Abstract
Thrombopoietin (TPO) is a hematopoietic growth factor that regulates megakaryocytopoiesis and platelet production through binding to its receptor, Mpl, encoded by the c-mpl proto-oncogene. Circulating levels of TPO are regulated by receptor-mediated uptake and degradation. To better understand this mode of TPO regulation, we examined whether expression of Mpl was regulated by its ligand. Using RNase protection analysis, we found no differences in the levels ofc-mpl transcripts in megakaryocytes (MKs) produced in vitro either in the presence or absence of TPO and in platelets (PLTs) obtained from mice hyperstimulated in vivo by ectopic secretion of TPO. Similarly, Western blot analysis of MKs produced in the presence or absence of TPO showed no difference in Mpl levels. Levels of Mpl, GpIIb, or P-selectin were virtually identical in platelet lysates obtained from normal, TPO knockout and mildly TPO-stimulated mice. In contrast, the expression of Mpl was significantly reduced in PLTs from severely thrombocythemic mice. These results show that TPO does not have a major effect on the transcription or translation of Mpl. However, they do suggest that an excess of circulating TPO can lead to the disappearance of Mpl from PLTs via catabolism.
THROMBOPOIETIN (TPO), the ligand for the Mpl receptor, is the major physiologic regulator of megakaryocytopoiesis and platelet production.1-4 The major sites of TPO production are the parenchymal and sinusoidal endothelial cells of the liver and the proximal convoluted tubules of the kidney.5-8 TPO is released into blood and circulates to reach target cells present in the hematopoietic organs. It has been clearly shown that circulating TPO levels are inversely correlated to platelet mass.9 By analogy with the transcriptional regulation of erythropoietin (EPO) mRNA levels by anemia,10 it has been suggested that TPO gene transcription might be upregulated in response to a decrease in platelet mass. Alternatively, it has been proposed that TPO production is constant, with TPO activity regulated by binding to platelets (PLTs) and catabolism. In recent studies of mice made profoundly thrombocytopenic or thrombocythemic, no changes were observed in liver or kidney TPO mRNA levels. In addition, no variations were detected in the major alternative splice forms encoding nonsecreted or inactive TPO proteins.11-15 It is still a matter of debate, however, whether TPO synthesis in stromal cells is regulated by platelet mass.16,17 Definitive evidence that there is no modulation of TPO mRNA levels in response to platelet demand has been provided by mice genetically altered to be defective in TPO.14,18 In contrast, PLTs have been shown to play a key role in the plasma clearance of TPO, as they actively bind TPO via Mpl, and internalize and degrade the protein.19,20 Megakaryocytes (MKs) may also have a role in the regulation of TPO. NF-E2 knockout mice and patients with idiopathic thrombocytopenic purpura (ITP) are both markedly thrombocytopenic, exhibit increased numbers of MKs in their marrow, and have normal or only slightly elevated plasma levels of TPO.21-25 Together, these results support a model in which plasma TPO levels are regulated both by circulating PLTs, the most mature cells of the megakaryocytic lineage, and by maturing MKs present in the hematopoietic organs. Thus, the number of Mpl receptors present on the surface of PLTs and MKs is an important parameter in the regulation of circulating TPO levels.
Upregulation of receptor by ligand has been shown in cells from different hematopoietic lineages. One well-documented example is the interleukin-2 receptor (IL-2R). It has been reported that IL-2 augments expression of the receptor α chain in human T and B cells26 and β and γ chain mRNA levels in human monocytes.27,28 Upregulation of IL-2R γ transcripts is associated with increased IL-2 binding activity on the surface of monocytes.28 Similarly, granulocyte colony-stimulating factor (G-CSF) specifically upregulates transcripts of its own receptor in normal murine bone marrow cells and in a granulocytic cell line,29 and granulocyte-macrophage (GM)-CSF positively regulates GM-CSF receptors in murine macrophages.30 Such a mechanism has also been suggested for the M-CSF receptor.31
The aim of this study was to analyze whether TPO could positively regulate the transcription and/or translation of its receptor, Mpl, on MKs and PLTs, a mechanism that would amplify Mpl-mediated uptake of TPO. c-mpl transcript levels were first compared in cultured murine MKs grown either in the presence or absence of murine recombinant TPO, and in PLTs isolated from normal and TPO-stimulated mice. Second, the level of Mpl protein was analyzed in MKs obtained in the presence and absence of TPO and in PLTs isolated from normal, TPO-stimulated, and TPO knockout mice. To allow comparison between the different samples, the expression of two glycoproteins highly expressed in PLTs, P-selectin and GpIIb, was also analyzed. The data strongly argue against regulation of Mpl by TPO in PLTs and MKs, either at the transcriptional or translational level. However, we observed that expression of the Mpl protein was significantly reduced in PLTs obtained from mice with severe thrombocythemia.
MATERIALS AND METHODS
Experimental murine models of thrombocytosis.
Two different approaches were used to produce thrombocythemic mice. In the first model, (C57Bl/6 × DBA/2) F1 mice (Janvier, Lyon, France) were subcutaneously transplanted with 1 × 106 tumorigenic factor-dependent cell-P1 (FDC-P1) cells engineered to secrete murine TPO.13 PLTs were increased twofold to fourfold 3 to 4 weeks after the graft. In the second model, C57Bl/6 mice were lethally irradiated and hematologically reconstituted by a syngeneic transplantation of hematopoietic progenitor cells infected with a MPZen retrovirus carrying the murine TPO cDNA, as previously described.32 The number of PLTs in blood from these reconstituted mice sharply increased and reached eightfold to 10-fold baseline levels between 4 and 10 weeks posttransplant.
Platelet preparation.
Mice were bled by cardiac puncture under ether anesthesia. Blood was collected on sodium citrate (3.8%, 1 vol citrate for 9 vol blood) and diluted sixfold in buffered saline-glucose-citrate (BSGC, pH 7.3). Diluted blood was first centrifuged at room temperature for 15 minutes at 200g to eliminate nucleated and red blood cells. Supernatant was then centrifuged for 15 minutes at 1,700g to spin down the PLTs.
Culture and purification of megakaryocytes.
Both femurs and tibias from C57Bl/6 adult mice (10 to 12 weeks old) were flushed in Mem α medium (α-MEM) (GIBCO-BRL, Eragny, France) containing 2% fetal bovine serum (FBS). Cells (6 × 108 in 6 mL) were incubated for 30 minutes at 4°C with a cocktail of monoclonal antibodies containing 10 μg/mL of CD45R-B220, 5 μg/mL of TER 119 and 10 μg/mL of GR-1, Mac-1 (1:3,000), Ly+1 (1:1,000), and GK 1.5 (1:10). CD45R-B220, TER 119, and GR-1 antibodies were obtained from Pharmingen and Becton Dickinson (Le Pont de Claix, France). Mac-1 and Ly+1 were obtained from ascites and GK 1.5 from culture supernatant. After one wash, cells were incubated for 30 minutes at 4°C with immunomagnetic beads coated with sheep anti-rat IgG (Dynal, Compiègne, France) in a ratio of 3 beads/mononuclear target cell. The Lin− cell fraction was isolated using a Dynal separator (Oslo, Norway). To ensure higher purity of the preparation, a second round of bead separation was used. The overall yield was 10%. All washing, incubation, and purification procedures were performed in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) (Sigma, St Quentin Fallavier, France). The Lin− cellular fraction was cultured in suspension at 1 × 106 cells/mL in α-MEM medium supplemented with 10% FBS and stimulated either with 10 ng/mL recombinant murine TPO (r-mu TPO; R & D Systems, Minneapolis, MN) or in the presence of a cocktail of recombinant cytokines composed of r-mu IL-3 (R & D Systems; 50 U/mL), r-hu IL-6 (10 ng/mL; a generous gift from Dr S. Burstein, William K. Warren Medical Research Institute, University of Oklahoma, Oklahoma City, OK), and r-mu stem cell factor (SCF) (R & D Systems; 10 ng/mL). On day 4 of culture, MKs were enriched (50% to 70% purity) on a BSA gradient as previously described.33
Ribonuclease protection assay.
A 32P uridine triphosphate (UTP)-labeled antisense probe was transcribed from the T3 promoter of an XbaI linearized plasmid (KS MPL 500) containing a murine c-mpl cDNA. To construct KS MPL 500, an SphI-StuI fragment containing nucleotides 230 to 734 of the Mpl coding sequence was inserted into the polylinker site of the pBluescript II KS vector (Stratagene, La Jolla, CA). A 32P-labeled antisense murine GpIIb probe transcribed from the T7 promoter of the HindIII linearized pCR GpIIb 185 plasmid was used as an internal control for each reaction sample. The plasmid pCR GpIIb 185 (kindly provided by J. Starck [CNRS UMR 5534, Villeurbanne, France] and G. Uzan [INSERM U506, Villejuif, France]) was constructed by inserting a polymerase chain reaction (PCR) product, containing exons 24 and 25 of GpIIb cDNA,34 into the polylinker site of the pCR TM II vector (Invitrogen, Groningen, The Netherlands). Total RNAs (2 μg) from PLTs and MKs were hybridized with radioactive probes at 50°C overnight. Nonhybridizing RNAs were digested with RNase A (10 μg/mL) and RNase T1 (1,000 U/mL) for 1 hour at 37°C. To inhibit RNase activity, sodium dodecyl sulfate (SDS) (0.6%) and proteinase K (145 μg/mL) were added for 15 minutes at 37°C. Protected fragments were extracted in the presence of 15 μg of carrier transfer RNA with phenol/chloroform/isoamyl alcohol and precipitated with absolute ethanol at −20°C. Fragments were resolved on a 4% polyacrylamide, 7 mol/L urea gel and autoradiographed on hyperfilm MP (Amersham, Buckinghamshire, UK). Fragment sizes were determined by labeled MspI-digested pBR322 (New England Biolabs, Beverly, MA). Quantification of protected fragments was performed with a phosphorimager system (MACBAS program; Fuji Photo Film Co, Japan).
Western blots.
Cells were lysed at 4°C in lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP40, 5% glycerol, and 1 mmol/L dithiothreitol) containing protease inhibitors (Complete; Boehringer Mannheim, Meylan, France). A colorimetric assay was used to determine the concentration of proteins in the different lysates (Bio-Rad DC Protein Assay; Bio-Rad, Hercules, CA). Proteins from total lysates (30 μg) were analyzed on 8% polyacrylamide gels with prestained molecular weight markers (Bio-Rad). Proteins were transferred to nitrocellulose membranes (Amersham). The membranes were probed successively with a rabbit anti-mouse Mpl polyclonal antibody (kindly provided by Dr S. Lok, ZymoGenetics, Seattle, WA), a rabbit anti-human P-selectin polyclonal antibody cross-reacting with murine P-selectin (Pharmingen), a rabbit anti-human GpIIb polyclonal antibody cross-reacting with murine GpIIb35 (obtained from Dr D. Pidard, Institut Pasteur, Paris, France) and, finally, a goat anti-mouse actin polyclonal antibody (TEBU, le Perray en Yvelines, France).
RESULTS
Quantitative measurement of c-mpl transcripts in MKs and PLTs.
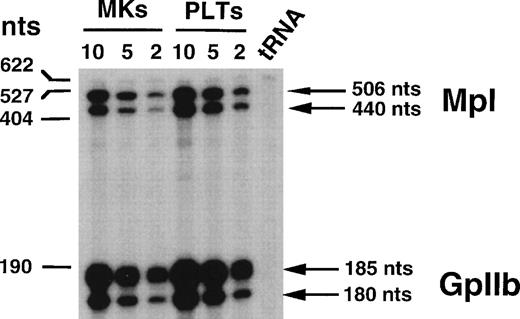
RNase protection assays were used to quantify the levels ofc-mpl transcripts in MKs and PLTs. To determine the threshold of the technique, a series of experiments was performed. A murinec-mpl antisense riboprobe was constructed that contained exon 3 (without the first 18 nucleotides), all nucleotides from exon 4, and 44 nucleotides from exon 5.36 To normalize c-mpltranscripts, an antisense riboprobe specific for the mouse megakaryocytic marker GpIIb spanning exons 24 and 2534 was introduced into the reaction samples. Various concentrations of total RNA prepared from MKs and PLTs were analyzed to measure the relative intensities of the protected fragments. Two protected fragments were obtained with the c-mpl riboprobe (Fig 1). The 440-bp protected fragment corresponds to an alternatively spliced form of c-mplcharacterized by a 24-nucleotide deletion in exon 4. The 506-bp protected fragment corresponds to the wild-type c-mplmRNA.37 Two protected fragments were also detected with the GpIIb riboprobe that probably corresponds to splice variants. As shown in Fig 1, the intensity of protected fragments varies in parallel with the concentration of total RNA from PLTs or MKs. We also confirmed that the ratio between c-mpl mRNA and GpIIb mRNA was independent of the total RNA concentration used in the protection assay. These results showed that this method could be used to compare levels of c-mpl mRNA obtained from different sources.
Ribonuclease protection analysis of RNA from MKs and PLTs. Decreasing amounts of RNA from 10 to 2 μg were used for MKs and PLTs. Transfer RNA (tRNA) was used as a negative control to verify specificity of protected fragments. The size of the markers is indicated on the left of the panel. Arrows on the right indicate the position of Mpl (top) and GpIIb (bottom) protected fragments.
Ribonuclease protection analysis of RNA from MKs and PLTs. Decreasing amounts of RNA from 10 to 2 μg were used for MKs and PLTs. Transfer RNA (tRNA) was used as a negative control to verify specificity of protected fragments. The size of the markers is indicated on the left of the panel. Arrows on the right indicate the position of Mpl (top) and GpIIb (bottom) protected fragments.
c-mpl mRNA levels in MKs grown in the presence or absence of TPO.
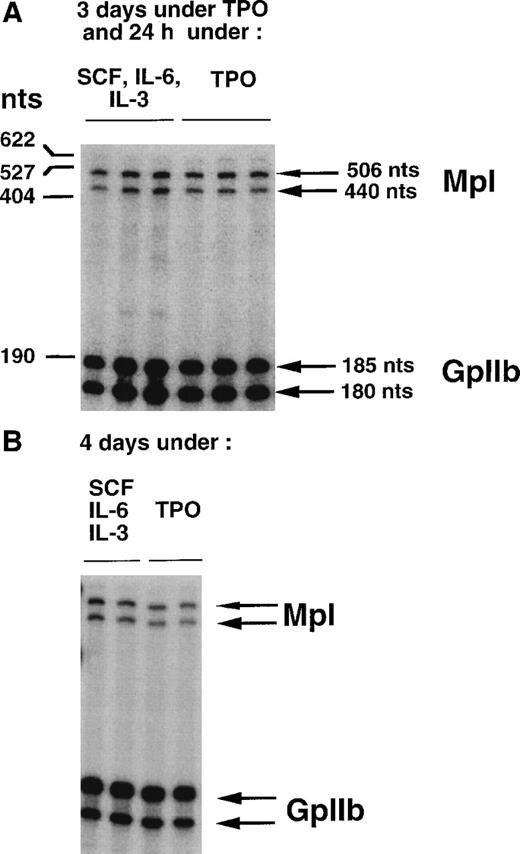
To study the effects of TPO on the stabilization of c-mpltranscripts and transcription of the c-mpl gene, two different protocols were used. For the first set of experiments, immature Lin− cells were grown in liquid medium in the presence of r-mu TPO. After 3 days of stimulation, cells were washed and MKs were enriched on a BSA gradient. The MK population was then divided into two samples. One was grown for an additional 24 hours in the presence of r-mu TPO, while the other one was stimulated with a cocktail of three cytokines that did not contain TPO. Three independent MK preparations were analyzed for each condition. Total RNA was extracted and RNase protection assay was performed. Results (Fig 2A and Table 1) show that the ratios betweenc-mpl- and GpIIb-protected fragments were similar in the two culture conditions, suggesting that TPO has no major effect onc-mpl or GpIIb mRNA levels.
Comparison of c-mpl transcript levels in MKs in the presence or absence of TPO, by ribonuclease protection analysis (2 μg of RNA). (A) MKs obtained in the presence of TPO (3 days) were either maintained in TPO or shifted for 24 additional hours to a medium deprived of TPO, but containing a combination of SCF, IL-6, and IL-3. For each condition, three independent MK preparations were made. (B) MKs were obtained either in the presence of TPO or in the presence of a combination of SCF, IL-6, and IL-3 (4 days). Two independent MK preparations were used for each condition. Arrows on the right indicate the position of the protected fragments.
Comparison of c-mpl transcript levels in MKs in the presence or absence of TPO, by ribonuclease protection analysis (2 μg of RNA). (A) MKs obtained in the presence of TPO (3 days) were either maintained in TPO or shifted for 24 additional hours to a medium deprived of TPO, but containing a combination of SCF, IL-6, and IL-3. For each condition, three independent MK preparations were made. (B) MKs were obtained either in the presence of TPO or in the presence of a combination of SCF, IL-6, and IL-3 (4 days). Two independent MK preparations were used for each condition. Arrows on the right indicate the position of the protected fragments.
Ratios of c-mpl mRNA to GpIIb mRNA Levels in PLTs From Normal and TPO-Stimulated Mice and in MKs in the Presence and Absence of TPO
| . | PLTs* . | ||
|---|---|---|---|
| Control Mice . | TPO-Stimulated Mice (model 1) . | TPO-Stimulated Mice (model 2) . | |
| 0.26 ± 0.02 | 0.28 ± 0.01 | 0.25 ± 0.01 | |
| . | PLTs* . | ||
|---|---|---|---|
| Control Mice . | TPO-Stimulated Mice (model 1) . | TPO-Stimulated Mice (model 2) . | |
| 0.26 ± 0.02 | 0.28 ± 0.01 | 0.25 ± 0.01 | |
| . | MKs (condition no. 1)† . | |
|---|---|---|
| SCF, IL-6, IL-3 . | TPO . | |
| 0.21 ± 0.02 | 0.20 ± 0.02 | |
| MKs (condition no. 2)‡ | ||
| SCF, IL-6, IL-3 | TPO | |
| 0.19 ± 0.01 | 0.18 ± 0.005 | |
| . | MKs (condition no. 1)† . | |
|---|---|---|
| SCF, IL-6, IL-3 . | TPO . | |
| 0.21 ± 0.02 | 0.20 ± 0.02 | |
| MKs (condition no. 2)‡ | ||
| SCF, IL-6, IL-3 | TPO | |
| 0.19 ± 0.01 | 0.18 ± 0.005 | |
The ratios were calculated from the quantification of protected fragments performed with a phosphorimager system. Each indicated ratio represents the mean (±SD) of ratios obtained with the independent preparations.
PLTs were isolated from control mice, from mice grafted subcutaneously with FDC-P1 cells producing TPO (model 1) or from mice in which the hematopoietic cells produce TPO (model 2).
MKs obtained in the presence of TPO (3 days) were either maintained in TPO or shifted for 24 additional hours to a medium deprived of TPO, but containing a combination of SCF, IL-6, and IL-3.
MKs were obtained either in the presence of TPO or in the presence of a combination of SCF, IL-6, and IL-3 (4 days).
To exclude the possibility that stimulation by TPO for 3 days had irreversibly induced c-mpl expression in MKs, we analyzed the levels of c-mpl transcripts in MKs grown for 4 days either in the presence of TPO alone or in the presence of the three-cytokine cocktail. Two independent MK samples were analyzed in each case. The levels of c-mpl and GpIIb mRNA remained similar whether MKs were grown in the presence or absence of TPO (Fig 2B and Table 1). In these studies, TPO and the three-cytokine cocktail had exactly the same effect on the level of expression of c-mpl transcripts. Together, these results strongly argue against positive regulation by TPO of c-mpl gene transcription or transcript stabilization in MKs.
c-mpl transcript levels in PLTs from control and TPO-stimulated mice.
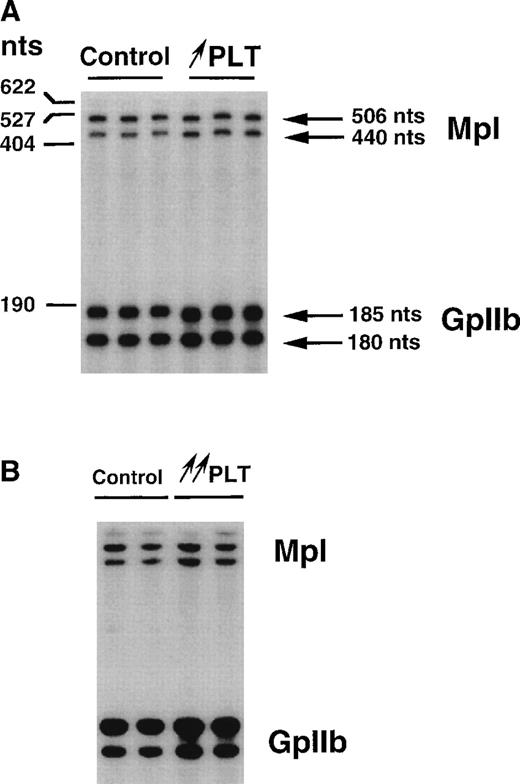
To study whether in vivo long-term stimulation of megakaryocytopoiesis by suprapharmacologic doses of TPO would affect c-mpltranscript levels, we used different animal models. In the first model, normal mice were subcutaneously injected with FDC-P1 tumorigenic cells engineered to secrete mu TPO. Three to 4 weeks after grafting, PLT counts were increased twofold to fourfold above baseline values. In the second model, the hematopoietic system of lethally irradiated mice was reconstituted with a bone marrow transplant infected with a retrovirus encoding mu TPO. Two months posttransplant, PLT counts were increased 8- to 10-fold, and the plasma TPO level was augmented by a factor of 1,000 to 5,000.32 In each model, independent PLT total RNA samples were analyzed by RNase protection assays. No variation in the ratios between c-mpl and GpIIb transcripts was detected when PLTs from normal mice were compared with PLTs from mildly or hyperstimulated animals (Fig 3A and B and Table 1). These data show that long-term in vivo stimulation of PLTs by high concentrations of TPO did not have a positive effect on the stabilization of c-mpl transcripts.
Comparison of c-mpl transcript levels in PLTs from control and TPO-stimulated mice by means of ribonuclease protection assays (2 μg). PLTs were isolated from mice grafted subcutaneously with FDC-P1 cells producing TPO (↑ PLT; A) or from mice in which the hematopoietic cells produce TPO (↑↑ PLT; B). Three (A) or two (B) independent platelet RNA preparations have been used.
Comparison of c-mpl transcript levels in PLTs from control and TPO-stimulated mice by means of ribonuclease protection assays (2 μg). PLTs were isolated from mice grafted subcutaneously with FDC-P1 cells producing TPO (↑ PLT; A) or from mice in which the hematopoietic cells produce TPO (↑↑ PLT; B). Three (A) or two (B) independent platelet RNA preparations have been used.
Levels of Mpl, GpIIb, P-selectin, and actin proteins in MKs grown in the presence or absence of TPO.
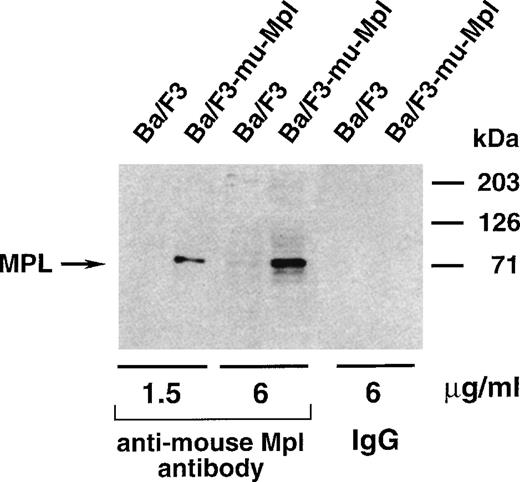
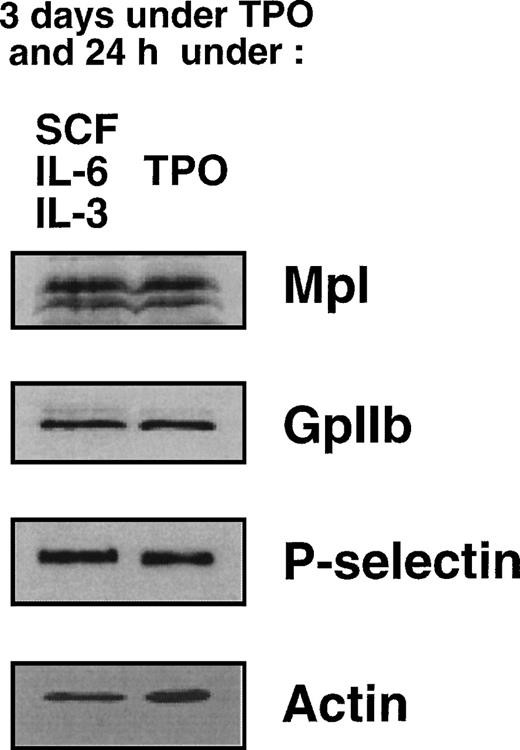
To determine whether TPO had any regulatory effect on the level of Mpl protein, we performed immunoblot experiments. The specificity of the anti-mouse Mpl polyclonal antibody was demonstrated on lysates prepared from Ba/F3 cells stably transfected with a murine c-mplexpression vector (Fig 4). MKs were prepared according to our first protocol (3 days with TPO followed by 24 hours with either TPO or IL-3, IL-6, and SCF) and the levels of Mpl in both samples were compared. Protein concentrations in MK lysates were determined by a colorimetric assay and equal amounts of protein (50 μg) were loaded on the gel. After protein transfer, membranes were successively incubated with antibodies directed against Mpl, GpIIb (CD41), P-selectin (CD62P) and, to ensure equivalent protein loading, actin. As shown in Fig 5, no significant difference in the levels of Mpl protein was detectable between MKs maintained in TPO and those shifted to the combination of SCF, IL-6, and IL-3. These data suggest that TPO does not have a major effect on the translation of Mpl.
Western blot analysis of the specificity of the rabbit polyclonal antibody directed against murine Mpl. The same amount of protein lysate from Ba/F3 and Ba/F3-mu-Mpl cells was analyzed, and two concentrations of the anti-Mpl antibody were used. Nonrelevant rabbit purified immunoglobulins were used as a control. The size of protein markers is indicated on the right of the figure.
Western blot analysis of the specificity of the rabbit polyclonal antibody directed against murine Mpl. The same amount of protein lysate from Ba/F3 and Ba/F3-mu-Mpl cells was analyzed, and two concentrations of the anti-Mpl antibody were used. Nonrelevant rabbit purified immunoglobulins were used as a control. The size of protein markers is indicated on the right of the figure.
Comparison of Mpl levels in MKs in the presence or absence of TPO. MKs obtained in the presence of TPO (3 days) were either maintained in TPO (right lane) or shifted to a medium without TPO and containing SCF, IL-6, and IL-3 (left lane). Immunoblot analysis of MK lysates using antibodies directed against Mpl, GpIIb, P-selectin, and actin, as described in Materials and Methods.
Comparison of Mpl levels in MKs in the presence or absence of TPO. MKs obtained in the presence of TPO (3 days) were either maintained in TPO (right lane) or shifted to a medium without TPO and containing SCF, IL-6, and IL-3 (left lane). Immunoblot analysis of MK lysates using antibodies directed against Mpl, GpIIb, P-selectin, and actin, as described in Materials and Methods.
Levels of Mpl, GpIIb, P-selectin, and actin proteins in PLTs from normal, TPO-stimulated, and TPO knockout mice.
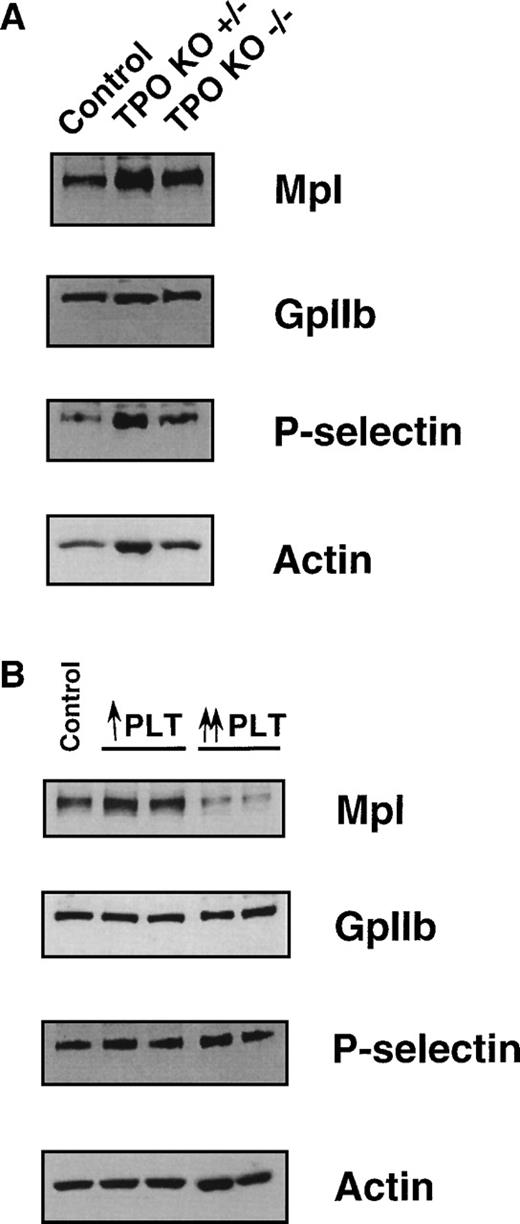
We further examined levels of Mpl protein in PLTs from normal, TPO knockout, and TPO-stimulated mice. Mpl expression was analyzed using PLT lysates prepared from heterozygous (TPO+/−) and homozygous (TPO−/−) TPO knockout mice and compared with normal mice. Whatever the phenotype of the mice, no significant difference was found in Mpl expression after normalization with actin and the two megakaryocytic markers (CD41 and CD62P). These results further indicate that TPO has no direct effect on the translation or stabilization of Mpl (Fig6A). The level of Mpl in TPO−/− mice argues strongly against TPO being necessary for high-level expression of its receptor. Similarly, the level of Mpl protein in PLTs from mice made moderately thrombocythemic (twofold to fourfold increase) by ectopic TPO expression was not different from that in PLTs from normal mice (Fig 6B). In contrast, when PLTs from severely thrombocythemic mice (8- to 10-fold increase) were analyzed, a reproducible and significant reduction in Mpl levels was seen, while the expression of GpIIb or P-selectin remained unchanged (Fig 6B).
Comparison of Mpl levels in PLTs isolated from control, TPO+/−, TPO−/−, and TPO-stimulated thrombocythemic mice. Immunoblot analysis of platelet lysates from (A) TPO+/− and TPO−/− mice; (B) the two types of TPO-stimulated mice, using antibodies directed against Mpl, GpIIb, P-selectin, and actin. For ↑ PLT and ↑↑ PLT, see legend to Fig 3. Two independent lysates were used for each TPO stimulation model.
Comparison of Mpl levels in PLTs isolated from control, TPO+/−, TPO−/−, and TPO-stimulated thrombocythemic mice. Immunoblot analysis of platelet lysates from (A) TPO+/− and TPO−/− mice; (B) the two types of TPO-stimulated mice, using antibodies directed against Mpl, GpIIb, P-selectin, and actin. For ↑ PLT and ↑↑ PLT, see legend to Fig 3. Two independent lysates were used for each TPO stimulation model.
DISCUSSION
Circulating levels of cytokines can be regulated by uptake and degradation when mature blood cells express the corresponding receptor.38-40 One well-documented example is the G-CSF/G-CSF–R model in which neutrophils play a key role in the clearance of G-CSF from plasma.40 G-CSF–R downregulation at the neutrophil membrane has been shown in vitro, as has internalization of G-CSF after interaction with its receptor.41 The mechanism of receptor downregulation and ligand-receptor complex internalization is a general feature of cytokine receptors,42 but in the case of G-CSF, it seems that this mechanism is largely involved in the in vivo clearance of G-CSF. It has also been reported that G-CSF upregulates G-CSF–R mRNA levels in a granulocytic precursor cell line.29 This process may be relevant to the in vivo increase of G-CSF clearance by neutrophils.40 Similar to the role played by neutrophils in the G-CSF/G-CSF–R system, PLTs and MKs are needed for clearance of circulating TPO.22,23,25,43 Binding and degradation of TPO by PLTs and MKs have also been documented,19,20 24 but downregulation of the Mpl receptor has not yet been shown.
In the Mpl/TPO system, precise regulation of platelet production is necessary to avoid significant fluctuations in platelet counts. In a manner similar to the G-CSF/G-CSF–R model, receptor-mediated TPO uptake by PLTs and MKs may be tightly controlled. Upregulation of Mpl by TPO could provide a mechanism for increased clearance of circulating TPO. To test this hypothesis, we determined if TPO was able to positively regulate c-mpl transcript and/or Mpl protein levels in MKs and PLTs, the two cell types involved in its clearance.
In this study, the megakaryocyte preparations had a purity of 50% to 70% with varying numbers of nonmegakaryocytic cells present. Therefore, we used two different megakaryocyte markers, GpIIb and P-selectin, to normalize the levels of c-mpl transcripts and Mpl protein in MK preparations.
We first investigated by RNase protection assay whether c-mpltranscripts could be modulated by TPO. Using MKs grown in vitro, we found that the levels of c-mpl transcripts were not modified by the presence of TPO in the culture medium. The same results were obtained when the bone marrow Lin− fraction cultured for 3 days in TPO was shifted to a TPO-containing or deprived medium for 1 additional day, or the Lin− fraction was cultured for 4 days in the presence or absence of TPO.
We next analyzed c-mpl transcript levels in PLTs stimulated by high doses of TPO. In two in vivo models of TPO overexpression,c-mpl transcript levels were similar to those found in normal PLTs. These results strongly suggest that TPO does not positively regulate c-mpl transcripts in PLTs and MKs, either by stabilization or by increased transcription. Surprisingly, the levels of GpIIb transcripts were the same whether MKs were obtained in the presence or absence of TPO, and whether PLTs were from normal or TPO-stimulated mice. These findings differ from previous studies looking at several human and murine GM-CSF or IL-3–dependent cell lines in which a shift to TPO induces expression of GpIIb. This increase in GpIIb expression is generally associated with an induction of MK maturation, including morphologic changes and polyploidization.44-46 Commitment to MK maturation appears to be related to prolonged activation of the ras pathway44,45 and to modification in the expression of transcription factors such as Spi1/PU1.47 In normal CD34+ cells, a three-cytokine combination (IL-3, IL-6, and SCF) mimics the effects of TPO on MK differentiation, inducing polyploidization, high-level expression of GpIIb, and cytoplasmic maturation.48 As recently reported, use of a constitutively activated H-Ras has similar effects on differentiation.45Our findings and those from these other studies imply that TPO does not directly affect the expression of individual MK-specific genes, such asc-mpl or GpIIb. Rather, TPO may allow the development of a complete megakaryocytic differentiation program, as does a combination of cytokines or an activated H-Ras.
To investigate if TPO has a regulatory effect on translation of Mpl, protein levels were compared in PLTs from normal, TPO−/−, TPO+/−, and mildly thrombocythemic mice. The data indicate that in these different samples, similar amounts of Mpl protein were present. In contrast, PLTs from thrombocythemic mice with very high and persistent levels of TPO expression had markedly decreased Mpl levels relative to PLTs from normal mice (Fig 6B). However, no significant differences were seen at the level of c-mpl transcripts (Fig 3B). In this model, the concentration of TPO in the plasma reached values 1,000-fold to 5,000-fold above normal.32 These results suggest that hyperstimulation of PLTs by very high TPO concentrations may result in increased internalization of Mpl followed by active degradation. TPO has been shown to be internalized and degraded after binding to Mpl expressed on platelet membranes.14,19 Both our results and these data suggest that the Mpl/TPO complex may be internalized and degraded. However, the hypothesis of Mpl recycling or shedding under physiologic conditions, as described for the IL-6 receptor,49 cannot be excluded.
In conclusion, our study strongly argues against TPO-mediated regulation of Mpl transcription or translation in MKs and PLTs.
ACKNOWLEDGMENT
We thank Drs S. Lok (ZymoGenetics, Seattle, WA), D. Pidard (Institut Pasteur, Paris, France), and S. Burstein (Oklahoma City, OK) for their gifts of antimouse Mpl polyclonal antibody, antihuman GpIIb polyclonal antibody, and recombinant human IL-6, respectively. We thank Dr F. de Sauvage for the TPO-deficient mice, and Drs J. Starck and G. Uzan for the plasmid pCR GpIIb 185.
Supported by the INSERM, the Institut Gustave Roussy, and by grants from the Association de Recherche contre le Cancer, La Ligue Nationale contre le Cancer and La ligue de Paris contre le Cancer. K.C.-S. was supported by a fellowship from La Ligue Nationale contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Karine Cohen-Solal, PhD, INSERM U 362, Institut Gustave Roussy, PR1, 39 Rue Camille Desmoulins, 94805 Villejuif, France; e-mail: kcohen@igr.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal