Abstract
This randomized study compared the number of leukaphereses required to collect an optimal target yield of 5 × 106CD34+ peripheral blood progenitor cells/kg, using either stem cell factor (SCF) at 20 μg/kg/d in combination with Filgrastim at 10 μg/kg/d or Filgrastim alone at 10 μg/kg/d, from 203 patients with high-risk stage II, III, or IV breast cancer. Leukapheresis began on day 5 of cytokine administration and continued daily until the target yield of CD34+ cells had been reached or a maximum of 5 leukaphereses performed. By day 5 of leukapheresis, 63% of the patients treated with SCF plus Filgrastim (n = 100) compared with 47% of those receiving Filgrastim alone (n = 103) reached the CD34+ cell target yield. There was a clinically and statistically significant reduction (P < .05) in the number of leukaphereses required to reach the target yield for the patients receiving SCF plus Filgrastim (median, 4 leukaphereses) compared with patients receiving Filgrastim alone (median, 6 or more leukapherses; ie, <50% of patients reached the target in 5 leukaphereses). All patients receiving SCF were premedicated with antihistamines, albuterol, and pseudoephedrine. Treatment was safe, generally well tolerated, and not associated with life-threatening or fatal toxicity. In conclusion, SCF plus Filgrastim is a more effective peripheral blood progenitor cell (PBPC)-mobilization regimen than Filgrastim alone. In addition to the potential for reduced leukapheresis-related morbidity and costs, SCF offers additional options for obtaining cells for further graft manipulation.
OVER THE PAST DECADE, peripheral blood progenitor cells (PBPCs) have replaced marrow as the major source of hematopoietic support, resulting in significantly faster rates of platelet and neutrophil engraftment after high-dose therapy for malignant diseases.1-3
The criteria defining an adequate or optimal leukapheresis product are of substantial interest to clinicians performing PBPC transplants. Although the number of mononuclear cells and/or colony-forming units granulocyte-macrophage (CFU-GM) were used previously to assess the adequacy of a PBPC product, the CD34+ cell content has recently evolved as the most consistent and clinically relevant measure available for this purpose.3-10 The methods for CD34+ cell analysis are variable, but published studies suggest that the vast majority of patients receiving PBPC autografts containing ≥5 × 106 CD34+ cells/kg experience prompt and durable platelet engraftment, whereas those receiving less than 1 to 2 × 106 CD34+cells/kg are at risk for delayed engraftment or, rarely, engraftment failure.3,6-10 Consequently, 5 × 106CD34+ cells/kg has been identified as an optimal target yield, associated with a high probability of rapid multilineage engraftment.3 6-8
Filgrastim (r-metHuG-CSF) is frequently used to mobilize hematopoietic progenitors from the marrow into the peripheral blood.1,2,4Patients then undergo leukaphereses until sufficient PBPCs for hematopoietic reconstitution are collected. The yields of CD34+ cells are adversely affected by factors including age, prior chemotherapy, prior radiation therapy, and tumor involvement in the bone marrow.3,4,6 To obtain sufficient CD34+ cells, multiple leukaphereses collections are often required. The morbidity and expense associated with leukapheresis have stimulated clinicians to develop strategies to improve PBPC mobilization and thereby reduce the number of leukaphereses performed. One such strategy involves the administration of chemotherapy plus cytokines, resulting in the collection of greater numbers of CD34+ cells than with either modality alone.11,12 Another approach is the use of multiple cytokines, such as Filgrastim combined with recombinant human stem cell factor (SCF; STEMGEN; US-adopted name, Ancestim)13-16; recombinant human Mpl ligands17,18; flt-3 ligand19; or interleukin-3 receptor ligands.20
The most extensively studied of these combinations is SCF plus Filgrastim. SCF is a cytokine that acts on primitive multilineage hematopoietic progenitor cells.21-23 In rats, dogs, and baboons the addition of species-specific SCF to granulocyte colony-stimulating factor (G-CSF) increases PBPC mobilization compared with G-CSF alone.24-28 In these animal models, the infusion of increased PBPC numbers resulted in more rapid engraftment after myeloablation.24,27 29
Recombinant human SCF has been shown in controlled clinical trials to enhance the Filgrastim-induced mobilization of CD34+ cells with and without chemotherapy.13,15,16 In a large, randomized phase 2 study of patients with breast cancer, Glaspy et al13 defined the optimal dose and schedule of SCF and Filgrastim when used in combination to mobilize PBPCs. The combination of SCF at 20 μg/kg/d plus Filgrastim at 10 μg/kg/d doubled the number of CD34+ cells that could be collected on days 5, 6, and 7 of cytokine administration, compared with Filgrastim alone. This study also supported 5 × 106 CD34+cells/kg as an optimal target yield. A central laboratory was used to determine CD34+ cell numbers, because standardization of target PBPC yields has been complicated by variation between laboratories performing CD34+ cell quantification.30 Infusion of greater than 5 × 106 CD34+ cells/kg resulted in rapid platelet engraftment (within 15 days) for all patients, whereas 23% of patients infused with less than 2 × 106 CD34+cells/kg experienced delayed platelet engraftment (≥28 days).
We report here a randomized phase 3 trial of breast cancer patients designed to determine whether the optimal target of 5 × 106 CD34+ cells/kg could be achieved with fewer leukaphereses when a combination of SCF plus Filgrastim was used for mobilization compared with Filgrastim alone.
MATERIALS AND METHODS
Patients.
The study protocol was approved by the Institutional Review Boards of the participating institutions, and all patients gave written informed consent before study enrollment. Patients with stage II or III breast cancer involving 10 or more axillary lymph nodes or chemotherapy-responsive metastatic disease were eligible for this study. Normal vital organ function and a Karnofsky performance status of ≥80% were required for study entry. Patients were excluded if breast cancer represented more than 10% of the overall cellularity of bilateral bone marrow biopsy specimens, as assessed by standard histological techniques. Patients were also excluded if they had received more than 12 cycles of prior chemotherapy, had documented brain metastases, or had a history of asthma or other significant IgE-mediated hypersensitivities.
Study design—Collection phase.
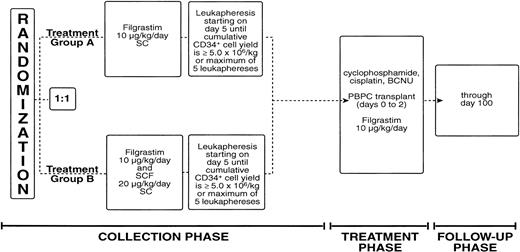
Eligible patients were randomized to receive either Filgrastim at 10 μg/kg/d alone or the combination of SCF at 20 μg/kg/d and Filgrastim at 10 μg/kg/d. The cytokine(s) was administered subcutaneously starting on day 1 and continued for up to 9 consecutive days (Fig 1). In the group that received both SCF and Filgrastim, the two cytokines were injected separately. Because of the potential for direct mast cell stimulation, all patients treated with SCF were to receive a premedication regimen consisting of diphenhydramine (50 mg orally every 6 hours), ranitidine (150 mg orally every 12 hours), two puffs of albuterol metered-dose inhaler before each injection, and pseudoephedrine (120 mg sustained release before each injection). Administration of diphenhydramine and ranitidine began 12 to 24 hours before the first dose of SCF and was timed so that a dose of all four medications was delivered approximately 1 hour before each injection of SCF. Diphenhydramine and ranitidine were administered for 48 hours after the last injection of SCF.
Patients eligible for study had baseline hematology and serum chemistry evaluations. Complete blood counts were evaluated daily until the final leukapheresis was performed. Leukapheresis began on day 5 of cytokine administration and was performed daily until either the optimal target of 5 × 106 CD34+ cells/kg was collected or a maximum of 5 leukaphereses was performed. Each leukapheresis processed approximately 10 L of blood using a Cobe Spectra apheresis device (COBE Laboratories, Lakewood, CO). The leukapheresis product was processed and cryopreserved on the day it was collected. If, after 5 leukaphereses, the cumulative CD34+ cell content of the PBPC graft was ≤1 × 106 CD34+ cells/kg, the patient was classified as a mobilization failure and withdrawn from the study.
Progenitor cell assays.
The CD34+ cell quantification was performed at a central laboratory (Cytometry Associates Inc, San Diego, CA) that was blinded to patient cytokine regimen. Samples obtained from each leukapheresis product were shipped immediately by courier to the central laboratory, where they were labeled with phycoerythrin (PE)-conjugated anti-CD34 (HPCA-2; Becton Dickinson, Mountain View, CA) and then analyzed for expression of CD34 using a FACScan (Becton Dickinson).
A bivariate plot of forward angle light scatter (FALS; ordinate) and orthogonal light scatter (SSC; abscissa) was created from the list mode data. A region was drawn on that plot that included all cells with the combined characteristics of lymphocytes. Within this region, the majority of cells with increased FALS and decreased SSC signal were included. A gate was then created from this region and applied to a bivariate plot of PE signal (ordinate) and SSC signal (abscissa). The cluster of the brightest CD34-staining cells was considered positive.
Study design—Transplant and follow-up.
Within 2 to 10 days of completing the leukapheresis procedures, patients were admitted to the hospital and treated with high-dose cyclophosphamide at 1,875 mg/m2/d administered intravenously over 1 hour on each of 3 days (days −5 through −3), cisplatin at 55 mg/m2/d administered by continuous intravenous infusion for 3 days (days −5 through −3), and carmustine at 600 mg/m2/d administered intravenously over 2 hours on day −2. After a 48-hour rest period, patients received an intravenous infusion of their PBPCs on days 0, 1, and 2, depending on their number of cryopreserved PBPC products. Patients received two PBPC products daily on days 0 and 1, and the fifth product was administered on day 2. Beginning on day 0, all patients received Filgrastim at 10 μg/kg/d, either by subcutaneous injection or intravenous infusion. Filgrastim therapy was continued until the absolute neutrophil count (ANC) was ≥5 × 109/L for 3 consecutive days or ≥10 × 109/L on one determination. Antibiotics, blood products, and intravenous fluids were administered according to institutional protocols. Complete blood counts were obtained daily until the ANC was ≥5 × 109/L and the platelet count (PLT) was ≥20 × 109/L and were obtained three times per week thereafter until the PLT was ≥50 × 109/L on two determinations separated by a minimum of 48 hours. All patients were eligible for discharge from the hospital when their ANC was ≥0.5 × 109/L and they were able to maintain adequate oral intake.
Study end-points and statistical analyses.
Primary end-points for this study included the number of leukaphereses required to reach an optimal target of 5 × 106CD34+ cells/kg, the number of days from the first day of PBPC infusion to neutrophil engraftment (≥0.5 × 109/L), and the number of days until transfusion-independent platelet engraftment (≥20 × 109/L). The safety profile of patients receiving SCF in combination with Filgrastim was compared with that of patients receiving Filgrastim alone.
The primary data set used for the analysis of this study included all patients who were randomized and underwent at least 1 leukapheresis (intent-to-treat). An efficacy-evaluable data set, from which patients with protocol violations were excluded, was also defined.
Efficacy analyses were performed on both the intent-to-treat and efficacy-evaluable data sets. All hypothesis testing and confidence interval estimates were two-sided and performed at a 5% significance level. All analyses were completed using SAS version 6.11 (SAS Institute Inc, Cary, NC) on a Sun Sparc Station, running under UNIX.
A Cox proportional hazards analysis was used to compare the number of leukaphereses required to reach the optimal target of 5 × 106 CD34+ cells/kg between treatment groups while controlling for a prospectively determined covariate, the number of cycles of prior chemotherapy. Values for patients whose leukapheresis schedule deviated from that of the protocol due to an adverse event were censored at ≥6 leukaphereses, regardless of the number of procedures performed. Values for all other patients who did not reach the target were censored at their last recorded leukapheresis.
The cumulative number and proportion of patients reaching the target yield were calculated by day using Kaplan-Meier methods. Odds ratios were calculated from the Kaplan-Meier event rates by day. The z-test was used to compare the total proportion of patients reaching the target by day 5 between the treatment groups. The rate of decrease in CD34+ cell yield over time was calculated for each treatment group to observe the comparative sustained yield. Also, for descriptive purposes, the total CD34+ cell yields were compared between treatment groups using the Wilcoxon rank sum test.
The number of days until neutrophil and platelet engraftment were assessed using Kaplan-Meier methods. Values for patients who failed to engraft were censored at their last recorded day or at day 100, whichever came first. Equivalence of engraftment was demonstrated using the Hodges-Lehmann median difference estimator and its corresponding 95% confidence interval estimate. The proportion of patients failing to achieve platelet engraftment within 14 days (and 28 days) of transplant was assessed, censoring all times greater than 14 days (and 28 days). These proportions are presented for patients transplanted with 1 to 2, 2 to 5, and greater than 5 × 106CD34+ cells/kg. The generalized Wilcoxon test was used to evaluate whether there was a significant difference between the proportion of patients failing to achieve platelet engraftment within 14 days (and 28 days) for those who yielded 2 to 5 × 106 CD34+ cells/kg versus those who yielded greater than 5 × 106 CD34+ cells/kg.
Safety.
During the collection phase, assessment of the safety profiles of SCF plus Filgrastim and Filgrastim alone included daily evaluation of vital signs, hematology laboratory values, and adverse events. Blood chemistry values were obtained before and upon completion of the collection phase.
During the treatment phase, assessment of safety after infusion of PBPCs mobilized by SCF plus Filgrastim or by Filgrastim alone included daily evaluation of vital signs during hospitalization, weekly monitoring of blood chemistry values until day 30 posttransplant, and frequent determination of hematology laboratory values (see section above entitled “Study design—Transplant and follow-up”). Long-term engraftment and patient survival were recorded at 60 and 100 days posttransplant.
Quality of life (QOL) study.
A companion study was conducted along with the clinical trial to assess patient QOL during PBPC mobilization and transplantation for both treatment groups. The total QOL study period for each patient was 160 days from the time of randomization. The QOL questionnaire consisted of questions selected from two generic instruments (SF-3631and EuroQOL32) and two disease-specific instruments (FACT-G33 and FACT-BMT34). The questionnaires were administered throughout the collection phase, during hospitalization, and at regular intervals during follow-up for a total of up to 20 planned assessments. Areas for assessment included general health perceptions, physical well-being, social well-being, functional well-being, emotional well-being, and relationship with physicians. In addition, questions specific to morbidity symptoms associated with bone marrow transplantation were included. The area under the curve (AUC) method was used for computing cumulative quality of life (AUC-QOL) scores over the entire study duration; these scores were divided by 160 days to obtain the daily QOL scores over the study duration.35 36
Study drug.
The SCF used in this trial was produced using genetically engineeredEscherichia coli, with retention of the initiating N-terminal methionine residue (Amgen Inc, Thousand Oaks, CA). The SCF was provided as a lyophilized powder that, after reconstitution, was administered at a concentration of 1.5 or 2.0 mg/mL. Filgrastim also was provided by Amgen Inc.
RESULTS
Collection phase—Groups analyzed.
Fourteen transplant centers participated in the study. A total of 203 breast cancer patients were randomized, received a cytokine(s), and underwent at least one leukapheresis (intent-to-treat data set); 103 received Filgrastim alone and 100 received the cytokine combination. The demographic and baseline disease characteristics are provided in Table 1. The two groups were evenly balanced for stage of disease, bone marrow involvement, and extent of prior treatment. There were protocol violations related to the primary end-points of the study for 28 patients. These patients were evenly balanced between the two treatment groups. Nine of the protocol violations were errors in patient enrollment (eg, recent or excessive prior chemotherapy), 14 were errors in following the protocol at study sites or at the central laboratory after patient enrollment (eg, leukapheresis stopped before target yield reached), and 5 were caused by logistical difficulties (eg, bad weather preventing further leukaphereses). When the protocol violations were excluded, 175 patients were evaluable for the primary study end-points (efficacy-evaluable data set), 90 in the Filgrastim alone group and 85 in the SCF plus Filgrastim group.
Patient Baseline Demographics and Disease Characteristics
| . | Filgrastim . | SCF + Filgrastim . | Total . | P Value . |
|---|---|---|---|---|
| Sex (n) | ||||
| Female | 103 | 100 | 203 | — |
| Age (yr) | ||||
| Median (min, max) | 45.5 (27.1, 62.6) | 46.4 (26.7, 63.1) | 46.1 (26.7, 63.1) | .492 |
| Weight (kg) | ||||
| Median (min, max) | 65.5 (42.0, 114.0) | 70.1 (37.0, 123.2) | 66.8 (37.0, 123.2) | .096 |
| Baseline hematology | ||||
| ANC (×109/L) | ||||
| Mean (SD) | 3.80 (1.60) | 3.52 (1.69) | 3.67 (1.65) | .052 |
| Median (min, max) | 3.73 (0.82, 11.75) | 3.11 (0.78, 11.47) | 3.38 (0.78, 11.75) | |
| PLT (×109/L) | ||||
| Mean (SD) | 252 (84) | 245 (96) | 249 (90) | .295 |
| Median (min, max) | 247 (98, 591) | 224 (128, 935) | 238 (98, 935) | |
| Bone marrow involvement (all <10% involvement) | ||||
| n (%) | ||||
| No | 92 (89.3) | 97 (97.0) | 189 (94.5) | .121 |
| Yes | 8 (7.8) | 3 (3.0) | 11 (5.5) | |
| Unknown | 3 (2.9) | 0 (0) | 3 (1.5) | |
| Prior cycles of chemotherapy | ||||
| Median (min, max) | 5 (2, 22) | 5 (2, 17) | 5.0 (2, 22) | .946 |
| Prior cycles of cyclophosphamide | ||||
| Median (min, max) | 4 (0, 19) | 4 (0, 13) | 4 (0, 19) | .658 |
| Prior radiotherapy | ||||
| n (%) | ||||
| Yes | 29 (28.2) | 27 (27.0) | 56 (27.6) | .855 |
| No | 73 (70.9) | 72 (72.0) | 145 (71.4) | |
| Unknown | 1 (1.0) | 1 (1.0) | 2 (1.0) | |
| Disease stage | ||||
| n (%) | ||||
| II | 10 (9.7) | 13 (13.0) | 23 (11.2) | .810 |
| IIIA | 24 (23.3) | 19 (19.0) | 43 (21.2) | |
| IIIB | 15 (14.6) | 14 (14.0) | 29 (14.3) | |
| IV | 54 (52.4) | 54 (54.0) | 108 (53.2) |
| . | Filgrastim . | SCF + Filgrastim . | Total . | P Value . |
|---|---|---|---|---|
| Sex (n) | ||||
| Female | 103 | 100 | 203 | — |
| Age (yr) | ||||
| Median (min, max) | 45.5 (27.1, 62.6) | 46.4 (26.7, 63.1) | 46.1 (26.7, 63.1) | .492 |
| Weight (kg) | ||||
| Median (min, max) | 65.5 (42.0, 114.0) | 70.1 (37.0, 123.2) | 66.8 (37.0, 123.2) | .096 |
| Baseline hematology | ||||
| ANC (×109/L) | ||||
| Mean (SD) | 3.80 (1.60) | 3.52 (1.69) | 3.67 (1.65) | .052 |
| Median (min, max) | 3.73 (0.82, 11.75) | 3.11 (0.78, 11.47) | 3.38 (0.78, 11.75) | |
| PLT (×109/L) | ||||
| Mean (SD) | 252 (84) | 245 (96) | 249 (90) | .295 |
| Median (min, max) | 247 (98, 591) | 224 (128, 935) | 238 (98, 935) | |
| Bone marrow involvement (all <10% involvement) | ||||
| n (%) | ||||
| No | 92 (89.3) | 97 (97.0) | 189 (94.5) | .121 |
| Yes | 8 (7.8) | 3 (3.0) | 11 (5.5) | |
| Unknown | 3 (2.9) | 0 (0) | 3 (1.5) | |
| Prior cycles of chemotherapy | ||||
| Median (min, max) | 5 (2, 22) | 5 (2, 17) | 5.0 (2, 22) | .946 |
| Prior cycles of cyclophosphamide | ||||
| Median (min, max) | 4 (0, 19) | 4 (0, 13) | 4 (0, 19) | .658 |
| Prior radiotherapy | ||||
| n (%) | ||||
| Yes | 29 (28.2) | 27 (27.0) | 56 (27.6) | .855 |
| No | 73 (70.9) | 72 (72.0) | 145 (71.4) | |
| Unknown | 1 (1.0) | 1 (1.0) | 2 (1.0) | |
| Disease stage | ||||
| n (%) | ||||
| II | 10 (9.7) | 13 (13.0) | 23 (11.2) | .810 |
| IIIA | 24 (23.3) | 19 (19.0) | 43 (21.2) | |
| IIIB | 15 (14.6) | 14 (14.0) | 29 (14.3) | |
| IV | 54 (52.4) | 54 (54.0) | 108 (53.2) |
Progenitor cell harvests.
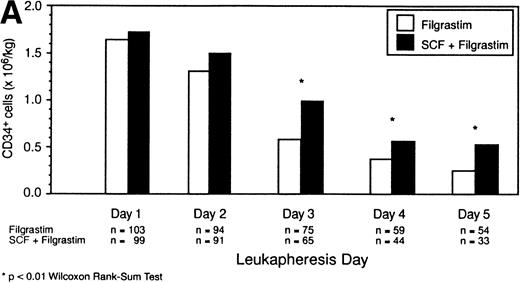
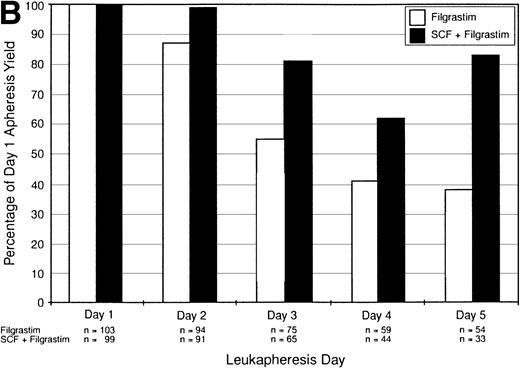
The daily median CD34+ cell yields for the intent-to-treat population are illustrated in Fig 2A. Patients receiving the combination of SCF plus Filgrastim had higher median yields of CD34+ cells on each day of leukapheresis compared with patients receiving Filgrastim alone; this difference was statistically significant (P < .01) on days 7, 8, and 9 of cytokine therapy (leukapheresis days 3, 4, and 5). Although patients in the SCF plus Filgrastim group underwent fewer leukaphereses than those in the Filgrastim alone group, the median total CD34+ cell yield was nevertheless higher for the SCF plus Filgrastim group (5.3 × 106/kg v 4.8 × 106/kg,P = .067). Note, as indicated in Fig 2A, that the patient numbers decreased with successive days as those who successfully reached the target yield no longer required leukapheresis; this decrease accounts for the decrease in medians cell yields across days of leukapheresis. It can be seen in Fig 2A that the rate of decrease in cell yield across days of leukapheresis was markedly lower in the SCF plus Filgrastim combination group compared with the Filgrastim alone group, indicating that CD34+ cell yields were sustained to a greater degree. This is further illustrated in Fig2B, which shows CD34+ cell yield values, only for the patients remaining on each successive day, as a percentage of their day 1 yields. For patients who underwent 5 leukaphereses, the median day-5 yield was 84% of the day-1 yield for the SCF plus Filgrastim group but only 38% for the Filgrastim alone group.
(A) Intent-to-treat population. Median CD34+ cell yield (×106/kg) by day of leukapheresis. (B) Intent-to-treat population. Median daily CD34+ cell yields (only for those patients remaining on each successive day) as a percentage of the day 1 values, which are shown as 100%.
(A) Intent-to-treat population. Median CD34+ cell yield (×106/kg) by day of leukapheresis. (B) Intent-to-treat population. Median daily CD34+ cell yields (only for those patients remaining on each successive day) as a percentage of the day 1 values, which are shown as 100%.
Proportion of patients reaching target yield and number of leukaphereses.
Table 2 shows the proportion of patients reaching the target yield of 5 × 106CD34+ cells/kg within 5 leukaphereses and the median number of leukaphereses required to reach the target by treatment group for the intent-to-treat and efficacy-evaluable data sets. For the intent-to-treat data set, 63% of the patients receiving SCF plus Filgrastim reached the target yield compared with 47% of patients receiving Filgrastim alone (P < .05). The number of leukaphereses required to reach the optimal target yield showed a clinically and statistically significant reduction (P < .05) for the cytokine combination group (median, 4 leukaphereses) compared with the Filgrastim alone group (median, 6 or more leukaphereses; ie, <50% of patients receiving Filgrastim alone reached the target in 5 leukaphereses). Thus patients mobilized with the cytokine combination required at least 2 fewer procedures to reach the target, compared with those receiving Filgrastim alone. Analyses of the 175 patients in the efficacy-evaluable data set yielded similar results. Sixty-seven percent of patients receiving SCF plus Filgrastim reached the target yield within 5 leukaphereses compared with 48% of patients receiving Filgrastim alone (P < .01). The median number of leukaphereses required to reach the target was 3 for efficacy-evaluable patients receiving SCF plus Filgrastim and ≥6 for the patients receiving Filgrastim alone (P < .01).
Proportion of Patients Reaching the Target CD34+ Cell Yield and the Median Number of Leukaphereses Required to Reach the Target Yield for Each Treatment Group
| Treatment Group . | Percentage of Patients Reaching the Target CD34+ Cell Yield . | Median No. of Leukaphereses Required to Reach the Target Yield . | ||
|---|---|---|---|---|
| ITT (n = 203) . | Evaluable (n = 175) . | ITT (n = 203) . | Evaluable (n = 175) . | |
| SCF + Filgrastim | 63 | 67 | 4 | 3 |
| Filgrastim | 47 | 48 | ≥6 | ≥6 |
| P value | <.05 | <.01 | <.05 | <.01 |
| Treatment Group . | Percentage of Patients Reaching the Target CD34+ Cell Yield . | Median No. of Leukaphereses Required to Reach the Target Yield . | ||
|---|---|---|---|---|
| ITT (n = 203) . | Evaluable (n = 175) . | ITT (n = 203) . | Evaluable (n = 175) . | |
| SCF + Filgrastim | 63 | 67 | 4 | 3 |
| Filgrastim | 47 | 48 | ≥6 | ≥6 |
| P value | <.05 | <.01 | <.05 | <.01 |
Abbreviation: ITT, intent-to-treat.
The number of prior cycles of chemotherapy was confirmed to be a statistically significant covariate for PBPC mobilization (P< .001); there was a direct positive correlation between the number of leukaphereses required to reach the target yield and the number of cycles of prior chemotherapy. Therefore, this prospectively identified covariate was included in the statistical analysis of the primary end-point.
A retrospective stepwise multiple regression was performed to look at factors that may affect PBPC mobilization (response variable = average CD34+ cell yield per leukapheresis) and to determine whether any subgroup of patients identified in terms of these factors benefit most from SCF. Thirty-three demographic factors, including age, stage of disease, prior chemotherapy, prior radiotherapy, baseline laboratory values, and treatment center, were evaluated. None of the factors, other than prior therapy, influenced the extent of PBPC mobilization, and there was no interaction between prior therapy and effect of SCF, indicating that SCF increases CD34+ cell yields in both the heavily and less heavily treated patients who received, respectively, greater than 5 or less than 5 cycles of prior therapy.
The median number of prior chemotherapy cycles for both treatment groups was 5. In patients with less than 5 cycles of prior chemotherapy, the median CD34+ cell yields per leukapheresis were 2.31 × 106/kg for the SCF plus Filgrastim group and 1.83 × 106/kg for the Filgrastim alone group. In patients with ≥5 cycles of prior chemotherapy, the corresponding values were 0.99 × 106/kg and 0.68 × 106/kg.
Seven patients (3 treated with SCF plus Filgrastim and 4 treated with Filgrastim alone) failed to achieve a CD34+ cell yield of ≥1.0 × 106/kg with 5 leukaphereses. They were deemed mobilization failures and were ineligible for transplant on study, as specified in the protocol. The 3 mobilization failures in the SCF plus Filgrastim group had all received 6 cycles of chemotherapy prior to enrollment on study, and 2 had received prior radiotherapy. Their mean baseline ANC and PLT were 3.91 (SD, 1.35) × 109/L and 192 (SD, 30) × 109/L, respectively. The 4 mobilization failures in the Filgrastim alone group had received 22, 7, 7, and 3 prior cycles of chemotherapy, respectively. The patient who had received 22 prior chemotherapy cycles, and 1 of the patients who had received 7 prior chemotherapy cycles, had also received prior radiotherapy. The mean baseline ANC and PLT for the 4 patients were 4.26 (SD, 1.67) × 109/L and 186 (SD, 48) × 109/L, respectively. With the small number of mobilization failures, their prior chemotherapy, prior radiotherapy, and baseline ANC and PLT did not differ significantly from those for the total study populations (Table 1).
Treatment phase—Groups analyzed.
One hundred eighty-nine (97 in the Filgrastim alone group and 92 in the SCF plus Filgrastim group) of the 203 patients underwent PBPC infusion and were included in the analysis for the transplant phase (intent-to-treat data set). Fourteen patients (6 in the Filgrastim alone group and 8 in the SCF plus Filgrastim group) were withdrawn before PBPC infusion on study, but after completing the leukapheresis schedule. Seven of these patients (mentioned in preceding section: 4 treated with Filgrastim alone and 3 treated with SCF plus Filgrastim) failed to achieve a CD34+ cell yield of ≥1.0 × 106/kg with 5 leukaphereses and were withdrawn as required by the protocol. The high-dose chemotherapy was not administered to 3 patients who had developed disease progression, 2 patients who had developed unrelated medical conditions, and 1 patient who voluntarily withdrew from study. One patient died during high-dose chemotherapy administration.
The efficacy-evaluable data set for the transplant phase excluded 28 patients with protocol violations (essentially the same 28 patients who incurred protocol violations in the collection phase; see section above entitled “Collection phase—Groups analyzed”), such that this data set was composed of 161 patients (85 in the Filgrastim alone group and 76 in the SCF plus Filgrastim group).
Engraftment.
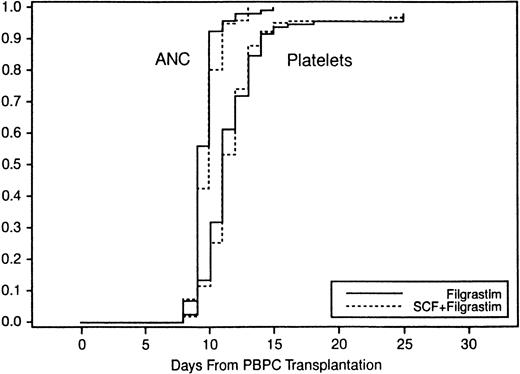
Engraftment was equivalent for the two intent-to-treat groups with respect to ANC and PLT recovery (Fig 3). Hodges-Lehmann estimates and distribution-free 95% confidence intervals for median differences indicated that there was, at most, a 1-day difference in median time to ANC recovery and no difference in PLT recovery between the two treatment groups. The median number of days to engraftment of ANC ≥0.5 × 109/L for patients mobilized with SCF plus Filgrastim and for patients mobilized with Filgrastim alone was 10 days (range, 8 to 13 days) and 9 days (range, 8 to 15 days), respectively. Both treatment groups required 11 days (range, 8 to 100+ days and 8 to 88+ days, respectively) to reach PLT ≥20 × 109/L. There was no difference in the requirements for red blood cell and platelet transfusions between the two treatment groups. Similar results were observed for the efficacy-evaluable population.
Probability of engraftment of ANC to 0.5 × 109/L and PLT to 20 × 109/L.
Delayed platelet engraftment.
Figure 4 shows the proportion of patients (overall in the top panel, and within each treatment group in the bottom panel) that failed to engraft to PLT ≥20 × 109/L by day 14 or day 28 as a function of number of CD34+ cells infused. Overall (top panel), for those patients receiving greater than 5 × 106CD34+ cells/kg, only 1% failed to recover by day 14, and all recovered by day 28. For those patients receiving 2 to 5 × 106 CD34+ cells/kg, 13% failed to recover by day 14, and 3% failed to recover by day 28. Thus, there were significantly less (P < .05) patients failing to engraft by day 14 or day 28 for the patients receiving greater than 5 × 106 CD34+ cells/kg compared with those receiving 2 to 5 × 106 CD34+ cells/kg. Similar engraftment results, as a function of CD34+ cells per kilogram infused, were documented for each treatment group (bottom panel); the differences in delayed engraftment between the two treatment groups are not statistically significant.
Proportion of patients from both groups failing to engraft to PLT ≥20 × 109/L within 14 or 28 days after transplant with respect to the number of CD34+ cells infused, overall (top panel) and by treatment group (bottom panel).
Proportion of patients from both groups failing to engraft to PLT ≥20 × 109/L within 14 or 28 days after transplant with respect to the number of CD34+ cells infused, overall (top panel) and by treatment group (bottom panel).
Safety.
As expected, peripheral white blood cell (WBC) counts increased in both treatment groups during the collection phase, plateauing at about 40 to 50 × 109/L. There was no difference between the treatment groups in this regard. Only one patient (combination group) had a WBC greater than 100 × 109/L. This patient’s baseline WBC was 14 × 109/L, increased to 110 × 109/L on day 7 of the collection phase, and returned to baseline after the cytokines were discontinued. There were no clinical sequelae associated with any level of leukocytosis.
Small increases from baseline were noted for several serum chemistry values in both treatment groups (Table 3). At the end of the collection phase, the increases in median values for alkaline phosphatase, lactic dehydrogenase, and uric acid were greater for the SCF plus Filgrastim group than for the Filgrastim alone group. These increases probably resulted from increased neutrophil production and turnover, as reported previously for Filgrastim,37 and may be further increased with the addition of SCF. None of the increases in serum chemistries was considered clinically significant, because no adverse clinical symptoms were associated with these transient elevations.
Median Serum Chemistry Values in the Collection Phase for Each Treatment Group
| . | Filgrastim . | SCF + Filgrastim . | P Value3-151 . | ||||
|---|---|---|---|---|---|---|---|
| n3-150 . | Pre . | Post . | n3-150 . | Pre . | Post . | ||
| AST (U/L) | 77 | 27 | 29 | 68 | 24 | 28 | .957 |
| ALT (U/L) | 62 | 27 | 43 | 57 | 25 | 34 | .177 |
| Alkaline phosphatase (U/L) | 77 | 81 | 131 | 66 | 86 | 150 | .504 |
| LDH (U/L) | 56 | 184 | 210 | 49 | 199 | 358 | .002 |
| Uric acid (μmol/L) | 76 | 238 | 268 | 61 | 274 | 309 | .083 |
| . | Filgrastim . | SCF + Filgrastim . | P Value3-151 . | ||||
|---|---|---|---|---|---|---|---|
| n3-150 . | Pre . | Post . | n3-150 . | Pre . | Post . | ||
| AST (U/L) | 77 | 27 | 29 | 68 | 24 | 28 | .957 |
| ALT (U/L) | 62 | 27 | 43 | 57 | 25 | 34 | .177 |
| Alkaline phosphatase (U/L) | 77 | 81 | 131 | 66 | 86 | 150 | .504 |
| LDH (U/L) | 56 | 184 | 210 | 49 | 199 | 358 | .002 |
| Uric acid (μmol/L) | 76 | 238 | 268 | 61 | 274 | 309 | .083 |
Abbreviations: AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactic dehydrogenase.
Represents those patients for whom both pre and post values were available.
P value for comparison from baseline between treatment groups, based on Wilcoxon rank sum test.
Generally, adverse events reported in the collection phase were mild to moderate in severity for both treatment groups. No fatal or life-threatening events were reported. Severe events were reported for 17% (17/100) of patients in the SCF plus Filgrastim group and for 12% (12/103) of patients in the Filgrastim alone group. Consistent with the known side effects of Filgrastim, mild to moderate musculoskeletal symptoms were reported at least possibly related to cytokine for 39% of patients in both treatment groups.
Overall incidence of adverse events, including all severities and relationships that were reported more frequently (≥5% difference) in the SCF plus Filgrastim group than in the Filgrastim alone group are provided in Table 4. These included injection site reactions, dizziness, tachycardia, dyspnea, hypocalcemia, and pruritus. The increased incidence of dizziness and tachycardia (heart rate, 90 to 145 beats/min) in the SCF plus Filgrastim group may be due to one or more of the premedications that these patients received. In 1 patient, mild tachycardia was reported as related to SCF. None of the other events of dizziness or tachycardia was reported as related to cytokine, and all were reported as mild in severity; no patients were treated for these symptoms. The highest heart rate recorded was 145 beats/min in a patient who had a baseline heart rate of 100 beats/min. Paresthesia, headache, and nausea were reported more frequently in the Filgrastim alone group (Table 4).
Adverse Events of All Severities and Treatment Relationship With a Difference of ≥5% in Incidence Between the Treatment Groups
| . | Filgrastim (n = 103) . | SCF + Filgrastim (n = 100) . | P Value4-150 . |
|---|---|---|---|
| Injection site reactions | 10% | 92% | <.0001 |
| Dizziness | 6% | 12% | .142 |
| Tachycardia | 0% | 8% | NA |
| Dyspnea | 2% | 8% | .055 |
| Hypocalcemia | 3% | 8% | .129 |
| Pruritus | 1% | 6% | .061 |
| Paresthesia | 35% | 29% | .453 |
| Nausea | 23% | 16% | .221 |
| Headache | 23% | 13% | .071 |
| . | Filgrastim (n = 103) . | SCF + Filgrastim (n = 100) . | P Value4-150 . |
|---|---|---|---|
| Injection site reactions | 10% | 92% | <.0001 |
| Dizziness | 6% | 12% | .142 |
| Tachycardia | 0% | 8% | NA |
| Dyspnea | 2% | 8% | .055 |
| Hypocalcemia | 3% | 8% | .129 |
| Pruritus | 1% | 6% | .061 |
| Paresthesia | 35% | 29% | .453 |
| Nausea | 23% | 16% | .221 |
| Headache | 23% | 13% | .071 |
Abbreviation: NA, P value could not be computed.
P value from Fisher Exact test.
Five patients (5%) who received SCF plus Filgrastim and no patients who received Filgrastim alone experienced serious adverse events that were considered at least possibly related to the cytokine. Three of these 5 patients experienced systemic allergic-like reactions attributed to SCF: 1 experienced dyspnea (plus cough with angiodema); 1 experienced throat tightness; and 1 developed generalized urticaria and pruritus. All symptoms resolved completely in these patients after SCF was discontinued and antihistamine and/or steroid was administered. The 2 additional patients experienced events that were reported as possibly related to SCF: 1 (who received multiple respiratory depressive medications) developed hypoxia and confusion; the other developed a dermatologic reaction manifested by edema and erythema of the hands, which culminated in skin exfoliation 10 days after the cessation of SCF. In both patients, the symptoms/clinical findings resolved completely.
No toxicities reported as related to SCF were observed upon infusion of PBPCs mobilized with SCF plus Filgrastim. In addition, through 100 days, no difference was noted in the incidence of disease progression or death between the two groups (data not shown).
QOL study.
Ninety-three percent of the patients enrolled in this clinical trial participated in the QOL study and patients in both groups completed 91% of the scheduled assessments. Table 5summarizes the QOL study results. Although the study was not powered to show a statistically significant difference in QOL end-points, patients receiving SCF plus Filgrastim had slightly higher QOL scores on all nine scales.
Summary of Daily QOL Results Over the Study Duration
| Assessment . | Filgrastim (n = 85) . | SCF + Filgrastim (n = 76) . | % Difference5-150 . | P Value5-151 . |
|---|---|---|---|---|
| VAS | .207 | |||
| Mean | 70.72 | 72.86 | +3.0 | |
| Median | 74.14 | 76.44 | +3.1 | |
| SF36-GH | .991 | |||
| Mean | 61.25 | 60.78 | −0.8 | |
| Median | 61.12 | 64.04 | +4.8 | |
| FACT-PW | .220 | |||
| Mean | 17.12 | 17.86 | +4.3 | |
| Median | 17.37 | 19.27 | +10.9 | |
| FACT-SW | .790 | |||
| Mean | 19.60 | 19.69 | +0.5 | |
| Median | 19.97 | 20.70 | +3.7 | |
| FACT-FW | .719 | |||
| Mean | 15.11 | 15.49 | +2.5 | |
| Median | 15.22 | 15.99 | +5.1 | |
| FACT-EW | .269 | |||
| Mean | 14.98 | 15.40 | +2.8 | |
| Median | 15.48 | 16.22 | +4.8 | |
| FACT-MD | .107 | |||
| Mean | 6.71 | 7.07 | +5.4 | |
| Median | 7.16 | 7.48 | +4.5 | |
| FACT-G | .251 | |||
| Mean | 73.41 | 75.44 | +2.8 | |
| Median | 74.62 | 78.46 | +5.1 | |
| FACT-BMT | .809 | |||
| Mean | 25.28 | 25.65 | +1.5 | |
| Median | 25.75 | 25.82 | +0.3 |
| Assessment . | Filgrastim (n = 85) . | SCF + Filgrastim (n = 76) . | % Difference5-150 . | P Value5-151 . |
|---|---|---|---|---|
| VAS | .207 | |||
| Mean | 70.72 | 72.86 | +3.0 | |
| Median | 74.14 | 76.44 | +3.1 | |
| SF36-GH | .991 | |||
| Mean | 61.25 | 60.78 | −0.8 | |
| Median | 61.12 | 64.04 | +4.8 | |
| FACT-PW | .220 | |||
| Mean | 17.12 | 17.86 | +4.3 | |
| Median | 17.37 | 19.27 | +10.9 | |
| FACT-SW | .790 | |||
| Mean | 19.60 | 19.69 | +0.5 | |
| Median | 19.97 | 20.70 | +3.7 | |
| FACT-FW | .719 | |||
| Mean | 15.11 | 15.49 | +2.5 | |
| Median | 15.22 | 15.99 | +5.1 | |
| FACT-EW | .269 | |||
| Mean | 14.98 | 15.40 | +2.8 | |
| Median | 15.48 | 16.22 | +4.8 | |
| FACT-MD | .107 | |||
| Mean | 6.71 | 7.07 | +5.4 | |
| Median | 7.16 | 7.48 | +4.5 | |
| FACT-G | .251 | |||
| Mean | 73.41 | 75.44 | +2.8 | |
| Median | 74.62 | 78.46 | +5.1 | |
| FACT-BMT | .809 | |||
| Mean | 25.28 | 25.65 | +1.5 | |
| Median | 25.75 | 25.82 | +0.3 |
Abbreviations: VAS, Euroquol-Visual Analog Scale; SF36-GH, SF-36 general health perceptions domain; FACT-PW, functional assessment of cancer treatment: physical well-being domain; FACT-SW, functional assessment of cancer treatment: social well-being domain; FACT-FW, functional assessment of cancer treatment: functional well-being domain; FACT-EW, functional assessment of cancer treatment: emotional well-being domain; FACT-MD, functional assessment of cancer treatment: relationship with doctors; FACT-G, functional assessment of cancer treatment: total summary score; FACT-BMT, functional assessment of cancer treatment: bone marrow transplant scale.
Defined as: [(Daily QOL of SCF + Filgrastim) − (Daily QOL of Filgrastim Alone)/(Daily QOL of Filgrastim Alone)] × 100.
Wilcoxon rank sum test.
DISCUSSION
The efficiency of PBPC mobilization may be quite variable, influenced by the patient’s underlying disease, by the amount of prior chemotherapy and/or irradiation, and by the mobilization regimen used.3,4,6 Correlations have been between particular PBPC graft parameters and rate of hematopoietic engraftment; the number of CD34+ cells in the PBPC harvest has emerged as the most reliable predictor of engraftment kinetics (ANC and PLT recovery).3-10
To avoid the risks of delayed or impaired hematopoietic engraftment, clinicians generally will not initiate high-dose chemotherapy unless a minimum number of CD34+ cells, ranging from 1 to 2.5 × 106 cells/kg, are available for infusion. Patients yielding less than 1 × 106 CD34+ cells/kg usually are considered mobilization failures and will typically not receive high-dose chemotherapy until and unless more cells are collected. Conversely, the infusion of 3 to 8 × 106 CD34+ cells/kg has been reported to be necessary for rapid platelet recovery.3,4,7-9,38 39
In the present study, 5 × 106 CD34+cells/kg was chosen as an optimal target yield for rapid engraftment, based on studies by a number of investigators.3,6-8 13 A central laboratory was used to standardize the determination of CD34+ cell numbers, and the analysis of engraftment kinetics as a function of CD34+ cell numbers infused supports the choice of 5 × 106 CD34+cells/kg as an optimal target. There were significantly fewer patients failing to engraft to platelets ≥20 × 109/L by day 14 or day 28 overall for patients receiving greater than 5 × 106 CD34+ cells/kg compared with those receiving 2 to 5 × 106 CD34+ cells/kg (P < .05).
The proficiency of mobilization dictates the number of leukaphereses required to achieve the desired PBPC yields. The use of Filgrastim alone for mobilization often requires multiple leukapheresis procedures.3 However, it is difficult to determine precisely from the literature how many leukaphereses are routinely performed. As mentioned, the PBPC yield and number of leukaphereses required to achieve the yield are influenced by mobilization regimen (eg, cytokine with or without chemotherapy), tumor type, extent of prior therapy, and variability in CD34+ cell measurement and leukapheresis procedures. However, in general, it would appear that 1 to 3 leukaphereses are routinely used for chemotherapy plus cytokine mobilization and 2 to 8 for cytokine-alone mobilization. The CD34+ cell yields for patients receiving Filgrastim alone in the present study are similar to those in a previous clinical study in breast cancer patients,13 and as we demonstrated, are less than the optimal target of 5 × 106/kg in more than half the patients.
Clinicians are interested in reducing the number of leukaphereses required for reasons of associated morbidity (hypocalcemia, paresthesia, thrombocytopenia, and catheter-related complications), convenience (hospital visits and time undergoing leukaphereses), and cost, but not at the risk of delayed engraftment. The cost of a single leukapheresis has been estimated as $2,266 (US).40
In the present study, in comparison with Filgrastim alone, the combination of SCF plus Filgrastim for PBPC mobilization resulted in significant reductions in the number of leukaphereses required to achieve CD34+ cell yields optimal for successful transplant in patients with breast cancer. The reductions in leukaphereses occurred because patients receiving SCF plus Filgrastim had higher median yields of CD34+ cells on each day of leukapheresis, such that the cumulative proportion of patients reaching the CD34+ cell target over 5 days of leukapheresis was higher in this group for both the intent-to-treat and efficacy-evaluable data sets. Patients in the SCF plus Filgrastim group also had CD34+ cell yields that were more sustained over the leukapheresis period than the yields for patients in the Filgrastim alone group. The CD34+ cell data suggested that patients who required more than 2 leukaphereses benefited most from the use of SCF along with Filgrastim.
These findings of increased and sustained leukapheresis CD34+ cell yields with SCF plus Filgrastim are consistent with the preclinical biology of SCF21-23 and with a previous clinical study in breast cancer patients in which the increases in PBPCs were sustained when the cytokine combination was administered for 7, 10, or 13 days.13 Similar results showing increased CD34+ cell yields with SCF plus Filgrastim have been obtained in patients with lymphoma, myeloma, and ovarian cancer.14,15,41,42 The addition of SCF to regimens using chemotherapy and Filgrastim for mobilization is also associated with substantial increases in the number of PBPCs harvested.15
The overall results show that approximately 20% more patients reached the optimal target of 5 × 106 CD34+cells/kg in 5 leukaphereses for the SCF plus Filgrastim group than for the Filgrastim alone group. However, it is also clear that for patients who did not reach the optimal target, those in the SCF plus Filgrastim group nevertheless yielded more CD34+ cells than those in the Filgrastim alone group, ie, median yields of CD34+cells were higher on each day of leukapheresis for the SCF plus Filgrastim group. Thus, the use of SCF along with Filgrastim can be expected to have a range of clinical benefits for patients, depending on their underlying mobilization potential. For poor mobilizers (<1 × 106 CD34+ cells/kg), the likelihood of reaching a minimum yield (1 to 2 × 106CD34+ cells/kg) to allow transplant would be expected to become greater.4 41 For medium mobilizers (2 to 5 × 106 CD34+ cells/kg), the likelihood of reaching an optimal yield (>5 × 106 CD34+cells/kg) would be expected to become greater. And for good mobilizers (>5 × 106 CD34+ cells/kg), there is a likelihood of reaching the optimal yield with fewer leukaphereses. Because leukapheresis to an optimal CD34+ cell target is increasingly adopted in transplant centers, the combination of SCF plus Filgrastim will have the added advantage, over Filgrastim alone, of sustained mobilization beyond day 5 of leukapheresis, ie, continued daily leukapheresis would be more feasible, decreasing the need for second mobilizations or back-up bone marrow harvests.
Engraftment was equivalent for the two treatment groups in this study. This result was expected because patients were mobilized to a target CD34+ cell yield. As discussed, rapidity of engraftment was directly related to the number of CD34+ cells infused.
The dosing of SCF in this study (ie, 20 μg/kg/d) was based on prior dose-finding clinical studies performed in combination with Filgrastim.13 The dosing of Filgrastim (ie, 10 μg/kg/d) was that most typical for mobilization with Filgrastim as a single agent and is in accordance with product labeling. Some studies43,44 have suggested that higher doses of Filgrastim as a single agent do not enhance mobilization, whereas other studies45 have suggested that they do. However, studies in baboons have shown that SCF in combination with Filgrastim enhances mobilization relative to Filgrastim alone, regardless of the Filgrastim dose.28 Thus, clinical trials of SCF in combination with higher doses of Filgrastim may be warranted.
SCF at 20 μg/kg/d, when administered with premedication as in this study, is safe and generally well tolerated. Three patients (3%) receiving SCF developed systemic allergic-like reactions that were considered to be related to the cytokine. These reactions were similar in character and severity to those reported in other SCF clinical trials and resolved completely with the administration of antihistamines/steroids and cessation of SCF treatment.
Ancillary studies have shown that there was no difference in the incidence of tumor contamination of the leukapheresis products of the two groups46 or in immune reconstitution of the grafts after transplantation.47
In addition to the benefits for patients undergoing conventional or multicycle PBPC transplant, an enhanced CD34+ cell yield has potential benefits for the further therapeutic manipulation of PBPC harvests. A number of procedures invariably result in loss of a substantial fraction of hematopoietic progenitor cells; these include CD34+ cell selection, tumor cell purging, and genetic manipulation. Other procedures that would benefit from higher starting cell numbers include ex vivo expansion of CD34+ cells, which has the potential to further decrease the duration and severity of myelosuppression after transplantation, and ex vivo manipulations to increase the number of effector cells and cycling stem cells, thereby facilitating immunotherapy and gene therapy, respectively.
ACKNOWLEDGMENT
The authors thank Angela Brame, Garinell Davis, Lisa Gaynes, Eric Guempel, Lisa Hami, Sharon Heister, Betty Hinshaw, Randi Isaacs, Kathy Jelaca-Maxwell, John Lu, Becky Malloy, Lisa Matta, Hillary O’Kelly, Sheryl Oliversen, Joseph Rosenblatt, Carol Rush, Anne Sharpe, Jeffrey Shogan, Sharon Taffs, Kate Tierney, Arianne Van Vliet, Jennifer Wang, and Marianne Zblyski for their contributions towards this research. We also thank Cheryl Garrison, Keith Langley, Melanie LaPolla, and MaryAnn Foote, PhD, for their assistance in the finalization of the manuscript.
Supported in part by National Institutes of Health/National Cancer Institute Grant No. R01-CA61508 (to E.J.S.); by Grant No. M01-RR00865 from The Revlon/UCLA Women’s Cancer Research Program (to J.A.G.); by Grants No. M01RR00046 and 5-P30-CA16086-23 from the University of North Carolina, Chapel Hill, NC (to T.C.S.); and by Amgen Inc.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Elizabeth J. Shpall, MD, Bone Marrow Transplant Program, University of Colorado Health Sciences Center, Denver, CO 80262.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal