Abstract
Circulating endothelial cells (CECs) have been detected in association with endothelial injury and therefore represent proof of serious damage to the vascular tree. Our aim was to investigate, using the technique of immunomagnetic separation, whether the pathological events in unstable angina (UA) or acute myocardial infarction (AMI) could cause desquamation of endothelial cells in circulating blood compared with effort angina (EA) and noncoronary chest pain. A high CEC count was found in AMI (median, 7.5 cells/mL; interquartile range, 4.1 to 43.5, P < .01 analysis of variance [ANOVA]) and UA (4.5; 0.75 to 13.25 cells/mL, P < .01) within 12 hours after chest pain as compared with controls (0; 0 to 0 cells/mL) and stable angina (0; 0 to 0 cells/mL). CEC levels in serial samples peaked at 15.5 (2.7 to 39) cells/mL 18 to 24 hours after AMI (P < .05 repeated measures ANOVA), but fell steadily after UA. Regardless of acute coronary events, the isolated cells displayed morphologic and immunologic features of vascular endothelium. The CECs were predominantly of macrovascular origin. They did not express the activation markers intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and E-selectin, although some were positive for tissue factor. CECs failed to exhibit characteristics of apoptosis (TUNEL assay) excluding this event as a possible mechanism of cell detachment. The presence of CECs provides direct evidence of endothelial injury in AMI and UA, but not in stable angina, confirming that these diseases have different etiopathogenic mechanisms.
ENDOTHELIAL INJURY represents a major initiating step in the pathogenesis of atherosclerosis that can lead to acute coronary syndromes such as acute myocardial infarction (AMI) and unstable angina (UA).1-3 These acute coronary pathologies represent high-risk processes with severe life-threatening thrombotic events. Various experimental models have been developed to improve our understanding of the importance of endothelial integrity and the pathogenic mechanisms of vascular alterations leading to these clinical situations.4 Fuster et al5 have proposed a classification of vascular injury that includes stages of increasing severity. Type I consists of functional alterations of endothelial cells without substantial morphologic changes. These alterations are followed by lipid accumulation, monocyte and platelet adhesion, and smooth muscle cell proliferation resulting in plaque formation. Type I injury can be followed by type II and type III injuries, defined as endothelial denuding, and intimal injury with or without medial damage. Finally, the process may be complicated by plaque ulceration, rupture or erosion, and thrombus formation, which can trigger a heart attack or sudden death.
Research in clinical settings has been hindered by the inaccessibility of vascular endothelium in both healthy subjects and patients. Circulating endothelial cells (CECs) may provide useful material for the study of vascular injury. They have been detected in the blood in diverse conditions such as coronary angioplasty, sickle cell disease, thrombotic thrombocytopenic purpura, and infection with Rickettsia conorii and cytomegalovirus.6-15 We therefore hypothesized that AMI and UA could present such a stress to the vascular system so that endothelial cells would be lost from the intima and thus appear in the blood. Because preliminary studies suggested that acute coronary syndromes could be associated with increased levels of cells presenting endothelial morphologic features,16,17we combined specific capture and immunological characterization to investigate CECs in AMI and UA. Consequently, using a previously developed immunomagnetic separation assay based on S-Endo 1 monoclonal antibody (MoAb) directed against the endothelial antigen CD146,10-12,18 19 we studied the number, origin, and surface phenotype of CECs. We also determined whether or not these CECs were apoptotic and would be simply shed from the intima. We controlled our AMI and UA specimens by obtaining blood from subjects with effort angina (EA) and from subjects with noncoronary chest pain.
MATERIALS AND METHODS
Patient selection and diagnosis.
Subjects were recruited from among those admitted to the coronary care unit at CHU Timone (Marseille, France). Local ethical committee approval was obtained according to the Declaration of Helsinki, and written informed consent was obtained from each subject. They were categorized into four groups: the target groups AMI and UA and the two control groups of EA and patients presenting with noncoronary chest pain. Characteristics and risk factors of patients are listed in Table 1. All of the patients selected for the study did not undergo catheterization and percutaneous angioplasty before and during the time of the study. For each patient included, standard coronary angiography was performed after the completion of the study, 8 days after admission for AMI, 5 days after for UA and controls, and 24 hours after for EA. Coronary artery aspect was analyzed according to the classification of Ambrose et al.20 Diagnosis and coronary angiography characteristics of patients are summarized in Tables 2 and3.
Clinical Characteristics of Patients
| Characteristics . | Patient Groups . | |||
|---|---|---|---|---|
| AMI . | UA . | EA . | C . | |
| (n = 26) | (n = 33) | (n = 13) | (n = 14) | |
| Age (yr)* | 66 ± 13 | 68 ± 11 | 64 ± 10 | 57 ± 15 |
| (36-86) | (44-85) | (44-85) | (26-74) | |
| No risk factors | 8 | 5 | 0 | 7 |
| Hypertension (BP > 160/90 Hg mm) | 10 | 14 | 7 | 1 |
| Smoking | 5 | 14 | 11 | 6 |
| Dyslipidemia† | 4 | 13 | 12 | 2 |
| Coronary heredity‡ | 2 | 8 | 6 | 1 |
| Diabetes mellitus1-153 | 2 | 7 | 2 | 1 |
| Obesity1-155 | 1 | 5 | 5 | 0 |
| Previous angina | 2 | 3 | 0 | 0 |
| Characteristics . | Patient Groups . | |||
|---|---|---|---|---|
| AMI . | UA . | EA . | C . | |
| (n = 26) | (n = 33) | (n = 13) | (n = 14) | |
| Age (yr)* | 66 ± 13 | 68 ± 11 | 64 ± 10 | 57 ± 15 |
| (36-86) | (44-85) | (44-85) | (26-74) | |
| No risk factors | 8 | 5 | 0 | 7 |
| Hypertension (BP > 160/90 Hg mm) | 10 | 14 | 7 | 1 |
| Smoking | 5 | 14 | 11 | 6 |
| Dyslipidemia† | 4 | 13 | 12 | 2 |
| Coronary heredity‡ | 2 | 8 | 6 | 1 |
| Diabetes mellitus1-153 | 2 | 7 | 2 | 1 |
| Obesity1-155 | 1 | 5 | 5 | 0 |
| Previous angina | 2 | 3 | 0 | 0 |
Cholesterolemia: normal range, 3.4 to 6.6 mmol/L.
The four groups are equivalent for age (ANOVA).
Known before the hospitalization.
Father or mother with AMI before the age of 60 years.
Noninsulin-dependent diabetes.
Body mass index >25 kg/m2 for women and >27 kg/m2 for men.
ECG and Enzyme Data
| Exploration . | Patient Groups . | |||
|---|---|---|---|---|
| AMI . | UA . | EA . | C . | |
| Creatinine kinase peak | 639 IU/mL (IQR 307-988) | <120 IU/mL | ND | <120 IU/mL |
| Electrocardiography | 17 Q wave | 4 Depressive ST | 11 Normal | 12 Normal |
| 9 Non-Q wave | 3 Elevated ST 26 Negative T wave | 2 Abnormal: Negative T wave | 2 Abnormal: Negative T wave | |
| —Segment* | 13 Anterior 10 Inferior 3 Lateral | 17 Anterior 9 Inferior 7 Lateral | Anterior and inferior | Anterior and inferior |
| Exploration . | Patient Groups . | |||
|---|---|---|---|---|
| AMI . | UA . | EA . | C . | |
| Creatinine kinase peak | 639 IU/mL (IQR 307-988) | <120 IU/mL | ND | <120 IU/mL |
| Electrocardiography | 17 Q wave | 4 Depressive ST | 11 Normal | 12 Normal |
| 9 Non-Q wave | 3 Elevated ST 26 Negative T wave | 2 Abnormal: Negative T wave | 2 Abnormal: Negative T wave | |
| —Segment* | 13 Anterior 10 Inferior 3 Lateral | 17 Anterior 9 Inferior 7 Lateral | Anterior and inferior | Anterior and inferior |
Abbreviation: ND, not determined.
Angiographic akinetic segment corresponding to electrocardiographic infarction zone for AMI and electrocardiographic ST changes in UA.
Coronary Data From Angiography
| . | AMI . | UA . | EA . | Controls . |
|---|---|---|---|---|
| Stenoses | N = 203-150 | N = 323-151 | N = 13 | N = 14 |
| 0 stenosis | 1 | 1 | 0 | 14 |
| 1 stenosis | 12 | 13 | 3 | 0 |
| 2 stenoses | 4 | 8 | 4 | 0 |
| 3 stenoses | 3 | 10 | 6 | 0 |
| Coronary aspect | ||||
| Concentric lesions | 1 | 4 | 3 | 0 |
| Eccentric lesions | 3 | 13 | 2 | 0 |
| Multiple irregular lesions | 7 | 9 | 4 | 0 |
| Occlusion | 6 | 4 | 4 | 0 |
| Clot | 1 | 0 | 0 | 0 |
| Spasm | 1 | 0 | 0 | 0 |
| Dissection | 1 | 0 | 0 | 0 |
| Left ventriculography | ||||
| All abnormal | 31 Normal 3 Abnormal3-152 | All normal | All normal |
| . | AMI . | UA . | EA . | Controls . |
|---|---|---|---|---|
| Stenoses | N = 203-150 | N = 323-151 | N = 13 | N = 14 |
| 0 stenosis | 1 | 1 | 0 | 14 |
| 1 stenosis | 12 | 13 | 3 | 0 |
| 2 stenoses | 4 | 8 | 4 | 0 |
| 3 stenoses | 3 | 10 | 6 | 0 |
| Coronary aspect | ||||
| Concentric lesions | 1 | 4 | 3 | 0 |
| Eccentric lesions | 3 | 13 | 2 | 0 |
| Multiple irregular lesions | 7 | 9 | 4 | 0 |
| Occlusion | 6 | 4 | 4 | 0 |
| Clot | 1 | 0 | 0 | 0 |
| Spasm | 1 | 0 | 0 | 0 |
| Dissection | 1 | 0 | 0 | 0 |
| Left ventriculography | ||||
| All abnormal | 31 Normal 3 Abnormal3-152 | All normal | All normal |
Six patients did not undergo coronary angiography because of age (5 patients, 79-86 years) and death (1 patient, heart failure).
One patient did not undergo the coronary angiography because of Alzheimer’s disease.
Lateral hypokinetic zone, anterior hypokinetic zone, one false inferior aneurysm.
Diagnosis of AMI was made within 24 hours after the onset of symptoms according to established criteria of the World Health Organization. At least two of the three following criteria were present: typical sustained chest pain, electrocardiogram (ECG) Q wave, and raised peak creatinine kinase (CPK). All patients showed raised CPK (peak median, 639 IU/mL; interquartile range [IQR], 307 to 988; 12 to 18 hours after admission) and coronary stenoses.
Diagnosis of UA was made within 12 hours after the onset of symptoms with electrocardiographic changes on ST segment during angina pectoris crisis. All patients presented creatinine kinase levels within normal range and subsequently showed coronary stenoses.
Diagnosis of EA was performed by exercise testing with a bicycle ergometer according to the protocol (30 W each for 3 minutes).21 Systemic arterial pressure and ECG were continuously supervised. Exercise was stopped when angina pectoris or ST segment depression appeared.
Diagnosis of control group was made within 12 hours after noncoronary chest pain. Creatinine kinase was normal. Coronary angiography showed no stenosis.
All patients from AMI, UA, and control groups received the same medical treatment: unfragmented heparin (choay: 5,000 IU as a bolus and 1,000 IU/h during 8 days adaptable in function of activated partial thromboplastin time [aPTT]), aspirin (160 mg/d), and β blockers (atenolol: 100 mg/d intravenous during 48 hours then orally during the hospitalization). Only four patients with AMI underwent an intravenous thrombolysis within 1 to 6 hours delay (tissue plasminogen activator [actilyse]), 15 mg as a bolus, 0.75 mg/kg over 30 minutes, and 0.50 mg/kg over 60 minutes because the standard clinical procedure followed in our hospital is angioplasty for AMI patients hospitalized within the 6 hours after the onset of symptoms. We have excluded such patients because in a previous report we have demonstrated that coronary angioplasty induces the release of endothelial cells in the circulation.11 Hence, AMI patients included in the present study were those hospitalized later than 6 hours and who followed the conventional pharmacologic treatment (unfragmented heparin, aspirin, and β blockers) and the four patients who underwent thrombolysis.
Blood collection.
For CEC quantitation, the first 2 mL of blood drawn were discarded to avoid contamination by endothelial cells from the punctured vessel wall. A total of 5 mL of blood was then collected into EDTA. For the AMI, UA, and control groups, a first blood sample was collected on arrival at hospital, within 24 hours of the onset of chest pain. Then, three or four other samples were drawn at intervals, such as every 6 hours, for up to 42 hours. For the EA group, one sample was collected before undertaking the exercise tolerance test, just after the chest pain, and again 4 hours later.
Antibodies.
For immunocapture of CECs from whole blood, S-Endo 1 (Biocytex, Marseille, France), a MoAb raised against human umbilical vein endothelial cells (HUVEC) in our laboratory,11 was used. This MoAb was selected because of its strong reactivity for endothelial cells from all vascular beds and its negative reaction with hematopoietic cells, mesothelial cells, or fibroblasts. This antibody also reacts moderately with smooth muscle cells.19 For the immunological characterization of the cells, we used the following antibodies: a rabbit polyclonal antibody against human von Willebrand factor (vWF, a kind gift from Y. Sultan, Laboratoire d’Hematologie, Hopital de la Miletrie, Poitiers, France),22 a murine MoAb against smooth muscle α-actin (IgG2a, clone 1A4; Sigma, St Quentin-Fallavier, France); MoAbs against the adhesion molecules intercellular adhesion molecule-1 (ICAM-1) (IgG1, clone F431C2/B7; Biocytex, Marseille, France), vascular cell adhesion molecule-1 (VCAM-1) (IgG1, clone 1G11; Immunotech, Marseille, France), and E-Selectin (IgG1, clone 1.2B6; Immunotech). Fluorescein isothiocyanate (FITC) conjugated MoAb against tissue factor (TF) (IgG1, clone TF9; Ortho Diagnostic System, Roissy, France) and CD36 (IgG1, clone FA6-152; Immunotech).
The antibodies used as controls were rabbit antiserum for the rabbit polyclonal antibody (Sigma) and isotype-matched antibodies with irrelevant binding specificities for the murine MoAbs (IgG1, clone MOPC-21 and IgG2a, clone UPC-10, Sigma, FITC-conjugated IgG1, clone 679.1MC7, Immunotech).
Goat anti-rabbit and goat anti-mouse antibodies conjugated to tetramethylrhodamine isothiocyanate (TRITC) (Immunotech) and to FITC (Silenus, Eurobio, Les Ullis, France), respectively, were used to show the unconjugated antibodies.
Preparation of immunomagnetic beads.
Monodispersed magnetizable beads (Dynabeads-M-450) were obtained from Dynal A.S. (Oslo, Norway). They are 4.5-μm diameter polystyrene beads with rat anti-mouse IgG1 covalently bound to the surface. They were coated with S-Endo 1 MoAb as a second layer as previously described.11
Immunomagnetic separation and counting of CECs.
Separation and quantitation of CECs were performed as previously described.11 A total of 1 mL of whole blood diluted 1:4 in phosphate-buffered saline (PBS)-0.1% bovine serum albumin-0.1% sodium azide were mixed with 20 μL of S-Endo 1-coated beads and submitted to gentle agitation for 30 minutes on a sample mixer (Robbins Scientific, Bio Techgen, Les Ullis, France). Magnetic beads and rosetted cells were separated from blood using the MPC6 concentrator (Dynal). After two washes, the rosetted cells were resuspended in acridin orange (10 μg/mL in PBS) (Sigma) for cell counting. Analysis was performed under an optical fluorescence microscope (λexc = 490 nm) using a Nageotte hemocytometer (CML, Nemours, France) for quantitation. The criteria retained for the identification of CECs were first, rosettes bearing more than 10 beads and a cell size in the range of 20 to 50 μm and second, cells with less than 10 beads, but with a well-preserved and recognizable morphology (clear nucleus in a well delimited cytoplasm and a size, which might correspond to endothelial cells). For aggregates, the number of cells was deduced from the number of nuclei or from the number of spherical rosetted features discriminated in the aggregate. When cell count was higher than 5 cells/mL, the rest of the blood was processed for immunomagnetic separation, and rosetted endothelial cells were either cytocentrifuged onto a glass slide at 200 rpm with low acceleration (Cytospin, Shandon, UK) or deposited on the slide, dried in air for further characterization including May-Grünwald-Giemsa staining, immunologic labeling, and TUNEL assay. They were kept at −80°C until used.
Immunofluorescence procedure.
Rosetted endothelial cells were rehydrated in PBS, fixed in paraformaldehyde (PFA; Fluka Chemie, Buchs, Switzerland) 3% and permeabilized with 0.1% Triton X100 (Prolabo, Vaulx-en-Velin, France) or 0.5% NP-40. Nonspecific sites were coated with PBS-0.5% milk powder, which was used for antibody dilutions. Slides were then proceeded for double immunostaining. They were incubated with anti-vWF antibody together with one of the following unconjugated MoAbs (antismooth muscle-specific α-actin, ICAM-1, VCAM-1, and E-selectin) previously described, or FITC-conjugated MoAbs against TF and CD36, during 3 hours. They were then washed and incubated with the second step reagents: TRITC-goat anti-rabbit for vWF antibody, FITC-goat anti-mouse for the unconjugated MoAbs, or PBS-milk for FITC-conjugated first MoAbs during 2 hours. The slides were then washed with PBS incubated 5 minutes with 1 μg/mL propidium iodide (Sigma), washed, and mounted in Moeviol. Cell preparations were analyzed with a Leica confocal microscope (Lyon, France).
Detection of apoptotic cells.
Apoptotic cells were identified by the detection of DNA strand breaks using the technique of in situ terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL)23 using a commercial kit: the TdT in situ apoptosis detection kit-fluorescein (Genzyme Diagnostics, Cambridge, UK). Biotinylated nucleotides were incorporated into the apoptically generated DNA ends using TdT. The covalently bound biotinylated nucleotides were detected using a streptavidin-fluorescein conjugate. Nuclei were counterstained with a solution of 1 μg/mL propidium iodide during 5 minutes. Cell preparations were analyzed with a Leica confocal microscope.
Statistical analysis.
The sign test was used to show that the distribution of the CEC data was nonnormal and is therefore presented as median and interquartile range (IQR). Data from the four groups of subjects (AMI, UA, EA, and controls) was analyzed by the Kruskal-Wallis test with three degrees of freedom. It was then log transformed to allow Tukey’s posthoc test to be applied. Serial data were analyzed by repeated measures (Friedman’s two-way) analysis of variance on Minitab 10 extra.
RESULTS
Cross-sectional estimation of CECs.
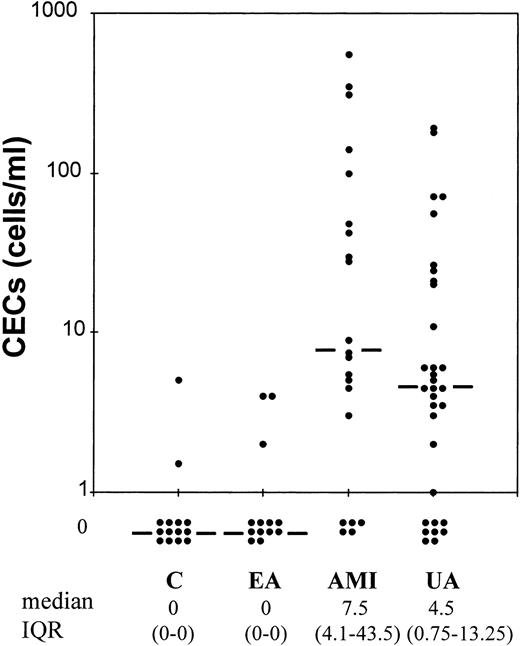
The levels of CECs were determined on blood samples obtained within 12 hours of the development of symptoms (Fig1). Median CEC count was 7.5 cells/mL (IQR, 1.5 to 43.5 cells/mL) in the AMI group and 4.5 cells/mL (IQR, 0.75 to 13.25) in UA. In both the EA (before test exercise) and control groups, no CECs were detected (median, both 0; IQR, both 0 to 0). Log transformation of these data followed by Tukey’s test showed levels to be higher in AMI than in both EA and controls (both P < .01). Levels in UA were higher than in both EA and controls (both P < .05). Levels between AMI and UA and between EA and controls were not significantly different. Our data does not support the hypothesis that pharmacologic treatment and the release of endothelial cells are linked because the four groups received the same medication, but only AMI and UA patients presented CECs (Table 4). It is noteworthy that among the four patients treated by thrombolysis, two presented high levels of CECs (100 and 550 cells/mL), while the remainder presented low levels (0 and 7 cells/mL within the first 12 hours).
Quantitation of CECs. Levels of CECs in patients with AMI (n = 21) and UA (n = 32) compared to subjects with EA (n = 13) and to subjects with noncoronary chest pain (C, n = 14) within 12 hours of the development of symptoms. Bar represents median value.
Quantitation of CECs. Levels of CECs in patients with AMI (n = 21) and UA (n = 32) compared to subjects with EA (n = 13) and to subjects with noncoronary chest pain (C, n = 14) within 12 hours of the development of symptoms. Bar represents median value.
Serial Changes in Levels of CECs After AMI, UA, and EA
| Patient Group . | Time Point . | Median CEC . | IQR . |
|---|---|---|---|
| AMI | <12 h | 7.5 | 1.5-43.5 |
| 12-18 h | 12.0 | 0.5-62.0 | |
| 18-24 h | 15.5 | 2.7-39.0 | |
| 24-30 h | 9.0 | 0.0-42.5 | |
| 30-42 h | 12.0 | 0.5-40.0 | |
| UA | <12 h | 4.5 | 0.75-13.25 |
| 12-18 h | 3.0 | 0.0-10.0 | |
| 18-24 h | 3.0 | 0.0-12.0 | |
| 24-36 h | 2.0 | 0.38-20.75 | |
| Controls | <12 h | 0.0 | 0.0-0.0 |
| 12-18 h | 0.0 | 0.0-2.0 | |
| 18-24 h | 0.0 | 0.0-0.25 | |
| 24-36 h | 0.0 | 0.0-0.0 | |
| EA | Before exercise | 0.0 | 0.0-0.0 |
| Immediately after exercise | 2.0 | 0.0-3.0 | |
| 4 h after exercise | 0.0 | 0.0-1.0 |
| Patient Group . | Time Point . | Median CEC . | IQR . |
|---|---|---|---|
| AMI | <12 h | 7.5 | 1.5-43.5 |
| 12-18 h | 12.0 | 0.5-62.0 | |
| 18-24 h | 15.5 | 2.7-39.0 | |
| 24-30 h | 9.0 | 0.0-42.5 | |
| 30-42 h | 12.0 | 0.5-40.0 | |
| UA | <12 h | 4.5 | 0.75-13.25 |
| 12-18 h | 3.0 | 0.0-10.0 | |
| 18-24 h | 3.0 | 0.0-12.0 | |
| 24-36 h | 2.0 | 0.38-20.75 | |
| Controls | <12 h | 0.0 | 0.0-0.0 |
| 12-18 h | 0.0 | 0.0-2.0 | |
| 18-24 h | 0.0 | 0.0-0.25 | |
| 24-36 h | 0.0 | 0.0-0.0 | |
| EA | Before exercise | 0.0 | 0.0-0.0 |
| Immediately after exercise | 2.0 | 0.0-3.0 | |
| 4 h after exercise | 0.0 | 0.0-1.0 |
Serial estimation of CECs.
Five serial blood samples were obtained from 26 patients suffering from an AMI, four samples from 20 patients in UA, and three samples from 13 patients undergoing the treadmill exercise test (Table 4). Data were analyzed by Friedman’s two-way repeated measures of analysis of variance (ANOVA). For the AMI, there was a significant peak in CECs at 18 to 24 hours (P = .009 overall repeated measures ANOVA,P < .05 peak v <12 hours sample and v 30 to 42 hours sample). There was no change in levels of CECs in UA (P = .451) or in EA, despite the immediate peak postexercise due to exercise testing (P = .417).
Morphologic aspects.
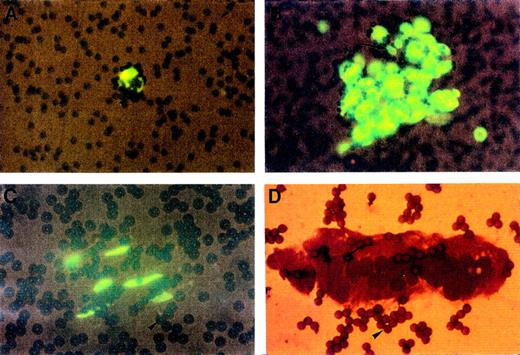
After immunomagnetic separation and cell observation in the hemocytometer, the same morphologic features were noticed between cells harvested from AMI and from UA. In both cases, different cytological patterns were observed after staining with the fluorescent probe acridin orange or with May-Grünwald-Giemsa: a majority of cells were 20 to 50 μm in diameter with a large nucleolated nucleus and a less fluorescent cytoplasm surrounded by beads; they were found alone or clumped together (Fig 2A and B). We sometimes observed spindle-shaped cells presenting elongated nuclear configuration (Fig 2C) and sheets of CECs remaining largely intact with well-preserved nuclei and well-delineated cytoplasm (Fig 2D).
Cytological analysis of CECs isolated with magnetic beads coated with S-Endo 1 antibody. Each panel shows CECs isolated from donors with AMI or UA. Panels A, B, and C show CECs stained with acridin orange presenting a round shape, clear nuclei and nucleoli (arrows) (A and B) or a spindle shape (C). Panel D shows a cell sheet stained with May-GrünwaldGiemsa. Numerous beads (focus or out of focus) are evident in all panels (arrowheads); they have a diameter of 4.5 μm.
Cytological analysis of CECs isolated with magnetic beads coated with S-Endo 1 antibody. Each panel shows CECs isolated from donors with AMI or UA. Panels A, B, and C show CECs stained with acridin orange presenting a round shape, clear nuclei and nucleoli (arrows) (A and B) or a spindle shape (C). Panel D shows a cell sheet stained with May-GrünwaldGiemsa. Numerous beads (focus or out of focus) are evident in all panels (arrowheads); they have a diameter of 4.5 μm.
Immunofluorescence staining.
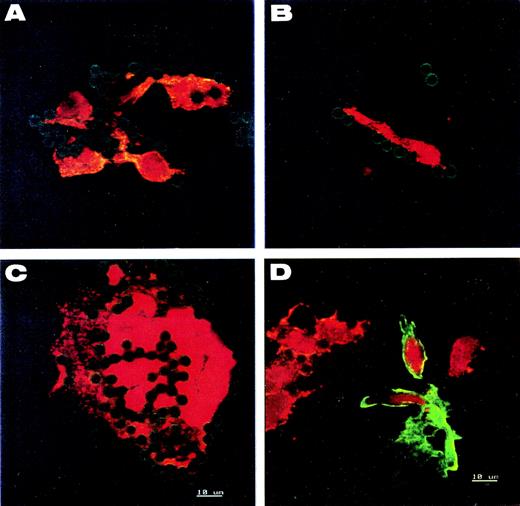
To confirm the endothelial origin of the circulating cells and exclude contamination by smooth muscle cells, double immunological stainings for vWF and smooth muscle-specific α-actin were performed on the recovered cells. On all cell types examined, vWF was detected as granular structures uniformly distributed in cytoplasm (Fig 3A through C), whereas antismooth muscle-specific α-actin labeling was negative. Cell staining was compared with control slides of mixed human umbilical vein endothelial cells (HUVEC) (vWF positive) and smooth muscle cells (α-actin positive) (Fig 3D).
Immunofluorescence analysis of CECs. (A, B, and C) CECs isolated from donors with AMI or UA. Cells of (D) are mixed endothelial and smooth muscle cells of human umbilical vein for control. All are stained for both intracellular vWF (red) and smooth muscle-specific actin (green). Round- and spindle-shaped cells (A and B), cell sheets (C), and umbilical vein endothelial cells (D) show the same granular pattern of vWF expression. The presence of smooth muscle-specific actin is detected only in control smooth muscle cells (D). Nuclei are counterstained red with propidium iodide. The green halo around beads comes from anti-mouse FITC-secondary antibody bound on the S-endo 1-coated beads.
Immunofluorescence analysis of CECs. (A, B, and C) CECs isolated from donors with AMI or UA. Cells of (D) are mixed endothelial and smooth muscle cells of human umbilical vein for control. All are stained for both intracellular vWF (red) and smooth muscle-specific actin (green). Round- and spindle-shaped cells (A and B), cell sheets (C), and umbilical vein endothelial cells (D) show the same granular pattern of vWF expression. The presence of smooth muscle-specific actin is detected only in control smooth muscle cells (D). Nuclei are counterstained red with propidium iodide. The green halo around beads comes from anti-mouse FITC-secondary antibody bound on the S-endo 1-coated beads.
Information regarding the origin of CECs was obtained by staining with CD36, a marker that is present predominantly in microvascular endothelium.24-26 By double-staining for CD36 and vWF, we showed that the cells isolated in samples from patients with AMI (n = 6) or with UA (n = 6) did not express CD36 and therefore were predominantly of macrovascular origin. Negative and positive controls were provided by lymphocytes and granulocytes (CD36 negative) and monocytes and platelets (CD36 positive) on blood smears. To assess whether endothelial cells circulate in an activated state, we analyzed on the same AMI and UA samples, the dual expression of vWF with molecules that appear on activated endothelial cells, namely ICAM-1, VCAM-1, E-selectin, and tissue factor. Only tissue factor was expressed by 25% of the CECs examined. Cell staining was compared with negative and positive control slides of resting and TNF (10 ng/mL) activated HUVEC.
Detection of DNA degradation.
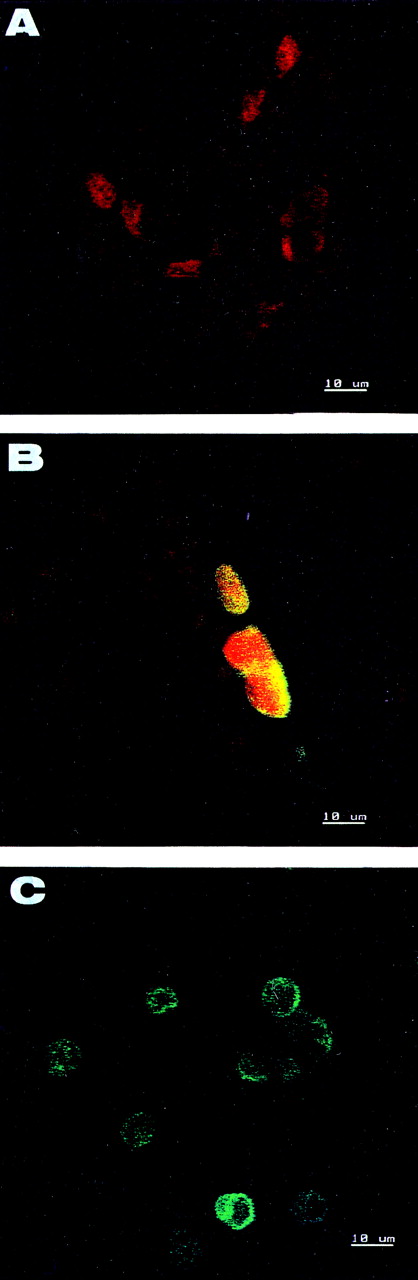
To assess if apoptosis accounted for cell desquamation, we investigated nuclear DNA fragmentation of CECs from four patients with AMI and four patients with UA displaying the highest levels of CECs. In each sample examined, less than 10% of cells was found to be positive. The majority, including the endothelial sheets, were devoid of green staining showing the lack of DNA breaks, but showed a uniform nuclear staining with propidium iodide (Fig 4).
Detection of apoptotic cells with TUNEL assay. (A and B) CECs isolated from donors with AMI or UA. (C) Control apoptotic HL60 cells. Nuclei of CECs are counterstained red with propidium iodide. A large majority of CECs including endothelial sheets are negative (A). Only rare cells show DNA breaks (B). The control apoptotic HL60 cells are positive (C).
Detection of apoptotic cells with TUNEL assay. (A and B) CECs isolated from donors with AMI or UA. (C) Control apoptotic HL60 cells. Nuclei of CECs are counterstained red with propidium iodide. A large majority of CECs including endothelial sheets are negative (A). Only rare cells show DNA breaks (B). The control apoptotic HL60 cells are positive (C).
DISCUSSION
To our knowledge, this is the first combined specific capture and immunologic demonstration of CECs in AMI and UA. Because CECs were not detected in the controls or in the EA group, their presence in blood is indicative of endothelial cell detachment in acute coronary syndromes with probable atheroma plaque rupture. Therefore, our data supplements preliminary reports that suggested the presence of cells or cell “carcasses” with endothelial morphological features in these two pathologies without demonstrating their endothelial nature and their viability.16 17
In certain other vascular diseases, CECs have been reported to be an “ex vivo” indicator of vascular injury. The level of CECs varies according to the extent of the endothelial lesion. A high endothelial cell count is found in widespread vascular damage associated with rickettsial vasculitis (2 to 1,600 cells/mL),10 sickle cell crisis (46 to 430 cells/mL),6-8 or cytomegalovirus infection (0 to 50 endothelial cells per 2 × 105ficoll-separated mononuclear cells).14,15 However, the number of CECs found in localized vessel damage, such as after coronary angioplasty, is just over the normal (mean values, approximately 5 to 10 cells/mL).11
In the present study, a large range of CEC counts was found in AMI as well as in UA, suggesting a wide variation of vessel injury associated with these events. Notably, some CECs were still observed in the blood in some patients up to 42 hours after the onset of chest pain. This may reflect the half-life of these cells, as already reported in patients undergoing coronary angioplasty (at least 24 hours),11 or it may reflect protracted vascular irritability or delayed endothelial cell detachment from the subendothelium of the vessel wall. No cells were found at rest in EA; this result was not significantly modified after exercise testing, suggesting that ischemia associated with stable angina does not induce significant endothelial cell desquamation. In addition, we can exclude the possibility that the level of CECs reflects drug treatment because controls (who suffered from a noncoronary chest pain) received the same medication as patients (including heparin therapy), but had no CECs. Furthermore, unpublished data of a group of patients not suffering an acute coronary disease and treated with various doses of unfractionated heparin (0.1 to 0.5 IU/mL) did not show any CECs.
The cytological heterogeneity of cells isolated both in AMI and UA is noteworthy. The majority of CECs presented classical endothelial morphologic features with rounded shapes. These cells showed the typical granular cytoplasmic distribution of vWF and the absence of smooth muscle-specific α actin consistent with their endothelial nature. The same typical granular staining was found on spindle-shaped cells and endothelial sheets. Such circulating endothelial sheets were previously described in a dog model of myocardial infarction and were shown to come from endocardial endothelium.27 Because no specific immunologic marker for this type of endothelium is available, it was not possible to show that the CECs are indeed from cardiovascular origin. These cells tend to be predominantly of macrovascular origin as defined by the negativity of CD36 staining. We cannot be completely confident that CD36− CECs are not microvascular, as some CD36− endothelial cells have been identified in dermal microvessels.26 The majority of the isolated cells did not present a proadhesive phenotype, as evidenced by the absence of expression of three adhesion molecules ICAM-1, VCAM-1, and E-selectin. This observation can be linked to the recent demonstration that endothelial cells differentially express cell adhesion molecules depending on the size of blood vessels, with the most prominent expression of E-selectin and ICAM in microvessels.28 Alternatively, it is also possible that CEC activation state does not accurately reflect the phenotype of the endothelium remaining attached in situ. Compared with data published by Solovey et al,7 all we can conclude is that the CECs recovered in acute coronary diseases and in sickle cell anemia are different in terms of origin and activated phenotype. A small proportion of CECs expressed tissue factor, suggesting that circulating endothelium may have a procoagulant phenotype, although this hypothesis clearly demands confirmation. Recently, a circulating population of endothelial putative progenitor cells that expressed vascular endothelial growth factor receptor were identified from peripheral blood of healthy subjects by CD34 magnetic beads.29 These cells should differ from those recovered in this study, as the CECs isolated with S-Endo 1 beads are not detectable in healthy individuals.
To assess the potential mechanism of cell detachment, we investigated whether apoptosis was involved. However, 90% of the CECs showed a remarkably good cytoplasmic and nuclear morphologic preservation without the usual morphologic features of apoptosis. Because one of the biochemical hallmarks of apoptosis is fragmentation of chromatin, we analyzed these cells with a TUNEL assay. In the majority, including the endothelial sheets, the CECs showed a lack of DNA breakage indicating that they were not in apoptosis when they detached from the vessel wall. Moreover, it has been reported that in advanced atheromatous lesions or in coronary restenosis, apoptosis concerns mostly smooth muscle cells and monocytes/macrophages.30 31 In the present study, less than 10% of CECs analyzed showed DNA signs of apoptosis. It is possible that the apoptotic changes in these cells occurred after detachment when they were already in the blood, which may be a function of the lag time between detachment and blood collection.
Apart from apoptosis, several other possibilities can be considered for the mechanisms responsible for endothelial cell detachment. It might be due to (1) mechanical dislodgment of cells during plaque rupture (as supported by angiographic data); (2) proteolysis of subendothelial matrix proteins triggered by u-PA or t-PA–mediated plasminogen activation32,33; (3) prolonged ischemia of the heart muscle can lead to the detachment of the most sensitive endothelial cells such as endocardial endothelial cells; and (4) oxidative burst occurring after blood reperfusion.34-36 Another possibility is that these cells represent cells released from the microcirculation during ischemia. The fact that CECs are CD36− and probably not of microvascular origin does not favor this hypothesis.
In conclusion, the measurement of CECs represents a direct evidence of injury of the endothelial lining among patients with acute coronary disease. This information can be obtained by a simple, noninvasive test that may be useful for endothelial exploration and may yield new insights in the pathophysiology of endothelial injury. Further study will determine if this endothelial marker can be used as a diagnostic index, especially in UA. Furthermore, the fact that CECs are not apoptotic suggests potential use as autologous vectors for diagnosis of endothelial abnormalities and for vascular therapy.
ACKNOWLEDGMENT
The authors are grateful to Drs Victor Gurewich and Valentin Fuster for suggestions and comments on the manuscript and to Biocytex and Immunotech companies for providing antibodies. We thank Dr Serge Yvorra for his contribution in the early phase of this study. We also thank Robert Pistoresi and Michel Dehri for confocal microscope analyses, Annie Bottari and Andrée Boyer for secretarial work.
Supported in part by Hoechst Laboratories.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Françoise Dignat-George, PhD, Laboratoire d’Hématologie et d’Immunologie, U.F.R. de Pharmacie, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France; e-mail: hematim@pharmacie.univ-mrs.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal