Abstract
In the absence of the hematopoietic transcription factor GATA-1, mice develop thrombocytopenia and an increased number of megakaryocytes characterized by marked ultrastructural abnormalities. These observations establish a critical role for GATA-1 in megakaryopoiesis and raise the question as to how GATA-1 influences megakaryocyte maturation and platelet production. To begin to address this, we have performed a more detailed examination of the megakaryocytes and platelets produced in mice that lack GATA-1 in this lineage. Our analysis demonstrates that compared with their normal counterparts, GATA-1–deficient primary megakaryocytes exhibit significant hyperproliferation in liquid culture, suggesting that the megakaryocytosis seen in animals is nonreactive. Morphologically, these mutant megakaryocytes are small and show evidence of retarded nuclear and cytoplasmic development. A significant proportion of these cells do not undergo endomitosis and express markedly lower levels of mRNA of all megakaryocyte-associated genes tested, including GPIb, GPIbβ, platelet factor 4 (PF4), c-mpl, and p45 NF-E2. These results are consistent with regulation of a program of megakaryocytic differentiation by GATA-1. Bleeding times are significantly prolonged in mutant animals. GATA-1–deficient platelets show abnormal ultrastructure, reminiscent of the megakaryocytes from which they are derived, and exhibit modest but selective defects in platelet activation in response to thrombin or to the combination of adenosine diphosphate (ADP) and epinephrine. Our findings indicate that GATA-1 serves multiple functions in megakaryocyte development, influencing both cellular growth and maturation.
THE DIFFERENTIATION of hematopoietic cells from pluripotent progenitors involves the progressive restriction of differentiation potential and the acquisition of lineage-specific patterns of gene expression.1 2 These patterns are coordinated by a number of tissue-restricted and general transcription factors that act in a combinatorial manner. In the megakaryocytic lineage, one can operationally divide the roles of these transcription factors into those required for the specification and maintenance of progenitors, maturation of megakaryoctyes, and terminal differentiation of megakaryocytes, including platelet production.
From analysis of expression patterns, of cis-elements important in the regulation of megakaryocyte-specific genes and of mouse null mutants of transcription factor genes, a number of tissue-restricted transcription factors are implicated in megakaryopoiesis. These include SCL/Tal-1, c-Myb, Ets family members, GATA-1, GATA-2, FOG, and NF-E2.3-11 Among these, GATA-1, FOG, and NF-E2 stand out for the nonredundant and essential roles they play in megakaryopoiesis.
Two lines of evidence have suggested that GATA-1 can participate in megakaryopoiesis. First, when overexpressed, either directly or indirectly, GATA-1 can induce megakaryocytic differentiation in a multipotential murine myeloid cell line, 416B.12,13Similarly, forced GATA-1 expression in Myb-Ets–transformed chicken myeloblasts induces differentiation into thromboblasts.14Second, in fetal liver of chimeric mice generated with GATA-1–null ES cells there is a fourfold increase in megakaryocyte number, suggesting that GATA-1 influences megakaryocyte homeostasis.15However, these studies do not clearly define a requirement for GATA-1 in megakaryocytes. Recently, we generated two mutant lines of mice with megakaryocyte-specific loss of GATA-1 expression, allowing a more direct evaluation of the importance of GATA-1 in this lineage.8 Preliminary analysis of these mice pointed to a critical role for GATA-1 in the proper proliferation and terminal maturation of megarkaryocytes and in platelet production.
Mice with megakaryocyte-specific GATA-1 deficiency have a platelet count of approximately 15% of normal with a markedly increased mean platelet volume, suggesting that the platelets may be structurally abnormal.8 There is also a remarkable megakaryocytosis in these animals, with a significant increase in megakaryocyte numbers in the spleen and bone marrow. Colony assays from yolk sac and fetal liver indicate that megakaryocyte progenitor numbers are normal but that some megakaryocyte progenitors exhibit marked hyperproliferation, producing abnormally large colonies principally composed of immature megakaryocytes similar to those seen in vivo. Ultrastructural examination of these megakaryocytes showed gross abnormalities with a small cytoplasm, excess rough endoplasmic reticulum, a reduction in the number of platelet granules, and underdeveloped and disorganized platelet demarcation membranes. Takahashi et al have also recently reported that when GATA-1 levels are reduced in vivo, heterozygous female mice display megakaryocytosis in the spleen.16
These studies lead to the question as to how GATA-1 orchestrates megakaryocyte development. To begin to address this issue, we have performed a detailed characterization of GATA-1–deficient megakaryocytes and platelets. These studies more accurately define where GATA-1 is likely to act in this lineage.
MATERIALS AND METHODS
Generation of neoΔHS and ΔneoΔHS mice.
NeoΔHS and ΔneoΔHS mice used in this study have been previously described.8 They were generated by a targeted deletion of an upstream region of the GATA-1 locus. The mutation removes at least one DNase I hypersensitive site (HS), which probably earmarks sequences important for GATA-1 expression. Two different lines of mutant mice were generated: neoΔHS, in which the PGK-neo selection cassette remains in the targeted locus, and ΔneoΔHS, in which the selection marker has been excised from the targeted locus. In neoΔHS, there is no detectable expression of GATA-1 in megakaryocytes, whereas in neoΔHS mice, GATA-1 is expressed at a level of approximately 1% to 5% of normal.8 The megakaryocyte and platelet phenotypes of the two mutant mice are indistinguishable, but as ΔneoΔHS mice are viable, they were used for studies involving adult mice.
Determination of primary megakaryocyte growth curves.
Fetal liver cells were isolated from 15 neoΔHS fetuses and 15 normal littermate controls at embryonic day 12.5 (E12.5). Single-cell suspensions from individual fetal livers were cultured in Dulbecco’s minimum essential medium (DMEM) with 10% fetal calf serum (FCS) supplemented with 1% recombinant human thrombopoietin (Tpo) tissue culture supernatant.17 A 50-μL quantity of the culture was taken every 2 days for cytocentrifugation and the slides assayed for acetylcholinesterase activity.18 The total number of acetylcholinesterase-positive cells was counted in 50 μL.
DNA content analysis of primary megakaryocytes.
Individual single-cell suspensions were made from bone marrow harvested from the femurs of eight ΔneoΔHS mice and six wild-type littermates aged 6 to 10 weeks, and resuspended and washed once in CATCH medium (0.38% Na citrate + 10−3 mol/L adenosine + 2 × 10−3 mol/L theophylline, in Ca2+/Mg2+-free Hanks’ balanced solution).19 To assay DNA content from cultured cells, individual single-cell suspensions were made from E12.5 fetal livers from three GATA-1–deficient embryos and three littermate control embryos. These suspensions were cultured in 10% FCS in DMEM supplemented with 1% recombinant human Tpo tissue culture supernatant.17 The cells were harvested on day 4 of culture. To label megakaryocytes, cells were incubated with a rabbit antimouse platelet antiserum (RAMPS), used at a dilution of 1:250.20 Cells were then incubated with a fluorescein-conjugated goat antirabbit IgGF(ab′)2antibody (Tago International, Biosource International, Camarillo, CA). Last, cells were stained with propidium iodide in hypotonic citrate.21 Using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and two-color flow cytometry, DNA content of all RAMP-positive cells was measured.22 The proportion of cells in each ploidy class was determined by integrating the area under each peak. Comparison of the percentage of 2N cells was performed by the Student’s paired t-test.
Semiquantitative reverse-transcriptase polymerase chain reaction analysis of megakaryocyte-associated genes.
Approximately 1 to 5 × 105 fetal liver cells from neoΔHS and normal littermate fetuses were plated in a methylcellulose mix, which included 1% recombinant human Tpo tissue culture supernatant.9 At day 5 to 6 colony-forming unit–megakaryocytes (CFU-Mk) were picked and placed in DMEM with 10% FCS. The cells were then washed once in phosphate-buffered saline (PBS). RNA was extracted using RNAZOL B (Tel-Test, Friendwood, TX) according to the manufacturer’s protocol and cDNA was made using an oligo(dT) primer. Semiquantitative reverse-transcriptase polymerase chain reaction (RT-PCR) was then performed.9 The amount of cDNA in the samples was normalized using primers for hypoxanthine phosphoribosyl transferase (HPRT). Reaction products were separated on 4% nondenaturing polyacrylamide gels. No reaction products were detected using RNA samples from which RT had been omitted (data not shown). Quantitation of fold-differences in mRNA levels was performed by densitometric analysis (Molecular Dynamics, Sunnyvale, CA) of autoradiographs. The sum of the average pixel intensity above background was determined for each of the bands representing different PCR cycle numbers; normalization was performed relative to the bands from the PCR reactions for HPRT. The primer pairs used for HPRT, platelet factor 4 (PF4), and c-mpl have been reported previously.9 23 The following primer pairs were used to amplify: GPIbα—5′ CCTGGAAGAAGCTCTGTTCCTCC 3′ and 5′ CATTGGTCTGCAGGCTCGTC 3′; GPIbβ—5′AGGACAGGACGCGGCATTCA 3′ and 5′ AGGCTTCTGGGAGGAAGGCG 3′; and p45 NF-E2—5′ AACTTGCCGGTAGATGACTTTAAT 3′ and 5′ CAGAGTGCGGTCAGCCTCCCCTCG 3′.
Electron microscopy.
Electron microscopy of platelets was performed according to a modification of the procedure of Stenberg et al.24 Briefly, whole blood, collected by cardiac puncture directly into syringes containing an excess of 1.5% glutaraldehyde in 0.01 mol/L cocadylate buffer, pH 7.4, was fixed overnight at 4°C. The blood was centrifuged at 800g for 15 minutes to isolate platelets, which were dehydrated through an ascending series of alcohols, infiltrated with propylene oxide, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with a JEOL 100CX-II transmission electron microscope (JEOL, Peabody, MA) at an accelerating voltage of 60 kV.
Western blot of platelets.
Platelets isolated from ΔneoΔHS and wild-type mice as previously described25 were directly resuspended in 2X protein lysis sample buffer (125 mmol/L Tris pH 6.8, 2% sodium dodecyl sulfate [SDS], 50% glycerol, 5% β-mercaptoethanol, 0.001% bromophenol blue). The samples were electrophoresed through 10% SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted onto nitrocellulose membranes. The membranes were blocked overnight at 4°C or for 2 hours at room temperature in Tris-buffered saline with Tween (TBST; 50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 1 mmol/L EDTA, 0.1% Tween-20) supplemented with 5% nonfat milk. Binding of the primary and secondary antibody was performed in TBST. Washes of the membranes after antibody binding were performed for 1 hour with TBST. One filter was sequentially used to detect expression of c-MPL, GPIIb, and c-src, and another to detect GPIb and c-src; we evaluated c-src expression to ensure equivalent sample loading. Commercially available antibodies to GPIIb, GPIb (Pharmingen, San Diego, CA), and c-src (Santa Cruz Biotechnology, Santa Cruz, CA) were used at dilutions of 1:500, 1:500, and 1:1,000. Monoclonal antibodies against c-mpl were a kind gift from Dr Federic de Sauvage and used at a dilution of 1:1,000. The secondary antibody was conjugated with HPRT and the signal detected with a commercial chemiluminescence kit (Amersham, Arlington Heights, IL).
Bleeding time studies.
Four ΔneoΔHS mice and six wild-type littermate controls at age 6 to 8 weeks were used to measure bleeding times as previously described.26
Platelet lifespan studies and platelet function tests.
RESULTS
Growth dysregulation of GATA-1–deficient megakaryocytes.
To quantitate the growth disturbance of primary GATA-1–deficient megakaryocytes, we compared the growth rates of GATA-1–deficient and normal megakaryocytes in vitro. Fetal liver cells isolated from neoΔHS mice and wild-type littermates at E12.5 were expanded in liquid culture in the presence of recombinant Tpo and the number of acetylcholinesterase-positive cells was determined every 2 days. The growth curves (Fig 1) show a dramatic proliferative advantage for GATA-1–deficient cells; not only are the maximal numbers of megakaryocytes 15-fold greater, but the GATA-1–deficient megakaryocytes continue in culture for up to 4 weeks, whereas normal megakaryocytes die after 12 to 14 days. These results extend the previous colony data8 by showing the greatly enhanced proliferative response of GATA-1–deficient megakaryocytes in response to Tpo and are in agreement with megakaryocytosis seen in mutant animals.
Proliferation profiles of normal (WT) and neo▵HS (MUT) primary megakaryocytes expanded from fetal liver cells in liquid culture in recombinant Tpo. The number of cells with acetylcholinesterase (AchE) activity in 50-μL aliquots of the culture is shown as a function of the duration of culture. The values shown are the mean ± 1 SD from separate cultures of 15 normal and 15 mutant fetal livers.
Proliferation profiles of normal (WT) and neo▵HS (MUT) primary megakaryocytes expanded from fetal liver cells in liquid culture in recombinant Tpo. The number of cells with acetylcholinesterase (AchE) activity in 50-μL aliquots of the culture is shown as a function of the duration of culture. The values shown are the mean ± 1 SD from separate cultures of 15 normal and 15 mutant fetal livers.
Nuclear and cytoplasmic arrest of GATA-1–deficient megakaryocytes.
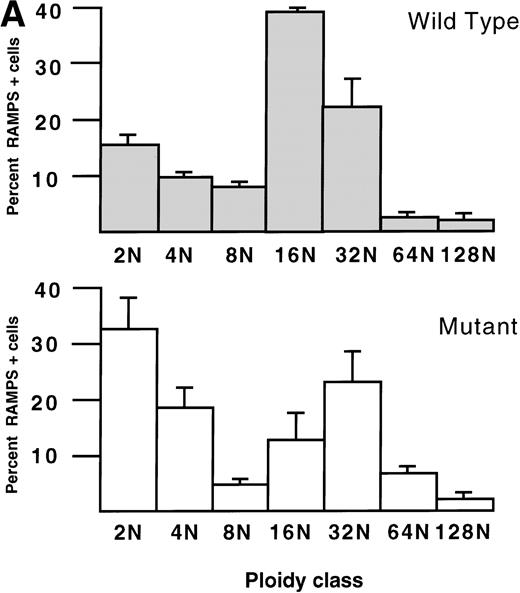
Associated with this hyperproliferation, GATA-1–deficient megakaryoctyes, as a population, show evidence of arrested nuclear and cytoplasmic development. DNA content analysis of eight ΔneoΔHS and six normal animals shows that the percentage of bone marrow megakaryocytes with 2N DNA content in the mutants is 32% compared with 15% in normal animals (P < .001; Fig2A). In addition, there is a reduction in the proportion of mutant megakaryocytes with 8N and 16N DNA content compared with controls. Nonetheless, a subpopulation of mutant megakaryocytes achieves a higher ploidy and the modal ploidy of these megakaryocytes is 32N, compared with 16N in wild-type megakaryocytes. Mirroring these findings, the proportion of cells with low DNA content is increased in cultured GATA-1–deficient megakaryocytes compared with controls (Fig 2B); controls also show a higher fraction of megakaryocytes with DNA content ≥8N.
Histograms of the DNA content of (A) bone marrow megakaryocytes from 6 normal (Wild Type, top) and 8 ▵neo▵HS (Mutant, bottom) weanling mice (age 4 weeks), and (B) megakaryocytes cultured ex vivo from E12.5 fetal livers of 3 normal (Wild Type, top) and 3 ▵neo▵HS (Mutant, bottom) embryos. The mean percentage (± 1 SD) of RAMPS-positive cells in each ploidy class
Histograms of the DNA content of (A) bone marrow megakaryocytes from 6 normal (Wild Type, top) and 8 ▵neo▵HS (Mutant, bottom) weanling mice (age 4 weeks), and (B) megakaryocytes cultured ex vivo from E12.5 fetal livers of 3 normal (Wild Type, top) and 3 ▵neo▵HS (Mutant, bottom) embryos. The mean percentage (± 1 SD) of RAMPS-positive cells in each ploidy class
Retardation of cytoplasmic maturation is indicated by semiquantitative RT-PCR analysis of megakaryocyte-specific transcripts. In these experiments, we used mutant and wild-type megakaryocytes of comparable morphology and size (colonies derived from CFU-Mk) as the source of mRNA. This was done to minimize differential patterns of gene expression being recorded solely because of differences in maturation state, rather than as a specific consequence of lack of GATA-1. GATA-1–deficient megakaryocytes showed significantly decreased mRNA expression of GPIbα (7.4-fold) and GPIbβ (3.6-fold), PF4 (3.4-fold) c-mpl (3.8-fold), and p45 NF-E2 (35-fold). Indeed, all of the markers studied showed decreased expression, albeit at different levels (Fig 3).
Semiquantitative RT-PCR analysis of GPIb, GPIbβ, PF4, c-mpl, and p45 NF-E2 mRNA transcripts from primary megakaryocytes derived from normal (WT) and neo▵HS (MUT) CFU-Mk. PCR reactions were performed with tracer [32P]dCTP for the indicated number of cycles before analysis by PAGE. HPRT signal was used to normalize the input cDNA.
Semiquantitative RT-PCR analysis of GPIb, GPIbβ, PF4, c-mpl, and p45 NF-E2 mRNA transcripts from primary megakaryocytes derived from normal (WT) and neo▵HS (MUT) CFU-Mk. PCR reactions were performed with tracer [32P]dCTP for the indicated number of cycles before analysis by PAGE. HPRT signal was used to normalize the input cDNA.
Platelet phenotype of GATA-1–deficient mice.
Despite the marked megakaryocyte abnormalities outlined above, some platelets (∼15% of normal) are produced in neoΔHS and ΔneoΔHS mice. In the following studies, we examined the ultrastructure of these platelets and sought to determine their competence in hemostasis.
Morphology of GATA-1–deficient platelets.
Electron microscopy of platelets isolated from the peripheral blood of ΔneoΔHS mice shows a number of gross morphologic abnormalities (Fig4). In addition to their larger volume, mutant platelets are uniformly spherical in shape, in contrast to the discoid shape of normal platelets. Rough endoplasmic reticulum and ribosomes are excessive. Most of the GATA-1–deficient platelets contain few platelet-specific granules or lack them entirely. There is marked heterogeneity in distribution of organelles, with some platelets harboring few organelles, while others are replete. Finally, many platelets have an increased internal canalicular membrane system. These findings are reminiscent of the ultrastructural abnormalities seen in the defective megakaryocytes from which these platelets are derived8 and indicate abnormal compartmentalization of organelles within GATA-1–deficient megakaryocytes before platelet release.
Representative electron micrographs of platelets produced by megakaryocytes deficient in GATA-1. Normal mouse platelets (A) have a uniform appearance and show the typical discoid shape with a normal complement of organelles, including platelet-specific granules. In contrast, GATA-1–deficient platelets (B and C) are large, invariably round, heterogeneous, and show a paucity of organelles, especially platelet-specific granules. In particular, these platelets have an excess of rough endoplasmic reticulum (C), similar to the megakaryocytes from which they are derived. Bars in (A) and (B) are 5 μm; in (C), 1 μm.
Representative electron micrographs of platelets produced by megakaryocytes deficient in GATA-1. Normal mouse platelets (A) have a uniform appearance and show the typical discoid shape with a normal complement of organelles, including platelet-specific granules. In contrast, GATA-1–deficient platelets (B and C) are large, invariably round, heterogeneous, and show a paucity of organelles, especially platelet-specific granules. In particular, these platelets have an excess of rough endoplasmic reticulum (C), similar to the megakaryocytes from which they are derived. Bars in (A) and (B) are 5 μm; in (C), 1 μm.
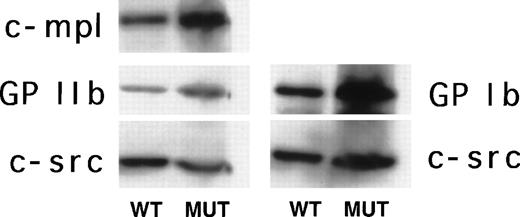
In surprising contrast to the mRNA expression data from GATA-1–deficient megakaryocytes, mutant platelets exhibit greater evidence of cytoplasmic maturity. This is evidenced by normal protein expression levels of GPIb, GpIIb, and c-mpl in GATA-1–deficient platelets compared with normal controls (Fig5). This apparent discrepancy between the maturation state of GATA-1–deficient megakaryocytes and platelets leads us to speculate that the platelets may be produced by a small subset of megakaryocytes that achieves the greatest degree of maturity in the bulk population of GATA-1–deficient megakaryocytes, presumably from among those cells with a higher DNA content.
Western blot analysis of c-mpl, GPIIb,and GPIb expression in platelets isolated from normal (WT) and ▵neo▵HS (MUT) adult mice. In the left panel, the same filter was sequentially used to assay expression for c-mpl, GPIIb,and c-src, whereas in the right panel a separate filter was sequentially used to detect expression of GPIb and c-src. The c-src signal was used to verify equivalent sample loading.
Western blot analysis of c-mpl, GPIIb,and GPIb expression in platelets isolated from normal (WT) and ▵neo▵HS (MUT) adult mice. In the left panel, the same filter was sequentially used to assay expression for c-mpl, GPIIb,and c-src, whereas in the right panel a separate filter was sequentially used to detect expression of GPIb and c-src. The c-src signal was used to verify equivalent sample loading.
The high ribosome and RNA content of GATA-1–deficient platelets is suggestive of young platelet age.29 Taken together with the thrombocytopenia, this raises the possibility that the abnormal GATA-1–deficient platelets have an increased susceptibility to destruction. To address this, we studied platelet lifespan in four ΔneoΔHS and four normal animals. No difference in platelet survival was observed between wild-type and mutant platelets (Table1), indicating that thrombocytopenia is due to a failure of platelet production.
Lifespan and Activation of GATA-1–Deficient and Wild-Type Platelets
| Variable . | Wild-Type . | Mutant . | P . |
|---|---|---|---|
| Platelet count (×10−6/μL) | 1.16 ± 0.12 | 0.18 ± 0.03 | <.00001 |
| Spontaneous activation (%) | 23.4 ± 11.7 | 18.9 ± 4.0 | NS |
| ADP activation (%) | 33.3 ± 7.9 | 32.2 ± 6.0 | NS |
| ADP + epinephrine activation (%) | 40.4 ± 8.4 | 23.5 ± 3.7 | .01 |
| Thrombin activation (%) | 92.9 ± 2.9 | 83.4 ± 2.7 | .002 |
| Platelet lifespan (hr) | 91.4 ± 17.3 | 105.4 ± 20.5 | NS |
| Variable . | Wild-Type . | Mutant . | P . |
|---|---|---|---|
| Platelet count (×10−6/μL) | 1.16 ± 0.12 | 0.18 ± 0.03 | <.00001 |
| Spontaneous activation (%) | 23.4 ± 11.7 | 18.9 ± 4.0 | NS |
| ADP activation (%) | 33.3 ± 7.9 | 32.2 ± 6.0 | NS |
| ADP + epinephrine activation (%) | 40.4 ± 8.4 | 23.5 ± 3.7 | .01 |
| Thrombin activation (%) | 92.9 ± 2.9 | 83.4 ± 2.7 | .002 |
| Platelet lifespan (hr) | 91.4 ± 17.3 | 105.4 ± 20.5 | NS |
Platelets isolated from 4 wild-type and 4 GATA-1–deficient ΔneoΔHS adult mice were tested for in vivo lifespan and activation in response to a variety of agonists.
Abbreviation: NS, not significant.
Function of GATA-1–deficient platelets.
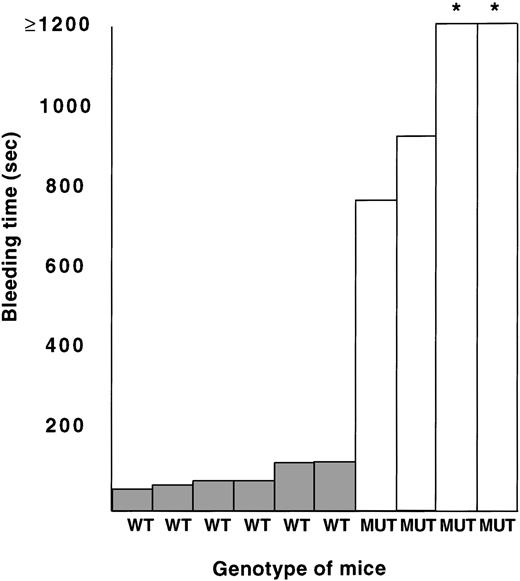
NeoΔHS and ΔneoΔHS mice do not exhibit a bleeding diathesis in the environment of a controlled animal care facility. However, when subject to trauma (tail biopsy), mutant mice frequently bleed excessively compared with wild-type littermates. To evaluate the function of GATA-1–deficient platelets, we measured bleeding times in four ΔneoΔHS and six normal animals. As shown in Fig 6, bleeding times are substantially prolonged in the mutant mice. It is helpful to compare these data with published results from Tpo-null mice, which have an equivalent degree of thrombocytopenia with functionally normal platelets and are reported to have an average bleeding time of 3 minutes.30 Thus, the excessively prolonged bleeding time of ΔneoΔHS mice points to a functional platelet defect superimposed on thrombocytopenia.
Bleeding times obtained from 6 normal (WT) and 4 ▵neo▵HS (MUT) adult mice. *Bleeding times >1,200 seconds.
Bleeding times obtained from 6 normal (WT) and 4 ▵neo▵HS (MUT) adult mice. *Bleeding times >1,200 seconds.
We performed platelet activation studies on four ΔneoΔHS mice and four normal control mice by measuring the appearance of P-selectin on the platelet cell surface in response to a variety of platelet agonists. This analysis showed a modest selective defect in activation of mutant platelets by either thrombin or a combination of adenosine diphosphate (ADP) and epinephrine, whereas activation by ADP alone was comparable to normal (Table 1).
DISCUSSION
The findings presented here indicate that megakaryocytes deficient in GATA-1 hyperproliferate, have a modal ploidy of 2N, express significantly lower levels of several megakaryocyte-specific genes, and produce large, structurally abnormal platelets. Connected with these abnormalities, platelet function is compromised in vivo and in vitro.
The combination of the colony and liquid culture data provide useful insights into the biology of the megakaryocytosis seen in neoΔHS and ΔneoΔHS mice. In liquid culture there is a 15-fold increase in the maximal number of mutant megakaryocytes and these cells persist or are produced for more than twice as long. However, megakaryocytosis is not driven by an increased number of CFU-Mk progenitors, the frequency of which is similar in wild-type and mutant mice.8 We interpret the striking differences between wild-type and mutant cultures to reflect hyperproliferation of the progeny of a subset of mutant progenitors (Fig7), which presumably give rise to the large abnormal colonies consisting of thousands of cells. There are two mutually nonexclusive possibilities for the different proliferative response in mutant and wild-type megakaryocyte progenitors. First, in the absence of GATA-1, there may be selective, abnormal expansion of an otherwise rare pool of normal progenitors with high proliferative capacity that is distinct from the pool of normal CFU-Mk. Alternatively, the large colonies could arise from abnormal CFU-Mk progenitors that are present only in mutant animals; however, mutant animals must also have normal CFU-Mk progenitors, as normal-sized megakaryocyte colonies can be cultured from their hematopoietic cells. Interestingly, May-Grünwald-Giemsa staining of the abnormally large colonies shows cells not only with megakaryocytic morphology, but also some that are blast-like8 and others that resemble proerythroblasts (data not shown). Moreover, the progeny of these abnormally large colonies have a low but definite secondary replating potential, producing mainly erythroid colonies arrested at the proerythroblast stage (data not shown). Taken together, these results suggest that the hyperproliferating cell is not a normal CFU-Mk, but rather represents an earlier progenitor, perhaps one with bilineage erythromegakaryocytic potential. Similar colonies are almost never seen in cultures of normal fetal livers in Tpo.
A hierarchy of transcription factors important in megakaryopoiesis. The normal progression of a multipotential progenitor to a platelet-producing megakaryocyte (MK) is shown. The upper set of arrows denotes the normal pattern of progression from one cell type to another, and the lower set of arrows illustrates the findings in a GATA-1–deficient environment. The thickness of the arrows represents the degree to which the progression from 1 cell type to another occurs. In GATA-1 deficiency, there is a marked increase of immature megakaryocytes relative to the wild type. Most normal immature megakaryocytes complete differentiation, whereas in GATA-1 deficiency, many immature megakaryocytes fail to complete the differentiation program, and the few that do are abnormal structurally. This results in a lower number of circulating platelets that are functionally and structurally abnormal. In the absence of FOG, either the megakaryocyte progenitor is not specified or fails to differentiate. Conversely, the requirement for NF-E2 is apparently limited to terminal megakaryocyte differentiation before platelet release.
A hierarchy of transcription factors important in megakaryopoiesis. The normal progression of a multipotential progenitor to a platelet-producing megakaryocyte (MK) is shown. The upper set of arrows denotes the normal pattern of progression from one cell type to another, and the lower set of arrows illustrates the findings in a GATA-1–deficient environment. The thickness of the arrows represents the degree to which the progression from 1 cell type to another occurs. In GATA-1 deficiency, there is a marked increase of immature megakaryocytes relative to the wild type. Most normal immature megakaryocytes complete differentiation, whereas in GATA-1 deficiency, many immature megakaryocytes fail to complete the differentiation program, and the few that do are abnormal structurally. This results in a lower number of circulating platelets that are functionally and structurally abnormal. In the absence of FOG, either the megakaryocyte progenitor is not specified or fails to differentiate. Conversely, the requirement for NF-E2 is apparently limited to terminal megakaryocyte differentiation before platelet release.
It is interesting that GATA-1 impinges upon growth control in both the megakaryocytic and erythroid lineages. In erythroid cells, absence of GATA-1 leads to maturation arrest and premature apoptosis at the proerythroblast stage in vitro.23 31 The clear hyperproliferative response seen in megakaryocytes deficient in GATA-1 both in vitro and in vivo suggests that further analysis of the mechanisms by which GATA-1 influences cell growth and apoptosis in blood cells is likely to be rewarding.
GATA-1–deficient megakaryocytes exhibit evidence of retarded nuclear development, reflected by the modal ploidy of 2N, compared with 16N in wild-type controls. However, a population of mutant megakaryocytes does undergo endoreduplication and achieves higher DNA content. It is unclear whether this suggests that GATA-1 is not required for the initiation of endomitosis or that most megakaryocytes do require GATA-1 for endoreduplication, but that a subset of megakaryocytes can become polyploid through GATA-1–independent mechanisms. Regardless of the putative mechanism, we speculate that the minority of the megakaryocytes with higher DNA content produce platelets.
The promoters of many megakaryocyte-expressed genes have been demonstrated to harbor functional GATA-1–binding sites, eg, in GPIbα and GPIbβ, GPIIb, c-mpl, PF4, and GPIX promoters.5,32-36 This consistent finding may be interpreted to suggest that GATA-1 regulates an entire program of megakaryocyte gene expression and differentiation, much as has been suggested in the related erythroid lineage.37,38 Indeed, GATA-1–deficient megakaryocytes show decreased expression of mRNAs encoding all cell surface, cytoplasmic, and nuclear megakaryocyte-specific genes tested, including GPIbα and GPIbβ, c-mpl, p45 NF-E2, and PF4 (Fig 3) and GPIIb and GPIX (data not shown). There are two caveats to the interpretation of these results. First, differential expression of these mRNAs does not necessarily mean that they are all direct transcriptional targets of GATA-1. Rather, differential expression may simply reflect immaturity of GATA-1–deficient megakaryocytes if levels of these mRNAs normally increase late in megakaryocyte maturation by GATA-1–independent means. We specifically attempted to avoid this bias by conducting expression studies on normal-size megakaryocyte colonies derived from cultured wild-type and mutant CFU-Mk. Second, it remains formally possible that the differences in mRNA profile observed in cultured megakaryocytes may not be reflected in vivo. However, GPIbβ is a good candidate for a true GATA-1 target gene. A patient with Bernard-Soulier syndrome (absence of GPIbβ expression) has been described with a deletion of the structural GPIbβ gene on one chromosome and a point mutation in a functional GATA-binding site in the GPIbβ promoter as the sole defect on the other, implying a critical role for GATA-1 in GPIbβ expression in vivo.39
Even though some platelets are produced in neoΔHS and ΔneoΔHS mice, they are abnormal in several respects. Morphologically, they are bigger, spherical, show an excess of rough endoplasmic reticulum and ribosomes, have few electron dense and α granules, and often have an excess of internal canalicular membranes. Ultrastructurally, the platelets are similar to the megakaryocytes from which they are released. These observations suggest that GATA-1 deficiency leads not only to defective organelle and granule formation, but also to abnormal compartmentalization of these structures in megakaryocytes, which is reflected in the platelets that are released. We further speculate that platelet release from GATA-1–deficient megakaryocytes is abnormal, as proplatelet formation in liquid culture is infrequent,40and the rare proplatelets produced by GATA-1–deficient megakaryocytes tend to be short buds, rather than the typical long filamentous structures that emanate from normal megakaryocytes.
Functionally, GATA-1–deficient platelets are also abnormal. Bleeding times in mutant animals are greatly prolonged, beyond that expected for the degree of thrombocytopenia. Platelet activation in vitro is modestly affected in response to either thrombin or a combination of ADP and epinephrine, but not to ADP alone. The latter findings suggest specific defects in the adrenergic and thrombin-induced pathways of platelet activation.
From the observations presented in this report, we present a model for the function of GATA-1 in megakaryocytes and platelets (Fig 7). In the absence of GATA-1, the majority of megakaryocytes are small and immature. These arise from hyperproliferation of megakaryocytes, probably from a small subset of progenitors, and many cells have 2N DNA content. Moreover, mature mutant megakaryocytes derived from CFU-Mk express lower levels of a number of megakaryocyte-associated mRNA transcripts. Nevertheless, a minority of megakaryocytes does undergo endoreduplication to have a ploidy profile that is comparable to normal and some of the mutant megakaryocytes are able to release platelets. Although these platelets display functional and structural abnormalities, they do express some platelet-associated proteins at apparently normal levels. We conclude that GATA-1 is required for the normal homeostasis of megakaryocyte numbers and nuclear and cytoplasmic maturation and platelet release.
It is unclear why some megakaryocytes are able to mature partially, albeit abnormally. This could occur if the mutations we have generated in mice (neoΔHS and ΔneoΔHS) are leaky with respect to GATA-1 expression in megakaryocytes, ie, a small proportion of cells is able to express GATA-1 to allow fuller differentiation. This is unlikely because we were unable to detect GATA-1 expression in megakaryocytes from neoΔHS mice by either immunofluorescence or RT-PCR.8Moreover, although megakaryocytes from ΔneoΔHS mice show a low level of GATA-1 mRNA expression (1% to 5% by RT-PCR analysis8), the megakaryocyte and platelet phenotype is identical in the two mutant strains.8 We hence favor the possibility that the partial maturation of megakaryocytes seen in neoΔHS and ΔneoΔHS mice occurs by GATA-1–independent means.
The GATA-1–deficient megakaryocyte phenotype contrasts markedly with the two other transcription factor knockouts that affect the megakaryocytic lineage. FOG was isolated as a protein that interacts physically with GATA-1 and enhances its capacity to drive megakaryocytic differentiation in the 416B myeloid cell line.10 Thus, it was surprising that FOG-null fetuses do not produce any megakaryocytic colonies, a defect in megakaryopoiesis that is distinct from neoΔHS and ΔneoΔHS mice.11 We have speculated that FOG has a GATA-1–independent function early in megakaryopoiesis, as well as a GATA-1–dependent role later in megakaryocyte maturation. NF-E2 is vital for terminal megakaryocyte maturation and platelet release.9 However, the megakaryocyte and platelet phenotypes between NF-E2–null and neoΔHS and ΔneoΔHS mice are also clearly distinguishable. As expression of p45 NF-E2 is significantly reduced in colonies derived from GATA-1–deficient CFU-Mk, it could be that part of the failure of these megakaryocytes to undergo proper terminal differentiation is related to this observation. It is likely that these three transcription factors regulate mutually exclusive sets of target genes, although some genes may be controlled in a complex manner requiring two or all three of these proteins.
ACKNOWLEDGMENT
We are grateful to Jerry Ware for providing unpublished sequence data to design GPIbα oligonucleotide primers for RT-PCR analysis, and to Yuhui Xu and Paula Stenberg for assistance with electron microscopy.
Supported in part by fellowships and grants from the Wellcome Trust, National Institutes of Health, and the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paresh Vyas, MD, PhD, Children’s Hospital Medical Center, 300 Longwood Ave, Boston, MA 02115; e-mail:vyas@rascal.med.harvard.edu.



![Fig. 3. Semiquantitative RT-PCR analysis of GPIb, GPIbβ, PF4, c-mpl, and p45 NF-E2 mRNA transcripts from primary megakaryocytes derived from normal (WT) and neo▵HS (MUT) CFU-Mk. PCR reactions were performed with tracer [32P]dCTP for the indicated number of cycles before analysis by PAGE. HPRT signal was used to normalize the input cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2867/4/m_blod40924003w.jpeg?Expires=1767724030&Signature=luKRT9ui6dL8emH6W98N8cO26tzOshrg8edbuZDStoQ8aKurDOWkZry7KO27UbbcLHOe237-n7NyjZAkRMzmy4kZrgkbDRbuettSTwFtO92f7JSbI655LE0cUcLhjkklBJPQW3-NE6WTTZh0UXIyKVB9ardxsGRORZr-E~f7ljFy27lTiKvg1eOqRgOtBQzm4mi1u-rrjv-4JpGrKge9KD2ExCalAu79t5KU7KDhi8iHLJDXwN4JTk5P3OQl-DZPmenQQDKGT0uNR9LbihLa80IBnJReeOqj3WP8~Ns~l9dv5A6uGZPacWv3CtOvImKG~DzmdPEsgcpPgfBNsSiarg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal