Abstract
We have previously demonstrated a significant inverse correlation between circulating thrombopoietin (TPO) levels and peripheral platelet (PLT) counts in patients with thrombocytopenia secondary to megakaryocytic hypoplasia but not in patients with immune thrombocytopenic purpura (ITP; Chang et al, Blood 88:3354, 1996). To test the hypothesis that the differences in the circulating TPO levels in these two types of thrombocytopenia are caused by differences in the total capacity of Mpl receptor-mediated TPO clearance, thrombocytopenia was induced in female CD-1 mice either by sublethal irradiation (irradiated) or rabbit antimouse PLT serum (RAMPS) for 1 day (1 d RAMPS) and 5 days (5 d RAMPS). A well-characterized murine model of autoimmune thrombocytopenic purpura, male (NZW × BXSB) F1 mice (W/B F1), was also included in this study. All thrombocytopenic mice and their controls received trace amounts of 125I-recombinant murine TPO (125I-rmTPO) intravenously and were killed 3 hours postinjection. Blood cell-associated radioactivity was significantly decreased in all 4 groups of thrombocytopenic mice. Significantly increased plasma and decreased whole spleen-associated radioactivity was observed in the irradiated group compared with controls (P < .05). While a lesser but still significant increase in plasma and decrease in whole spleen-associated radioactivity was observed in the 1 d RAMPS mice (P < .05), there were no significant differences between the 5 d RAMPS nor the W/B F1 male mice compared with controls, although whole spleen-associated radioactivity was higher in the W/B F1male. A significant inverse correlation of plasma and whole spleen-associated radioactivity was demonstrated in W/B F1male mice (r = −.91, n = 6, P < .05). There was also a decrease in bone (femur)/blood-associated radioactivity in the irradiated group compared with controls (P < .05), but a significant increase in 1 d and 5 d RAMPS mice (P < .01). Furthermore, the 125I-rmTPO uptake capacity within the spleen and marrow of immune thrombocytopenic mice appeared to be associated with a higher megakaryocytic mass when tissue samples were examined by light microscopy. Internalization of 125I-rmTPO by megakaryocytes and PLTs in the spleens and marrows of ITP mice was also demonstrated directly using electron microscopic autoradiography. Labeled PLTs were also found within splenic macrophages. Additionally, the mean PLT volumes of RAMPS mice were significantly higher than those of the control and irradiated mice (P < .05), as was the bound 125I-rmTPO (cpm) per million PLT (P < .05). Finally, significantly decreased 125I-rmTPO degradation products were only found in the plasma of the irradiated mice compared with control animals (P < .05). These data suggest that the lack of Mpl+ cells in the mice with thrombocytopenia secondary to megakaryocytic hypoplasia (irradiated) results in decreased uptake and degradation of TPO and higher circulating TPO levels. Furthermore, these data also suggest that, after a brief TPO surge in response to immune thrombocytopenia (1 d RAMPS), the lack of an inverse correlation of circulating TPO with PLT counts during steady-state immune thrombocytopenic mice (5 d RAMPS + W/B F1 male) is due, at least in part, to its uptake and degradation by the high PLT turnover and increased mass of megakaryocytes.
THE PRIMARY REGULATOR of platelet (PLT) production, thrombopoietin (TPO), identified as the ligand for the c-mpl proto-oncogene product, has been isolated and cloned from several species.1-6 Recombinant TPO enhances megakaryocyte colony formation; increases the size, number, and ploidy of developing megakaryocytes; and results in increased PLT production in vitro and in vivo.3-5,7,8 Injection of recombinant TPO into mice, neonatal rats, and nonhuman primates causes a 400% increase in the circulating PLT count and increases spleen and bone marrow megakaryocytes and their precursors.4,7,8 Additionally, in both c-mpl and TPO-deficient mice, there is a significant decrease in both circulating PLTs and spleen and marrow megakaryocytes.9-11
If TPO is the primary physiological regulator of PLT production, circulating TPO levels would be expected to vary inversely with PLT demand. We have previously measured circulating TPO levels in patients with comparable degrees of thrombocytopenia secondary to two different mechanisms: (1) decreased PLT production due to megakaryocytic hypoplasia (myeloablative therapy, submyeloablative therapy, and Fanconi’s Anemia) and (2) decreased PLT life span with normal or increased megakaryocyte mass (immune thrombocytopenic purpura [ITP]).12 Circulating levels of TPO in the plasma of all of the thrombocytopenic patients with megakaryocytic hypoplasia were markedly elevated. A significant inverse correlation between endogenous TPO levels and peripheral PLT counts was observed in this group of patients. However, the circulating levels of TPO present in the plasma of ITP patients with severe thrombocytopenia remained undetectable. Similar differences in circulating TPO levels between megakaryocyte hypoplastic and ITP patients have been independently reported by other laboratories.13-15
One of several different mechanisms that may regulate circulating TPO levels16-18 was originally proposed by de Gabriele and Penington,19 and then by Kuter and Rosenberg16after TPO was isolated, and suggested that TPO production is constitutive and that plasma levels are controlled by the circulating PLT levels. Based on the recent finding that PLTs express Mpl receptors for TPO, Fielder et al20 further demonstrated that plasma TPO levels are controlled by the circulating PLT levels through Mpl receptor-mediated uptake and metabolism using mice lacking the Mpl receptor. Consequently, a lower circulating PLT mass would have less capacity for TPO uptake and metabolism, resulting in higher circulating TPO levels. However, recent findings that Mpl receptor is expressed not only on PLTs, but also on the megakaryocyte and its progenitors21-23 suggest that circulating TPO levels may not always depend exclusively on the absolute numbers of circulating PLTs but may actually be regulated by the total cell mass of the megakaryocyte lineage that expresses Mpl receptor. Therefore, circulating TPO levels would be significantly increased in thrombocytopenic patients with megakaryocytic hypoplasia due to the low quantity of total Mpl receptor-expressing cellular mass. In contrast, despite similar degrees of acute thrombocytopenia, the total number of Mpl-positive cells, and consequently TPO uptake capacity, would be higher in ITP patients due to increased numbers of megakaryocyte progenitor cells and megakaryocytes and the continuous production of new PLTs, which may still be able to take up TPO before antibody-facilitated reticuloendothelial clearance, resulting in low or undetectable levels of TPO.
To test this hypothesis, we developed several murine models that mimic various forms of human thrombocytopenia. CD-1 female mice were treated with either sublethal irradiation, which induced thrombocytopenia secondary to megakaryocytic hypoplasia, or rabbit antimouse PLT serum (RAMPS) for 1 day and 5 days, which shortened PLT half-life. One-day RAMPS treatment represents a model of pre–steady-state ITP conditions. Five-day RAMPS treatment more closely mimics steady-state conditions of ITP in which megakaryocytopoiesis and thrombopoiesis are already significantly accelerated in response to immune thrombocytopenia.24,25 In addition, another well-characterized murine model of autoimmune thrombocytopenic purpura, male W/B F1 mice, which spontaneously develop thrombocytopenia with age, showing reduced PLT life-spans, increased PLT-associated autoantibodies, and PLT-binding serum antibodies,26 27 was also included in the study together with their nonthrombocytopenic female littermates as controls. Radiolabeled TPO uptake capacity and organ distribution between normal controls and these thrombocytopenic models were compared. The results are consistent with the hypothesis that TPO uptake and, consequently, catabolic capacity during steady states of immune thrombocytopenia remained significantly higher than that of megakaryocyte hypoplastic hosts, which could account, at least in part, for the low levels of circulating TPO demonstrated in humans during steady-state immune-mediated thrombocytopenia.
MATERIALS AND METHODS
Animals.
Eight-week-old female CD-1 mice (Charles River Laboratories, Hollister, CA) were maintained at constant room temperature with free access to food and water for at least 1 week before use. Approval for this study was granted by the Vivarium Committee at Children’s Hospital of Orange County (CHOC; Orange, CA). Thrombocytopenia was induced in 1 group of CD-1 mice by 550 cGy total body irradiation from a 6 mV x-ray linear accelerator at 60 cGy/min (Clinac 6/100, Palo Alto, CA). Thrombocytopenia was induced in a second group of mice by intraperitoneal injection for 1 day or every other day for a 5-day period with 50 μL RAMPS that had been adsorbed against erythrocytes and leukocytes (Inter-Cell Technologies Inc, Hopewell, NJ). Immediately before irradiation or antiserum injection, and at intervals thereafter, 10 μL of mice blood was collected by nicking the tail veins of the animals with a sterile 27-gauge needle. Blood samples were electronically counted (Serano-Baker Diagnostics, Allentown, PA) using a mouse cell discriminator to determine PLT counts and mean PLT volume.
Idiopathic thrombocytopenic purpura-prone male W/B F1 mice were produced by cross-breeding NZW female and BXSB male (Jackson Laboratories, Bar Harbor, ME) in the animal facility of CHOC. These animals were raised under specific pathogen-free conditions.
Tissue distribution of 125I-recombinant murine TPO (125I-rmTPO).
Full-length, biologically active rmTPO was iodinated using the Indirect Iodogen method described previously.20,23 The specific activity of the 125I-rmTPO was 80 μCi/μg protein. Iodinated rmTPO retained approximately full biological activity as determined by its ability to bind to the c-Mpl receptor on mouse PLTs (data not shown).23 To test the stability of the labeled protein, purified 125I-rmTPO was incubated with murine PLT-poor plasma for more than 40 hours at 37°C. Examination using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography showed virtually no degradation (data not shown).20 Administration of sodium iodine to prevent accumulation of 125I in the thyroid of the animals and administration of 125I-rmTPO intravenously into control and thrombocytopenic mice were as described previously.20,23 CD-1 thrombocytopenic mice received125I-rmTPO either 10 days postirradiation (irradiated group), 1 day (20 to 24 hours) after single injection of RAMPS (1 d RAMPS group), or 5 days after initial injection of RAMPS, which was repeated every other day during that period of time. W/B F1male and female mice received 125I-rmTPO at 4 to 5 months of age. All mice received 0.1 mL of 125I-rmTPO (4 μCi) and were killed 3 hours postinjection. The following tissues were collected immediately after the animals were killed: blood (0.38% citrate), femur, heart, kidney, liver, lung, spleen, and sternum. Tissues were sectioned, rinsed, and weighed as described previously.20 23 Tissue-associated radioactivity was determined by counting for 1 minute in a gamma counter (LKB/Wallac 1282; Wallac, Inc, Turku, Finland).
Whole blood (0.1 mL) or plasma (0.05 mL) was counted to obtain the total cpm per sample. Whole blood (0.1 mL) was diluted to 1 mL in phosphate-buffered saline (PBS), vortexed, and centrifuged at 2,950g for 10 minutes, and the remaining blood cell-associated radioactivity was counted after aspiration of the liquid phase. To determine protein (rm-TPO)-associated radioactivity in the plasma, plasma samples (0.05 mL) were diluted to 0.5 mL in PBS containing 1.0% bovine serum albumin, mixed with 0.5 mL of 20% trichloroacetic acid (TCA), and incubated at 4°C for 15 minutes. After centrifugation at 2,950g for 10 minutes and aspiration of the liquid phase, the remaining pellets were counted to obtain the TCA-precipitable counts per 0.05 mL plasma. TCA nonprecipitable counts (degradation products of125I-rmTPO as free isotope) in the plasma were defined as the difference between total plasma cpm and TCA-precipitable cpm. The bound radioactivity per million PLT (cpm/million) was defined as the ratio between blood cell-associated radioactivity per milliliter of blood and PLT count × 10−6 per milliliter of blood, assuming blood cell-associated radioactivity was primarily PLT associated.23
Histopathology.
Zinc formalin-fixed, paraffin-embedded tissues were sectioned (5-μm thickness) and stained with hematoxylin and eosin. Histologic sections were examined by light microscopy with a Zeiss microscope (standard 16). In spleen and marrow sections, the number of megakaryocytes was evaluated using the 40× objective and 10× eyepiece (∼0.1 mm2 per field) with random selection and counting in a minimum of 40 fields. Megakaryocyte counts were performed by observers blinded to the different subgroups of animals. Counts are expressed as number of megakaryocytes per 10 high power fields (hpf).
Electron microscopic autoradiography.
Spleen and sternum collected at autopsy from both control and thrombocytopenic mice that received a single intravenous bolus injection of 125I-rmTPO were processed for electron microscopy autoradiography.23 28 Briefly, the tissues were cut into approximately 2-mm cubes, fixed in Karnovsky’s fixative (2% paraformaldehyde, 2.5% glutaraldehyde in cacodylate buffer), postfixed in osmium tetroxide, dehydrated, and embedded in Eponate 12 media (Ted Pella Inc, Redding, CA). Thin sections were coated with Ilford L4 EM autoradiography emulsion (Ilford, Warrington, PA) and exposed for 3 to 8 weeks. Developed sections were stained with lead citrate and uranyl acetate before observation in a CM12 Philips electron microscope (Philips Corp, Eindhoven, The Netherlands).
Statistical analysis.
All results are expressed as the mean values plus or minus standard deviation (SD) of three or more samples. The probability of significant differences when examining multiple treatments was determined using one-way analysis of variance (ANOVA) or analogous nonparametric tests (Kruskal-Wallis test), followed by Tukey’s or Dunn’s multiple comparison test to define unique subsets within the study. The correlation between two sets of variables was determined by linear correlation test (Pearson’s test). P values less than .05 were considered significant. Statistical analyses were performed using the Instat (Graphpad, San Diego, CA) or Sigmastat (Jandel Scientific, San Rafael, CA) statistical software programs.
RESULTS
PLT values in normal and thrombocytopenic mice.
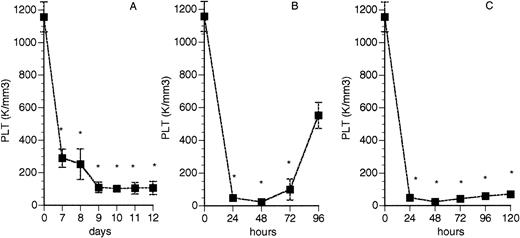
Control CD-1 mice had a mean PLT count of 1,157.5 ± 91.6 × 103/μL (n = 8). The posttreatment PLT counts from irradiated and 2 RAMPS mice are shown in Fig 1. Both radiation and RAMPS treatments resulted in severe thrombocytopenia. W/B F1 males at an age of about 4 months showed a marked reduction in PLT count (476.7 ± 260.94 × 103/μL; range, 836 to 232 × 103/μL) compared with those at an age of 1 month (1,105 ± 134 × 103/μL),29 which was also significantly lower than that of their nonthrombocytopenic female littermates (1,313.2 ± 287.2 × 103/μL, n = 11,P < .0001) and CD-1 controls (1,157.5 ± 91.64 × 103/μL, n = 8, P < .05).
PLT counts in thrombocytopenic CD-1 mice. Mice were treated with 550 cGy total body irradiation (A) or a single injection of 50 μL RAMPS (B) or injection of 50 μL RAMPS every other day (at 0, 48, and 96 hours) for a 5-day period (C). Values represent the mean ± SD for at least 3 animals in each group. *P < .05 when compared with day (hour) 0. K = 103.
PLT counts in thrombocytopenic CD-1 mice. Mice were treated with 550 cGy total body irradiation (A) or a single injection of 50 μL RAMPS (B) or injection of 50 μL RAMPS every other day (at 0, 48, and 96 hours) for a 5-day period (C). Values represent the mean ± SD for at least 3 animals in each group. *P < .05 when compared with day (hour) 0. K = 103.
Tissue distribution of 125I-rmTPO in normal and thrombocytopenic mice.
The in vivo tissue content of 125I-rmTPO in various organs of both control and thrombocytopenic mice is presented in Table 1. Tissue distribution of125I-rmTPO in both control groups was very similar to that reported previously.23 The whole blood and the spleen contained the most radioactivity, and greater than half of the radioactivity in the whole blood was due to 125I-rmTPO binding to washed blood cell fractions. Although radioactivity was found in all of the highly perfused tissues, the spleen was the only organ which contained a greater amount per gram than did blood (Table1).
Comparison of Tissue-Associated Radioactivity in Control and Thrombocytopenic Mice
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 8) . | Irradiated (n = 5) . | 1 d RAMPS (n = 6) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female Control (n = 3) . | |
| Femur | 14.9 ± 2.3 | 12.6 ± 2.7 | 24.8 ± 5.1*,† | 19.6 ± 6.5 | 15.8 ± 7.5 | 17.5 ± 3.3 |
| Heart | 17.6 ± 3.2 | 19.7 ± 3.0 | 15.9 ± 2.7 | 12.4 ± 2.8 | 14.9 ± 5.2‡ | 33.4 ± 6.5 |
| Kidney | 25.9 ± 2.6 | 31.2 ± 3.8 | 29.2 ± 5.0 | 22.6 ± 3.8 | 28.9 ± 4.5‡ | 40.4 ± 7.7 |
| Liver | 15.9 ± 2.2 | 17.1 ± 1.2 | 19.0 ± 3.5 | 14.7 ± 2.0 | 19.5 ± 4.75‡ | 28.6 ± 5.7 |
| Lung | 47.3 ± 9.7 | 43.7 ± 12.4 | 42.7 ± 13.6 | 33.7 ± 6.8 | 33.5 ± 10.5‡ | 63.3 ± 15.3 |
| Spleen | 160 ± 24.7 | 18.0 ± 3.01-153 | 65.1 ± 24.1 | 46.0 ± 11.7* | 43.9 ± 13.1‡ | 180.9 ± 51.7 |
| Sternum | 12.8 ± 3.9 | 13.4 ± 3.5 | 21.5 ± 4.9*,† | 17.2 ± 5.16 | 15.8 ± 7.6 | 17.3 ± 0.7 |
| Whole blood | 92.1 ± 9.7 | 131.3 ± 14.61-155 | 97.8 ± 22.0 | 74.0 ± 15.9† | 69.9 ± 17.1‡ | 128.0 ± 18.3 |
| Washed cells | 49.9 ± 7.9 | 5.2 ± 1.5* | 3.6 ± 1.2* | 5.3 ± 1.6* | 16.6 ± 10.4‡ | 61.5 ± 16.8 |
| Plasma ppt | 35.1 ± 9.0 | 105.4 ± 12.5* | 72.0 ± 16.1* | 42.4 ± 9.6† | 38.8 ± 10.7 | 39.5 ± 11.0 |
| PLT nadir | 1,157.5 ± 91.6 | 105.7 ± 35.5 | 48.4 ± 14.3 | 75.2 ± 17.3 | 476.7 ± 260.9 | 1,313 ± 287 |
| Body weight | 27.0 ± 0.9 | 27.0 ± 0.6 | 26.5 ± 2.1 | 27.0 ± 1.0 | 31.0 ± 2.2 | 26.8 ± 2.2 |
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 8) . | Irradiated (n = 5) . | 1 d RAMPS (n = 6) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female Control (n = 3) . | |
| Femur | 14.9 ± 2.3 | 12.6 ± 2.7 | 24.8 ± 5.1*,† | 19.6 ± 6.5 | 15.8 ± 7.5 | 17.5 ± 3.3 |
| Heart | 17.6 ± 3.2 | 19.7 ± 3.0 | 15.9 ± 2.7 | 12.4 ± 2.8 | 14.9 ± 5.2‡ | 33.4 ± 6.5 |
| Kidney | 25.9 ± 2.6 | 31.2 ± 3.8 | 29.2 ± 5.0 | 22.6 ± 3.8 | 28.9 ± 4.5‡ | 40.4 ± 7.7 |
| Liver | 15.9 ± 2.2 | 17.1 ± 1.2 | 19.0 ± 3.5 | 14.7 ± 2.0 | 19.5 ± 4.75‡ | 28.6 ± 5.7 |
| Lung | 47.3 ± 9.7 | 43.7 ± 12.4 | 42.7 ± 13.6 | 33.7 ± 6.8 | 33.5 ± 10.5‡ | 63.3 ± 15.3 |
| Spleen | 160 ± 24.7 | 18.0 ± 3.01-153 | 65.1 ± 24.1 | 46.0 ± 11.7* | 43.9 ± 13.1‡ | 180.9 ± 51.7 |
| Sternum | 12.8 ± 3.9 | 13.4 ± 3.5 | 21.5 ± 4.9*,† | 17.2 ± 5.16 | 15.8 ± 7.6 | 17.3 ± 0.7 |
| Whole blood | 92.1 ± 9.7 | 131.3 ± 14.61-155 | 97.8 ± 22.0 | 74.0 ± 15.9† | 69.9 ± 17.1‡ | 128.0 ± 18.3 |
| Washed cells | 49.9 ± 7.9 | 5.2 ± 1.5* | 3.6 ± 1.2* | 5.3 ± 1.6* | 16.6 ± 10.4‡ | 61.5 ± 16.8 |
| Plasma ppt | 35.1 ± 9.0 | 105.4 ± 12.5* | 72.0 ± 16.1* | 42.4 ± 9.6† | 38.8 ± 10.7 | 39.5 ± 11.0 |
| PLT nadir | 1,157.5 ± 91.6 | 105.7 ± 35.5 | 48.4 ± 14.3 | 75.2 ± 17.3 | 476.7 ± 260.9 | 1,313 ± 287 |
| Body weight | 27.0 ± 0.9 | 27.0 ± 0.6 | 26.5 ± 2.1 | 27.0 ± 1.0 | 31.0 ± 2.2 | 26.8 ± 2.2 |
Tissue-associated radioactivity is presented as cpm × 10−4/per gram (mL) of tissue (whole blood or plasma; mean ± SD). PLT nadir is presented as count × 10−3/mL (mean ± SD). Body weight is presented in grams (mean ± SD).
Abbreviation: plasma ppt, TCA-precipitable plasma-associated radioactivity.
P < .05 when compared with CD-1 control.
P < .05 when compared with irradiated.
P < .05 when compared with F1 female control.
P < .001 when compared with CD-1 control.
P < .01 when compared with CD-1 control.
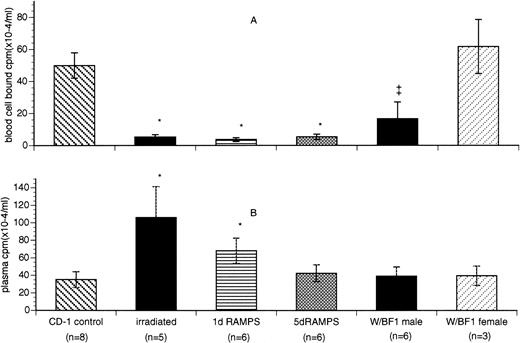
Significantly decreased blood cell-associated radioactivity, compared with those of controls, was observed in all 4 groups of thrombocytopenic mice, consistent with their corresponding degrees of thrombocytopenia (P < .05; Table 1 and Fig 2A). However, levels of plasma-associated radioactivity (TCA-precipitable cpm) among these groups differed significantly (Table 1 and Fig 2B). Irradiated thrombocytopenic mice had significantly elevated plasma associated radioactivity compared with control animals, the highest among all groups (P < .05). In marked contrast, the 5 d RAMPS mice and W/B F1 males had normal levels of plasma-associated radioactivity compared with controls. One d RAMPS mice had a plasma-associated radioactivity significantly higher than that of control (P < .05) but still much lower than that of irradiated mice (Table 1 and Fig 2B).
Comparison of blood cell-bound and plasma-associated radioactivity in control and thrombocytopenic mice. (A) Comparison of blood cell-bound radioactivity. (B) Comparison of plasma-associated radioactivity (TCA-precipitable cpm). Blood cell-bound radioactivity is presented as cpm × 10−4/mL blood (mean ± SD). Plasma-associated radioactivity is presented as cpm × 10−4/mL plasma (mean ± SD). *P < .05 when compared with CD-1 control. ‡P < .05 when compared with F1 female control.
Comparison of blood cell-bound and plasma-associated radioactivity in control and thrombocytopenic mice. (A) Comparison of blood cell-bound radioactivity. (B) Comparison of plasma-associated radioactivity (TCA-precipitable cpm). Blood cell-bound radioactivity is presented as cpm × 10−4/mL blood (mean ± SD). Plasma-associated radioactivity is presented as cpm × 10−4/mL plasma (mean ± SD). *P < .05 when compared with CD-1 control. ‡P < .05 when compared with F1 female control.
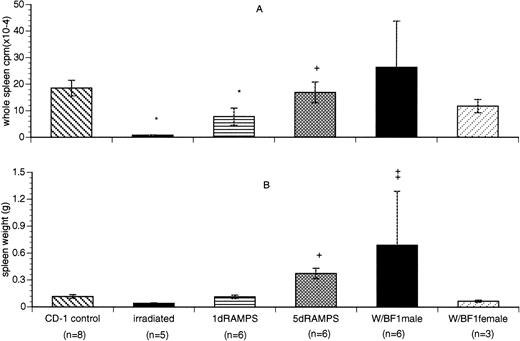
The tissue distribution pattern of 125I-rmTPO in the irradiated group was very similar to that observed in c-mpl−/− mice.20 Irradiated mice had the lowest spleen-associated radioactivity normalized by weight among the various mice (11% of the normal CD-1 control value,P < .001; Table 1) and, due to the reduced spleen weight in this group (Fig 3B), also had whole spleen-associated radioactivity that was only 3.9% of the control group (P < .05; Fig 3A). Conversely, the spleen-associated radioactivities of all 3 groups of mice with immune thrombocytopenia were much higher than that of the irradiated group when normalized for tissue weight (Table 1). Both 5 d RAMPS and W/B F1 male groups had significantly increased spleen size and weight (P< .05), contributing to a whole spleen-associated radioactivity that was either comparable to (5 d RAMPS) or higher than (W/B F1male) that of the controls (Fig 3). Whole spleen-associated radioactivity in the 5 d RAMPS mice was also significantly higher than that of irradiated mice (P < .05). The 1 d RAMPS mice with normal spleen weights, again, had a whole spleen-associated radioactivity intermediate to that of irradiated and 5 d RAMPS mice (Fig 3). The levels of whole blood-associated radioactivity varied significantly among the thrombocytopenic groups (Table 1). To account for possible differences in the amount of radioactivity associated with blood trapped within the tissue, whole spleen-associated radioactivity was also expressed as the percentage of the whole blood-associated radioactivity. This further reduced the value for the irradiated group to only 2.5% of the control. Conversely, the value for the 5 d RAMPS group was increased to 22% higher than that of the control (data not shown). However, the statistical significance of the normalized differences comparing the thrombocytopenic groups with controls or with each other (data not shown) remained the same as the results described in Fig 3.
Comparison of whole spleen-associated radioactivity in control and thrombocytopenic mice. (A) Comparison of whole spleen-associated radioactivity in control and thrombocytopenic mice. (B) Comparison of the spleen weight among control and thrombocytopenic mice. Whole spleen-associated radioactivity is presented as cpm × 10−4 (mean ± SD). Spleen weight is presented in grams (mean ± SD). *P < .05 when compared with CD-1 control. †P < .05 when compared with irradiated. ‡P< .05 when compared with F1 female control.
Comparison of whole spleen-associated radioactivity in control and thrombocytopenic mice. (A) Comparison of whole spleen-associated radioactivity in control and thrombocytopenic mice. (B) Comparison of the spleen weight among control and thrombocytopenic mice. Whole spleen-associated radioactivity is presented as cpm × 10−4 (mean ± SD). Spleen weight is presented in grams (mean ± SD). *P < .05 when compared with CD-1 control. †P < .05 when compared with irradiated. ‡P< .05 when compared with F1 female control.
Femur- and sternum-associated radioactivity in the RAMPS and W/B F1 male mice, whether normalized by weight (Table 1) or whole bone associated (data not shown), was also higher than in irradiated mice. Femur and sternum weights varied only slightly among different mice groups (∼10% of the total bone weight; data not shown). Tissue/blood-associated radioactivity of the sternum was decreased and femur was significantly decreased in the irradiated group, whereas both were significantly increased in 1 d and 5 d RAMPS (P < .001) versus the CD-1 control animals (Table 2). The W/B F1 male group, which did not develop thrombocytopenia as severe as the RAMPS mice, also had increased tissue/blood-associated radioactivity in both the sternum and femur compared with their female controls (22.5± 4.4 v 13.6 ± 0.3%; 22.4% ± 4.4%v 13.7% ± 1.55%; P = not significant; Table 2).
Comparison of Bone/Blood-Associated Radioactivity in Control and Thrombocytopenic Mice
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 8) . | Irradiated (n = 5) . | 1 d RAMPS (n = 6) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female Control (n = 3) . | |
| Femur | 15.7 ± 3.4 | 9.6 ± 1.5* | 25.5 ± 3.1† | 26.3 ± 6.1† | 22.3 ± 7.0 | 13.8 ± 2.3 |
| Sternum | 13.1 ± 3.9 | 10.4 ± 3.1 | 22.1 ± 2.3† | 23.1 ± 3.3† | 22.2 ± 7.7 | 13.7 ± 1.5 |
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 8) . | Irradiated (n = 5) . | 1 d RAMPS (n = 6) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female Control (n = 3) . | |
| Femur | 15.7 ± 3.4 | 9.6 ± 1.5* | 25.5 ± 3.1† | 26.3 ± 6.1† | 22.3 ± 7.0 | 13.8 ± 2.3 |
| Sternum | 13.1 ± 3.9 | 10.4 ± 3.1 | 22.1 ± 2.3† | 23.1 ± 3.3† | 22.2 ± 7.7 | 13.7 ± 1.5 |
Femur and sternum associated radioactivity is expressed as the percentage of whole blood-associated radioactivity (cpm per gram of tissue/cpm per mL blood × 100%, mean ± SD).
P < .05 when compared with CD-1 control.
P < .01 when compared with CD-1 control.
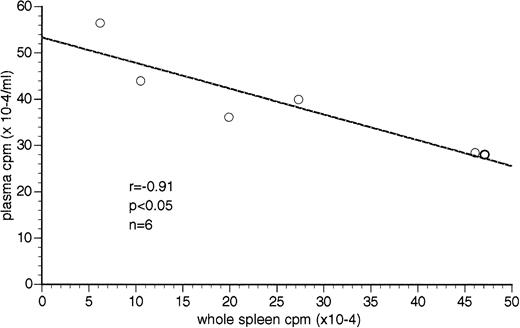
The plasma-associated radioactivity appears to be inversely related to the whole spleen-associated radioactivity (r = −.9106,P < .05, n = 6) in the W/B F1 male mice (Figs 2B,3A, and 4). None of the other soft organ-associated radioactivity, whether normalized by weight or whole organ-associated radioactivity, had a significant inverse correlation with plasma-associated radioactivity (data not shown).
Inverse correlation between plasma-associated radioactivity (TCA-precipitable cpm) and whole spleen-associated radioactivity in W/B F1 male mice. Plasma-associated radioactivity is presented as cpm × 10−4/mL of plasma. Whole spleen-associated radioactivity is presented as cpm × 10−4. A significant correlation between plasma and whole spleen-associated radioactivity was found (r = −.91,P < .05) using Pearson correlation analysis.
Inverse correlation between plasma-associated radioactivity (TCA-precipitable cpm) and whole spleen-associated radioactivity in W/B F1 male mice. Plasma-associated radioactivity is presented as cpm × 10−4/mL of plasma. Whole spleen-associated radioactivity is presented as cpm × 10−4. A significant correlation between plasma and whole spleen-associated radioactivity was found (r = −.91,P < .05) using Pearson correlation analysis.
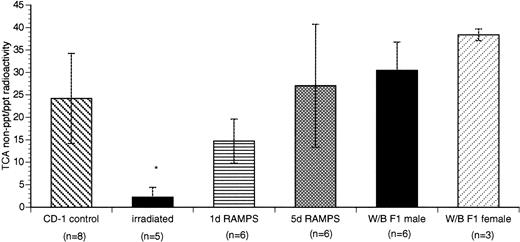
We also compared the ratio between plasma TCA nonprecipitable radioactivity (representing low molecular weight degradation products of 125I-rmTPO) and TCA-precipitable radioactivity (representing intact 125I-rmTPO) among the 6 different groups of mice (Fig 5). Whereas 5 d RAMPS and W/B F1 mice had ratios comparable to those of their corresponding controls (Fig 5), the irradiated group had a significantly lower ratio of plasma TCA nonprecipitable radioactivity versus TCA-precipitable radioactivity (P < .05; Fig 5). The ratio of 1 d RAMPS was, again, intermediate among the 4 groups of thrombocytopenic mice (Fig 5).
Comparison of degraded versus intact125I-rmTPO in the plasma of control and thrombocytopenic mice. Data are presented as the ratio between TCA nonpreciptable radioactivity and TCA-precipitable radioactivity in plasma (mean ± SD). *P < .05 when compared with CD-1 control.
Comparison of degraded versus intact125I-rmTPO in the plasma of control and thrombocytopenic mice. Data are presented as the ratio between TCA nonpreciptable radioactivity and TCA-precipitable radioactivity in plasma (mean ± SD). *P < .05 when compared with CD-1 control.
Megakaryocytes in the spleens and marrows of control and thrombocytopenic mice.
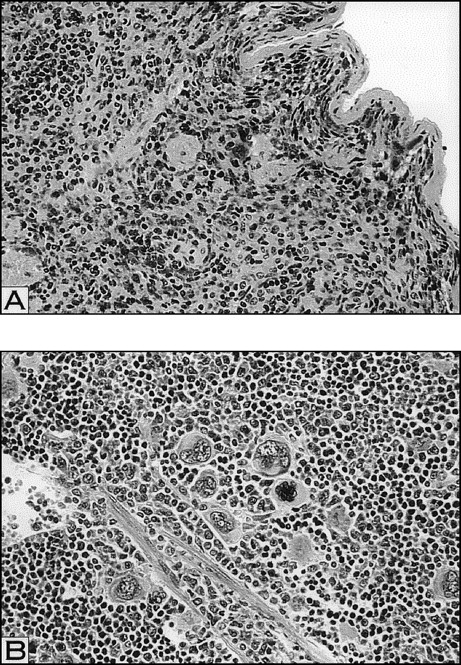
We next examined the number and morphology of megakaryocytes within the spleens and marrows of the various thrombocytopenic mice. Compared with the control spleen samples, the spleens of the irradiated groups were hypocellular. Thorough examination of the entire spleen sections from the irradiated mice demonstrated very few, if any, visible megakaryocytes (Fig 6 and Table 3). In contrast, examination of spleen sections taken from both 5 d RAMPS and W/B F1 males showed a substantial increase in overall cellularity and megakaryocyte numbers compared with controls (Fig 6 and Table 3). This, together with their larger megakaryocyte size and increased spleen weight (Fig 3), indicated that megakaryocyte mass in the entire spleens of these mice was the highest among the thrombocytopenic mice. Examination of the spleens from 1 d RAMPS mice showed a slightly increased number of megakaryocytes per 10 hpf compared with that of controls (Table 3). The number of megakaryocytes per field within the marrow of the control mice was markedly higher than that of the spleen. Megakaryocytes within the marrow of 5 d RAMPS and W/B F1 males were also approximately 90% and 75% greater in number, respectively, and larger in size than controls (data not shown), consistent with earlier studies,25 26 whereas megakaryocyte numbers were only approximately 40% higher in 1 d RAMPS mice than in controls (data not shown). Although megakaryocytes could be observed in some portions of the marrow in the irradiated mice, the overall number was reduced compared with controls (data not shown). In general, changes in the marrow megakaryocyte mass of thrombocytopenic mice seemed to be less pronounced than in the spleens.
Histopathological analysis of megakaryocytes in spleens of irradiated (A) and 5 d RAMPS mice (B). Megakaryocyte numbers appear markedly decreased in irradiated mice, whereas the 5 d RAMPS mice appear to have marked increases in the spleen sections. Increased numbers of megakaryocytes appear associated with increased size (hematoxylin and eosin; original magnification × 100).
Histopathological analysis of megakaryocytes in spleens of irradiated (A) and 5 d RAMPS mice (B). Megakaryocyte numbers appear markedly decreased in irradiated mice, whereas the 5 d RAMPS mice appear to have marked increases in the spleen sections. Increased numbers of megakaryocytes appear associated with increased size (hematoxylin and eosin; original magnification × 100).
Megakaryocyte Profile in the Spleen of Control and Thrombocytopenic Mice
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 5) . | Irradiated (n = 5) . | 1 d RAMPS (n = 5) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female Control (n = 3) . | |
| Megakaryocytes (per 10 hpf) | 10.2 ± 1.4 | 0.5 ± 0.73-150 | 14.6 ± 2.8 | 29.8 ± 8.13-150,3-151 | 20.9 ± 4.63-152 | 6.32 ± 2.45 |
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 5) . | Irradiated (n = 5) . | 1 d RAMPS (n = 5) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female Control (n = 3) . | |
| Megakaryocytes (per 10 hpf) | 10.2 ± 1.4 | 0.5 ± 0.73-150 | 14.6 ± 2.8 | 29.8 ± 8.13-150,3-151 | 20.9 ± 4.63-152 | 6.32 ± 2.45 |
Data were determined from histologic sections at 400× magnification with a minimum of 40 hpf counted per mouse (mean ± SD).
P < .05 when compared with CD-1 control.
P < .05 when compared with irradiated.
P < .05 when compared with F1 female control.
Electron microscopic autoradiograph.
The localization of 125I-rmTPO retained in the spleen and sternum of control and thrombocytopenic mice was analyzed using ultrastructural autoradiography. Autoradiographic silver grains indicating the presence of 125I-rmTPO were found associated mainly with PLTs in the spleen and megakaryocytes in the marrow of W/B F1 female control mice, consistent with a previous report.23 Close examination of the autoradiographs indicated that 125I-rmTPO had been internalized by the PLTs (data not shown). In W/B F1 male mice,125I-rmTPO was also associated with the PLTs and the megakaryocytes in the spleen and marrow. The labeled PLTs could be found within splenic macrophages (data not shown). The spleens of irradiated mice contained no morphologically identifiable PLTs or megakaryocytes and, as expected, only background levels of autoradiographic silver grains were found in these spleens (data not shown). 125I-rmTPO could not be specifically detected in neutrophils, lymphocytes, erythrocytes, stromal cells, or endothelial cells (data not shown).23
Relative PLT 125I-rmTPO binding capacity of control and thrombocytopenic mice.
To test the hypothesis that accelerated thrombopoiesis in ITP mice could lead to the formation of abnormally large PLTs, as in human ITP,30 and that these larger PLTs may bind and take up more TPO than those of controls,13 the mean PLT volumes of the control, irradiated, and various immune thrombocytopenic mice were measured. The mean PLT volumes in all 3 groups of immune thrombocytopenic mice, but not in the irradiated mice, were indeed significantly higher than that of the control (P < .05; Table 4). We further compared the bound radioactivity per million PLT of each of these groups. Bound radioactivity per million PLT was significantly higher in the 2 RAMPS groups compared with the control (P < .05; Table 4). Similar to spleen and bone, PLTs in W/B F1 male mice also seemed to take up an amount of 125I-rmTPO comparable to that of controls (Table 4), even though the labeling levels of their whole blood and their nonhematopoietic tissues were only half of the female controls (Table 1).
Comparison of Bound Radioactivity Per Million PLT and Mean PLT Volume in Control and Thrombocytopenic Mice
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 8) . | Irradiated (n = 5) . | 1 d RAMPS (n = 6) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female (n = 3) . | |
| cpm/million PLT | 429 ± 63 | 490 ± 144.4 | 730.5 ± 274-150 | 761.2 ± 1154-150 | 348 ± 47.8 | 468.4 ± 128 |
| Mean PLT volume | 4.19 ± 0.25 (n = 7) | 6.05 ± 0.93 (n = 4) | 8.71 ± 0.814-150 (n = 9) | 8.5 ± 0.764-150 (n = 6) | 7.5 ± 1.04-150 (n = 6) | 4.5 ± 0.2 (n = 3) |
| . | CD-1 Mice . | W/B F1 . | ||||
|---|---|---|---|---|---|---|
| Control (n = 8) . | Irradiated (n = 5) . | 1 d RAMPS (n = 6) . | 5 d RAMPS (n = 6) . | Male (n = 6) . | Female (n = 3) . | |
| cpm/million PLT | 429 ± 63 | 490 ± 144.4 | 730.5 ± 274-150 | 761.2 ± 1154-150 | 348 ± 47.8 | 468.4 ± 128 |
| Mean PLT volume | 4.19 ± 0.25 (n = 7) | 6.05 ± 0.93 (n = 4) | 8.71 ± 0.814-150 (n = 9) | 8.5 ± 0.764-150 (n = 6) | 7.5 ± 1.04-150 (n = 6) | 4.5 ± 0.2 (n = 3) |
Bound radioactivity per million PLT (cpm/million) is defined as described in the text (mean ± SD). Mean PLT volume is presented as cubic microns (mean ± SD).
P < .05 when compared with control.
DISCUSSION
We hypothesized that differences between the circulating TPO levels in thrombocytopenia secondary to megakaryocyte hypoplasia versus shortened platelet life span are caused by differences in the total mass of residual Mpl+ cells and, consequently, total capacity of Mpl receptor-mediated TPO uptake and clearance during thrombocytopenia. To test this hypothesis, we compared the 125I-rmTPO uptake capacity and organ distribution in control, irradiated, and various forms of immune thrombocytopenic mice. As in thrombocytopenic humans, the level of plasma-associated 125I-rmTPO in mice with thrombocytopenia secondary to megakaryocytic hypoplasia (irradiated group) was significantly higher than that of the immune thrombocytopenic mice (Table 1 and Fig 2B), despite the absence of significant differences in the PLT counts or the blood cell/PLT-associated 125I-rmTPO between the irradiated and immunothrombocytopenic mice (Table 1 and Fig 2A). Among all of the tissues studied, only the spleen- and plasma-associated radioactivity levels were inversely correlated (r = −.91, P < .05; Fig 4). Bone-associated radioactivity was also higher in immune thrombocytopenic mice than in irradiated mice (Tables 1 and 2). In contrast, the amount of 125I-rmTPO in tissues other than spleen and bone had no inverse correlation with the125I-rmTPO levels in plasma and was generally proportional to the corresponding whole blood labeling levels: higher in the irradiated group and lower in the 5 d RAMPS and W/B F1 male mice compared with that of the controls (Table 1). These results suggest that, despite significant thrombocytopenia, specific uptake of125I-rmTPO still occurs in the spleen and bone of the immune thrombocytopenic but not of the irradiated mice.
The specific uptake of 125I-rmTPO in the hematopoietic tissues of immune thrombocytopenic mice appeared to be associated with a higher megakaryocyte mass when the tissue samples were examined using light microscopy (Table 3). Electron microscopic autoradiography further confirmed the binding and internalization of125I-rmTPO by megakaryocytes and PLTs in the spleens and marrows of the immune thrombocytopenic mice (data not shown). Labeled PLTs were also found engulfed by splenic macrophages (data not shown). However, the spleen of irradiated mice contained no morphologically identifiable PLTs or megakaryocytes. Although it has been previously suggested that other cells, such as endothelial and stromal cells, of hematopoietic tissue may be involved in the regulation of circulating TPO levels in ITP,31 32 our data did not suggest a significant association between these cells and the uptake of125I-rmTPO (data not shown). Taken together, these results suggest that higher Mpl+ cell mass, consisting of higher megakaryocyte mass and increased production of PLTs, which are rapidly cleared by reticulo-endothelial destruction, seems to be responsible for the higher hematopoietic tissue-based uptake of125I-rmTPO in 5 d RAMPS and W/B F1 male mice compared with irradiated mice.
In addition to higher hematopoietic tissue-based uptake, our data also indicated significantly greater 125I-rmTPO uptake per million circulating PLT in the 2 RAMPS groups compared with the control (Table 4). PLTs in W/B F1 male mice also seemed to take up more 125I-rmTPO than expected (Tables 1 and 4). Because all of these mice also had higher mean PLT volumes, these results seem to be consistent with the previous speculation13 that larger PLTs, also frequently observed during human acute ITP, may take up more TPO and, thus, contribute more to the regulation of circulating TPO levels than their absolute number would indicate. However, further comparison of the number and affinity of TPO receptor sites present on normal PLTs and the larger PLTs from immune thrombocytopenic subjects is needed to verify this hypothesis.
If the lower circulating levels of 125I-rmTPO in ITP models are indeed associated with higher total mass of Mpl+ cells compared with the irradiated mice, one would expect this mechanism to be independent of the process that triggers an increase in Mpl+ cell mass. Our data are consistent with such a mechanism. Despite differences in the cause of immune thrombocytopenia between the 5 d RAMPS and W/B F1 males, both groups had the highest total mass of Mpl+ cells and the lowest circulating125I-rmTPO among all groups of thrombocytopenic mice. Conversely, although both 1 d and 5 d RAMPS had the same type of immune thrombocytopenia after receiving the same antibody treatment, 1 d RAMPS mice only had intermediate levels of Mpl+ cells and circulating 125I-rmTPO levels to those of the irradiated and 5 d RAMPS mice. These results, together with the findings that the tissue distribution pattern of 125I-rmTPO in the irradiated group was very similar to that observed in c-mpl−/− mice (increased plasma-associated radioactivity, dramatically reduced blood cell as well as spleen-associated radioactivity,20 and significantly lower amounts of 125I-rmTPO degradation products), are all consistent with the hypothesis that a lack of Mpl+ cells in mice with thrombocytopenia secondary to megakaryocytic hypoplasia results in higher circulating TPO levels. Conversely, the Mpl+ cellular mass, and consequently TPO uptake and catabolic capacity, in steady-state immune thrombocytopenic mice (5 d RAMPS and W/B F1 male) remained significantly higher than that of megakaryocyte hypoplastic mice after a brief TPO surge (1 d RAMPS) in response to the initial thrombocytopenia that induced megakaryocytopoiesis and thrombopoiesis. This could account, at least in part, for the low levels of circulating TPO during the steady state of immune thrombocytopenia.
The relative significance of megakaryocyte versus PLT receptors for TPO clearance in immune thrombocytopenic mice remains unclear. Increased megakaryocyte mass in these mice suggests that megakaryocyte-associated TPO clearance may also be significantly increased. However, quantitative evaluation of 125I-rmTPO associated with the large number of megakaryocytes is needed to determine whether the increased uptake capacity is indeed quantitatively proportional to the increased cell mass in either pre–steady-state or steady-state ITP models. In the current study, the femur- and sternum-associated radioactivity in 1 d RAMPS mice was comparable to those of the 5 d RAMPS and W/B F1 male mice (Table 2). However, megakaryocytic mass within the marrow of those mice was greater than that of the 1 d RAMPS (data not shown). This raises the question of whether the greater number of Mpl receptor in steady-state ITP models25 may result in a shortage of the tracer and, therefore, less saturation of the available receptor by the labeled ligand, causing the bone-associated radioactivity in those mice to appear lower than might be expected. Techniques to scan through larger pieces of tissue, such as light microscopic autoradiography, may be helpful in future studies to verify this hypothesis.33 Furthermore, ITP in humans and mice is associated with accelerated new PLT formation as well as destruction compared with controls.34 It is possible that the kinetics of the TPO clearance process in immune thrombocytopenic humans and mice may be increased35 due to the higher turnover of PLTs in these hosts that could also account, in part, for the low circulating TPO levels. Because the current data represent only a glimpse of this highly dynamic process, PLT-associated TPO clearance over time is likely to be underestimated. Future studies are needed to quantitatively evaluate the kinetics of TPO clearance in immune thrombocytopenic versus control and irradiated models. Finally, our results are consistent with recent reports that splenic megakaryocytopoiesis in mice seems to be more responsive to induction or repression than that of the marrow under certain experimental conditions.11,36 However, these observations do not contradict other previous evidence demonstrating that the bone marrow, not the spleen, is probably the production site for the majority of megakaryocytes, as well as PLTs, in rodents.37 Because it has been previously shown that the control spleen contributes less than 2% of the total megakaryocyte mass,37 small changes in local marrow megakaryocyte production are undoubtedly greatly amplified throughout the total bone marrow. Therefore, although the megakaryocyte mass increase within the spleen of steady-state ITP models was higher than that of the marrow, the total bone marrow still probably produces a majority of megakaryocytes.
In summary, the data in this study provide evidence that, as in c-mpl−/− mice, decreased Mpl+cellular mass in mice with thrombocytopenia secondary to megakaryocytic hypoplasia (irradiated mice) is associated with higher circulating TPO levels, whereas the lack of an inverse correlation of circulating TPO with PLT counts during steady-state ITP may be due, at least in part, to its uptake and degradation by higher PLT turnover and the increased mass of megakaryocytes.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr Mitchell S. Cairo (Georgetown University) for his support, laboratory facilities, and helpful discussions; Drs Paul Fielder, Eric Stenfanich, Gilbert-Andre Keller, and Ramon Widmer (Genentech) for the generous gift of125I-rmTPO, assistance with electron microscopic autoradiography, and helpful discussions; Dr Yu Suen, Eva Knoppel, Azita Nourani, and Sara Fernandez for their technical expertise in animal experiments; Dr Mark Lones for evaluation and photography of the histological sections of murine hematopoietic tissues; Judy Petella for preparation of histopathological specimens; Lorie Higgins for scoring of megakaryocyte numbers; Dr Bruce Liming for assisting with the animal irradiation; Carmella van de Ven, Sandra Kulczyk, and Dr Francisco Bracho for helpful discussions; and Sally Anderson and Linda Rahl for editorial assistance in the preparation of this manuscript.
Supported by grants from the Pediatric Cancer Research Foundation, the Walden W. and Jean Young Shaw Foundation, and the Children’s Hospital of Orange County Research and Education Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mei Chang, PhD, Hematology/Oncology Research, Children’s Hospital of Orange County, 455 S Main St, Orange, CA 92868.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal