Polystyrene microtiter wells (high binding, type I; Costar, Badhoevedorp, The Netherlands) were coated at a concentration of 5 μg/mL with purified soluble uPAR dissolved in 15 mmol/L Na2CO3, 35 mmol/L NaHCO3, pH 9.6, and subsequently blocked with 3% (wt/vol) BSA. Binding of125I-VN to the immobilized receptor was performed in a final volume of 50 μL in the absence or presence of competitors as indicated in the figure legends. Incubation was performed in Tris-buffered saline, containing 0.05% (wt/vol) Tween 20, 0.3% (wt/vol) BSA for 18 hours at 4°C, after which the wells were washed and counted in a γ-counter. Nonspecific binding of125I-VN to BSA-coated wells (in the absence of the immobilized receptor) was used as an additional blank in all experiments.
Cell adhesion assays.
Cell adhesion to immobilized VN or FBG was performed according to previously described protocols.6 Briefly, multiwell plates were coated with 2 μg/mL VN or 10 μg/mL FBG and blocked with 3% (wt/vol) BSA. BSA coated wells were used as controls to measure nonspecific adhesion. Differentiated U937 cells (24-hour preincubation with 100 nmol/L vitamin D3 and 2 ng/mL transforming growth factor-β) or BAF-3 cells were washed in serum-free medium and plated onto VN- or FBG-coated wells for 60 to 90 minutes at 37°C in the absence or presence of additives in serum-free medium containing 0.3% (wt/vol) BSA. In some experiments, HEPES-buffered saline (25 mol/L HEPES, pH 7.4, and 130 mmol/L NaCl) with or without Ca2+ (1 mmol/L) and Mg2+ (1 mmol/L) was used as the adhesion buffer and the adhesion assay was limited to 30 minutes. In case of adhesion to FBG, differentiated U937 cells were also incubated with 20 ng/mL phorbol ester (PMA) as a positive control of β2-integrin activation. Thereafter, the wells were washed and the number of adherent cells was measured by quantifying the absorbance of crystal violet staining at 590 nm.
Adhesion of myelomonocytic HL60 cells to cultured human umbilical vein endothelial cells was performed according to a previously published protocol.17 Briefly, differentiated HL60 cells were preincubated with different stimulators in the absence or presence of MoAb 60.3 as outlined in the legend to Fig 6 and then allowed to adhere to confluent endothelial cell monolayers. After incubation and washing, adherent HL60 cells were quantified.
Zn2+ affinity chromatography.
Metal affinity chromatography resin (Pharmacia) was charged with Zn2+ and used as described before for uPA purification.34 Batch chromatography was performed with 5 μg each of multimeric VN, uPAR, and uPA. Proteins were bound in phosphate-buffered saline and after washing they were eluted with the same buffer containing 0.05 mol/L EDTA. The flow-through fraction and the eluate were analyzed by denaturing polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate followed by silver staining.
Flow cytometry.
HL60 cells (2.5 × 105) were washed twice with HEPES-buffered saline and incubated in the absence or presence of 100 μmol/L Zn2+ or other test substances for 20 minutes at room temperature. After washing, the cells were incubated with saturating concentrations of mouse–anti-human MoAb 24 or MoAb 2LPM19c for 30 minutes on ice. Cells were washed again, resuspended in HEPES buffer and phycoerythrin-conjugated (Fab′)2 fragment of goat–anti-mouse IgG (Dianova, Hamburg, Germany) was added in saturating concentrations for 30 minutes on ice. After washing and resuspension, mean fluorescence of 10,000 cells was measured in a flow cytometer (Becton Dickinson, Heidelberg, Germany). Nonspecific fluorescence was determined using an isotype-matched mouse IgG as primary antibody.
RESULTS
Zn2+-induced adhesion of U937 cells on VN.
Adhesion of myelomonocytic cells to VN is mediated via uPAR. uPA or its isoforms containing the uPAR binding domain increase the affinity of this interaction, thereby enhancing adhesion of monocytic cells to VN.6 13 U937 or HL60 cells were differentiated for 24 hours with vitamin D3 and transforming growth factor-β along the monocyte lineage, which results in a concomitant upregulation of uPAR on these cells, as well as their adhesiveness to VN.
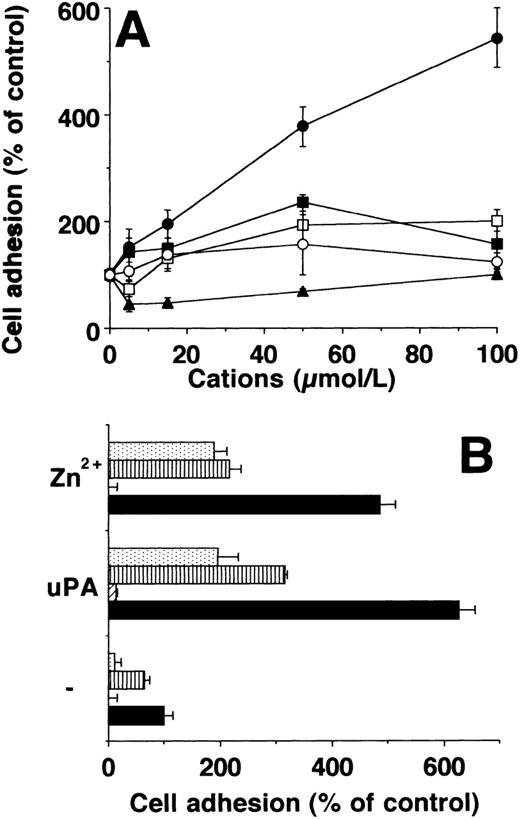
The effect of divalent cations Mn2+, Fe2+, Co2+, Cu2+, Ni2+, Cd2+, Hg2+, and Zn2+ in a concentration range between 5 and 100 μmol/L on adhesion of U937 cells to VN was tested. From these divalent cations, only Zn2+ induced a substantial increase in adhesion up to fivefold to sixfold, whereas Mn2+ had a much smaller effect (Fig1A). Zn2+ did not influence the adhesion on a control substrate (BSA) and there was no toxicity of these low concentrations of divalent cations used. The adhesion-promoting effect of Zn2+ at 100 μmol/L almost reached that of uPA at 50 nmol/L (Fig 1B). The adhesive effects of Zn2+ and uPA were additive (not shown). Adhesion due to Zn2+ and uPA on VN was inhibited by PAI-1 and MoAb R3 directed against domain 1 of uPAR, as well as by MoAb 13H1 against VN, which blocks the interaction of VN with uPAR6 (Fig 1B), whereas a control monoclonal antibody was ineffective.
Adhesion of U937 cells on vitronectin in response to divalent cations and uPA. (A) Differentiated U937 cells were allowed to adhere on VN in response to Zn2+ (•), Mn2+ (▪), Co2+ (□), Ni2+(○), or Cu2+ (▴). The data are expressed as percent of control as represented by adhesion of U937 cells in the absence of any stimulus (mean ± SEM, n = 3). Similar results were obtained in three separate experiments. (B) The adhesion of U937 on VN was performed without stimulus (−) or in the presence of 50 μmol/L Zn2+ or 50 nmol/L uPA respectively. In parallel wells, no other additives (▩) or 20 μg/mL of MoAb R3 against uPAR (▨), 10 μg/mL of MoAb 13H1 against VN (▥), or 100 nmol/L PAI-1 () were present. A control antibody (20 μg/mL) did not affect adhesion (not shown). Data are the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three independent experiments.
Adhesion of U937 cells on vitronectin in response to divalent cations and uPA. (A) Differentiated U937 cells were allowed to adhere on VN in response to Zn2+ (•), Mn2+ (▪), Co2+ (□), Ni2+(○), or Cu2+ (▴). The data are expressed as percent of control as represented by adhesion of U937 cells in the absence of any stimulus (mean ± SEM, n = 3). Similar results were obtained in three separate experiments. (B) The adhesion of U937 on VN was performed without stimulus (−) or in the presence of 50 μmol/L Zn2+ or 50 nmol/L uPA respectively. In parallel wells, no other additives (▩) or 20 μg/mL of MoAb R3 against uPAR (▨), 10 μg/mL of MoAb 13H1 against VN (▥), or 100 nmol/L PAI-1 () were present. A control antibody (20 μg/mL) did not affect adhesion (not shown). Data are the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three independent experiments.
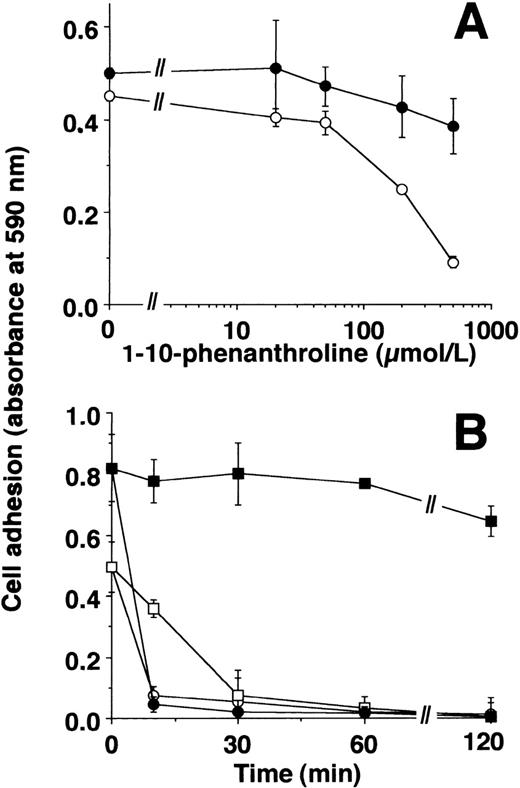
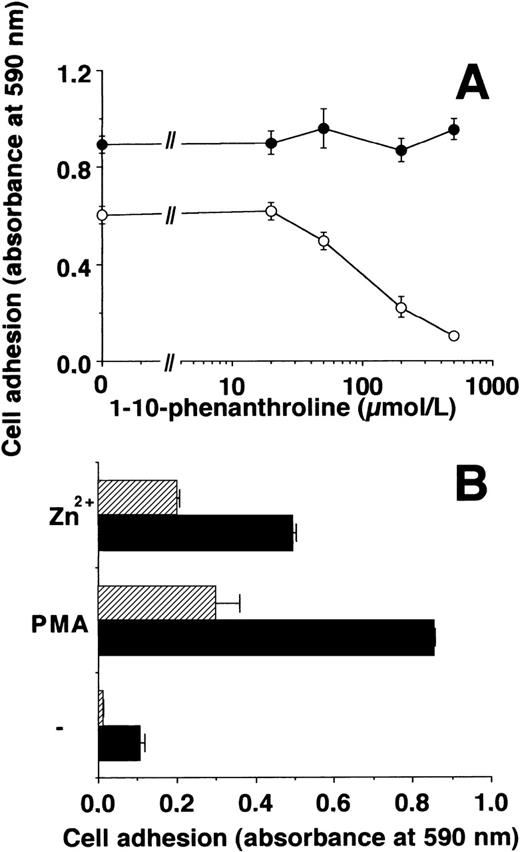
The cation-chelators, 1-10-phenanthroline or captopril, blocked the effect of Zn2+ on cell adhesion, whereas uPA-induced adhesion was only marginally affected (Fig2A, data for captopril not shown). Zn2+-induced adhesion was reversible: after the adhesion experiment for 2 hours, addition of PAI-1 led to an immediate dissociation of cells irrespective of Zn2+- or uPA-induced adherence. 1-10-Phenanthroline induced a slower detachment of Zn2+-stimulated, adherent cell, but did not affect the cells that adhered in response to uPA (Fig 2B).
Zn2+- and uPA-induced adhesion of U937 cells: effect of inhibitors. (A) Adhesion of U937 cells on VN-coated wells in response to 50 μmol/L Zn2+ (○) or 50 nmol/L uPA (•) was performed in the absence or presence of various concentrations of 1-10-phenanthroline. Basal adhesion in the absence of uPA or Zn2+ was 0.05 to 0.1 units of absorbance. Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments. (B) The adhesion of U937 cells in response to 50 nmol/L uPA (filled symbols) or 50 μmol/L Zn2+ (open symbols) was performed for 2 hours. Thereafter, 100 nmol/L PAI-1 (circles) or 500 μmol/L 1-10-phenanthroline (squares) was added for different time intervals as indicated, and residual adherent cells were quantitated. Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Zn2+- and uPA-induced adhesion of U937 cells: effect of inhibitors. (A) Adhesion of U937 cells on VN-coated wells in response to 50 μmol/L Zn2+ (○) or 50 nmol/L uPA (•) was performed in the absence or presence of various concentrations of 1-10-phenanthroline. Basal adhesion in the absence of uPA or Zn2+ was 0.05 to 0.1 units of absorbance. Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments. (B) The adhesion of U937 cells in response to 50 nmol/L uPA (filled symbols) or 50 μmol/L Zn2+ (open symbols) was performed for 2 hours. Thereafter, 100 nmol/L PAI-1 (circles) or 500 μmol/L 1-10-phenanthroline (squares) was added for different time intervals as indicated, and residual adherent cells were quantitated. Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Zn2+-induced adhesion of uPAR-transfected BAF-3 cells.
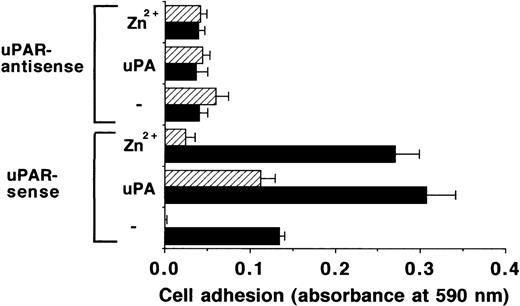
To further define the specificity of the effects of Zn2+, the adhesion on VN of BAF-3 cells (devoid of endogenous uPAR) transfected with sense or antisense uPAR cDNA was investigated. uPAR-expressing BAF-3 cells adhered to VN after stimulation by uPA or Zn2+, and this effect could be attributed to uPAR as evidenced by inhibition with MoAb R3 (Fig3). Moreover, BAF-3 cells with uPAR-antisense expression, either under control conditions or upon stimulation with uPA or Zn2+, respectively, showed no adherence to VN. This provides strong evidence that the adhesion-promoting activity of Zn2+ is due to increasing the strength of the VN-uPAR interaction.
Adhesion of uPAR-transfected BAF-3 cells on VN. The adhesion of BAF-3 cells transfected with uPAR-sense or uPAR-antisense cDNA as indicated was performed in response to 50 μmol/L Zn2+ or 50 nmol/L uPA, respectively, in the absence (▩) or presence of 20 μg/mL of MoAb R3 against uPAR (▨). Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Adhesion of uPAR-transfected BAF-3 cells on VN. The adhesion of BAF-3 cells transfected with uPAR-sense or uPAR-antisense cDNA as indicated was performed in response to 50 μmol/L Zn2+ or 50 nmol/L uPA, respectively, in the absence (▩) or presence of 20 μg/mL of MoAb R3 against uPAR (▨). Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Influence of Zn2+ on the VN-uPAR interaction.
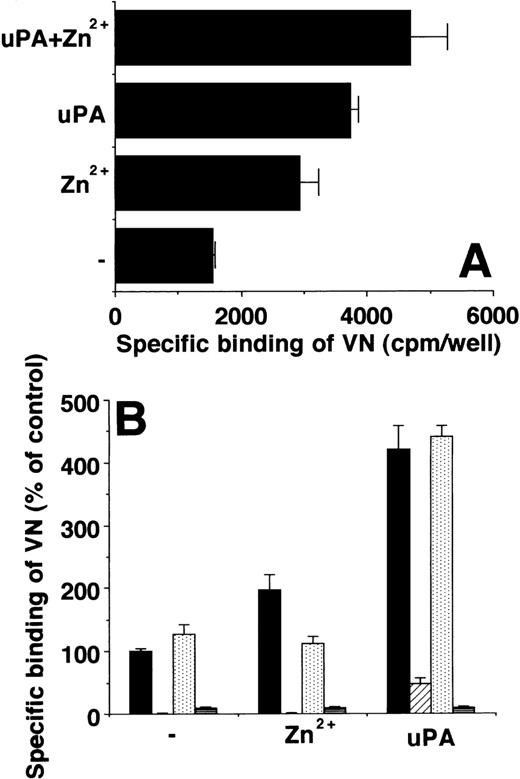
The effect of Zn2+ on the VN-uPAR interaction was tested on cells and in an in vitro system with isolated components. Like uPA, Zn2+ potentiated the direct binding of radiolabeled VN to U937 cells, and the effects of Zn2+ and uPA were additive (Fig 4A). Zn2+ increased binding of 125I-VN to immobilized uPAR in a manner comparable with uPA, whereas other cations were far less effective (not shown). Again, the effects of Zn2+ and uPA on VN binding to uPAR were additive. Both PAI-1 or MoAb R3 inhibited the effect of uPA and Zn2+, while a control MoAb had no influence. Zn2+ chelation with 1-10-phenanthroline abolished the increase in 125I-VN binding to uPAR, whereas the increase in binding mediated by uPA was not influenced (Fig 4B). These in vitro results with isolated components indicated that Zn2+ has a direct influence on VN-uPAR interaction by binding to these proteins and changing their conformation. This possibility was confirmed by testing the binding of these proteins to a Zn2+-Sepharose column as has been previously demonstrated for uPA.34 Both VN and uPAR are able to bind to the Zn2+-containing matrix directly and could be eluted as intact proteins (Fig5).
Influence of Zn2+ on VN binding to uPAR. (A) Binding of 125I-VN to U937 cells was performed in the absence or presence of 50 μmol/L Zn2+ or 20 nmol/L uPA alone or added together as indicated. Data (mean ± SEM, n = 3) are expressed as specific binding (as determined by subtracting binding in the presence of excess unlabeled VN). (B) Binding of125I-VN to immobilized uPAR was performed in the absence or presence of 50 μmol/L Zn2+ or 20 nmol/L uPA, respectively. Either no additives (▩) or 20 μg/mL MoAb R3 against uPAR (▨), 500 μmol/L 1-10-phenanthroline (), or 100 nmol/L PAI-1 (▤) were included as competitors. Data (mean ± SEM, n = 3) are expressed as specific binding (represented as percent of control). Similar results were obtained in at least three separate experiments.
Influence of Zn2+ on VN binding to uPAR. (A) Binding of 125I-VN to U937 cells was performed in the absence or presence of 50 μmol/L Zn2+ or 20 nmol/L uPA alone or added together as indicated. Data (mean ± SEM, n = 3) are expressed as specific binding (as determined by subtracting binding in the presence of excess unlabeled VN). (B) Binding of125I-VN to immobilized uPAR was performed in the absence or presence of 50 μmol/L Zn2+ or 20 nmol/L uPA, respectively. Either no additives (▩) or 20 μg/mL MoAb R3 against uPAR (▨), 500 μmol/L 1-10-phenanthroline (), or 100 nmol/L PAI-1 (▤) were included as competitors. Data (mean ± SEM, n = 3) are expressed as specific binding (represented as percent of control). Similar results were obtained in at least three separate experiments.
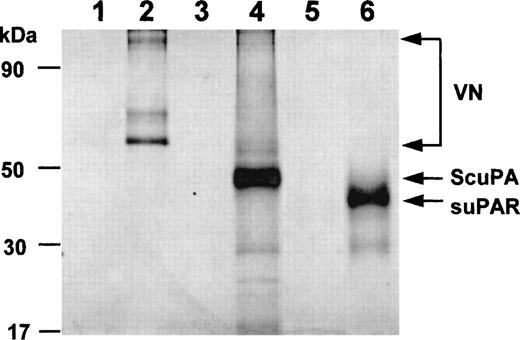
Binding of isolated proteins to Zn2+. After metal-ion affinity columns were charged with excess Zn2+or buffer alone, VN, uPAR, or single-chain urokinase type plasminogen activator (ScuPA), respectively, were allowed to bind to the column. Subsequent elution was performed with either binding buffer alone (lanes 1, 3, 5) or containing 0.05 mol/L EDTA (lanes 2, 4, 6). VN, uPAR, or ScuPA bound to the Zn2+ charged column, but not to a control column (not shown). The respective authentic protein bands are indicated by arrows on the right (monomeric and dimeric VN).
Binding of isolated proteins to Zn2+. After metal-ion affinity columns were charged with excess Zn2+or buffer alone, VN, uPAR, or single-chain urokinase type plasminogen activator (ScuPA), respectively, were allowed to bind to the column. Subsequent elution was performed with either binding buffer alone (lanes 1, 3, 5) or containing 0.05 mol/L EDTA (lanes 2, 4, 6). VN, uPAR, or ScuPA bound to the Zn2+ charged column, but not to a control column (not shown). The respective authentic protein bands are indicated by arrows on the right (monomeric and dimeric VN).
Zn2+-induced adhesion of monocytic cells on FBG and endothelial cells.
Since β2-integrins have been reported to be activated by divalent cations5,35 36 such as Mn2+ and Zn2+, the possibility that Zn2+ increases leukocytic cell adhesion on FBG was tested. Incubation of U937 cells with physiologic concentrations of Zn2+ resulted in a substantial induction of adhesion on FBG by threefold to fourfold (Fig6) similar to Mn2+, whereas other cations such as Fe2+, Co2+, Cu2+, Ni2+, Cd2+, and Hg2+ were far less effective (data not shown). 1-10-Phenanthroline inhibited the effect of Zn2+ without influencing the PMA-induced adhesion (Fig 6A). The effect of Zn2+ was comparable to that of PMA and was mainly mediated by β2-integrins, since MoAb 60.3 against the human β2-subunit inhibited Zn2+-induced adhesion (Fig 6B).
Zn2+-induced adhesion of U937 cells on FBG. (A) Adhesion of U937 cells on FBG-coated plates induced by 50 μmol/L Zn2+ (○) or 20 ng/mL PMA (•) in the absence or presence of various concentrations of 1-10-phenanthroline was measured. Data represent the mean ± SEM (n = 3) of a typical experiment. The basal adhesion in the absence of PMA or Zn2+ was 0.05 to 0.1 absorbance units. Similar results were obtained in three separate experiments. (B) The adhesion of U937 cells on FBG induced by 50 μmol/L Zn2+ or 20 ng/mL PMA was measured in the absence (▩) or presence of 10 μg/mL MoAb 60.3 against β2-integrin (▨). Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Zn2+-induced adhesion of U937 cells on FBG. (A) Adhesion of U937 cells on FBG-coated plates induced by 50 μmol/L Zn2+ (○) or 20 ng/mL PMA (•) in the absence or presence of various concentrations of 1-10-phenanthroline was measured. Data represent the mean ± SEM (n = 3) of a typical experiment. The basal adhesion in the absence of PMA or Zn2+ was 0.05 to 0.1 absorbance units. Similar results were obtained in three separate experiments. (B) The adhesion of U937 cells on FBG induced by 50 μmol/L Zn2+ or 20 ng/mL PMA was measured in the absence (▩) or presence of 10 μg/mL MoAb 60.3 against β2-integrin (▨). Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
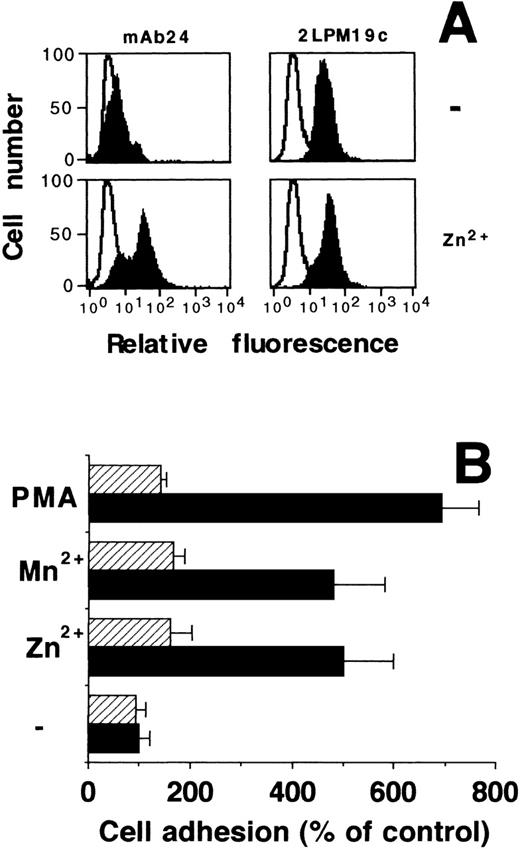
As previously reported5 for Mn2+, Zn2+ induced the activation of β2-integrins by exposing an activation-dependent epitope on the β2-subunit without changing its surface expression on HL60 cells as evidenced by flow cytometry (Fig7A). Activation of β2-integrins also regulates the adhesion of leukocytes to the endothelium, which takes place via the interaction between β2-integrins and counter-receptors such as ICAM-1 on endothelial cells. In this system, Zn2+ promoted a fivefold increase in HL60 cell adhesion to an endothelial monolayer, similarly to the effect of Mn2+ or PMA (Fig 7B), while MoAb 60.3 inhibited cell adherence in all cases to baseline values indicative of β2-integrin dependency.
Zn2+-dependent activation of β2-integrins and adhesion to endothelial cells. (A) Human myelomonocytic HL60 cells were incubated in the absence or presence of 100 μmol/L Zn2+ for 20 minutes at room temperature, and surface expression of an activation-dependent epitope (MoAb 24) on β2-subunit was quantitated by fluorescence-activated cell sorter (FACS) analysis (filled curves). For comparison, quantitative cell surface expression of β2-subunit was analyzed using MoAb 2LPM19c, which recognizes an epitope irrespective of the activation state of the integrin. Nonspecific fluorescence was determined using an isotype-matched mouse-IgG (open curves). The figure shows one of three representative experiments. (B) Differentiated HL60 cells, preincubated for 30 minutes in the absence or the presence of 100 μmol/L Zn2+, 0.5 mmol/L Mn2+, or 10 ng/mL PMA as indicated, were allowed to adhere to confluent endothelial cell monolayers in the absence (▩) or presence (▨) of MoAb 60.3 against β2-integrin. Data represent the mean ± SD (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Zn2+-dependent activation of β2-integrins and adhesion to endothelial cells. (A) Human myelomonocytic HL60 cells were incubated in the absence or presence of 100 μmol/L Zn2+ for 20 minutes at room temperature, and surface expression of an activation-dependent epitope (MoAb 24) on β2-subunit was quantitated by fluorescence-activated cell sorter (FACS) analysis (filled curves). For comparison, quantitative cell surface expression of β2-subunit was analyzed using MoAb 2LPM19c, which recognizes an epitope irrespective of the activation state of the integrin. Nonspecific fluorescence was determined using an isotype-matched mouse-IgG (open curves). The figure shows one of three representative experiments. (B) Differentiated HL60 cells, preincubated for 30 minutes in the absence or the presence of 100 μmol/L Zn2+, 0.5 mmol/L Mn2+, or 10 ng/mL PMA as indicated, were allowed to adhere to confluent endothelial cell monolayers in the absence (▩) or presence (▨) of MoAb 60.3 against β2-integrin. Data represent the mean ± SD (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
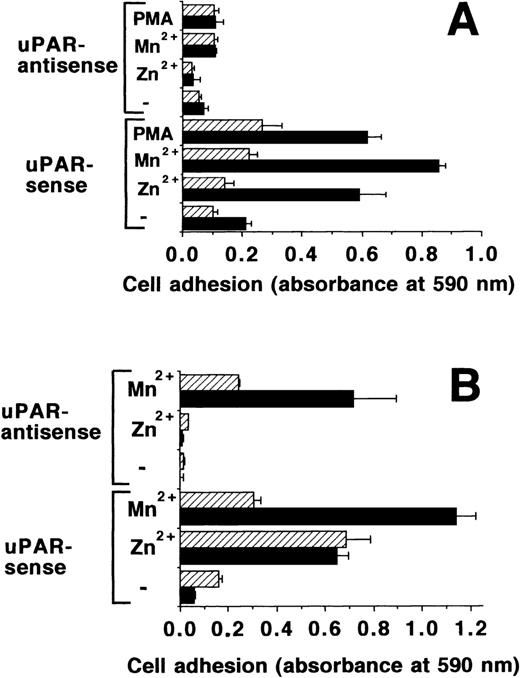
The β2-integrins have been shown to contain a divalent cation binding site that binds both Mn2+ and Zn2+, and their binding is negatively influenced35,36 by Ca2+ and Mg2+. Moreover, the activation state of β2-integrins is strongly influenced by uPAR.14,16,17 BAF-3 cells, which express β2-integrins37 transfected with uPAR in the sense and antisense orientation, were tested for adhesion on FBG. Zn2+, Mn2+, and PMA induced the adhesion of uPAR transfected cells, but not that of control transfected cells in normal adhesion buffer containing physiologic concentrations of Ca2+ and Mg2+ (Fig8A). This adhesion was inhibited by a blocking MoAb for mouse β2-integrins, Game 46 (Fig 8A), but not a control MoAb (data not shown). In a divalent cation-free buffer, Mn2+could stimulate the adhesion of both uPAR sense and antisense transfected cells to FBG, but Zn2+ could activate the adhesion of sense transfected cells only (Fig 8B). This indicates that Mn2+ can directly activate β2-integrins, whereas Zn2+ needs the intermediate uPAR. This hypothesis is also supported by the fact that the Mn2+ effect is strongly inhibited in the presence of physiological Ca2+ and Mg2+, whereas these two divalent cations have no influence on the effect of Zn2+(Fig 8B). These findings strongly imply that Zn2+ binding to uPAR regulates the activation status of β2-integrins in these model systems.
Adhesion of uPAR-transfected BAF-3 cells on FBG. (A) Adhesion of BAF-3 cells, transfected with uPAR in the sense or antisense orientation, was tested on FBG in response to Mn2+ (50 μmol/L), Zn2+ (50 μmol/L), or PMA (20 ng/mL), respectively, in the absence (▩) or presence of 10 μg/mL of MoAb Game 46 (▨) against mouse β2-integrins. (B) A similar adhesion assay was performed, except that either HEPES-buffered saline without (▩) or with Ca2+ and Mg2+ (▨) were used as the adhesion buffer instead of RPMI 1640. Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Adhesion of uPAR-transfected BAF-3 cells on FBG. (A) Adhesion of BAF-3 cells, transfected with uPAR in the sense or antisense orientation, was tested on FBG in response to Mn2+ (50 μmol/L), Zn2+ (50 μmol/L), or PMA (20 ng/mL), respectively, in the absence (▩) or presence of 10 μg/mL of MoAb Game 46 (▨) against mouse β2-integrins. (B) A similar adhesion assay was performed, except that either HEPES-buffered saline without (▩) or with Ca2+ and Mg2+ (▨) were used as the adhesion buffer instead of RPMI 1640. Data represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in three separate experiments.
Zn2+-induced adhesion of blood monocytes.
Monocytic cells were isolated from peripheral blood and their adhesion to VN- or FBG-coated plates was investigated. Monocyte adhesion to VN was induced twofold by 50 nmol/L uPA and more than 2.5-fold by 100 μmol/L Zn2+, whereas the effect of Mn2+ was much smaller (Table 1). As demonstrated above for myelomonocytic cell lines, the adhesion-stimulating effect of Zn2+ on peripheral blood monocytes was also dose-dependent (not shown). When FBG was used as a substrate, Zn2+promoted monocyte adhesion similar to Mn2+ or PMA (Table 1).
Adhesion of Peripheral Blood Monocytes to Provisional Matrix Proteins in Response to Different Stimuli
| Additives . | Monocyte Adhesion Onto . | |
|---|---|---|
| VN . | FBG . | |
| None | 100 ± 13 | 100 ± 12 |
| 100 μmol/L Zn2+ | 257 ± 23 | 277 ± 26 |
| 100 μmol/L Mn2+ | 149 ± 19 | 302 ± 35 |
| 50 nmol/L uPA | 192 ± 30 | ND |
| 20 ng/mL PMA | ND | 194 ± 15 |
| Additives . | Monocyte Adhesion Onto . | |
|---|---|---|
| VN . | FBG . | |
| None | 100 ± 13 | 100 ± 12 |
| 100 μmol/L Zn2+ | 257 ± 23 | 277 ± 26 |
| 100 μmol/L Mn2+ | 149 ± 19 | 302 ± 35 |
| 50 nmol/L uPA | 192 ± 30 | ND |
| 20 ng/mL PMA | ND | 194 ± 15 |
The adhesion of peripheral blood monocytes on VN- or FBG-coated wells in the absence or presence of Zn2+ and Mn2+ was compared. uPA was used as a positive control for adhesion on VN and PMA for adhesion on FBG. Data are represented as percent of control and represent the mean ± SEM (n = 3) of a typical experiment. Similar results were obtained in 3 separate experiments.
Abbreviation: ND, not determined.
DISCUSSION
Cell adhesion to the endothelium and the provisional matrix, mainly composed of VN or FBG/fibrin, is pivotal for leukocyte recruitment to inflamed or injured tissue in order to initiate an adequate inflammatory or wound healing response. These adhesive interactions involve, largely, the uPAR system for VN and the β2-integrin-system for FBG/fibrin or the endothelial monolayer as substrates.8,9,38 In the present work, we demonstrate that physiologic concentration39 of the trace element Zn2+ induces these different cell-matrix interactions, as well as the firm adhesion of leukocytes to the endothelium. Hence, Zn2+ could be a key regulator of leukocyte adhesion in vivo.
In particular, Zn2+ enhanced the adhesion of leukocytic cells on VN up to fivefold to sixfold, and this effect was specific for Zn2+, as it could not be mimicked by other divalent cations and was abolished by chelation with 1-10-phenanthroline or captopril. Zn2+-dependent adhesion engaged the uPAR system as deduced from several lines of evidence: (1) a MoAb against uPAR blocked the effect of Zn2+ on U937 cell adhesion; (2) Zn2+induced the adhesion of uPAR-sense transfected BAF-3 cells, whereas BAF-3 cells transfected with uPAR-antisense did not respond; and (3) in a purified system, Zn2+ promoted 125I-VN binding to uPAR-expressing cells and to immobilized uPAR. The adhesion promoting effect of Zn2+ in the VN-uPAR system was similar to that of uPA, and both components were additive in their function. We hypothesize that uPA and Zn2+ can induce a conformational alteration in VN and/or uPAR, thus favoring their interaction. This is also supported by our observation that both VN and uPAR can directly bind to Zn2+. Elucidation of the Zn2+ binding site(s) on uPAR and VN may explain how these two proteins interact in order to mediate cell adhesion. In an analogous manner, Zn2+ increased the β2-integrin–mediated adhesion of leukocytic cells on FBG/fibrin and endothelial cells, which was inhibited by MoAb 60.3 or Game 46 against β2-integrins. The specificity of Zn2+ was demonstrated by its ability to expose an activation-dependent epitope on this integrin.
Although Zn2+ may stimulate monocytic cell adhesion to FBG and the endothelium by direct activation of β2-integrins, Zn2+-dependent engagement of uPAR appears to be crucial, as deduced from our data. In a completely divalent cation-free buffer, β2-integrin–expressing BAF-3 cells without uPAR could adhere strongly to FBG in the presence of Mn2+ (inhibited by MoAb Game 46), but not in the presence of Zn2+. Hence, Zn2+ binding to β2-integrins35,36was not sufficient to stimulate adhesion to FBG. However, in uPAR-transfected BAF-3 cells, Zn2+ induced a robust increase in adhesion under the same divalent cation-free conditions, indicating that Mn2+ activates β2-integrins directly, while Zn2+ does so via uPAR activation. Zn2+, Mn2+, Cu2+, Ca2+, Mg2+, and Co2+ bind the I domain of the α-chain of β2-integrins with high affinity.35,36 Ca2+ and Mg2+ can inhibit Mn2+-stimulated β2-integrin–mediated events based on direct competition at the divalent cation binding site.35,36 Ca2+ and Mg2+ could inhibit the adhesion of Mn2+ stimulated BAF-3 cells (with or without uPAR) as expected, whereas Zn2+-induced adhesion of uPAR transfected BAF-3 cells could not be influenced by Ca2+ and Mg2+ at all, indicating that Zn2+ does not activate adhesion by the same mechanism as did Mn2+. Thus, Zn2+ induced β2-integrin-dependent adhesion to FBG or the endothelium through the activation of uPAR. We also observed some Mn2+-induced cell adhesion to VN, reflecting a “reverse” regulation of uPAR by β2-integrins, corroborating earlier studies that showed uPAR-dependent cell adhesion16 is influenced by activation of β2-integrins with Mn2+.
In a more complex physiologic environment, the cofactor role of Zn2+ for other biologic systems such as the matrix metalloproteinases should not be neglected. In a regulated manner, these Zn2+-dependent endopeptidases efficiently degrade matrix/tissue components required for cell migration and invasion and for the release of stored growth factors.22,40 Furthermore, the cell contact-dependent production and secretion of large quantities of various matrix metalloproteinases by macrophages41 may involve Zn2+as well. The administration of Zn2+chelators such as captopril to inhibit matrix metalloproteinases was used to prevent cancer cell invasion42 or tumor angiogenesis43 in different models. Yet, it remains to be clarified to what extent the presented functions of Zn2+ as an inducer of cell adhesion are affected during therapy with cation chelators.
In addition to the plasma physiologic concentration of Zn2+, platelets contain substantial quantities44 of stored Zn2+ and release this pool into regions of tissue damage, where Zn2+ may exert its proadhesive and chemotactic effects for recruiting inflammatory blood cells. Moreover, Zn2+ from implanted metal biomaterials in the vasculature may also accumulate at the sites of implantation.30 Conversely, in Zn2+ deficiency states, there is a decline in the B- and T-cell–dependent immune responses with increased susceptibility to bacterial, viral, and parasitic challenges,45 and more relevant to the current study, there is a decline in neutrophil adhesion.46 These causally linked observations, together with the data presented here, suggest that Zn2+ may be a crucial regulator of leukocyte adhesion and the extravasation of phagocytic cells into inflamed or injured tissue.
ACKNOWLEDGMENT
The technical assistance of Barbara Yutzy, Uwe Schubert, and Thomas Schmidt is greatly appreciated. We also acknowledge the generous gift of reagents from Drs H.R. Lijnen (University of Leuven, Leuven, Belgium), and G. Hoyer-Hansen and N. Behrendt (Finsen Laboratory, Copenhagen, Denmark).
Supported by Grant No. Pr 327/1-4 from the Deutsche Forschungsgemeinschaft, Bonn, Germany, and the Novartis Foundation.
This work is part of the MD thesis of T.C. at the Department of Medicine, Justus-Liebig-Universität Giessen, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sandip M. Kanse, PhD, MPI, Kerckhoff-Klinik, Sprudelhof 11, 61231 Bad Nauheim, Germany; e-mail:sandip.kanse@kerckhoff.med.uni-giessen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal