Abstract
Granulocyte colony-stimulating factor (G-CSF) enhances neutrophil functions in vitro and in vivo. It is known that neutrophil-derived products can alter the hemostatic balance. To understand whether polymorphonuclear leukocyte (PMN) activation, measured as PMN degranulation and phenotypical change, may be associated to hemostatic alterations in vivo, we have studied the effect of recombinant human G-CSF (rHuG-CSF) administration on leukocyte parameters and hemostatic variables in healthy donors of hematopoietic progenitor cells (HPCs). Twenty-six consecutive healthy donors receiving 10 μg/kg/d rHuG-CSF subcutaneously for 5 to 7 days to mobilize HPCs for allogeneic transplants were included in the study. All of them responded to rHuG-CSF with a significant white blood cell count increase. Blood samples were drawn before therapy on days 2 and 5 and 1 week after stopping rHuG-CSF treatment. The following parameters were evaluated: (1) PMN activation parameters, ie, surface CD11b/CD18 antigen expression, plasma elastase antigen levels and cellular elastase activity; (2) plasma markers of endothelium activation, ie, thrombomodulin (TM) and von Willebrand factor (vWF) antigens; (3) plasma markers of blood coagulation activation, ie, F1+2, TAT complex, D-dimer; and (4) mononuclear cell (MNC) procoagulant activity (PCA) expression. The results show that, after starting rHuG-CSF, an in vivo PMN activation occurred, as demonstrated by the significant increment of surface CD11b/CD18 and plasma elastase antigen levels. Moreover, PMN cellular elastase activity, which was significantly increased at 1 day of treatment, returned to baseline at day 5 to 6, in correspondence with the elastase antigen peak in the circulation. This change was accompanied by a parallel significant increase in plasma levels of the two endothelial and the three coagulation markers. The PCA generated in vitro by unstimulated MNC isolated from rHuG-CSF–treated subjects was not different from that of control cells from untreated subjects. However, endotoxin-stimulated MNC isolated from on-treatment individuals produced significantly more PCA compared with both baseline and control samples. All of the parameters were decreased or normal 1 week after stopping treatment. These data show that rHuG-CSF induces PMN activation and transiently affects some hemostatic variables in healthy HPC donor subjects. The clinical significance of these findings remains to be established.
GRANULOCYTE colony-stimulating factor (G-CSF) stimulates the proliferation and differentiation of hematopoietic progenitor cells (HPCs) and the release of mature granulocytes from bone marrow. It also induces mobilization of CD34+ HPCs in circulating blood.1,2 These properties justify the expanding use of recombinant human G-CSF (rHuG-CSF) in clinical conditions, including chronic idiopathic neutropenia, chemotherapy-induced myelosuppression, and mobilization of HPCs in the circulation with or without prior chemotherapy.3 Recently, it has been administered to normal subjects to mobilize (and collect) HPCs for allogeneic transplantation.3 4
Several studies in vitro5-7 and in vivo8 9 have documented the neutrophil-activating effect of G-CSF, indicating that it should be considered a potent immediate activator of mature circulating cells capable of priming respiratory burst, inducing the release of secretory vesicles and cytoplasmic granules, and modulating expression of surface adhesion molecules.
Upon activation, polymorphonuclear leukocytes (PMN) release reactive oxygen species and intracellular proteases that possess several activities on endothelial cells and platelets and may modify the hemostatic balance towards a prothrombotic state. In vitro experiments have indeed shown that leukocyte elastase and cathepsin G can induce detachment10,11 or even lysis12,13 of endothelial cells. In addition, independently from a cell-damaging effect, these proteases may modify endothelial cell function involved in thromboregulation. In this respect, PMN-derived proteases have been reported to prevent thrombin-induced prostacyclin production by endothelial cells14; cathepsin G has also been shown to induce the plasminogen activator inhibitor release by the endothelium.15 Proteolysis of components of the endothelial surface, namely heparan-sulphated proteoglycans16 or thrombomodulin,17 by elastase and cathepsin G also may contribute to impair the nonthrombogenicity of the endothelium. Furthermore, the potential thrombogenic effects of PMN-derived proteases include the direct potent platelet activation elicited by cathepsin G18,19 and the enhancing effect of elastase.20 Finally, besides the cellular effects, elastase can directly proteolyze and inactivate natural inhibitors of blood coagulation, including protein C,21 protein S,22 tissue factor pathway inhibitor,23antithrombin,24 and heparin cofactor II,25 thus impairing potent antithrombotic mechanisms.
In addition to endogenous products release, G-CSF–activated neutrophils present phenotype changes involving the expression of surface adhesion molecules,7-9 including integrins (eg, CD11b/CD18) and selectins (eg, L-selectin), which mediate PMN adhesion to endothelial cells and platelets.26,27 It has been shown that G-CSF can increase PMN adhesive function in vitro and in vivo.7,28,29 Besides other implications, this property is relevant for the in vivo biological activity of PMN-derived proteases. In fact, this phenomenon allows the formation of a close microenviroment in which these enzymes are released and protected against inhibitors and their interaction with the substrate facilitated.30-32
Taking into account all the observations reported above, we evaluated in an established in vivo model in which G-CSF injection in healthy humans results in neutrophil activation whether endothelial cell perturbance and imbalance of the hemostatic system may also occur. We report here on the evaluation of PMN activation status in vivo, as measured by degranulation parameters and phenotypical change, and a series of variables reflecting perturbance of the endothelium and activation of blood coagulation and monocytes in a group of healthy donors receiving rHuG-CSF to mobilize HPCs for allografting.
MATERIALS AND METHODS
Donor Subjects
Twenty-six consecutive healthy individuals (sex, 13 male and 13 female; median age, 40 years; age range, 8 to 61 years) received rHuG-CSF (Neupogen; Amgen-Roche, Basel, Switzerland) for HPC harvesting from peripheral blood and use for allogeneic transplant. Detailed written informed consent was obtained from each individual donor before starting the procedure. Results of an examination of medical history, physical examination, and routine laboratory investigation were normal in all subjects. These subjects were negative for previous history of thrombosis and other known risk factors for thrombosis. rHuG-CSF (10 μg/kg/d) was administered subcutaneously for 5 to 6 consecutive days; the daily dose was administered in two half doses, each administered every 12 hours. Leukapheresis was performed on a Cobe Spectre continous flow cell separator on day 5 or 6 of rHuG-CSF administration (when CD34+ cells were >0.5%). rHuG-CSF was stopped on the day of leukapheresis.
Thirty-one healthy non-HPC donor subjects (15 male and 16 female; median age, 40 years; age range, 13 to 64 years) acted as control group for the laboratory parameters studied.
Samples
Venous blood was collected into sterile siliconized tubes containing trisodium citrate (0.129 mol/L, 1:10 vol/vol). In selected patients, blood samples for the analysis of plasma multimeric vWF were also obtained in an anticoagulant and proteinase inhibitor mixture (0.125 mmol/L citrate, 5 mmol/L EDTA, 6 mmol/L n-ethilmaleimide, and 500 kIU/mL aprotinin). Blood samples were drawn before the morning injection of rHuG-CSF at the following time intervals: (1) before starting rHuG-CSF (time 0 = T0); (2) on the second consecutive day (time 1 = T1) of rHuG-CSF administration; (3) on the fifth or sixth consecutive day (time 2 = T2) of rHuG-CSF administration (corresponding to the day of leukapheresis); and (4) 1 week after leukapheresis and stopping rHuG-CSF (time 3 = T3).
Plasma was obtained by centrifugation of anticoagulated whole blood at 3,000g for 20 minutes at room temperature and stored in aliquots at −80°C until assays (within 2 months).
Whole blood was used for cytofluorimetric evaluation of PMN surface CD11b/CD18.
The PMN and mononuclear cell (MNC) fractions (for the analysis of PMN cellular elastase and MNC procoagulant activity [PCA], respectively) were prepared from citrated fresh venous blood immediately after venipuncture. Whole blood was layered onto Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient system and centrifuged at 1,500 rpm for 15 minutes at 4°C. The MNC fraction was recovered at the interface, whereas the PMN cell fraction and erythrocytes separated in the bottom pellet. Isolated MNC were washed, resuspended in RPMI 1640 (2 × 106 cells/mL) and used for the PCA assay. The pellet was washed and the PMN cell-enriched fraction was obtained after dextrane erythrocyte sedimentation. Isolated PMN were washed and resuspended in 10 mmol/L HEPES-Tyrode buffer, pH 7.4, containing 1% Triton X100. PMN lysates (by 3 cycles of freezing and thawing) were used for the elastase proteolytic activity assay. Cell viability was assessed by the trypan blue exclusion dye test and found to be greater than 90%.
Routine Hematological Assays
White blood cell (WBC) differential counts, hematocrit counts, hemoglobin counts, red blood cell (RBC) counts, and platelet counts were determined by automated methods by a NE800 Analyzer (Dasit, Milan, Italy).
Assays of PMN Activation
PMN membrane CD11b/CD18.
The expression of PMN CD11b/CD18 antigen was measured in whole blood by direct immunofluorescence using flow cytometry analysis and monoclonal antibodies (MoAbs). Cells were incubated with phycoerythrin (PE)-conjugated mouse antihuman CD11b MoAb (Becton Dickinson, Mountain View, CA) or with isotype-identical negative control MoAb (IgG2a PE; Becton Dickinson) for 30 minutes at 4°C in the dark. After erythrocyte lysis, samples were analyzed by a FACScan flow cytometer (Becton Dickinson). PMN were selectively gated using their forward and side scatter properties. The results were expressed as mean fluorescence units (MIF).
Plasma PMN elastase antigen.
This is the plasma marker to test the release of azurophil granule content. Elastase coupled to its natural inhibitor α1-antitrypsin (α1AT) was detected in plasma as elastase-α1AT complex by an immunoassay procedure (PMN Elastase IMAC; Merck, Darmstad, Germany).
PMN elastase proteolytic activity assay.
Elastase activity was measured in PMN lysates by monitoring the rate of release of p-nitroanilide from the specific chromogenic substrate N-Succinil-Ala-Ala-Val-p-nitroanilide (Sigma, St Louis, MO). Five microliters of a 100 mmol/L solution of the specific synthetic substrate dissolved in N-methylpirrolidinone (final concentration, 0.5 mmol/L) was added to the cell lysate and its cleavage was monitored spectrophotometrically by following the release of p-nitroanilide at 410 nm. The activity of the samples was then compared with a standard curve prepared with different concentrations of purified elastase.
Markers of Endothelial Cell Activation
The parameters of endothelium damage studied were the plasma concentrations of thrombomodulin (TM) and von Willebrand factor (vWF).33 TM is the endothelial membrane receptor for thrombin.34 vWF is a multimeric protein synthesized and released from endothelial cells, which are important for the mechanism of platelet adhesion to the vessel wall.35 The multimeric structure of vWF determines its biological activity, ie, high molecular weight (HMW) vWF multimers are functionally more potent than other forms of vWF.
Plasma TM antigen was quantitated by an enzyme-linked immunosorbent assay (ELISA) kit from Diagnostica Stago (Boheringer Mannheim, Milan, Italy) according to the manufacturer’s instructions.
Plasma vWF antigen was determined by an electro-immunodiffusion procedure using reagents from Diagnostica Stago (Assera-Plate vWF; Boheringer Mannheim). The values were expressed as the percentage of vWF by comparison with a standard curve obtained by serial dilutions of pooled normal plasma (undiluted plasma = 100% vWF).
In selected patients (n = 6) the multimeric pattern of vWF was analyzed by discontinuous sodium dodecyl sulfate (SDS) agarose gel electrophoresis [1.5% HGT(P)], as described,35 with minor modifications.36 Densitometric analysis to quantitate the multimers was performed with a computer-based digital image processing, by the software Image 1.55 (National Institutes of Health, Bethesda, MD). Low molecular weight (LMW) multimers were defined as the area of two peaks at the bottom of the gel, and HMW multimers were defined as the area after the first 10 peaks resolved. LMW and HMW were expressed as a percentage of the total area of sample. Studies of vWF fragments were also conducted, as previously described.37vWF was immunoisolated by anti-vWF MoAb (kindly provided by Dr Z.M. Ruggeri, The Scripps Research Institute, La Jolla, CA).
Markers of Hypercoagulation
As markers of in vivo clotting activation, the plasma levels of prothrombin fragment F1+2 (F1+2), thrombin-antithrombin (TAT) complex, and D-dimer were measured. These are enzyme-inhibitor complexes or byproducts of the coagulation reactions, liberated during clotting activation, which provide a biochemical tool for the definition of the hypercoagulable state and are modulated by therapy.38 39
Plasma F1+2 and TAT complex concentrations were determined by ELISA methods, using commercially available kits (Enzygnost F1+2 and Enzygnost TAT, respectively; Istituto Behring, Scoppito, Italy). Plasma D-dimer levels were determined by a commercial ELISA method (Asserachrom D-dimer; Boheringer Mannheim). Calibration was performed using the standards provided with the commercial kits.
PCA of MNC
The PCA expressed by circulating MNC, identified as tissue factor (TF), represented the parameter of white blood cell-mediated clotting activation. This activity plays an important role in physiological and pathological conditions.40
Isolated MNC from healthy donors (n = 6) and control subjects were washed and resuspended in RPMI 1640 (2 × 106cells/mL). Final cell preparation contained greater than 97% MNC and less than 1 platelet per nucleated cell. Monocytes were 12% to 30% (mean, 19%) of total MNC. PCA of MNC suspensions was measured immediately after isolation and after 4 hours of incubation at 37°C with or without stimulus (1 μg/mL Salmonella Enteritidislipopolysaccharide [LPS]) under an atmosphere consisting of 5% CO2. Cells were lysed by 3 cycles of freezing and thawing and PCA was measured by the one-stage recalcification assay of normal human plasma.41 Briefly, 0.1 mL of sample was added to 0.1 mL of citrated normal human plasma; after 1 minute of incubation at 37°C, 0.1 mL 0.025 mol/L CaCl2 was added and the clotting time was recorded. PCA activity was quantitated by reference to a calibration curve (log-log plot) constructed with different dilutions (from 10−1 to 10−6) of a standard rabbit brain thromboplastin (RBT; Sigma). Results were expressed as arbitrary units, with 1 unit equal to the activity of 1 mEq/mL RBT in the one-stage clotting assay. PCA was identified as TF by the clotting assay of factor VII-, VIII-, or X-deficient human plasmas (Istituto Behring). In some experiments, TF activity (TF:Act.) was further characterized by incubating the samples for 15 minutes at 37°C with an antihuman TF purified polyclonal IgG (1 mg/mL final concentration; American Diagnostica Inc, Greenwich, CT) before the clotting assay. A normal nonimmune rabbit IgG was the negative control in this assay.
Statistical Analysis
The Mann Whitney U-test was used to assess the significance of differences between the mean baseline values of the donors versus the untreated control group. To test the intragroup differences over time, the ANOVA one-way analysis of variance for repeated measures and Fisher’s test for multiple comparisons were used. Differences were considered significant at a P value less than .05.
Regression analysis was performed by the method of the least squares and the correlation coefficient (r) was calculated.
RESULTS
Table 1 shows the variations of the healthy donors’ hematological parameters at the different times of the study. At baseline (T0), before the start of rHuG-CSF treatment, none of the subjects had hematological abnormalities. Then, all of them responded to rHuG-CSF administration, with a significant increase in WBC count (on T1 and T2: P < .01 v T0). Accordingly, the circulating PMN fraction was significantly increased. Also, the monocytic fraction resulted increased. WBC, PMN, and monocytes were back to the basal values 1 week after HPC collection and rHuG-CSF discontinuation (T3). No significant modifications occurred to the other parameters during treatment, except for the median platelet count, which tended to decrease on T2, at the peak of rHuG-CSF–induced leukocytosis. After apheresis (T3), this reduction became statistically significant. Also at this time, the hematocrit, hemoglobin, and RBC counts were slightly, but significantly decreased compared with the baseline.
Effect of rHuG-CSF on Routine Hematological Parameters
| . | T0 . | T1 . | P<* . | T2 . | P<* . | T3 . | P<* . |
|---|---|---|---|---|---|---|---|
| WBC (×109/L) | 5.9 (4.3-10.3) | 28.35 (16.3-42.0) | .01 | 44.36 (22.6-74.63) | .01 | 4.8 (2.92-11.4) | NS |
| PMN (×109/L) | 3.1 (1.9-6.3) | 23.1 (13.4-33.2) | .01 | 37.6 (17.1-67) | .01 | 2.8 (1-6.6) | NS |
| Monocytes (×109/L) | 0.4 (0.1-1.0) | 1.2 (0.6-5.6) | .01 | 2.3 (1.2-6.0) | .01 | 0.2 (0.1-2.0) | NS |
| Hematocrit (%) | 42 (33.8-45.7) | 40.1 (33.5-47.0) | NS | 41.4 (34.3-48.0) | NS | 38.4 (30.3-48.1) | .01 |
| Hemoglobin (g/L) | 14.1 (11.5-16) | 14 (11.7-16.6) | NS | 13.8 (11.1-16.5) | NS | 13.0 (10.7-16.8) | .01 |
| RBC (×1012/L) | 4.72 (3.7-5.3) | 4.68 (3.99-5.22) | NS | 4.63 (3.66-5.25) | NS | 4.39 (3.41-5.5) | .01 |
| Platelets (×109/L) | 218 (146-296) | 205 (129-279) | NS | 199 (98-341) | NS | 189 (114-344) | .05 |
| . | T0 . | T1 . | P<* . | T2 . | P<* . | T3 . | P<* . |
|---|---|---|---|---|---|---|---|
| WBC (×109/L) | 5.9 (4.3-10.3) | 28.35 (16.3-42.0) | .01 | 44.36 (22.6-74.63) | .01 | 4.8 (2.92-11.4) | NS |
| PMN (×109/L) | 3.1 (1.9-6.3) | 23.1 (13.4-33.2) | .01 | 37.6 (17.1-67) | .01 | 2.8 (1-6.6) | NS |
| Monocytes (×109/L) | 0.4 (0.1-1.0) | 1.2 (0.6-5.6) | .01 | 2.3 (1.2-6.0) | .01 | 0.2 (0.1-2.0) | NS |
| Hematocrit (%) | 42 (33.8-45.7) | 40.1 (33.5-47.0) | NS | 41.4 (34.3-48.0) | NS | 38.4 (30.3-48.1) | .01 |
| Hemoglobin (g/L) | 14.1 (11.5-16) | 14 (11.7-16.6) | NS | 13.8 (11.1-16.5) | NS | 13.0 (10.7-16.8) | .01 |
| RBC (×1012/L) | 4.72 (3.7-5.3) | 4.68 (3.99-5.22) | NS | 4.63 (3.66-5.25) | NS | 4.39 (3.41-5.5) | .01 |
| Platelets (×109/L) | 218 (146-296) | 205 (129-279) | NS | 199 (98-341) | NS | 189 (114-344) | .05 |
Abbreviation: NS, not significant.
Anova one-way analysis of variance test.
PMN Activation Markers
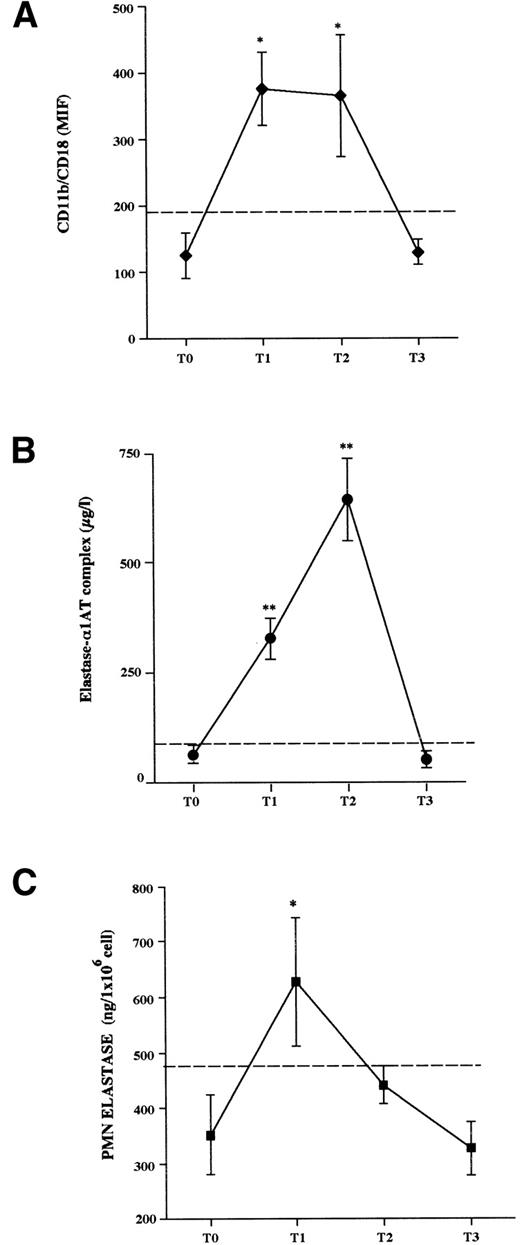
Cytofluorimetric analysis (Fig 1A).
The baseline expression of the surface β2 integrin CD11b/CD18 by donor PMN was not different from that of control subject PMN (donorv control, MIF values: 137.62 ± 21 v 125 ± 32;P = not significant [NS]). In response to rHuG-CSF, the expression of CD11b/CD18 by donor PMN significantly increased over the pretreatment values (T1 = 376 ± 55 MIF; and T2 = 366 ± 92 MIF;P < .05). However, 1 week after stopping rHuG-CSF (T3), the membrane CD11b/CD18 was decreased and returned to baseline (186 ± 20 MIF, P =NS).
Changes of PMN activation parameters in healthy HCP donors before (T0), during (T1 and T2), and 1 week after stopping (T3) rHuG-CSF administration. (A) Flow cytometry analysis of the expression of CD11b/CD18 on PMN cell surface; results are expressed as the mean intensity fluorescence (MIF). (B) Plasma concentration of elastase-1AT complex during rHuG-CSF administration. (C) Elastase activity of lysed PMN isolated from citrated whole blood. The results are the mean ± SEM. Dashed lines represent the cut-off of normal control values (mean + 2SD). Statistical analysis by ANOVA for repeated measures and Fisher’s test for multiple comparisons is shown. *P < .05, **P < .01 v T0.
Changes of PMN activation parameters in healthy HCP donors before (T0), during (T1 and T2), and 1 week after stopping (T3) rHuG-CSF administration. (A) Flow cytometry analysis of the expression of CD11b/CD18 on PMN cell surface; results are expressed as the mean intensity fluorescence (MIF). (B) Plasma concentration of elastase-1AT complex during rHuG-CSF administration. (C) Elastase activity of lysed PMN isolated from citrated whole blood. The results are the mean ± SEM. Dashed lines represent the cut-off of normal control values (mean + 2SD). Statistical analysis by ANOVA for repeated measures and Fisher’s test for multiple comparisons is shown. *P < .05, **P < .01 v T0.
Plasma elastase antigen levels (Fig 1B).
The concentration of this marker (at T0 = 59.9 ± 28.4 μg/L,P = NS v normal controls) increased after starting rHuG-CSF administration, peaking at T2 (635.5 ± 60.6 μg/L,P < .01). It decreased to 45.9 ± 27.7 μg/L after stopping the growth factor administration (T3 v T0:P = NS).
PMN cellular elastase activity (Fig 1C).
At T0, cellular elastase activity was in the normal control range values; rHuG-CSF administration resulted in a significant elevation of this activity from T0 to T1 (T1 v T0: 626 ± 116 v352 ± 72 ng/106 cells; P < .05). Thereafter, the enzyme activity started to decrease on T2, before leukapheresis (441 ± 35 ng/106 cells; T2 v T0,P = NS), and even more on T3 (327 ± 48 ng/106cells; T3 v T0, P = NS).
Endothelial Cell Markers
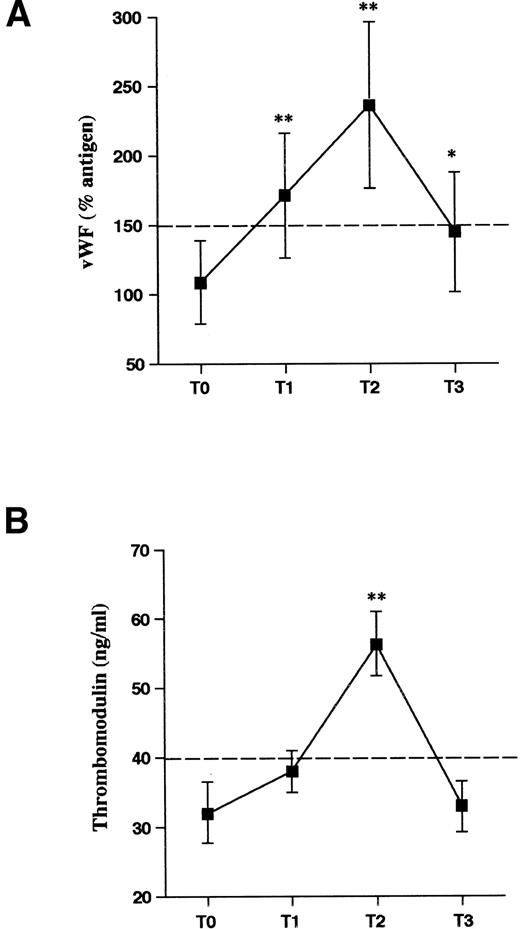
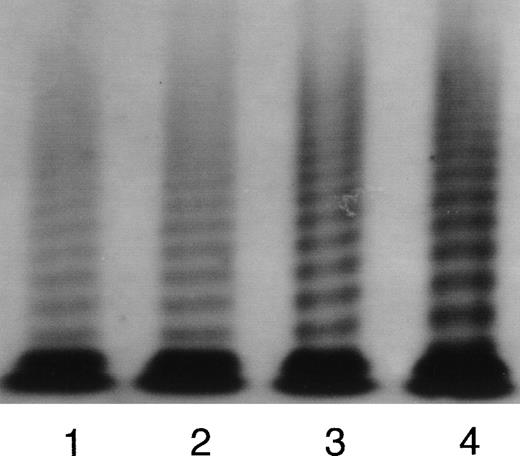
At T0, the levels of circulating endothelial cell markers of the donors were within the normal range values. A significant increment of TM and vWF was observed during rHuG-CSF treatment (Fig 2A and B). In both cases, the increments peaked at T2, before HPC apheresis (T2 v T0: TM = 65 ± 22 v 32 ± 12 ng/mL, P < .01; vWF:Ag = 241% ± 72% v 114% ± 26%, P < .01). At T3, both parameters were decreased back to the baseline values. In 6 subjects, the multimeric structure of vWF was analyzed at the different time intervals. vWF multimeric analysis showed a normal pattern of multimeric distribution as shown by autoradiography of a representative donor (Fig 3) and by densitometric analysis. The percentage of LMW multimer fraction was comparable in normal controls (21.6% ± 2.4%) and in donors on rHuG-CSF (T2: 21.7% ± 3.2%) as well as the area of HMW fraction (controls: 20.6% ± 2.1%; donors [T2]: 19.1% ± 1.8%). In the same plasma samples obtained during rHuG-CSF administration were found the 225-kD subunit as well as the normal 189-, 176-, and 140-kD fragments. No new fragments were detected. The proportion of the intact 225-kD subunit was comparable, at the different times, when calculated by densitometric analysis (T0: 71.4% ± 4.2%; T2: 67.2% ± 6.3%; T3: 71.5% ± 4.5%).
Markers of endothelial cell activation in HPC donors receiving rHuG-CSF. Plasma levels of vWF (A) and TM (B) were determined before (T0), during (T1 and T2), and 1 week after stopping (T3) rHuG-CSF administration. Mean values and SD are shown. Dashed lines represent the cut-off of normal control values. Statistical analysis as in Fig 1. *P < .05, **P < .01 v T0.
Markers of endothelial cell activation in HPC donors receiving rHuG-CSF. Plasma levels of vWF (A) and TM (B) were determined before (T0), during (T1 and T2), and 1 week after stopping (T3) rHuG-CSF administration. Mean values and SD are shown. Dashed lines represent the cut-off of normal control values. Statistical analysis as in Fig 1. *P < .05, **P < .01 v T0.
Representative autoradiographic image of vWF multimers from a subject receiving rHuG-CSF. The origin of the gel is at the top. Lane 1, normal plasma pool; lane 2, plasma from the subject before the rHuG-CSF administration (T0); lane 3, plasma from the subject after 2 days rHuG-CSF administration (T1); lane 4, plasma from the subject after 5 days rHuG-CSF administration (T2).
Representative autoradiographic image of vWF multimers from a subject receiving rHuG-CSF. The origin of the gel is at the top. Lane 1, normal plasma pool; lane 2, plasma from the subject before the rHuG-CSF administration (T0); lane 3, plasma from the subject after 2 days rHuG-CSF administration (T1); lane 4, plasma from the subject after 5 days rHuG-CSF administration (T2).
Plasma Markers of Hypercoagulation
HPC donors showed pretreatment normal values of plasma F1+2, TAT complex, and D-dimer. During the days of rHuG-CSF administration, a gradual increment of these markers was observed (Fig 4A, B, and C). On T2, the levels of all three parameters were all significantly greater as compared with T0 (T2 v T0: F1+2 = 2.17 ± 0.39 v 0.95 ± 0.09 nmol/L, P < .01; TAT complex = 4.72 ± 0.39 v 3.55 ± 0.25 ng/mL, P < .05; D-dimer = 0.37 ± 0.04v 0.29 ± 0.04 μg/mL; P < .05). However, the increment of the plasma D-dimer remained within the range of normal control values. On T3, the mean plasma levels of F1+2 and D-dimer were both decreased back to pretreatment values, whereas the TAT complex levels, although decreased, remained above the upper limit of normal control values.
Markers of hypercoagulation. Plasma levels of F1+2 (A), TAT complex (B), and D-dimer (C) before (T0), during (T1 and T2), and after the end (T3) of rHuG-CSF in HPC donors. Data are expressed as the mean values ± SEM. Dashed lines represent the cut-off of normal control values. Statistical analysis as in Fig 1. *P < .05, **P < .01 v T0.
Markers of hypercoagulation. Plasma levels of F1+2 (A), TAT complex (B), and D-dimer (C) before (T0), during (T1 and T2), and after the end (T3) of rHuG-CSF in HPC donors. Data are expressed as the mean values ± SEM. Dashed lines represent the cut-off of normal control values. Statistical analysis as in Fig 1. *P < .05, **P < .01 v T0.
Statistical Correlations
As shown in Table 2, at any time of observation there was a significant positive correlation between the plasma level of elastase-α1AT complex and the number of circulating PMN cells. Furthermore, the elastase-α1AT complex concentration positively correlated to the plasma levels of both endothelial markers, vWF and TM, and to the levels of F1+2. Significant positive correlations also were found between TM and vWF and F1+2 versus both TAT complex and D-dimer.
Correlations Between Plasma Elastase-1AT Complex Levels and (1) PMN Cell Count, (2) Plasma Markers of Endothelial Cell Damage (TM and vWF), and (3) Plasma Markers of Clotting Activation (F1 + 2, TAT Complex, and D-Dimer)
| Elastase Versus . | r . | P< . |
|---|---|---|
| PMN | .79 | .001 |
| vWF | .54 | .001 |
| TM | .50 | .001 |
| F1 + 2 | .40 | .01 |
| TAT | .11 | NS |
| D-dimer | .19 | NS |
| Elastase Versus . | r . | P< . |
|---|---|---|
| PMN | .79 | .001 |
| vWF | .54 | .001 |
| TM | .50 | .001 |
| F1 + 2 | .40 | .01 |
| TAT | .11 | NS |
| D-dimer | .19 | NS |
Abbreviations: r, correlation coefficient; NS, not significant.
MNC PCA
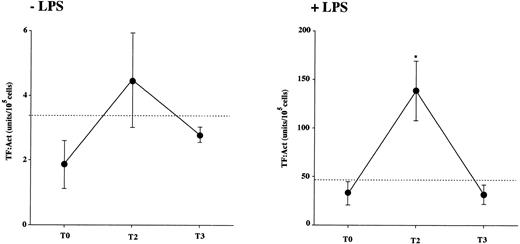
MNC were freshly isolated from 6 donor subjects on T0, T2, and T3. The PCA was evaluated before and after 4 hours of incubation with and without 1 μg/mL LPS. The results indicate that freshly isolated donors’ MNC as well as control MNC had virtually no PCA (not shown). After 4 hours of incubation at 37°C, in LPS-stimulated samples (+LPS; Fig 5, right panel) there was a significant increase in the PCA generated in vitro by donors’ MNC obtained at T2 (during rHuG-CSF administration), compared with that of MNC at T0 (before starting) and T3 (after stopping treatment). Instead, in the absence of stimulus (−LPS; Fig 5, left panel), there were no significant differences between the PCA of donor MNC from all of the time points.
MNC PCA. MNC were isolated from 6 healthy donors at baseline (T0), after 5 days of rHuG-CSF administration (T2), and 1 week after stopping the drug (T3). The dashed line indicates the mean PCA of MNC from the normal control subject group. The PCA of lysed MNC suspensions was tested after 4 hours of incubation with 1 μg/mL LPS (+LPS, right panel) or the vehicle (−LPS, left panel) by the one-stage recalcification assay of normal human plasma. Results are expressed as arbitrary units obtained from a calibration curve of standard thromboplastin (RBT, from 10−1 to 10−6); 1 unit equals the activity of 1 mEq/mL RBT in the one-stage clotting assay. PCA was identified as TF, as indicated in Materials and Methods. Statistical analysis as in Fig 1. *P < 0.05 v T0.
MNC PCA. MNC were isolated from 6 healthy donors at baseline (T0), after 5 days of rHuG-CSF administration (T2), and 1 week after stopping the drug (T3). The dashed line indicates the mean PCA of MNC from the normal control subject group. The PCA of lysed MNC suspensions was tested after 4 hours of incubation with 1 μg/mL LPS (+LPS, right panel) or the vehicle (−LPS, left panel) by the one-stage recalcification assay of normal human plasma. Results are expressed as arbitrary units obtained from a calibration curve of standard thromboplastin (RBT, from 10−1 to 10−6); 1 unit equals the activity of 1 mEq/mL RBT in the one-stage clotting assay. PCA was identified as TF, as indicated in Materials and Methods. Statistical analysis as in Fig 1. *P < 0.05 v T0.
DISCUSSION
A variety of functions of activated PMN can interfere with the hemostatic system. To test the hypothesis as to whether PMN activation is associated with hemostatic changes in vivo, we have analyzed in a group of healthy HPC donors receiving rHuG-CSF the PMN activation status, as measured by parameters of PMN degranulation and phenotypical change, simultaneously to a series of plasma variables reflecting ongoing endothelial and clotting activation.
First of all, besides the WBC count (which was significantly increased in all subjects), other hematological parameters, including hematocrit, hemoglobin, RBC, and platelets, were analyzed during treatment. Among them, only the median platelet count tended to decrease at the peak of rHuG-CSF–induced leukocytosis. After apheresis, the platelet reduction became statistically significant, and the hematocrit, hemoglobin, and RBC also appeared to be significantly reduced. This finding is in agreement with previous observations3 and is interpreted as a complication of large-volume leukapheresis.
The study of leukocytes confirmed the occurrence of activated PMN in the circulation of the healthy donors during treatment with rHuG-CSF. The PMN surface antigen CD11b/CD18 expression and the plasma elastase-α1AT complex levels were increased, as reported by others.3,9 In parallel, we also evaluated the elastase content of circulating neutrophils. Interestingly, this parameter significantly increased from baseline to the second day of treatment (T1), probably due to the presence of a large population of young neutrophils with no prior experience of activation into the circulation. Indeed, after 5 to 6 days of treatment (T2), when circulating elastase-α1AT complex levels were maximal, the cellular elastase was already reduced towards baseline, indicating that PMN had partially secreted their elastase content. This observation is in agreement with the hypothesis that elastase release by G-CSF–activated neutrophils requires an additional stimulus probably occurring in the vascular environment.9 An alternate explanation for the elastase content reduction at T2 might be the accelerated formation and transit of PMN from the marrow to the blood. However, this was ruled out because, in that case, the lowest levels of PMN elastase should have been found at T1, when the number of circulating PMN is increased about 7 times from the basal level (corresponding to the highest rate of PMN production), compared with T2 (when the number of PMN increases <2 times v T1) and with the basal (T0) pretreatment value (corresponding to a normal condition of PMN formation and transit). On the other hand, the finding that the PMN-associated elastase reaches the highest levels at T1 (concomitantly with the highest rate of PMN production) suggests rather that the fresh PMN population contains more elastase than the basal population. These considerations, together with the finding that the plasma levels of elastase-α1AT complex reach the maximal value at T2, support that the most plausible hypothesis to explain the observed results remains that the reduced levels of PMN-associated elastase at T2 result from PMN degranulation.
In the same subjects, we then have measured a series of plasma parameters to detect the occurrence of endothelial cell and coagulation system activation in vivo.
High levels of markers of endothelial cell damage/activation, TM and vWF, were found in the plasma, suggesting an in vivo endothelial cell dysfunction condition.33 TM can be synthesized also by other cells, including PMN42; therefore, a possible contribution of PMN to the increased TM plasma levels during G-CSF treatment cannot be completely excluded. However, PMN-associated TM would be in an intracellular compartement not under the regulation of secretory agonists. Moreover, it is not known whether G-CSF may stimulate the release of TM from PMN. In this study, the levels of TM paralleled the levels of circulating vWF, which is a more specific marker of endothelial perturbance and is 80% to 90% derived from the endothelium, with the remaining being from platelets.33 The positive correlation between the plasma levels of these variables is in favor of the hypothesis that endothelial cells are the common source of the two proteins. The levels of vWF and TM positively correlated also to the plasma levels of elastase. This type of study does not allow us to directly demonstrate a cause-effect relationship between neutrophil activation and endothelial cell dysfunction. However, on the basis of the in vitro results showing that elastase is able to stimulate vWF release13 and to remove proteolytically TM from endothelial surface,17 it is reasonable to speculate that this is the case. In this condition, other factors, such as the production and release of inflammatory cytokines (eg, tumor necrosis factor α),43-46 may also contribute to the increment of plasma vWF and TM levels, as well to the changes of other coagulation parameters. Finally, a direct effect of G-CSF on endothelial TM and vWF release could be hypothesized on the basis that endothelial cells express G-CSF receptors47; however, no studies have been conducted on the effect of G-CSF on these specific proteins. No alterations in the vWF subunits and fragments were found, indicating that, after secretion outside the cells, this factor is no further proteolyzed by neutrophil proteases. This is in agreement with the interpretation that neutrophil proteases may act on endothelial cell when released by adherent neutrophils, but are inactivated by inhibitors when released in the circulation. The normal multimeric pattern of the vWF protein also shows that in these subjects there is no increase of the HMW multimers with greater proadhesive and proaggregating activity.
According to the hypothesis that the hemostatic balance could be altered in this condition, we found that the markers of clotting activation (F1+2, TAT complex, and D-dimer) were increased during rHuG-CSF, with a time course superimposable to that of neutrophil activation markers. An increase of these markers shows increased thrombin generation (F1+2 and TAT complex) and fibrin formation (D-dimer) in vivo.38,39 The increments of these parameters from basaline values were moderate, but statistically significant. The D-dimer concentrations never exceeded the normal control cut-off value. Interestingly, only the F1+2 level positively correlated with the plasma elastase levels, suggesting that this parameter may be also a product of the lytic effect of elastase. However, the pattern of changes of the three markers during the observation period paralleled each other, with a positive correlation of the plasma F1+2 to the levels of both TAT and D-dimer. Particularly, the relation with the TAT complex concentration definitely provides evidence that part of F1+2 takes origin from the activation of the clotting cascade with actual thrombin formation. This is the first study using very sensitive markers to test the activation of blood coagulation in vivo in individuals receiving rHu-G-CSF. Other investigators have reported no changes in hemostatic variables during therapy with rHu-G-CSF.48 The discrepancy can be explained by various differences with our study. The type and the number of subjects analyzed were very different (6 cancer patients in remission, mostly with hematological malignancies). The types of parameters tested, with the exception of vWF antigen, were all different (ie, assays of coagulation factors activity, in vitro platelet aggregation, and analysis of platelet surface antigens). The dose of rHuG-CSF was not the same. Interestingly, in another study conducted on normal individuals receiving rHuG-CSF, increased in vitro platelet aggregation was documented.49
Finally, we have studied the PCA (TF) expressed by MNC, because this WBC property is relevant for the activation of the clotting system in humans.40 In addition, it is not known whether this activity is affected by G-CSF administration. We found that MNC of rHuG-CSF–treated subjects were primed to expose their TF, as an increase of the PCA generated in vitro upon LPS stimulus was recorded, whereas basal TF PCA was not modified. This effect on MNC PCA also terminated after the growth factor administration was stopped. This is the first report on an effect of G-CSF on monocyte PCA. Whether this effect is due to a direct or an indirect action of G-CSF is not established, with both possibilities being plausible. In fact, although no functional effect of G-CSF on monocytes has been described, a subset of these cells possess G-CSF receptor.50 The indirect effect may be hypothesized to take place in the network of cellular interactions occurring in the circulation or it may be induced by other cytokines, ie, tumor necrosis factor α, produced by activated PMN.43-46 Platelets also possess a functional G-CSF receptor that seems to transduce a priming effect and makes the platelets more responsive to additional agonists.51Although we did not directly investigate the activation status of platelets in the study subjects, it is possible that also platelets per se or via the interaction with neutrophils and monocytes19may contribute to the imbalance of the hemostatic system observed in this study.
In conclusion, this study provides the first evidence that the changes in the activation status of PMN observed in healthy donors receiving G-CSF are associated with changes of endothelial cell and clotting activation markers and a change of the procoagulant response of circulating MNC. The abnormalities of plasma markers and MNC PCA are transient and related to the drug administration. One week after the end of the therapy, all of the parameters were not significantly different from those before rHuG-CSF therapy.
No thrombotic events have been reported in normal subjects during G-CSF administration, suggesting that the transient, although significant, alteration of the hemostatic markers reported here does not indicate a real thrombotic risk for healthy persons. Recently, 2 cases of acute arterial thrombosis in cancer patients receiving rHuG-CSF have been reported.52,53 However, in malignancy, a number of other variables (eg, tumor cell procoagulants, cytokines, chemotherapy, etc.) can influence blood coagulation54 55 and contribute to the occurrence of thrombotic complications in course of G-CSF.
ACKNOWLEDGMENT
The authors are grateful to S. Bertoletti and A. Vignoli (Hematology Division, Ospedali Riuniti Bergamo, Bergamo, Italy) and to C. Rossi (Mario Negri Institute, Bergamo Laboratories, Bergamo, Italy) for technical assistance.
Supported in part by the Italian National Research Council (Convenzione CNR-Consorzio Mario Negri Sud). M.M. is a recipient of a fellowship of the Associazione Italiana Ricerca sul Cancro (AIRC).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A. Falanga, MD, Hematology Division, Ospedali Riuniti Bergamo, Largo Barozzi 1, 24100 Bergamo, Italy.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal