Abstract
6-Mercaptopurine (6MP) and methotrexate are the backbone of continuation therapy for childhood acute lymphoblastic leukemia (ALL). In studies of oral 6MP and methotrexate, indices of chronic systemic exposure to active metabolites of these agents, namely, red blood cell (RBC) concentrations of methotrexate polyglutamates (MTXPGs) and thioguanine nucleotides (TGNs) have positively correlated with event-free survival (EFS). Our objective was to evaluate whether MTXPGs, TGNs, and the dose intensity of administered methotrexate and 6MP were prognostic in the setting of a treatment protocol in which all treatment was coordinated through a single center, and the weekly doses of methotrexate were given parenterally. On protocol Total XII, 182 children achieved remission and received weekly methotrexate 40 mg/m2 parenterally and daily oral 6MP, interrupted every 6 weeks during the first year by pulse chemotherapy. A total of 709 TGN, 418 MTX-PG, and 267 thiopurine methyltransferase (TPMT) measurements, along with complete dose intensity information (dose received divided by protocol dose per week) for 19,046 weeks of 6MP and methotrexate, were analyzed. In univariate analyses, only higher dose intensity of 6MP and of weekly methotrexate were significant predictors of overall EFS (P = .006 and .039, respectively). The occurrence of neutropenia was associated with worse outcome (P = .040). In a multivariate analysis, only higher dose intensity of 6MP (P = .020) was a significant predictor of EFS, with lower TPMT activity (P = .096) tending to associate with better outcome. 6MP dose intensity was also associated (P = .007) with EFS among patients with homozygous wild-type TPMT phenotype. Lower 6MP dose intensity was primarily due to missed weeks of therapy and not to reductions in daily dose. We conclude that increased dose-intensity of oral 6MP is an important determinant of EFS in ALL, particularly among those children with a homozygous wild-type TPMT phenotype. However, increasing intensity of therapy such that neutropenia precludes chemotherapy administration may be counterproductive.
THE “BACKBONE” of continuation chemotherapy for childhood acute lymphoblastic leukemia (ALL) protocols comprises oral daily 6-mercaptopurine (6MP) and weekly methotrexate (MTX). It is widely held that treatment outcome is related to treatment intensity in many drug sensitive cancers, including childhood ALL. In this regard, several treatment groups have positively correlated indices of red blood cell (RBC) antimetabolite concentrations, either thioguanine nucleotides (TGNs)1 or methotrexate polyglutamates (MTXPGs),2,3 with long-term event-free survival (EFS). Because two of these prior treatment protocols involved oral administration of both weekly methotrexate and daily 6MP, it is possible that the association of higher RBC TGNs and/or MTXPGs with favorable outcome was due to patient compliance in taking oral medications. In addition, in patients experiencing hematopoietic toxicity due to a genetic defect in thiopurine methyltransferase (TPMT), dosages of methotrexate may have been reduced along with the dosages of 6MP, thus further compromising delivery of therapy.2 4 The importance of RBC indices of 6MP and methotrexate active metabolites has not previously been evaluated in a setting in which the delivery of all other components of ALL therapy are documented and incorporated into the outcome analysis.
In St Jude Children’s Research Hospital (SJCRH) protocol Total XII, we prospectively measured RBC TGNs and MTXPGs, along with plasma exposure to every dose of “pulse” chemotherapy, in 182 children with ALL. We documented and analyzed the exact dosage of weekly parenteral methotrexate and daily oral 6MP for the entire 120 weeks of chemotherapy and assessed whether RBC TGNs or MTXPGs have prognostic significance in this setting.
MATERIALS AND METHODS
Treatment.
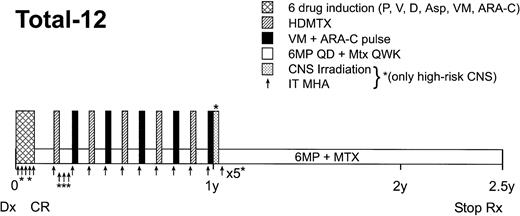
Children with ALL were treated on SJCRH Protocol Total XII after informed consent was obtained from the parent or guardian (as appropriate). All research procedures were approved by our institutional review board for ethical standards. Therapy has been described previously5 and is outlined in Fig 1. Briefly, after six-drug remission induction therapy, patients were randomized to receive every-6-week pulses of high-dose methotrexate alternating with teniposide plus cytarabine that were either dosed conventionally (based on body surface area) or individualized (based on pharmacokinetic parameters).5 Other than the weeks of pulse therapy, patients received weekly methotrexate 40 mg/m2(intravenously [IV] or intramuscularly [IM]) and daily oral 6MP 75 mg/m2. Complete blood counts were obtained weekly. Chemotherapy was given every week, provided that the absolute neutrophil count (ANC) was >300 cells/μL and that the patient did not exhibit other complications such as mucositis, fever, or hepatotoxicity. If toxicity or neutropenia in any given week precluded administration of chemotherapy, the scheduled pulse therapy was delayed until the patient recovered, whereas the scheduled low-dose methotrexate plus 6MP was omitted altogether.
Schema for Total XII protocol continuation therapy. Abbreviations: P, prednisone; V, vincristine; D, daunomycin; Asp,Escherichia coli asparaginase; VM, teniposide; ARA-C, cytarabine; HDMTX, high-dose methotrexate; 6MP QD, daily oral 6-mercaptopurine; Mtx QWK, methotrexate given IV or IM every week; CNS, central nervous system; IT MHA, intrathecal methotrexate, hydrocortisone, and cytarabine.
Schema for Total XII protocol continuation therapy. Abbreviations: P, prednisone; V, vincristine; D, daunomycin; Asp,Escherichia coli asparaginase; VM, teniposide; ARA-C, cytarabine; HDMTX, high-dose methotrexate; 6MP QD, daily oral 6-mercaptopurine; Mtx QWK, methotrexate given IV or IM every week; CNS, central nervous system; IT MHA, intrathecal methotrexate, hydrocortisone, and cytarabine.
RBC thiopurine metabolites.
RBC concentrations of TGNs and of thioinosine monophosphate (TIMP) were measured by hydrolyzing RBC lysates with acid and heat to the respective 6TG and 6MP bases, as described.6 Methylated metabolites of TIMP (meTIMP) were measured by hydrolyzing to methyl-6MP in a subset of patients in a separate high-performance liquid chromatography (HPLC) assay.7 Patients were scheduled to have thiopurine metabolite concentrations measured in RBCs at weeks 7, 31, 55, 82, 106, and 120 of continuation therapy. At each of these times, the treatment protocol specified that patients should have received daily 6MP for at least the prior 5 weeks. Patients were instructed to take their 6MP on an empty stomach in the evening, and all samples were obtained at least 8 hours after the preceding 6MP dose. Because of acute toxicity, noncompliance, or other unusual reasons (eg, misunderstanding directions, vacations, etc), some patients might not have received 6MP daily during this time period. Lack of dosing was not a reason for not obtaining a sample at the scheduled time. For each sample, a research nurse reviewed with the patient and his/her guardian the dosing history of 6MP for the preceding 6 weeks, the reason for obtaining the RBC sample (specified by the protocol or for suspected toxicity or noncompliance), and the time of day that the child had taken 6MP in the prior dosing interval. For purposes of evaluating RBC TGN, TIMP, and MeTIMP as possible prognostic factors, only samples that were collected as specified by the protocol were included in the analysis.
RBC MTXPG.
MTXPGs were measured as previously described8 in the same RBC samples used for thiopurine metabolite measures. If the volume of RBCs was inadequate, processing for TGNs took precedence over MTXPGs. At each sampling time, the treatment protocol specified that patients should have received weekly low-dose parenteral methotrexate (40 mg/m2; IV or IM for the first 60 weeks and IM thereafter in those who received cranial irradiation at 60 weeks) for every week for at least the prior 5 weeks. All samples were obtained at least 6 days after the last dose of methotrexate.
Plasma area under the curve (AUC).
Plasma area under the concentration versus time curve for methotrexate, cytarabine, and teniposide was measured in patients for every pulse chemotherapy as previously described.5
TPMT phenotype and genotype.
RBC TPMT activity was measured using blood collected in heparinized tubes as previously described.9 RBC TPMT activity measurements were made ≥90 days after the last RBC transfusion in 109 patients during their continuation therapy (1 to 843 days after achievement of complete remission) and in 45 patients after completion of continuation therapy (499 to 1,602 days after achievement of complete remission). If a patient had TPMT measured while on therapy, the lowest value was used to assign phenotype as follows: ≤4 U/mL packed RBCs, homozygous mutant; 4 to 13.5 U/mL packed RBCs, heterozygotes; ≥13.5 U/mL packed RBCs, wild-type. If the TPMT was measured only after completion of continuation therapy, the lowest value was used to assign phenotype as follows: ≤4 U/mL packed RBCs, homozygous mutant; 4 to 10.2 U/mL packed RBCs, heterozygous; ≥10.2 U/mL packed RBCs, homozygous wild-type. If a patient had no TPMT measured either during or after completion of therapy, but had RBC TGN concentrations above the 90th percentile for maximum TGNs for the entire group (1,120 pmol/8 • 108 RBCs), they were considered heterozygotes. For outcome analyses, TPMT homozygous mutant and heterozygous individuals were pooled into a single group (termed “TPMT defective”). Of the 182 patients who entered remission, either TGN or TPMT activity was evaluable in 180 patients (for purposes of assigning phenotype). TPMT genotype was evaluated, using somatic cell DNA, from a subset of patients, using polymerase chain reaction (PCR)-based methods specific for theTPMT*2, *3A, *3B, and *3C mutant alleles as previously described.10
Dosages of continuation chemotherapy.
A patient-specific treatment calendar, specifying dosages of chemotherapy for all 120 weeks of continuation therapy, was kept in the patient’s medical record and updated regularly by clinical and research staff. All pulses of high-dose methotrexate, teniposide, and cytarabine were administered at SJCRH. The exact dosages of every dose of every antileukemic medication were compiled into an institutional database, with reasons for any deviations from the planned protocol therapy documented at each week. One of the patients with extreme intolerance to continuation chemotherapy was identified to be homozygous deficient for TPMT.11 A drastic dosage reduction (from 75 mg/m2/d given daily to 10 mg/m2 given 3 days per week) resulted in excellent tolerance and allowed administration of full dosages of the remainder of continuation therapy medications. From that point forward, if physicians asked for a pharmacokinetic consult on the TPMT status and TGN concentrations of a patient experiencing unusual toxicity, consults on thiopurine status were provided. Dosages of 6MP were decreased gradually in patients with likely heterozygous status until reaching a dosage of 6MP that resulted in the desired degree of leukopenia (<4,000 cells/μL, but ANC >300 cells/μL) and allowed for full dosages of other antileukemic agents. Dosages were only decreased in those experiencing myelosuppression. In addition, 6MP dosage was increased after week 60 of continuation therapy in case of persistently high leukocyte counts (≥4,000/μL and ANCs ≥1,500/μL for 4 consecutive weeks). No dosage changes in weekly methotrexate were dictated by the protocol.
Dose intensity for 6MP and methotrexate.
To assess the importance of maintaining dosages of 6MP and low-dose methotrexate, dose-intensity variables were estimated for each drug. The ratio of the cumulative dose of 6MP (or methotrexate) actually received to the maximum dose that could have been received was calculated for each patient for each week, so that the information could be properly entered into a time-dependent Cox proportional hazards model. The numerator for estimating 6MP dose intensity was defined as the mg/m2/d dose of 6MP • the number of days that 6MP was taken that week. The denominator for 6MP dose intensity was the planned protocol dosage (75 mg/m2/d • 7 = 525 mg/m2/wk). The numerator for methotrexate dose intensity was defined as the mg/m2 dose per week of methotrexate actually administered; the denominator for methotrexate dose intensity was the planned protocol dosage (40 mg/m2/wk). The denominator excluded only the weeks of the pulses (ie, for a patient who completed therapy and received all 10 pulses, the denominator for the final cumulative methotrexate dose intensity was 120 − 10 = 110 weeks • 40 mg/m2 = 4,400 mg/m2).
Statistical analyses.
The Cox Proportional Hazards model12 was used to assess the prognostic significance of pharmacologic parameters on the complete remission experiences (because the analysis is restricted to patients who entered remission, EFS is used to indicate complete remission). Parameters that were measured repeatedly were treated as time-dependent covariates and included: average and maximum TGN levels, TIMP, MeTIMP; average MTXPG, methotrexate, teniposide, and cytarabine AUCs; and cumulative dose intensities of weekly 6MP and methotrexate. TPMT phenotype was considered a fixed covariate. Factors that were significant at the 0.20 level in univariate analyses and TPMT phenotype were included in the multivariate analyses. In addition, the occurrence of neutropenia in any given week, defined as an ANC of <300 cells/μL that precluded administration of therapy, was evaluated as a predictor of EFS. EFS duration was defined as the time between achievement of complete remission to the first adverse event: leukemic relapse, second malignancy, or death, for those who failed, or to the date of last contact for those patients who were censored. Models were stratified according to treatment arm (individualized or conventional dosing of the pulses) and risk group (better or worse; better risk was defined as initial leukocyte count <50,000 cells/mL in conjunction with either ETV6/CBFA2 [TEL/AML1] gene rearrangement or DNA index 1.16 to 1.60, and the absence of central nervous system [CNS] disease at diagnosis, T lineage, Philadelphia chromosome positivity and MLL rearrangement). Models involving methotrexate, teniposide, and cytarabine AUCs were stratified by risk criteria only because the plasma AUCs were significantly associated with treatment arm.5 Kaplan-Meier curves of the estimated complete remission duration starting from the end of continuation therapy for the 142 patients who completed the 120-week continuation treatment were constructed using the coefficients and the baseline survivor function13 from a Cox proportional hazards model that included TPMT phenotype, treatment arm, and cumulative dose intensity of weekly 6MP at the end of continuation therapy, and then selecting two hypothetical dose intensity levels of 6MP (70% and 85%). The curves represent the weighted average of the estimates among the two risk groups. The prognostic significance of the product of the mean MTXPG and the mean TGN dichotomized at its median, among the subset of patients aged 1 to 15 years who had a minimum of three TGN and three MTX-PG measurements (as reported by Schmiegelow et al2), was assessed using the Cox model.
RESULTS
Of the 188 patients enrolled on Total XII, 182 achieved a complete remission. Their demographic characteristics have been described previously.5 The number of patients with pharmacologic variables measured and the total number of measurements are indicated in Table 1.
Pharmacologic Variables as Predictors of EFS
| . | Median . | Range . | P Value for Univariate Analysis . | P Value From Multivariate Analysis . | Association With Improved EFS . |
|---|---|---|---|---|---|
| Average TGN*(pmol/8 · 108 RBCs) (n = 709) | 401 | 0-1,498 | .611† | — | |
| Maximum TGN (pmol/8 · 108 RBCs) (n = 180) | 594 | 0-4,472 | .429† | — | |
| Average TIMP*(pmol/8 · 108 RBCs) (n = 709) | 69 | 0-256 | .640† | — | |
| Maximum TIMP (pmol/8 · 108 RBCs) (n = 180) | 131 | 0-456 | .509† | — | |
| Average MeTIMP*(pmol/8 · 108 RBCs) (n = 152) | 14,212 | 0-59,379 | .252† | — | |
| Maximum MeTIMP (pmol/8 · 108RBCs) (n = 86) | 16,532 | 0-68,371 | .287† | — | |
| TPMT activity (units/mL packed RBCs)* (n = 267) | 18.0 | 0.4-30.4 | .363† | .096† | Lower activity |
| Average MTXPGs* (pmol/109 RBCs) (n = 418) | 24.8 | 4.9-58.0 | .664† | — | |
| Maximum MTXPGs (pmol/109 RBCs) (n = 156) | 33.0 | 4.9-85.3 | .196† | .405† | |
| Average MTX AUC* (μmol · h) (n = 842) | 674 | 350-1,024 | .520‡ | — | |
| Average teniposide AUC* (μmol · h) (n = 830) | 731 | 235-1,454 | .914‡ | — | |
| Average cytarabine AUC*(μmol · h) (n = 811) | 58 | 12.7-443 | .362‡ | — | |
| Dose intensity 6MP1-153 (n = 19,046) | 83% | 11-170% | .006† | .022† | Higher dose intensity |
| Dose intensity MTX1-153 (n = 19,046) | 83% | 30-102% | .039† | .557† |
| . | Median . | Range . | P Value for Univariate Analysis . | P Value From Multivariate Analysis . | Association With Improved EFS . |
|---|---|---|---|---|---|
| Average TGN*(pmol/8 · 108 RBCs) (n = 709) | 401 | 0-1,498 | .611† | — | |
| Maximum TGN (pmol/8 · 108 RBCs) (n = 180) | 594 | 0-4,472 | .429† | — | |
| Average TIMP*(pmol/8 · 108 RBCs) (n = 709) | 69 | 0-256 | .640† | — | |
| Maximum TIMP (pmol/8 · 108 RBCs) (n = 180) | 131 | 0-456 | .509† | — | |
| Average MeTIMP*(pmol/8 · 108 RBCs) (n = 152) | 14,212 | 0-59,379 | .252† | — | |
| Maximum MeTIMP (pmol/8 · 108RBCs) (n = 86) | 16,532 | 0-68,371 | .287† | — | |
| TPMT activity (units/mL packed RBCs)* (n = 267) | 18.0 | 0.4-30.4 | .363† | .096† | Lower activity |
| Average MTXPGs* (pmol/109 RBCs) (n = 418) | 24.8 | 4.9-58.0 | .664† | — | |
| Maximum MTXPGs (pmol/109 RBCs) (n = 156) | 33.0 | 4.9-85.3 | .196† | .405† | |
| Average MTX AUC* (μmol · h) (n = 842) | 674 | 350-1,024 | .520‡ | — | |
| Average teniposide AUC* (μmol · h) (n = 830) | 731 | 235-1,454 | .914‡ | — | |
| Average cytarabine AUC*(μmol · h) (n = 811) | 58 | 12.7-443 | .362‡ | — | |
| Dose intensity 6MP1-153 (n = 19,046) | 83% | 11-170% | .006† | .022† | Higher dose intensity |
| Dose intensity MTX1-153 (n = 19,046) | 83% | 30-102% | .039† | .557† |
Abbreviations: RBC, red blood cell; TGN, thioguanine nucleotides; TIMP, thioinosine monophosphate; MeTIMP, methylthioinosine monophosphate; TPMT, thiopurine methyltransferase; MTXPG, methotrexate polyglutamates; AUC, plasma area under the concentration-times-time curve; MTX, methotrexate; 6MP, 6-mercaptopurine; n = number of measurements for each variable used in the analysis.
For those variables for which >1 measurement was taken per patient, the median and range of all possible values are indicated.
Stratified for risk group and protocol treatment arm (conventionalv targeted doses of pulse therapy).
Stratified for risk group only.
Dose intensity was estimated as the mg/m2/week of each drug administered ÷ the protocol scheduled dosage for all eligible weeks up until elective cessation of therapy or an event · 100%; n = number of weeks of therapy evaluated for the analysis.
TPMT was measured in 154 patients; 26 other patients never had an interpretable RBC TPMT measurement, but did have TGN measurements that were used to classify them as either TPMT homozygous wild-type or heterozygous individuals. Of the 182 patients achieving complete remission, two were homozygous mutants, 17 were heterozygotes, 161 were homozygous wild-type, and two were not classified because they had neither informative TPMT nor RBC TGN measured. TPMT genotype was evaluated in 28 patients and was in complete concordance with assigned phenotype (18 homozygous wild-type [all TPMT*1/*1], eight heterozygous [all TPMT*1/*3A], and two homozygous mutant individuals [one *2/*2, one *3A/*2]).
Descriptive summaries for TGNs, TIMP, MeTIMP, MTXPGs, methotrexate AUC, teniposide AUC, cytarabine AUC, 6MP dose intensity, methotrexate dose intensity, and RBC TPMT activity are provided in Table 1. Univariate analyses showed that the only factors associated with EFS for the entire follow-up period (Table 1) were 6MP (P = .006) and methotrexate dose intensity (P = .039). When the analysis was restricted to a follow-up period only extending to the end of the pulse therapy, higher methotrexate AUC was significantly (P = .0187) associated with improved EFS among the B-lineage cases, as previously reported.5 Because doses of 6MP were adjusted in some patients based on TPMT defects (heterozygotes and homozygous mutants), the impact of 6MP dose intensity was also evaluated among only the 161 patients with homozygous wild-type TPMT phenotypes, and it maintained prognostic importance (P = .007).
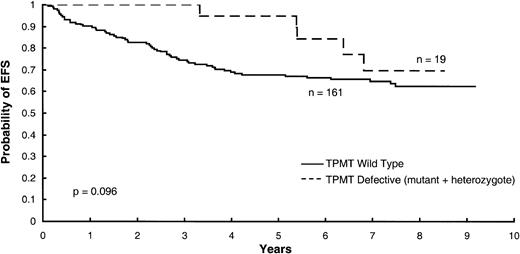
In a multivariate analysis, including 6MP dose intensity, methotrexate dose intensity, and maximum RBC concentrations of methotrexate polyglutamates, adjusting for TPMT status, only 6MP dose intensity (P = .022) was a significant predictor of EFS (Table 1). Patients with defects in TPMT activity (homozygous mutant and heterozygous patients) tended to have improved EFS in this model (P = .096, Fig 2). When MTX AUC was forced into a multivariate model for predicting overall EFS, it was not a statistically significant predictor (P = .143) and did not substantially change the prognostic importance of 6MP dose intensity (P = .001) and TPMT activity (P = .053).
Kaplan-Meier curves for EFS according to TPMT status (homozygous mutant plus heterozygotes versus homozygous wild-type phenotype), indicating a tendency for better outcome among those with TPMT defects (P = .096) in a multivariate model in which dose intensity for 6MP was the most important predictor (P = .022).
Kaplan-Meier curves for EFS according to TPMT status (homozygous mutant plus heterozygotes versus homozygous wild-type phenotype), indicating a tendency for better outcome among those with TPMT defects (P = .096) in a multivariate model in which dose intensity for 6MP was the most important predictor (P = .022).
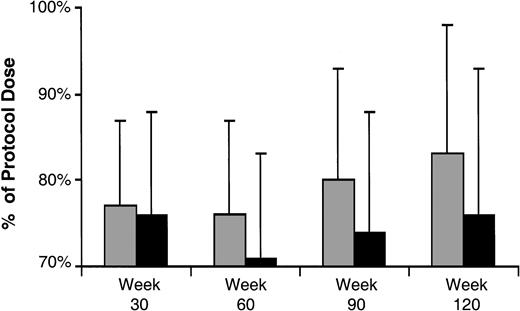
Because the pharmacologic predictors evaluated, including the cumulative 6MP dose intensity variable, were time-dependent variables, it is not possible to construct Kaplan-Meier survival curves that visually depict the importance of 6MP dose intensity to risk of failure. Therefore, to depict the influence of 6MP dose intensity on treatment outcome, two approaches were taken. First, patients were divided into those who remain in continuous remission and those who eventually failed. At 30, 60, 90, and 120 weeks of continuation therapy, the cumulative dose intensity for 6MP for each patient was determined, and the results are depicted in Fig 3. This figure illustrates that the average 6MP dose intensity was slightly higher among those who never failed than those who eventually failed at all time points in therapy.
Average (standard deviation) cumulative 6MP dose intensity, expressed as a percentage of the protocol-specified dosage of 6MP, evaluated at 30, 60, 90, and 120 weeks from the start of continuation therapy in those who remained in continuous remission (□) and those who eventually failed (▪). The numbers of patients at each of the four time points are 165, 156, 150, and 142, respectively.
Average (standard deviation) cumulative 6MP dose intensity, expressed as a percentage of the protocol-specified dosage of 6MP, evaluated at 30, 60, 90, and 120 weeks from the start of continuation therapy in those who remained in continuous remission (□) and those who eventually failed (▪). The numbers of patients at each of the four time points are 165, 156, 150, and 142, respectively.
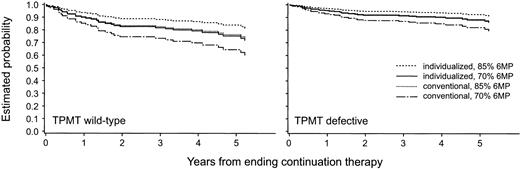
For the second approach, an analysis was conducted that was limited only to those patients who completed all 120 weeks of therapy (n = 142). The median (range) cumulative 6MP dose intensity was 84% (57% to 170%) in the 124 patients with wild-type TPMT phenotype and 70% (11% to 100%) in the 18 patients with a TPMT defect (heterozygotes and homozygous mutant). 6MP cumulative dose intensity at 120 weeks remained a significant predictor of EFS (P = .014) in a multivariate analysis including TPMT phenotype. Using estimates from the Cox proportional hazards model, Kaplan-Meier curves were estimated separately within each TPMT phenotypic group (wild-type vdefective), pulse treatment arms (conventional v targeted), and at two cumulative 6MP dose intensities (70% and 85%) (Fig 4). It can be observed that within each treatment arm, patients receiving a higher 6MP dose intensity are estimated to have an improved EFS compared with those receiving a lower 6MP dose intensity. Moreover, at any given 6MP dose intensity, patients in the TPMT defective group do better than those who are wild-type for TPMT. Finally, patients who were randomized to the targeted systemic therapy arm do better than those randomized to the conventional arm, in accordance with our prior analysis.5
Using estimates from the Cox proportional hazards model limited to the patients who completed all 120 weeks of continuation therapy, Kaplan-Meier curves were projected for each TPMT phenotypic group and for each treatment arm (conventional v targeted) at each of two cumulative 6MP dose intensities (70% v 85% of possible 6MP dosages). The curves projected for those with wild-type TPMT phenotype are depicted in the left panel, and those with either heterozygous or mutant TPMT phenotype are depicted in the right panel.
Using estimates from the Cox proportional hazards model limited to the patients who completed all 120 weeks of continuation therapy, Kaplan-Meier curves were projected for each TPMT phenotypic group and for each treatment arm (conventional v targeted) at each of two cumulative 6MP dose intensities (70% v 85% of possible 6MP dosages). The curves projected for those with wild-type TPMT phenotype are depicted in the left panel, and those with either heterozygous or mutant TPMT phenotype are depicted in the right panel.
Mean thiopurine pharmacologic parameters in patients who received the lowest, intermediate, and highest 6MP dose intensity are summarized in Table 2. Patients with wild-type TPMT phenotype who were in the lowest dose intensity quartile had (on average) over three times as many weeks with no 6MP therapy as those who got the highest dose intensity (24% v 7% of weeks missed). However, the average 6MP weekly dose, estimated only for the weeks that 6MP was given, was only marginally lower (504 v 561 mg/m2/wk) in those in the lowest versus the highest quartiles for dose intensity. Thus, the major reason that dose intensity was compromised was due to missing entire weeks of therapy, not due to reduced daily 6MP doses.
Mean (Standard Deviation) Thiopurine Pharmacologic Parameters by Dose Intensity Quartiles and TPMT Phenotype in Patients Who Completed 120 Weeks of Therapy
| Parameter . | Quartile . | Wild-Type n = 124 . | Heterozygote n = 16 . | Mutant n = 2 . |
|---|---|---|---|---|
| RBC TGN (pmol/8 · 108RBCs) | <25th 25th-75th >75th | 447 (172) 390 (164) 413 (168) | 1,026 (212) 754 (409) 499 (338) | 1,328 (42) — — |
| Dose intensity for 6MP | <25th 25th-75th >75th | 73% (6) 88% (4) 100% (16) | 38% (24) 76% (8) 83% (19) | 19% (10) — — |
| Proportion of weeks with no 6MP | <25th 25th-75th >75th | .24 (.05) .12 (.04) .07 (.03) | .24 (.08) .20 (.03) .15 (.12) | .16 (6) — — |
| Average weekly 6MP dose (mg/m2/wk), including only those weeks that 6MP was given | <25th 25th-75th >75th | 504 (35) 523 (5) 561 (82) | 270 (198) 494 (42) 507 (78) | 120 (69) — — |
| Parameter . | Quartile . | Wild-Type n = 124 . | Heterozygote n = 16 . | Mutant n = 2 . |
|---|---|---|---|---|
| RBC TGN (pmol/8 · 108RBCs) | <25th 25th-75th >75th | 447 (172) 390 (164) 413 (168) | 1,026 (212) 754 (409) 499 (338) | 1,328 (42) — — |
| Dose intensity for 6MP | <25th 25th-75th >75th | 73% (6) 88% (4) 100% (16) | 38% (24) 76% (8) 83% (19) | 19% (10) — — |
| Proportion of weeks with no 6MP | <25th 25th-75th >75th | .24 (.05) .12 (.04) .07 (.03) | .24 (.08) .20 (.03) .15 (.12) | .16 (6) — — |
| Average weekly 6MP dose (mg/m2/wk), including only those weeks that 6MP was given | <25th 25th-75th >75th | 504 (35) 523 (5) 561 (82) | 270 (198) 494 (42) 507 (78) | 120 (69) — — |
Missing entire weeks of therapy was often due to neutropenia (accounting for about 45% of all the weeks that therapy was missed). Neutropenia tended to be associated with worse EFS in a stratified univariate analysis (P = .040). In a multivariate analysis with 6MP dose intensity and TPMT activity, only 6MP dose intensity remained a significant predictor (P = .004 for dose intensity, P= .119 for neutropenia, and P = .067 for TPMT).
To enable a comparison of these results with prior studies,1,2 we analyzed our data using procedures similar to those used previously. A total of 73 patients met the age and sampling criteria set out by Schmiegelow et al.2 In these patients, the product of the median RBC MTX-PG and the median TGN was not a predictor of EFS (P = .95) or of CNS relapse-free survival (P = .24). In addition, EFS was not different between patients whose maximum TGN reading fell above or below our median value of 594 pmol/8 • 108 RBCs (P = .64) or when divided according to a value of 284 pmol/8 • 108 RBCs (previously reported to be associated with EFS1) (P= .99). These results are consistent with our Cox proportional hazards model analysis in which TGN values were evaluated as a continuous variable (Table 1).
DISCUSSION
These data represent a comprehensive analysis of pharmacologic determinants of outcome in childhood ALL. Systemic exposure to every dose of the three agents (methotrexate, cytarabine, and teniposide) repeatedly given as pulse therapy during year 1, as well as multiple measures of chronic exposure to thiopurine and methotrexate active metabolites (RBC TGN and MTX-PG concentrations), were evaluable in 180 of 182 patients who entered complete remission on a single protocol. Among all of these pharmacologic parameters, the most important determinant of overall EFS was the 6MP dose intensity. This treatment protocol, SJCRH Total XII, was highly dependent on daily 6MP and weekly low-dose methotrexate, as these two drugs were the only chemotherapy administered from weeks 60 to 120 of continuation therapy. Having more weeks in which chemotherapy is administered or receiving higher dosages has been suggested to be important in other studies of ALL that are heavily antimetabolite-based.14-16
In contrast to prior reports,1 we did not find that RBC TGNs (or other 6MP metabolite indices) were predictive of EFS. Several reasons may account for this. First, the median and maximum RBC TGNs in our population were substantially higher than levels reported previously.1 Thus, it is possible that the vast majority of the patients reported herein had active thiopurine metabolite concentrations which exceeded a minimum threshold value for efficacy. Second, it is possible that prior associations of RBC thiopurine1 and MTX-PGs2,3 with EFS were more indicative of good compliance with chemotherapy (including 6MP, methotrexate, and other elements of ALL therapy) than they were indicators of a minimum required level of exposure to antimetabolite therapy. This latter explanation is particularly a possibility because low-dose weekly methotrexate was given orally in the prior protocols,1-3 but was given solely by the parenteral route on the protocol reported herein. Thus, RBC thiopurine metabolite indices would not be expected to reflect compliance with methotrexate therapy in our study.
It is not clear why we observed relatively high TGN concentrations in our patients. It is possible that the close follow-up with medication histories by our research nurses, pharmacists, and physicians resulted in improved compliance with oral 6MP. It is also possible that the increased exposure to low-dose methotrexate, due to parenteral dosing of 40 mg/m2 (rather than 20 mg/m2 used by most other groups), was more effective at inhibiting de novo purine synthesis, resulting in increased levels of phosphoribosyl pyrophosphate, and thereby increasing availability of a rate-limiting cofactor for TGN formation by hypoxanthine phosphoribosyl transferase.17 In any case, the high TGNs we observed in our patients may indicate that we exceeded a “threshold” value for TGNs in most patients, and may partly explain why 6MP dose intensity was prognostically important, whereas TGNs were not. Because our assessment of dose intensity provides data on all weeks of continuation therapy and TGNs were only measured a few times (and were, putatively, above some minimum threshold when they were measured), it is plausible that 6MP dose intensity could provide more information on overall treatment intensity and correlate with EFS, while TGNs did not.
There has been controversy as to whether EFS can be improved by advancing the weekly dosage of 6MP to the point of toxicity (in which case subsequent weeks of therapy may be omitted due to neutropenia), or whether it is better to proceed with smaller dosage increments, thereby causing less severe neutropenia.1,14-16,18-21 Our study shows that among patients with wild-type TPMT phenotype who were in the lowest quartile for dose intensity of 6MP (and thus most likely to fail), 24% of weeks of therapy were completely omitted, compared to only 7% of weeks of therapy omitted among those with higher dose intensity (see Table 2). However, the weekly dose of 6MP (when 6MP was given) among such children with low-dose intensity appeared to be only modestly lower compared to those with the highest dose intensity (504v 561 mg/m2/wk). Was this lower dose intensity due to our failure to prescribe enough 6MP, or because neutropenia precluded administration of 6MP? To partially address the latter possibility, we examined whether neutropenia was associated with outcome and found that in a univariate analysis, neutropenia was associated with worse EFS. In addition, we evaluated an alternative estimate of 6MP dose intensity, ie, each week was scored dichotomously as to whether any dose of 6MP was given for at least 3 of the 7 days or not. This measure of 6MP dose intensity was also prognostic for EFS (P = .0295, data not shown). Thus, although caution must be used in drawing conclusions from these retrospectively analyzed data, our findings are consistent with the notion that neutropenia which compromises the ability to deliver 6MP worsens EFS, and that EFS is improved by ensuring that some 6MP is given every week. We hypothesize that every effort should be made to administer maximal doses of 6MP, especially in individuals with TPMT wild-type phenotype, but that such dosing should not be advanced at the expense of toxicity which precludes administration of subsequent therapy. The notion that too much toxicity may compromise outcome by resulting in too many interruptions of therapy has been suggested by other investigators, as well.16 20 Efficacy of 6MP therapy appears to be dependent on adequate chronic exposure to the drug over a high percentage of the weeks of continuation therapy.
The importance of dose intensity of 6MP therapy as a determinant of ALL outcome is somewhat surprising, given that the difference in the 6MP dose intensity between children who remained in remission and those who failed, examined at any time point in therapy, appears to be very modest (Fig 3). These data suggest that intensive monitoring and scrupulous attention to weekly continuation therapy dosing may be necessary to optimize therapy for children with ALL. Whether intensifying the administration of 6MP will be necessary in the context of contemporary ALL protocols, which often include therapy other than antimetabolites, such as monthly pulses of glucocorticoid and vincristine, is uncertain. However, cumulative dose of 6MP was an important prognostic determinant in at least one study16which included monthly glucocorticoid plus vincristine.
Our data indicate that dosage individualization of 6MP therapy in ALL is dependent on TPMT phenotype. TPMT is subject to a genetic polymorphism, with about 10% of white and American black populations exhibiting a heterozygous phenotype.9,22 Despite the fact that heterozygous and homozygous mutant patients received lower 6MP dose intensity than patients with wild-type TPMT, TPMT defective phenotype tended to be associated with improved EFS (Figs 2 and 4). Our hypothesis is that advancing 6MP dose intensity may be more important for patients with wild-type phenotype. We11 and others23 24 have demonstrated that substantial (≈10-fold) dosage decreases are required in patients with homozygous mutant TPMT phenotype; we caution that the appropriate degree of dosage alteration in those with heterozygous phenotype is not known.
Our data support a balanced approach to dosage individualization in childhood ALL. Administration of maximum doses of 6MP is important in childhood ALL, particularly in patients with wild-type TPMT phenotype, but dosage increases should be tempered by the fact that resulting toxicity may compromise the ability to give 6MP during all scheduled weeks, thereby reducing overall dose intensity and worsening outcome.
ACKNOWLEDGMENT
We thank our clinical staff for scrupulous attention to patient care and documenting dosages; Nancy Kornegay for computer assistance; our research nurses, Sheri Ring, Lisa Walters, Terri Kuehner, and Margaret Edwards; our technical staff, YaQin Chu, Eve Su, Natasha Lenchik, and May Chung; and the patients and their parents for their participation in these studies.
Supported by Grants No. CA51001, CA20180, and CA36401 from the National Institutes of Health; Cancer Center CORE Grant No. CA21765; by a Center of Excellence grant from the State of Tennessee; and by American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mary V. Relling, PharmD, St Jude Children’s Research Hospital, 332 N Lauderdale, Memphis, TN 38105.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal