Abstract
Studies in cell lines have indicated that expression of the BCL-2 family of proteins is an important determinant of chemotherapy-induced apoptosis; however, the level of expression of these proteins in childhood acute lymphoblastic leukemia (ALL) has not been extensively reported. Using quantitative Western blotting we have determined the level of expression of BCL-2, BAX, MCL-1, and BCL-X in lymphoblasts from 47 children with ALL (33 at presentation only, 4 at relapse only, and 10 at both presentation and on relapse). Results were determined as a ratio to actin as an internal control. BCL-2, BAX, and MCL-1 were detected in all samples. BCL-XL was only detected in 6 cases (4 at presentation and 2 at relapse) and BCL-XS in none. No correlation was found between expression and white blood cell count, age at diagnosis, gender, or blast karyotype. BCL-2 levels and the BCL/BAX and MCL-1/BAX ratios were found to be significantly higher in B-lineage as compared with T-lineage disease (P < .003, .02, and .02, respectively). No consistent pattern of change in expression was noted in the 10 cases studied at both presentation and relapse. Kaplan-Meier analysis showed a significant correlation between high BAX expression and an increased probability of relapse (P< .05 by the log rank test), suggesting that chemosensitivity in leukemic blasts may be regulated by factors that override the BCL-2 pathway.
CONSIDERABLE evidence now exists that cytotoxic drugs used in the treatment of malignancies exert their effects through the triggering of apoptotic pathways.1,2Preclinical studies have indicated that alterations in the apoptotic threshold may be a cause of drug resistance, but the direct relevance of this to clinical practice is, as yet, uncertain. Although apoptosis involves a complex series of intracellular molecular interactions, cell line studies have indicated that, in most systems, alterations in the expression of BCL-2 and related proteins can have a marked influence on chemosensitivity.3 The aim of the study reported here is to establish if measurement of expression of these proteins could be of prognostic relevance in childhood acute lymphoblastic leukemia (ALL).
The BCL-2 oncoprotein is a suppressor of apoptosis,3,4initially described in human B-cell follicular lymphomas with the t(14;18) chromosomal translocation, where its juxtaposition with the JH region of the Ig heavy chain results in deregulated overexpression and elevated levels of a 26-kD protein.5-7 However, BCL-2 has also been found in normal T- and B-lymphoid cells and in a variety of lymphoproliferative disorders in which t(14;18) is not present.5,8 In follicular lymphomas, BCL-2 suppresses apoptosis mediated by several agents9,10 and also correlates with poor treatment outcome.10 BCL-2 has now been shown to inhibit apoptosis in many different cell types and under a variety of conditions, such as treatment with γ-irradiation, chemotherapeutic drugs, glucocorticoids, cytotoxic lymphokines, and heat shock,11-14 suggesting that it inhibits cell death triggered by multiple routes and acts at a step common to many pathways. Protection by BCL-2 against inhibitors of thymidylate synthase and also DNA-damaging agents, such as nitrogen mustard, camptothecin, or etoposide, showed that BCL-2 is not involved in the reduction of drug-induced DNA damage, alterations in the rates of DNA repair, inhibition of drug-induced alterations in nucleotide pools, or changes in cell cycle kinetics.15-17 Overall, these findings suggest that BCL-2 acts downstream of these events.
Since the discovery of BCL-2, a large number of genes have been identified whose products have homology with BCL-2 and have been termed the BCL-2 family of proteins. These proteins either suppress or promote apoptosis. Those, like BCL-2, that suppress apoptosis include BCL-XL, MCL-1, A1, BCL-W, NR-13, and BFL-1, and those that promote apoptosis include BAX, BCL-XS (a splice variant of BCL-XL), BAD, BAK, NBK/BIK, BID, HRK,18,19 and BOK.20
The BCL-2 family of proteins appears to regulate apoptosis by a process involving complex protein-protein interactions. They can form homodimers or heterodimers with each other and in some cases with structurally unrelated proteins.21 There is evidence that the relative expression and competitive dimerization between these proteins may influence response of a cell to apoptotic stimuli. Although a number of studies have been reported in which the expression of BCL-2 has been related to clinical outcome in hematological malignancies, few of these have included measurement of the relative expression of other members of the BCL-2 family. However, recently, Kaufmann et al22 published data describing the expression of BCL-2, BCL-XL, and MCL-1 in the blasts of adults presenting with acute myeloid leukemia (AML) or ALL in which they reported an increase in MCL-1 in the majority of cases at relapse (10 of 19 matched pairs for AML and 3 of 4 matched pairs for ALL).22 We extend here these observations to childhood ALL and link measurement of expression in blasts at presentation to long-term survival.
MATERIALS AND METHODS
Patients.
Forty-seven children with de novo ALL, referred to the Royal Victoria Infirmary (RVI; Newcastle upon Tyne, UK) between 1986 and 1997, were used in this study. Forty-three samples were presentation blasts and 14 samples were obtained at first relapse. Children who failed to achieve remission were excluded from the study. Samples obtained at relapse included 10 from children who were also analyzed at presentation. The diagnosis was established by cytological examination of bone marrow (BM) smears according to the French-American-British Group recommendations23 and immunophenotyping of leukemic cells.24 Immunophenotype was assessed by flow cytometry (FACScan; Becton Dickinson, San Jose, CA) and immunocytochemistry, using antibodies against terminal deoxynucleotidyl transferase (TdT), cytoplasmic μ (cyt-μ), surface membrane Ig (SIg), CD2, CD3, CD7, CD10, CD13, CD19, CD20, CD33, and CD34 by the Department of Haematology, RVI, Newcastle upon Tyne. Within the cases diagnosed as ALL, immunologic subgroups were defined as follows: null ALL (CD19+, CD20±, CD10−, cyt-μ−, SIg−), common ALL (cALL; CD19+, CD20±, CD10+, cyt-μ−, SIg−), pre-B ALL (CD19+, CD20±, CD10±, cyt-μ+, SIg−), B-ALL (CD19+, CD20+, CD10±, cyt-μ+, SIg+), and T-ALL (CD2±, CD3+, CD7+).
Cytogenetic analysis was performed in the Department of Cytogenetics, RVI, Newcastle upon Tyne, using standard G-banding techniques to stain metaphase preparations obtained from unstimulated cultures of BM cells. A complete karyotype analysis was obtained in 34 presentation samples and 11 relapse samples, and incomplete karyotype analysis was obtained in 4 presentation samples and 1 relapse sample.
Of the 43 presentation childhood ALL cases studied, 25 were male and 18 were female. The median age was 5 years (age range, 0.8 to 16 years). The median presenting white blood cell (WBC) count was 17 × 109/L (range, 0.3 × 109/L to 1,000 × 109/L). There were 30 common ALL, 3 pre-B–cell ALL, 1 null cell ALL, and 9 T-cell ALL.
Eighteen of the 43 patients studied at diagnosis relapsed. First relapses included 11 BM, 3 central nervous system (CNS), 1 testicular, 2 with joint BM and CNS relapse, and 1 with joint BM and testicular relapse. The median follow-up of patients was 36 months (range, 1 to 136 months).
Children in this study had been entered into one of three trials administered by the UK Medical Research Council (MRC): UKALL X (1995 to 1992, 8 patients), UKALL X1 (1992 to 1997, 33 patients), or ALL97 (1997 to date, 2 patients). Remission induction was achieved with prednisolone (or dexamethasone in the case of ALL97), vincristine, L-asparaginase, and intrathecal methotrexate. Daunorubicin was also used in UKALLX. Intensification therapy consisted of prednisolone (or dexamethosone in ALL97), etoposide, vincristine, cytarabine, thioguanine, intrathecal methotrexate, and daunorubicin. Continuing therapy consisted of prednisolone (or dexamethasone in ALL97), methotrexate, vincristine, and 6-mercaptopurine (or 6-thioguanine in ALL97).
Preparation of samples.
All samples were processed within 5 hours of BM aspiration. Lymphoblasts and mononuclear cells were separated by centrifugation over Ficoll. The percentage of leukemic lymphoblasts in the Ficoll prepared samples was determined by cytological examination of cells stained with May Grunwald-Giemsa stain. Cells were cryopreserved at −135°C before analysis.
Cells were thawed at 37°C and 10 vol of RPMI 1640 (Dutch modification; GIBCO-BRL Life Technologies Ltd, Paisley, UK) containing 15% heat-inactivated fetal calf serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine (RF15) added slowly. Cells were pelleted by centrifugation at 230g for 5 minutes, resuspended in 5 mL of RF15, and incubated at 37°C in 5% CO2 for 90 minutes. An aliquot was taken to determine the percentage of viable and apoptotic cells using Hoescht and trypan blue staining or acridine orange and ethidium bromide.25 Samples containing more than 15% of dead or apoptotic cells were excluded from further study.
The remaining cells were spun at 230g for 5 minutes, and the pellet was resuspended in 2 mL of 100 μmol/L EDTA, 1 mmol/L KHCO3, and 0.17 mol/L NH4Cl in deionized water (pH7.3) for 4 minutes to lyse any contaminating red blood cells.26 Twenty milliliters of ice-cold phosphate-buffered saline (PBS) was added; the sample was centrifuged at 230g for 4 minutes; and the pellet was lysed in sodium dodecyl sulfate (SDS) sample buffer containing 62.5 mmol/L Tris/HCl, pH 6.8, 2% SDS, and 20% glycerol at 100 μL per 5 million cells and was heated at 100°C for 5 minutes. The sample was sonicated for 5 seconds at 15 μm and microcentrifuged at 14,000 rpm for 10 minutes, and the pellet was discarded. An aliquot of the supernatant was diluted 1:9 with water and used to estimate the protein level using a commercially available kit using the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Samples were stored at −80°C until ready for analysis. Immediately before use, 2-mercaptoethanol (5%) and bromophenol blue (0.005%) were added and the samples were diluted to a concentration of 0.5 mg/mL in SDS sample buffer containing 2-mercaptoethanol and bromophenol blue before being heated at 50°C for 5 minutes.
Determination of BCL-2, BAX, MCL-1, and BCL-X by immunoblotting.
The Epstein-Barr virus-immortalized human Philadelphia chromosome-positive ALL cell line, SD-1 (kindly provided by Dr S. Dhut27 ICRF, Medical Oncology Unit, St Bartholomew’s Hospital, London, UK) was used as a positive control for BCL-2, BAX, and actin. The erythroleukemia K562 cell line28was used as a control for MCL-1 and BCL-X. Cells were lysed in SDS sample buffer as described above for patient lymphoblasts and diluted to give a five-point standard curve for each blot by loading 20, 15, 10, 5, and 2.5 μg of total cellular protein.
Immunoblotting.
Twenty microliters of sample (equivalent to 10 μg of total protein) was subjected to SDS-polyacrylamide gel electrophoresis according to the method of Laemmli29 using minigel equipment supplied by Bio-Rad Laboratories Ltd (Hertfordshire, UK). Twelve percent acrylamide gels were used for analysis of BCL-2 and BAX expression, and 10% gels were used for MCL-1 and BCL-X. For an individual experiment, 5 standards were loaded on the same gel as 4 lymphoblast samples and a molecular weight (MW) marker (SeeBlue Pre-Stained Standard; Novex Experimental Technology, San Diego, CA).
After electrophoresis, proteins were transferred to nitrocellulose membranes (Hybond-C; Amersham, Little Chalfont, UK) using miniblot equipment supplied by Bio-Rad Laboratories Ltd (Hertfordshire, UK) as previously described.30 After transfer, blots for BCL-2, BAX, and actin analysis were divided between the 30- and 36-kD MW markers. One half was probed for actin (MW 42 kD) and the other for BCL-2 (MW 26 kD). After developing the BCL-2 blot, it was washed with two changes of Tris-buffered saline (TBS; 0.01 mol/L Tris, pH 7.5, containing 0.1 mol/L NaCl) and stored at 4°C before reprobing for BAX within 1 month. One part of the MCL-1/BCL-X blot was probed for MCL-1 (41 kD) and the other part for BCL-X (BCL-XL 29 kD, BCL-XS 20 kD).
Immunocomplexes were detected using enhanced chemoluminescence. Blots were immersed for 1 hour in a blocking buffer of 5% instant dried skimmed milk (Boots Co PLC, Nottingham, UK) in TBS containing 0.05% tween 20 (TBS-T). Primary antibody was diluted in blocking buffer and incubated with the blots for 1 hour as follows: BCL-2 (mouse monoclonal; Dako, Cambridge, UK) at 1/200; BAX (rabbit polyclonal; Pharmingen, San Diego, CA) at 1/100; BCL-X and MCL-1 (rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA) at 1/100; and actin (mouse monoclonal; Sigma-Aldrich Chemical Co Ltd, Dorset, UK) at 1/1,000. Blots were washed thoroughly with TBS-T and incubated in secondary antibody (biotinylated swine antirabbit or rabbit antimouse, diluted 1 in 1,000 in 0.5% casein in TBS-T) for 30 minutes. After further washing in TBS-T, blots were incubated for 30 minutes in streptavidin-conjugated horseradish peroxidase (HRP; Dako) diluted 1 in 1,000 in 0.5% casein in TBS-T. After a final wash in TBS-T, HRP was detected using ECL reagent (Amersham Life Science Ltd, Buckinghamshire, UK) as described in the manufacturer’s instructions. Chemiluminescence was detected by autoradiography using x-ray film (Fuji, Gateshead, UK). The integrated optical density (IOD) of the resulting bands was determined by densitometry using a Bio-Image system (Millipore UK Ltd, Hertfordshire, UK).
Statistical analysis.
Survival analysis was performed using the Kaplan-Meier method and survival curves were compared using the log-rank test. Protein expression in different prognostic groups was compared using the Mann-Whitney U test. Expression was correlated with WBC count using Spearman rank analysis. P values of less than .05 were considered to be statistically significant.
RESULTS
Percentage of blasts and apoptosis in lymphoblast samples.
The blast count after mononuclear cell separation was greater than 90% in most cases. No cases were analyzed that contained fewer than 80% blasts.
Reproducibility of immunoblots.
To check intrablot reproducibility, 9 identical samples were run on 10% polyacrylamide gels. As 10 or 20 μL of samples was loaded on polyacrylamide gel, reproducibility was checked for loading both of these amounts. On one gel was loaded 20 μL of a 1 μg/μL MCL-1 standard and on another 10 μL of a 2 μg/μL standard. The coefficient of variation for loading 10 μL samples was 10.8% and for loading 20 μL samples was 8.7%. To check interblot reproducibility, one sample was loaded on five separate gels with standards, blotted, and probed for BCL-2 and actin. The coefficient of variation for BCL-2 and actin measurements and the BCL-2/actin ratio were 15.7%, 12.3%, and 12.7%, respectively.
Expression of BCL-2, BAX, MCL-1, and BCL-X in lymphoblasts at diagnosis and on relapse.
Examples of immunoblots for BCL-2, BAX, MCL-1, and actin are shown in Fig 1. The identity of the target protein was determined on the basis of MW where multiple bands were present. A standard curve was produced for each blot by plotting the IOD of each standard band against the amount of cell lysate loaded. For the patient samples, these standard curves were used to determine the amount of cellular protein in the standards required to produce an equivalent IOD. These values were finally expressed as a ratio to actin used as an internal control for the amount of cell lysate loaded. Because actin has approximately the same MW as MCL-1, actin and MCL-1 could not be probed on the same blots. Hence, MCL-1 levels were expressed as a ratio to actin measured on blots probed for BCL-2 and BAX. Because the ratios of BCL-2 to BAX and MCL-1 to BAX have been suggested as determinants of apoptotic threshold, these were determined for each sample.
Immunoblot showing level of expression of BCL-2, BAX, MCL-1, and BCL-XL with corresponding standards from SD1 cells (in the case of BCL-2 and BAX) and K562 cells (in the case of MCL-1 and BCL-XL). Actin is included as a loading control.
Immunoblot showing level of expression of BCL-2, BAX, MCL-1, and BCL-XL with corresponding standards from SD1 cells (in the case of BCL-2 and BAX) and K562 cells (in the case of MCL-1 and BCL-XL). Actin is included as a loading control.
BCL-XL expression was only detected in four lymphoblast samples at presentation and 2 relapse samples. Levels of expression were less than the 2.5 μg standard except for one patient that was between the 2.5 and 5 μg standard. BCL-XS was not detected in any of the samples analyzed.
Relationship between relapse-free survival and expression of BCL-2, BAX, and MCL-1 at diagnosis.
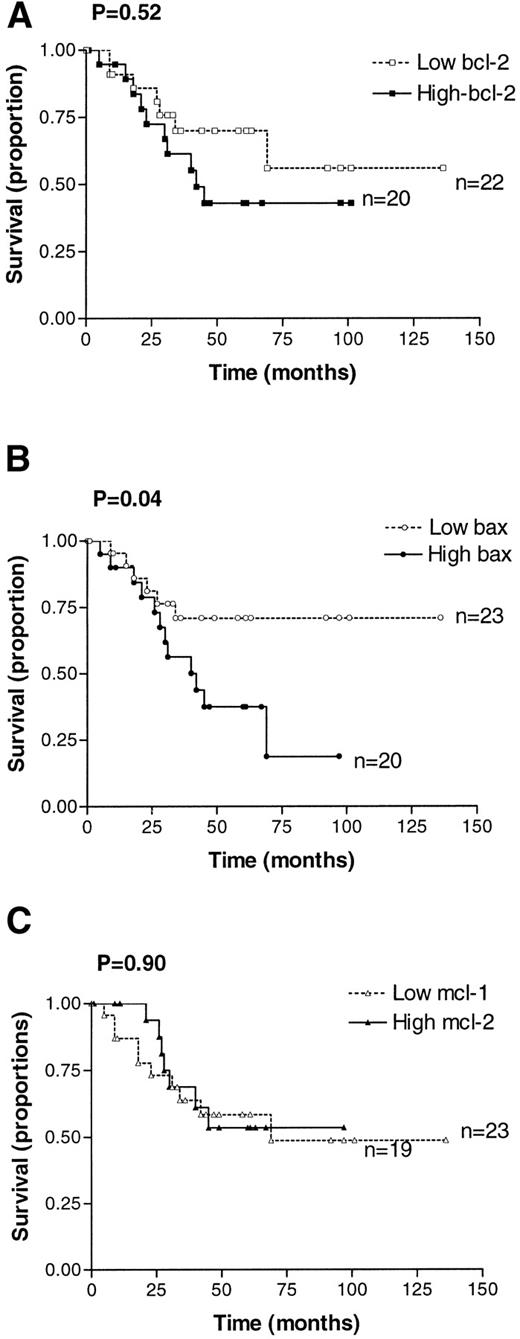
Relapse-free survival was compared in cases in which expression of BCL-2 family proteins was above the median for the whole group (high expressers) with those in which expression was below or equal to the median value (low expressers). Survival curves were compared using the log rank test. Relapse-free survival did not differ with expression of BCL-2, BCL-2/BAX ratio, MCL-1, or MCL-1/BAX ratio. However, there was a significant correlation between high levels of BAX expression and an increased probability of relapse (P = .04), as shown in Fig 2.
Kaplan-Meier analysis of relapse-free survival for patients with high or low expression of BCL-2 (A), BAX (B), and MCL-1 (C). High expression is defined as greater than the median, and low expression is defined as less than or equal to the median value obtained by quantitative Western blotting.
Kaplan-Meier analysis of relapse-free survival for patients with high or low expression of BCL-2 (A), BAX (B), and MCL-1 (C). High expression is defined as greater than the median, and low expression is defined as less than or equal to the median value obtained by quantitative Western blotting.
Expression of BCL-2 family proteins in lymphoblasts at diagnosis and correlation with presenting WBC count, gender, and age at diagnosis.
There was no significant relationship between expression of BCL-2, BAX, BCL-2/BAX, MCL-1, and MCL-1/BAX levels with presenting WBC count (data not shown). Expression of these BCL-2 family proteins was compared between age groups associated with a poor prognosis (ie, <2 years and >10 years) and age associated with good prognosis (ie, >2 years and <10 years). Correlations were also made with gender, because males with ALL have been reported to have a poorer prognosis than females. However, there was no correlation between levels of expression of BCL-2, BAX, BCL-2/BAX, MCL-1, or MCL-1/BAX with age at diagnosis or gender (data not shown).
Immunophenotype, karyotype, and expression of BCL-2, BAX, MCL-1, bcl2/BAX, and MCL-1/BAX ratios in lymphoblasts at diagnosis.
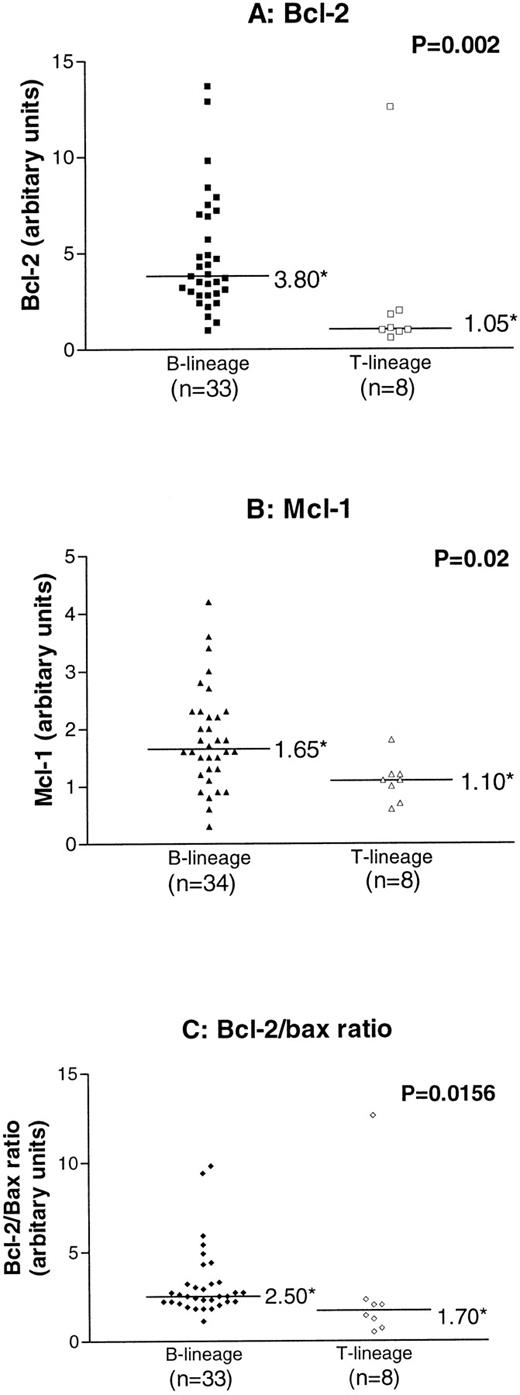
Levels of expression of BCL-2, BAX, BCL-2/BAX, MCL-1, and MCL-1/BAX were compared in B-lineage versus T-lineage ALL. There was no correlation with BAX or the MCL-1/BAX ratio with the immunophenotype (data not shown). However, BCL-2 and MCL-1 expression and the BCL-2/BAX ratio were significantly higher in B-lineage ALL compared with T-lineage ALL cases (P values of .002, .016, and .012, respectively; Fig 3).
Comparison of expression of BCL-2, MCL-1, and the BCL-2/BAX ratio in B-lineage and T-lineage childhood ALL (*median value). P values refer to results of analysis by the Mann Whitney U test.
Comparison of expression of BCL-2, MCL-1, and the BCL-2/BAX ratio in B-lineage and T-lineage childhood ALL (*median value). P values refer to results of analysis by the Mann Whitney U test.
Patients were divided into good (hyperdiploid, >50 chromosomes), standard (diploid), and poor prognosis (pseudodiploid) groups according to karyotype analysis. Because only 2 patients fell into the poor prognostic group, as determined by karyotype, the levels of BCL-2, BAX, BCL-2/BAX, MCL-1, and MCL-1/BAX ratio were correlated only in good versus standard karyotype prognostic groups. There was no significant correlation between BCL-2 family protein expression and karyotype (data not shown).
Expression of BCL-2, BAX, BCL-2/BAX ratio, MCL-1, and MCL-1/BAX in lymphoblasts at diagnosis and on relapse.
Comparison of expression of BCL-2, BAX, BCL-2/BAX, MCL-1, and MCL-1/BAX levels in lymphoblasts at diagnosis and on first relapse showed no significant difference between the two groups (data not shown). Although there were differences in some cases between expression of these proteins in paired presentation and first relapse lymphoblasts from the same patient, there was no consistent pattern of change (data not shown).
DISCUSSION
High levels of expression of BCL-2 have been shown to correlate with poor treatment outcome in some hematological malignancies, including follicular lymphoma,10 chronic lymphocytic leukemia (CLL),31 and AML.32 Results from the present study show no correlation between BCL-2 expression in childhood ALL and known prognostic features, including age at diagnosis, gender, karyotype, or presenting WBC count. In the present study, no correlation was found between BCL-2 expression and relapse-free survival. This is in agreement with several other reports, including a study of 43 adult ALL cases,33 a study of 40 childhood and adult ALL cases,34 and studies of childhood ALL involving 5235 and 338 patients.36 Although in the latter study high BCL-2 levels predicted slow early response in T-lineage but not B-lineage ALL, as judged by the number of blasts in a BM aspirate at day 28 of induction therapy, it had no impact on short-term event-free survival within the first 2 years of diagnosis.
Although BCL-2 is a suppressor of apoptosis and hence may be expected to promote drug resistance in childhood ALL, increased expression has been found to be associated with certain favorable prognostic features, such as non–T-lineage ALL. In this study, BCL-2 was found to be significantly lower in T-lineage compared with B-lineage cases of ALL, with a 3.2-fold difference in the median levels of expression. Similar differences have been reported by others.36-38 High levels have also been related to expression of the CD10 antigen in B-lineage ALL (common ALL)38; however, because there were only 3 pre-B ALL cases compared with 30 common ALL cases in our patient cohort, we could not make this comparison. Increased BCL-2 expression has been found by others to be associated with a relatively low WBC count,36 38 another favorable prognostic feature. This association was not found in our study.
Because the ability of BCL-2 to modulate the apoptotic threshold is affected by the relative expression of BAX,39-41 several studies have attempted to assess the relationship between the BCL-2/BAX ratio and clinical outcome. A high BCL-2/BAX ratio has been reported to correlate with an inability to achieve complete remission in AML42 and with in vitro resistance to drug-induced apoptosis in CLL.43,44 We could find no correlation between BAX expression or the BCL-2/BAX ratio and age at diagnosis, gender, karyotype, or presenting WBC count. Although there was no significant difference in BAX expression between B-lineage and T-lineage ALL, the BCL-2/BAX levels were significantly lower in T-lineage compared with B-lineage ALL, as was found with BCL-2 expression alone. The BCL-2/BAX ratio did not predict relapse-free survival. However, high BAX expression alone was shown to be associated with an increased probability of relapse (P = .04). From in vitro data, this result was unexpected, although a comparable association between high BAX expression and unfavorable prognostic features has been reported for cancers of the breast45 and ovary.46
The unexpected relationship between high BAX expression and poor prognosis may be explained by the observation that BCL-2 and BAX have a role in the control of proliferation as well apoptosis. Mature T cells overexpressing BAX have been shown to have lower levels of p27Kip1 and enter S phase more rapidly in response to interleukin-2 stimulation than control T cells. The converse is true for BCL-2–transfected T cells.47 Transfection of several mammalian cell lines with BCL-2 has been associated with reduced cell proliferation and prolongation of the G1 phase of the cell cycle,48,49 both of which could be abrogated by the coexpression of BAX.48 A relationship between elevated BCL-2 expression and a reduced rate of disease progression has been shown in studies of clinical samples from patients with ovarian, non-small cell lung cancer, and stage II carcinoma of the colon.46,50,51 In addition, expression of BAX has been associated with increased proliferative capacity in ovarian and breast cancer.45,46 We did not assess cell proliferation in the current study; however, we did demonstrate that BCL-2 was lower in T-lineage disease, which has, in turn, been associated with a higher WBC count than B-cell ALL.52 Although the generalized correlation between high WBC count and T-lineage ALL was not statistically apparent in the patients analyzed here (data not shown), Salomons et al,38 who did report a correlation with T-cell lineage and WBC count, found that, after controlling for WBC count, the association between low BCL-2 and expression of T-cell markers disappeared.
Because BCL-2 family members other than BCL-2 and BAX may be important in regulating apoptosis in ALL, levels of expression of MCL-1, BCL-XL, and BCL-XS were also assessed in the current study. There was a 14-fold variation in MCL-1 expression and ninefold variation in MCL-1/BAX expression. MCL-1 and MCL-1/BAX expression did not correlate with age at diagnosis, gender, karyotype, presenting WBC count, or event-free survival. BCL-XL was only detected in 4 cases at diagnosis. Two of these cases have relapsed (1 after 15 months and the other after 26 months) and 2 remain in remission (after a follow-up of 96 and 116 months). BCL-XLwas only detected in 2 of 15 cases at relapse, although BCL-XL was not detected in lymphoblasts from these same patients at diagnosis. BCL-XS was not detected in any of the samples analyzed. Hence, this study does not suggest that MCL-1, BCL-XL, or BCL-XS are of prognostic significance in childhood ALL. However, it is possible that other BCL-2 family members, not assessed in this study, may be important in regulating apoptosis and determining prognosis in ALL.
There is evidence that posttranscriptional modification regulates the function of BCL-2 and possibly other family members. Several studies have suggested that phosphorylation of BCL-2 affects its function, although there have been conflicting reports that this may enhance53,54 or reduce55,56 its ability to suppress apoptosis. It is also possible that mutations may alter the function of these proteins by, for example, affecting protein stability. A variety of BAX mutations have been reported in cell lines derived from hematological malignancies, including ALL57 58; hence, the high level of BAX in some cases reported in our series may have been due to the increased transcription of nonfunctional protein, analogous to the increases seen for some p53 mutations.
In summary, we have shown that, whereas expression of BCL-2 and determination of the BCL-2/BAX ratio do not appear to have prognostic significance in childhood ALL, high expression of BAX at diagnosis is associated with a significant increase in the probability of relapse. Studies using a larger cohort of uniformly treated patients and multivariate analysis will need to be performed to determine if this is an independent prognostic variable that may be used to aid treatment stratification.
ACKNOWLEDGMENT
The help of Mike Reid, Elizabeth Matheson, and Jill Robson in the collection and preparation of samples is gratefully acknowledged.
Supported by grants from the North of England Children’s Cancer Research Fund and the Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Andrew G. Hall, PhD, MD, Cancer Research Unit, Medical School, Framlington Place, Newcastle Upon Tyne, NE2 4HH, UK.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal