Abstract
Telomere shortening may reflect the total number of divisions experienced by a somatic cell and is associated with replicative senescence. We found that the average rate of telomere shortening in peripheral blood mononuclear cells (PBMCs) obtained longitudinally from nine different infants during the first 3 years of life (270 bp per year) is more than fourfold higher than in adults and does not correlate with telomerase activity. These results show that the rate of telomere loss changes during ontogeny, suggesting the existence of periods of accelerated cell division. Because human immunodeficiency virus (HIV) preferentially infects actively dividing cells, our observation suggesting accelerated cell division in children may provide an explanation for some of the distinctive pathogenic features of the HIV disease in infants, including higher viral loads and more rapid progression to acquired immunodeficiency syndrome (AIDS).
BECAUSE TELOMERES in several different cell types1-3 shorten as a function of cell doublings in vitro and of patient age in vivo, telomere length has been proposed to be a potentially useful marker for the total number of divisions experienced by a cell and hence as a correlate of the aging process. Telomere length may serve as a mitotic clock limiting the replicative capacity of somatic cells.4 However, most studies concerning telomere length and aging have examined telomere length in adults, particularly elderly adults. Few studies have specifically examined telomere lengths, or other correlates of aging, in the very young, even though infancy is the stage in life when the most profound developmental changes occur and when cellular replication peaks. Molecular correlates of the aging process might be expected to show their most dramatic changes during infancy, particularly for organ systems, which display the most dramatic developmental changes during infancy, such as the immune system. If rates of cell turnover vary during growth and development, the rates of telomere shortening should also change correspondingly. We therefore hypothesized that these changes should be greatest during the periods of life and in the tissues in which cellular replication is maximal. To explore this hypothesis and to obtain baseline values for the rate of telomere shortening in infants, we measured telomere length in the peripheral blood mononuclear cells (PBMCs) of infants.
Because the immune system undergoes such significant changes during the first years of life and because telomere lengths and the association of age with telomere length have been extensively studied in the PBMCs of adults, we determined telomere lengths from serial samples of pediatric patients over the first years of life. We found that PBMC telomeres shorten substantially faster in infants than in adults, and that infant PBMCs contain little telomerase, suggesting that rapid cell turnover may accompany the developmental processes occurring during infancy in the cell populations sampled using PBMCs. The infant telomere shortening rates determined in this study may also serve as a baseline for comparing telomere shortening rates in patients in which PBMC turnover may be altered, for example human immunodeficiency virus (HIV) infection or congenital immunodeficiency syndromes. The implied elevation in cell turnover observed in infants may have additional ramifications for the pathogenesis of HIV disease in pediatric patients.
MATERIALS AND METHODS
Patients and cells.
Samples from nine children were collected at multiple times during the first 3 years of life. These children were born to HIV-infected mothers and hence at risk for vertically acquired HIV infection. They were therefore closely followed with a variety of clinical and immunologic measures, including serial blood sampling (such serial blood samples would be difficult to obtain from other non-HIV exposed normal children for ethical reasons). All of the children in this study were determined not to be infected with HIV and have had clinically normal growth and development. Samples from two adults followed longitudinally over 8 and 10 years, respectively, because they were considered to be at risk for HIV infection, but found to be repeatedly uninfected, were used for comparison. PBMCs were separated with Ficoll-Hypaque (Amersham Pharmacia, Piscataway, NJ), frozen in cryopreservative in a controlled rate freezer, and stored in liquid nitrogen. The studies were approved by the Institutional Review Board (IRB) at the National Cancer Institute and the IRB at the University of Medicine and Dentistry of New Jersey.
Telomere length measurements.
The telomere length assay was similar to those already described.5 In brief, high molecular weight genomic DNA was prepared (Puregene, Gentra Systems, Inc, Minneapolis, MN) and quantitated spectrophotometrically. Equal amounts of DNA (1 μg) were digested with AluI (New England Biolabs, Beverly, MA) to produce a terminal restriction fragment (TRF), an approximation of the telomere-containing DNA. Equal quantities of digested DNA were loaded on a 1% 20 × 25 cm agarose gel in 0.5X Tris-Borate-EDTA (TBE) buffer. A pulsed-field at 6 V/cm for 20 hours at 15°C was used for the electrophoretic separation, using a pulse sequence designed to ensure good separation for sizes between 1 and 37 kb (PPI-200 Programmable Power Inverter; MJ Research, Watertown, MA). Additional precautions taken to enhance the accuracy and precision of the TRF measurements included loading three different markers (for short, 1 to 12 kb, intermediate, 4 to 23 kb, and relatively high, 10 to 50 kb, sizes) in 12 different lanes of the gel to control for region to region nonuniformities, which may diminish the accuracy of the TRF measurements. Images of the gels stained with ethidium bromide were acquired by a quantitative imaging system constructed in our laboratory using a video camera and image analysis software to provide length calibrations and to correct for any inhomogeneities and nonuninformities across the gel. The DNA was transferred by standard Southern blotting,6 and the blotted membranes were hybridized with an alkaline phosphatase (AP)-linked telomere probe and a probe for the DNA length calibrating standards (Quick-Light Hybridization Kit; Lifecodes Corp, Stamford, CT). The blots were exposed to a chemiluminescent AP substrate, and the resulting signal was acquired with a Bio-Rad Molecular Imager (Bio-Rad, Hercules, CA) at a resolution of 0.1 mm. The acquired 16-bit images were initially quantitated using the Bio-Rad Molecular Analyst software and further analyzed with Scientist (MicroMath Scientific Software, Salt Lake City, UT).
TRF image analysis.
TRF length was determined by an analysis of the gel images using a slight modification of a previously published procedure.5In brief, the signal intensity measured by the Bio-Rad Molecular Imager was determined for each length element at the highest resolution of 100 μm along each gel lane. The mean telomere length, m, was calculated using the formula m = ΣSl/ΣS, where S is the signal intensity of the telomere length element and l is the size of the telomere length element calculated by a nonlinear regression analysis as a function of the distance from the wells. The calibration was performed using the molecular weight standards, which translated the migration distances into molecular size (kb) and included an additional correction for possible nonuniformities across the gel.
Telomerase activity assay.
Telomerase activity was quantified by using a polymerase chain reaction (PCR)-based assay,7 (Oncor, Gaithersburg, MD). The intensity of the bands produced by the assay was measured and the relative activity was normalized to that of a human T-lymphocytic cell line (CEM). The activities assayed in 104 infant PBMC are described as −, +, or ++, corresponding to the activities observed in <1, 1, or 2 to 3 CEM cells.
RESULTS
Rapid changes of telomere length in children.
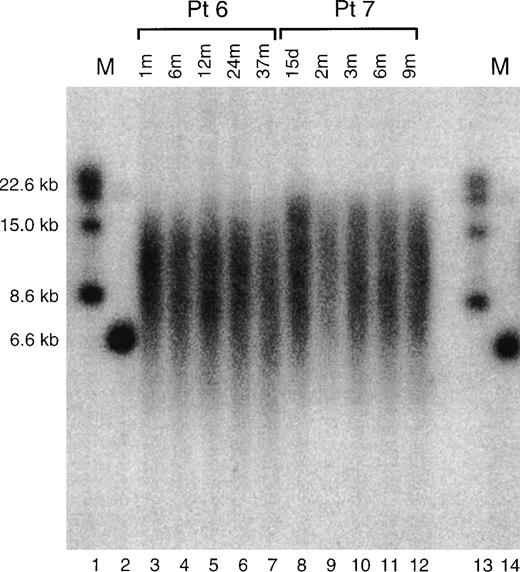
Figure 1 shows an example of Southern blot for samples from two children. On gross inspection, changes of telomere lengths are evident. TRF length data was extracted from such gel images as described in Materials and Methods and compiled in Tables 1 and2.
Shown is an example of a Southern blot telomere length determination for two patients, patient 6 and patient 7, described in the text and tables. Lanes 1 and 13 and 14 are marker lanes, with the sizes of the markers indicated at left. Lanes 3 to 7 show the TRFs from the cells of pediatric patient 6 obtained at the times after birth, indicated at the top of the figure. Lanes 8 to 12 show the TRFs from the cells of pediatric patient 7 obtained at the times indicated at the top of the figure.
Shown is an example of a Southern blot telomere length determination for two patients, patient 6 and patient 7, described in the text and tables. Lanes 1 and 13 and 14 are marker lanes, with the sizes of the markers indicated at left. Lanes 3 to 7 show the TRFs from the cells of pediatric patient 6 obtained at the times after birth, indicated at the top of the figure. Lanes 8 to 12 show the TRFs from the cells of pediatric patient 7 obtained at the times indicated at the top of the figure.
Pediatric Patient Ages, TRF Lengths, and Telomerase Activities
| Patient No. . | Pediatric Patients . | ||
|---|---|---|---|
| Time After Birth (mo) . | TRF Length (kb) . | Telomerase Activity . | |
| Patient 1 | 0.0 | 7.40 | − |
| 1.0 | 10.04 | − | |
| 4.0 | 9.57 | + | |
| 6.0 | 9.22 | − | |
| 9.0 | 9.15 | − | |
| 12.0 | 8.52 | − | |
| 18.0 | 8.66 | − | |
| 24.0 | 8.68 | − | |
| Patient 2 | 0.0 | 11.54 | − |
| 1.0 | 10.95 | − | |
| 6.0 | 11.22 | + | |
| 9.0 | 10.05 | − | |
| 12.0 | 11.02 | − | |
| 15.0 | 11.18 | − | |
| 24.0 | 10.54 | − | |
| 36.0 | 10.80 | − | |
| Patient 3 | 0.7 | 9.81 | − |
| 3.0 | 10.28 | − | |
| 6.0 | 9.94 | − | |
| 9.0 | 10.30 | − | |
| 17.0 | 9.77 | − | |
| 24.0 | 9.55 | ND | |
| Patient 4 | 0.0 | 8.09 | ++ |
| 0.3 | 9.88 | + | |
| 1.5 | 10.02 | + | |
| 3.0 | 10.80 | + | |
| 6.0 | 10.32 | + | |
| 9.0 | 10.48 | + | |
| 12.0 | 10.73 | + | |
| 18.0 | 10.31 | + | |
| 24.0 | 9.89 | − | |
| 36.0 | 9.15 | + | |
| Patient 5 | 1.0 | 10.98 | − |
| 2.0 | 11.05 | − | |
| 5.0 | 10.92 | − | |
| 12.0 | 10.73 | − | |
| 16.0 | 10.35 | − | |
| 27.0 | 10.50 | − | |
| Patient 6 | 1.0 | 9.89 | + |
| 6.0 | 9.68 | ++ | |
| 12.0 | 9.57 | + | |
| 24.0 | 9.62 | − | |
| 37.0 | 9.18 | ++ | |
| Patient 7 | 0.5 | 10.63 | ++ |
| 2.0 | 10.93 | + | |
| 3.0 | 10.39 | + | |
| 6.0 | 10.11 | − | |
| 9.0 | 10.67 | − | |
| Patient 8 | 1.0 | 11.30 | + |
| 6.0 | 10.90 | + | |
| 9.0 | 11.25 | − | |
| 36.0 | 11.24 | − | |
| Patient 9 | 0.0 | 11.04 | + |
| 2.0 | 11.07 | − | |
| 8.0 | 10.71 | − | |
| 12.0 | 10.75 | − | |
| 18.0 | 10.85 | − | |
| 26.0 | 10.78 | − | |
| Patient No. . | Pediatric Patients . | ||
|---|---|---|---|
| Time After Birth (mo) . | TRF Length (kb) . | Telomerase Activity . | |
| Patient 1 | 0.0 | 7.40 | − |
| 1.0 | 10.04 | − | |
| 4.0 | 9.57 | + | |
| 6.0 | 9.22 | − | |
| 9.0 | 9.15 | − | |
| 12.0 | 8.52 | − | |
| 18.0 | 8.66 | − | |
| 24.0 | 8.68 | − | |
| Patient 2 | 0.0 | 11.54 | − |
| 1.0 | 10.95 | − | |
| 6.0 | 11.22 | + | |
| 9.0 | 10.05 | − | |
| 12.0 | 11.02 | − | |
| 15.0 | 11.18 | − | |
| 24.0 | 10.54 | − | |
| 36.0 | 10.80 | − | |
| Patient 3 | 0.7 | 9.81 | − |
| 3.0 | 10.28 | − | |
| 6.0 | 9.94 | − | |
| 9.0 | 10.30 | − | |
| 17.0 | 9.77 | − | |
| 24.0 | 9.55 | ND | |
| Patient 4 | 0.0 | 8.09 | ++ |
| 0.3 | 9.88 | + | |
| 1.5 | 10.02 | + | |
| 3.0 | 10.80 | + | |
| 6.0 | 10.32 | + | |
| 9.0 | 10.48 | + | |
| 12.0 | 10.73 | + | |
| 18.0 | 10.31 | + | |
| 24.0 | 9.89 | − | |
| 36.0 | 9.15 | + | |
| Patient 5 | 1.0 | 10.98 | − |
| 2.0 | 11.05 | − | |
| 5.0 | 10.92 | − | |
| 12.0 | 10.73 | − | |
| 16.0 | 10.35 | − | |
| 27.0 | 10.50 | − | |
| Patient 6 | 1.0 | 9.89 | + |
| 6.0 | 9.68 | ++ | |
| 12.0 | 9.57 | + | |
| 24.0 | 9.62 | − | |
| 37.0 | 9.18 | ++ | |
| Patient 7 | 0.5 | 10.63 | ++ |
| 2.0 | 10.93 | + | |
| 3.0 | 10.39 | + | |
| 6.0 | 10.11 | − | |
| 9.0 | 10.67 | − | |
| Patient 8 | 1.0 | 11.30 | + |
| 6.0 | 10.90 | + | |
| 9.0 | 11.25 | − | |
| 36.0 | 11.24 | − | |
| Patient 9 | 0.0 | 11.04 | + |
| 2.0 | 11.07 | − | |
| 8.0 | 10.71 | − | |
| 12.0 | 10.75 | − | |
| 18.0 | 10.85 | − | |
| 26.0 | 10.78 | − | |
Shown are the data obtained from all nine of our pediatric patients, including the time the samples were obtained after birth, the measured TRF length, and whether telomerase activity was detected in the sample.
Abbreviation: ND, not determined.
Adult Patient Ages and TRF Lengths
| Patient No. . | Adult Patients . | |
|---|---|---|
| Age (yr) . | TRF Length (kb) . | |
| Patient 1 | 28 | 8.55 |
| 32 | 8.34 | |
| 36 | 8.23 | |
| Patient 2 | 30 | 8.91 |
| 35 | 8.32 | |
| 38 | 8.53 | |
| 40 | 8.22 | |
| Patient No. . | Adult Patients . | |
|---|---|---|
| Age (yr) . | TRF Length (kb) . | |
| Patient 1 | 28 | 8.55 |
| 32 | 8.34 | |
| 36 | 8.23 | |
| Patient 2 | 30 | 8.91 |
| 35 | 8.32 | |
| 38 | 8.53 | |
| 40 | 8.22 | |
Shown are the TRF lengths determined for our two adult patients and the ages at which these determinations were made.
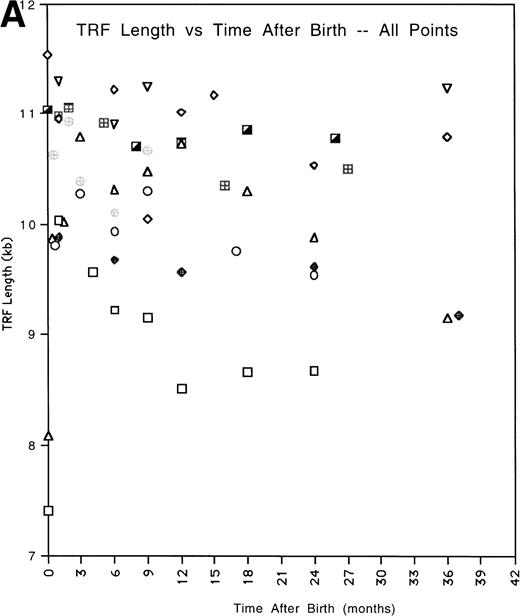
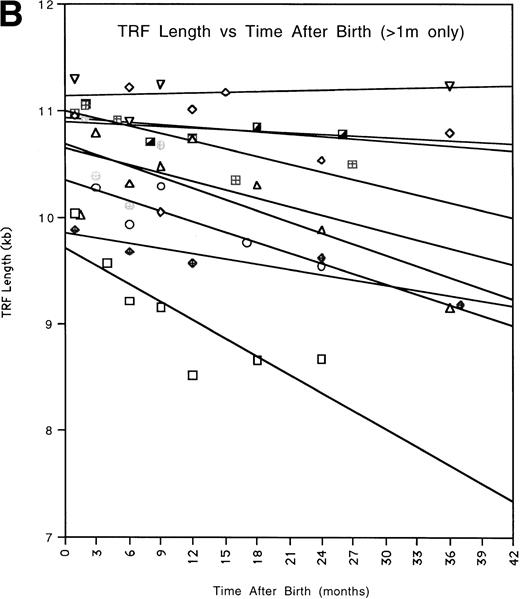
To obtain an overall expression for the rate of telomere change with time, we fitted the data for each patient by a linear regression analysis (Fig2A) and calculated the extrapolated values of the TRF mean lengths for time 1 month and 36 months (Fig 2B, Table3). The average TRF lengths were in the same ranges as previously reported from cross-sectional studies.3 The difference between the telomere lengths at 1 month and 36 months was statistically significant (P = .006, two tailed t-test for paired samples). The average rate of telomere length shortening between 1 month and 36 months was 270 bp per year. We omitted data obtained at <1 month from this analysis because several children showed a substantial increase in TRF length during the first month of life. However, including these points still produced an accelerated shortening rate compared with adults, 170 bp per year, and the difference in extrapolated TRF length between 0 months and 36 months was also still significant (P = .0015).
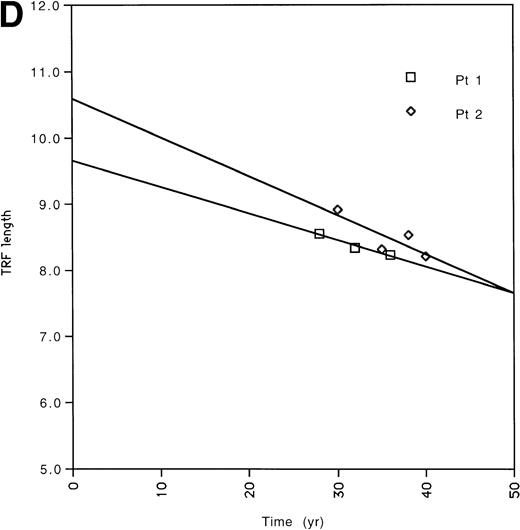
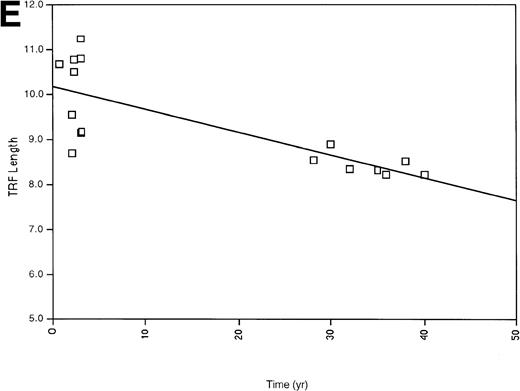
(A) All of the TRF measurements as a function of time for all of the pediatric patients is shown. (B) TRF measurements as a function of time plotted for the pediatric patients are shown. TRF measurements obtained at times <1 month after birth are omitted from this figure because some patients showed increases in TRF length in the immediate postnatal period. Least squares linear regression lines are plotted for each of the patients. Symbols for (A) and (B): (□), patient 1; (◊), patient 2; (○), patient 3; (▵), patient 4; (⊞), patient 5; (▨), patient 6; (⊕), patient 7; (▿), patient 8; (┌), patient 9. (C) TRF lengths for the nine pediatric patients were predicted from the equations of the lines of (B) and plotted for the 1-month and 36-month time points. (D) TRF lengths as a function of age were plotted for two uninfected adult patients. (E) TRF lengths as a function of age were plotted for all of the points obtained from our two adult patients and for the TRF length obtained at the oldest available age for each of our pediatric patients.
(A) All of the TRF measurements as a function of time for all of the pediatric patients is shown. (B) TRF measurements as a function of time plotted for the pediatric patients are shown. TRF measurements obtained at times <1 month after birth are omitted from this figure because some patients showed increases in TRF length in the immediate postnatal period. Least squares linear regression lines are plotted for each of the patients. Symbols for (A) and (B): (□), patient 1; (◊), patient 2; (○), patient 3; (▵), patient 4; (⊞), patient 5; (▨), patient 6; (⊕), patient 7; (▿), patient 8; (┌), patient 9. (C) TRF lengths for the nine pediatric patients were predicted from the equations of the lines of (B) and plotted for the 1-month and 36-month time points. (D) TRF lengths as a function of age were plotted for two uninfected adult patients. (E) TRF lengths as a function of age were plotted for all of the points obtained from our two adult patients and for the TRF length obtained at the oldest available age for each of our pediatric patients.
TRF Lengths of Infants Extrapolated to 1 Month and 36 Months
| Patient No. . | Pediatric Patients . | |
|---|---|---|
| TRF at 1 mo . | TRF at 36 mo . | |
| 1 | 9.66 | 7.68 |
| 2 | 10.89 | 10.71 |
| 3 | 10.32 | 9.18 |
| 4 | 10.65 | 9.44 |
| 5 | 10.98 | 10.14 |
| 6 | 9.83 | 9.26 |
| 7 | 10.63 | 9.72 |
| 8 | 11.15 | 11.22 |
| 9 | 10.92 | 10.66 |
| Patient No. . | Pediatric Patients . | |
|---|---|---|
| TRF at 1 mo . | TRF at 36 mo . | |
| 1 | 9.66 | 7.68 |
| 2 | 10.89 | 10.71 |
| 3 | 10.32 | 9.18 |
| 4 | 10.65 | 9.44 |
| 5 | 10.98 | 10.14 |
| 6 | 9.83 | 9.26 |
| 7 | 10.63 | 9.72 |
| 8 | 11.15 | 11.22 |
| 9 | 10.92 | 10.66 |
Shown are the TRF lengths for our nine pediatric patients extrapolated to 1 month and 36 months after birth. The data presented in Table 1 were used in a linear regression model to obtain estimates of the dependence of TRF length on age. The linear model was used to obtain estimates of the TRF length at 1 month and 36 months after birth. Those estimates are presented in the table and graphically represented in Fig 2C.
Telomere length shortening in adults, including shortening in PBMCs, is in the range of 30 to 50 bp per year.1,3 8 However, these results were derived from cross-sectional studies. To confirm our ability to measure small changes in TRF length in longitudinally obtained serial samples, we obtained blinded cryopreserved PBMCs from 2 HIV-uninfected adults followed prospectively (initial ages, 28 years and 30 years, seen for 8 and 10 years, respectively). These individuals were followed because they were judged to be at risk for HIV infection, but remained uninfected over the course of the study (Fig 2D, Table 2). The TRF shortening rate for these adults was 40 and 59 bp per year, with a composite average of 49 bp per year, which agrees well with previously found rates of telomere shortening from the cross-sectional studies. These adult data thus support the observation that the rate of TRF shortening is accelerated during the first 3 years of life.
Our observations of TRF shortening rate for the children and adults suggested that the two populations had substantially different shortening rates. However, it is possible that telomere length shortening in older children and young adults might also be accelerated compared with that of older adults. We therefore calculated an average shortening rate using the sample obtained at the oldest age from each of our infants and the adult samples (see Tables 1 and 2) described above. Using this analysis, the average TRF change (50 bp per year) was similar to that calculated from the longitudinal samples from the two adults and the rates previously described. These results support the estimates for the rate of telomere length changes from the longitudinal samples of adults and indicate that after approximately the age of 3, the rate of telomere length change appears to be relatively constant and similar to the rate in adults.
Two children showed relatively rapid changes in TRF length during the first several days of life. In these two newborns, the telomere length was initially short relative to the other patients and then significantly increased during the first month to reach a maximal value, after which it began to decline. While it is not clear why the telomere length increased during the first month of life, it may be due either to redistribution of new naive cells or to a transient activation of telomerase. Due to the small number of cells available from the banked infant samples, it was not possible to subfractionate the cells and determine the telomere length in different subfractions. However, we did measure the telomerase activity by a PCR-based assay, which requires only a small number of cells.
Telomerase activity in PBMCs of newborn children.
The telomerase activity of 104 PBMCs was undetectable (the level of detection corresponded to the activity of one cell from the continuous cell line CEM) in the majority of samples (38 of 58) (Table1). It was at about the limit of detection in 16 of 58 samples, and in several (four) samples it was relatively high, corresponding to that of 2 to 3 CEM cells. Thus, as in adults, telomerase activity varied between individuals. We did not observe a correlation of telomerase activity with absolute TRF length or rate of decline of TRF length. This, together with the observation that telomerase activities were generally low, suggests that observed TRF length changes in the infant cells are unrelated to telomerase activity, thus TRF length changes may indeed reflect the replicative history of the cells.
DISCUSSION
Our results indicate that the TRF lengths of PBMCs shorten more rapidly during the first years of life than do the TRF lengths of PBMCs and other cell types of adults. If telomeres do shorten by some relatively constant amount with each somatic cell division, these differences in shortening rates could either be due to an increased cell replication rate in infants or to the rapid disappearance during infancy of a long telomere-containing cell population. Although we can not formally exclude the second possibility with experimental evidence from fractionated infant PBMCs, due mainly to the small blood sample volumes that can be ethically obtained from infants and the present requirements for reasonably large amounts of chromosomal DNA for the TRF length assays, we believe that this second possibility is unlikely. Previously published work has not shown large differences in TRF length in different cell populations in a given individual.9 Our experiments (not shown) using fractionated adult PBMCs also failed to find differences in TRF length in different cell populations.
While our report was in preparation, Frenck et al10reported a cross-sectional study of TRF lengths in families. Although Frenck et al mention that they could not perform the ideal study, involving measurements of TRF length on serial blood samples from normal children because the IRB at their institution would not approve the study due to ethical considerations, their study found that TRF shortening rates were significantly increased during the first few years of life. The value for the shortening rate obtained in their study, more than 1,000 bp per year, was somewhat more than the value we determined, 270 bp per year. Nevertheless, both values are substantially higher than the ≈50 bp per year typically found in studies of adult TRF shortening. Although our larger number of samples obtained in the first few years of life, our modified electrophoresis and image analysis techniques (see Materials and Methods), and the longitudinal nature of the serial sampling strategy used in our study may have yielded a more refined estimate of child TRF shortening rates, the findings of elevated child TRF shortening rates from both studies may hold important implications for immune system development, aging, and the pathogenesis of immune system and infectious diseases.
The human immune system undergoes striking changes during the first years of life.11 The neonate has functional deficits in both the cellular and humoral arms, as well as the phagocytic arm of the immune system, but by 1 to 3 years of life, the infant immune system has generally acquired its mature functional capacity. For example, neonates have a decreased ability to produce antibodies, particularly antibodies against carbohydrate antigens and neonatal T cells have poor helper function, and a lower proportion of the cells can produce various cytokines. The gross anatomical features of the immune system also change significantly during the first years of life. For example, infants have a comparatively large fraction of body mass devoted to the lymphoid system, particularly the thymus, which declines relative to total body mass during childhood. In addition to functional and anatomical changes in the immune system, lymphocyte numbers change substantially during the first few years of life. Although the values display substantial interpatient variability, compared with adults, infants are born with relatively higher numbers of total lymphocytes. The median counts increase during the first approximately 6 to 7 months of life, from about 2,800 in the immediate postnatal period, peaking at around 3,200 at about 6 months, and then begin to decline, approaching adult levels after 3 to 4 years. In addition, the relative ratio of CD4 to CD8 cells is somewhat higher at birth (≈2.5), declining steadily to about 1.8 by 3 to 4 years.12,13 At the same time, T cells represent a relatively smaller fraction of the PBMC population of infants than of adults and the proportion of cells bearing markers associated with immaturity (eg, CD45RA) decline as infants age.14 15 These substantial changes in the characteristics of the infant immune system are likely accompanied by increased rates of cell turnover.
If the relatively rapid decrease in TRF length during the first months of life does reflect a real acceleration in the cell replication rate during infancy, our results support the notion that varying rates of cellular replication occur at different stages of development or, perhaps more graphically, that ‘aging’ is not a constant throughout life. These varying rates of replication may be particularly important for the development of the immune system, when rapid cell turnover likely accompanies immune system maturation. Because the rate of decrease in telomere length is not constant during life, our data from normal children may serve as a helpful baseline for comparison with certain pathological conditions, such as HIV infection or congenital immunodeficiency syndromes, in which the rates of cell turnover may be either increased or decreased. Several studies have used TRF shortening to investigate cell turnover rates in adult patients with HIV infection.16-18 Several features distinguish HIV disease in infants compared with the disease in adults and because some of these features may be reflected in varying rates of cell turnover, it may be helpful to assess cell turnover rates in HIV-infected infants using a variety of techniques, including TRF shortening rates.
The observation that the rates of TRF decrease are accelerated in infants and hence, that cell turnover may also be increased in infants, suggests a hypothesis that may to help explain some of the puzzling features of pediatric HIV disease. Infants with vertically-acquired HIV disease often suffer from a much more rapid course of disease progression than adult patients, and infants with vertically-acquired HIV typically have substantially higher blood viral RNA concentrations than adults.19-24 HIV preferentially replicates in activated and dividing cells.25,26 If the PBMCs of infants turn over more rapidly than those of adults, then the pool of cells vulnerable to a productive HIV infection should also be increased. This increased pool of vulnerable cells naturally present in infants may support increased levels of viral replication, higher amounts of circulating virus, and therefore more rapid disease progression.23,24 27-30
ACKNOWLEDGMENT
The authors thank Drs James Oleske and Robert Yarchoan for encouragement and support.
Supported in part by grants from the Elizabeth Glaser Pediatric AIDS Foundation and from the Centers for Disease Control and Prevention.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Steven L. Zeichner, MD, PhD, HIV and AIDS Malignancy Branch, National Cancer Institute, National Institutes of Health, Bldg 10, Room 13N240, Bethesda, MD 20892; e-mail:zeichner@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal