Abstract
Allogeneic bone marrow transplantation (BMT) is a common treatment of hematologic malignancies. Recurrence of the underlying malignancy is a major cause of treatment failure. Donor-derived cytotoxic T lymphocytes (CTLs) specific for patients’ minor histocompatibility antigens (mHags) play an important role in both graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) reactivities. mHags HA-1 and HA-2 induce HLA-A*0201-restricted CTLs in vivo and are exclusively expressed on hematopoietic cells, including leukemic cells and leukemic precursors, but not on fibroblasts, keratinocytes, or liver cells. The chemical nature of the mHags HA-1 and HA-2 is known. We investigated the feasibility of ex vivo generation of mHag HA-1– and HA-2–specific CTLs from unprimed mHag HA-1– and/or HA-2–negative healthy blood donors. HA-1 and HA-2 synthetic peptide-pulsed dendritic cells (DCs) were used as antigen-presenting cells (APC) to stimulate autologous unprimed CD8+ T cells. The ex vivo–generated HA-1– and HA-2–specific CTLs efficiently lyse leukemic cells derived from acute myeloid leukemia (AML) and acute lymphoid leukemia (ALL) patients. No lytic reactivity was detected against nonhematopoietic cells. Sufficient numbers of the CTLs can be obtained for the adoptive immunotherapy purposes. In conclusion, we present a feasible, novel therapy for the treatment for relapsed leukemia after BMT with a low risk of GVHD.
BONE MARROW transplantation (BMT) is the present treatment for hematologic malignancies.1,2 One of the major drawbacks of BMT is leukemia relapse.3,4 Donor lymphocyte infusions (DLI) as a treatment for relapsed leukemia appeared successful at least in patients with chronic myeloid leukemia (CML).5-7 However, DLI therapy is associated with graft-versus-host disease (GVHD) and is far less effective in acute lymphoid leukemia (ALL) and acute myeloid leukemia (AML) patients.6,7 The DLI-induced graft-versus-leukemia (GVL) effect may result from T-cell responses against minor histocompatibility antigens (mHags).6,7 Human mHags are polymorphic antigens that are inherited independent from HLA, show broad or restricted tissue distribution, and are recognized by alloreactive T cells.8-15 The mHags HA-1 and HA-2 are expressed exclusively on hematopoietic cells.9-13HLA-A*0201–restricted, HA-1–, and HA-2–specific cytotoxic T lymphocytes (CTLs) effectively lyse leukemic cell precursors and circulating myeloid and lymphoid leukemic cells,9-12 but not cells derived from GVHD target organs such as skin fibroblasts, keratinocytes, or liver cells.13 Because the hematopoietic cell-restricted and patient-specific target cell damage, HA-1– and HA-2–specific CTLs can be used to specifically eliminate the leukemia with a low risk of GVHD. We have recently identified the peptide sequences of HA-1 and HA-2 antigens.14 15 Here, we investigated the feasibility of ex vivo generation of mHag HA-1– and HA-2–specific CTLs from unprimed mHag HA-1– and/or HA-2–negative healthy blood donors using HA-1 and HA-2 synthetic peptide-pulsed antigen-presenting cells (APCs).
Dendritic cells (DCs) are specialized APCs for the induction of T-cell responses from naive T-cell precursors.16 DCs were successfully used to generate human immunodeficiency virus (HIV)-specific CTLs that lysed peptide-pulsed as well as virally infected target cells.17,18 In some studies, other types of APCs, such as unfractionated peripheral blood mononuclear cells (PBMC), monocytes, or even activated T cells, were used for the in vitro induction of antigen-specific CTLs.19 20 We aimed at using HA-1– and HA-2–specific CTLs for the adoptive immunotherapy purposes. Therefore, we searched for the optimal APC that can be easily obtained, and can reproducibly induce HA-1– and HA-2–specific CTLs with good expansion capacity and strong lytic and specific reactivity against mHag-positive AML and ALL cells.
MATERIALS AND METHODS
Peptides.
Cellular material.
All cells were obtained from HLA-typed healthy blood bank donors, volunteers, or healthy bone marrow donors after informed consent. Peripheral blood was collected with manual leukapheresis. PBMC and bone marrow mononuclear cells (BMMC) were isolated by Ficoll density gradient separation.
Monocytes.
Monocytes were isolated by 2-hour plastic adherence from PBMC.
Peripheral blood DC.
Low-density peripheral blood DC (PBDC) were enriched from PBMC as described earlier.21 Briefly, PBMC were depleted from T cells by sheep red blood erythrocyte rosetting. Non-T cells were cultured for 36 hours at 37°C in RPMI plus 10% autologous plasma. After depleting monocytes, nonadherent cells were layered on 14.5% metrizamide (Sigma-Aldrich, Zwijndrecht, The Netherlands) gradients and the light-density PBDC were recovered from the interphase after centrifugation at 600g for 10 minutes. PBDC were identified by fluorescence-activated cell sorting (FACS) being negative for CD3, CD14, CD16, and CD19, and positive for HLA-DR. The preparations contained 2 to 6 × 106 cells with a DC content of 20% to 50%. In some cases, the light-density cells were further depleted from CD14 and CD19 cells using antibody-coated magnetic beads (DYNAL, Oslo, Norway).
BMDC.
BMDC were differentiated from bone marrow CD34+ cells (isolated using a CD34+ isolation kit; MACS, Bergisch Gladbach, Germany) by culturing with 100 ng/mL FLT3 ligand (Genzyme; Leuven, Belgium), 30 ng/mL interleukin-3 (IL-3), 25 ng/mL stem cell factor (SCF; Genzyme), 50 U/mL tumor necrosis factor-α (TNF-α; Genzyme), and 250 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Genzyme) for 10 to 14 days. The cultures contained 20% to 60% DC as detected by high levels of DR and negative expression of CD3, CD14, CD16, and CD19.
Ex vivo induction of HA-1– and HA-2–specific CTLs.
APC were pulsed with HA-1 or HA-2 peptides (both 10 μg/mL) for 90 minutes at 37°C in serum-free AIM-V medium (GIBCO-BRL, Breda, The Netherlands). After washing, APC and 10 to 15 × 106 responder cells (CD4-depleted autologous PBMC) were cultured at different APC:responder cell ratios depending on the type of APC (5:1, 1:3, and 1:10 for PBMC, monocytes, and DC, respectively) in 24-well culture plates. Culture medium was RPMI-supplemented with 10% autologous plasma, 1 U/mL IL-2 (Cetus, Emeryville, CA), and 1 U/mL IL-12 (Genzyme). The cells were kept at 37°C in an humidified, 5% CO2− air mixture. At day 5, 10 U/mL of IL-2 was added. From day 7 on, the T-cell cultures were restimulated weekly with peptide-pulsed autologous monocytes. Twenty-four hours after each restimulation, 10 U/mL IL-2 was added. The T-cell lines were expanded with 10 to 20 U/ml IL-2–containing culture medium.
In vivo–induced mHag-specific T-cell clones.
Cytotoxicity (chromium-51 release) assays.
Standard, 4-hour 51Cr release assays using phytohemagglutinin (PHA)-blasts, Epstein-Barr virus–transformed B-cell lines (EBV-LCL), and fibroblasts and leukemic cells as target cells were performed as described previously.13 The percent specific lysis was calculated using the following formula: 100 × (cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release).
Target cells.
EBV-LCL were cultured in RPMI plus 10% fetal calf serum (FCS). PHA-activated T-cell blasts (PHA-blasts) were obtained by stimulation of PBMC with 0.1 μg/mL PHA (Murex, Dartford, UK) over 72 hours. PHA-blasts were expanded with medium containing 20 U/mL IL-2. Skin fibroblasts of an HLA-A*0201–positive, HA-1–positive, HA-2–positive healthy individual were isolated, cultured, and tested as described previously.13 In short, fibroblasts were trypsinized and cultured in the wells of 96-well flat-bottomed microtiter culture plates at a concentration of 3 × 103 cells/well with or without addition of interferon-gamma (IFN-γ) and TNF-α (both 300 U/mL) during 72 hours. When indicated, target cells were pulsed with HA-1 or HA-2 peptides (both 10 μg/mL) during 51Cr labeling.
Leukemia patients’ (AML or ALL) PBMC or bone marrow containing greater than 95% morphologically recognizable malignant cells were assigned as leukemic cells. Leukemic cells were thawed and cultured in RPMI plus 10% human serum for 72 hours with or without addition of IFN-γ and TNF-α (both 300 U/mL) before using as target cells.
RESULTS
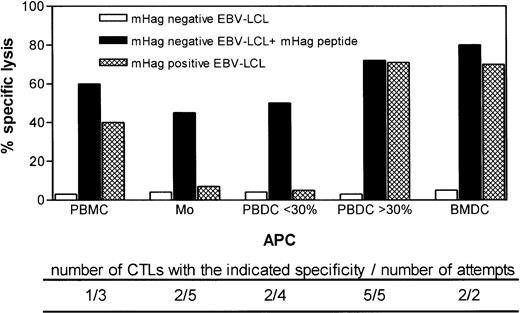
To define the optimal APC for ex vivo generation of HA-1– and HA-2–specific CTLs, we prepared PBMC, monocytes, PBDC, or BMDC from 15 HLA-A*0201–positive, HA-1– or HA-2 –negative healthy individuals. These APCs were pulsed with HA-1 and/or HA-2 synthetic peptides and used to stimulate autologous unprimed CD8+ T cells (Fig 1). The attempts to induce HA-1– or HA-2–specific CTLs using monocytes or PBMC as APC were unsuccessful. Using PBMC, mHag-specific CTLs could be generated in one of three attempts only. Monocytes induced HA-1–specific CTLs in two of five attempts. However, the HA-1–specific CTLs induced by monocytes failed to lyse HA-1–positive target cells, suggesting that monocytes induced CTLs with low-affinity T-cell receptors for the naturally expressed HA-1 antigen (Fig 1).
Target cell reactivity of HA-1– and HA-2–specific ex vivo–induced CTLs using various types of APCs pulsed with synthetic peptides. The type of APC used to induce CTLs is indicated on the x-axis. Below the x-axis, the number of CTLs generated using the same type of APC (but in all cases from a different individual) and the total number of attempts are indicated. Effector-to-target ratio was 20:1.
Target cell reactivity of HA-1– and HA-2–specific ex vivo–induced CTLs using various types of APCs pulsed with synthetic peptides. The type of APC used to induce CTLs is indicated on the x-axis. Below the x-axis, the number of CTLs generated using the same type of APC (but in all cases from a different individual) and the total number of attempts are indicated. Effector-to-target ratio was 20:1.
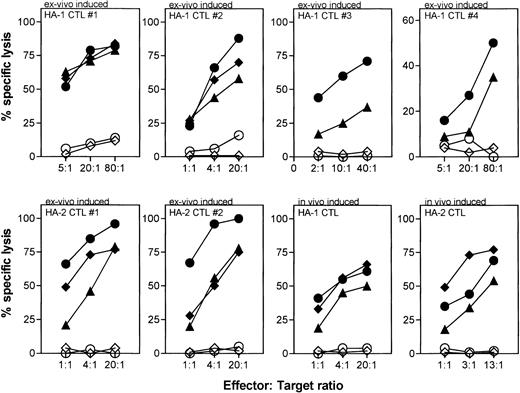
PBDC were enriched from in total nine different individuals to induce HA-1– or HA-2–specific CTLs. In the two of four cases where the DC preparations had a purity of less than 30%, we induced CTLs that lysed peptide-pulsed target cells but not mHag-positive target cells (Fig 1). In contrast, in five cases of five attempts where PBDC purity was 30% or greater, we generated CTLs that recognized not only peptide-pulsed target cells, but also mHag-positive EBV-LCL, demonstrating the recognition of the naturally expressed ligand (Fig 1). Similarly, in two of two attempts where BMDC were used as APCs, CTLs were induced that recognized both peptide-pulsed target cells and HA-1–positive target cells (Fig 1). For six of these ex vivo–generated HA-1– or HA-2–specific CTLs (HA-1 CTL no. 1 through 4 and HA-2 CTL no. 1 and 2) using PBDC or BMDC, the actual lytic activity against several mHag-positive, peptide-pulsed, or unpulsed mHag-negative target cells is shown in Fig 2. The cytotoxic activity of two in vivo–generated HA-1– and HA-2–specific CTLs against same targets are shown as comparison. Similar to the in vivo–generated CTLs, all ex vivo–generated CTLs showed strong lysis against peptide-pulsed and mHag-positive target cells. Some CTLs (HA-1 CTL no. 1 and 2, HA-2 CTL no. 1 and 2) effectively lysed target cells even in effector-to-target ratios as low as 4:1 (Fig 2). No cytotoxic activity was observed against autologous PHA-blasts or against several mHag-negative EBV-LCL. Thus, neither autoreactivity nor “third-party” alloantigen reactivity could be detected (Fig 2). Several HA-1– or HA-2–specific CTL clones isolated from these CTLs showed the same target cell reactivity (data not shown).
CTL activity against peptide-pulsed and mHag-positive target cells by ex vivo–induced HA-1– and HA-2–specific CTLs. HA-1 CTLs no. 1, 2, 3, and 4 and HA-2 CTLs no. 1 and 2 were generated from different individuals using PBDC (HA-1 CTLs no. 1, 4 and HA-2 CTLs no. 1, 2) or BMDC (HA-1 CTLs no. 2, 3). The cytotoxic activities of in vivo–induced HA-1– and HA-2–specific CTLs against the same target cells are shown as comparison. Target cells: autologous PHA-blasts (◊); autologous PHA-blasts pulsed with peptide (⧫; EBV-LCL positive for HA-1 (n = 4) or HA-2 (n = 3) (▧); EBV-LCL negative for HA-1 (n = 3) or HA-2 (n = 3) (◍); HA-1– or HA-2–negative EBV-LCL pulsed with HA-1 or HA-2 peptide (◍).
CTL activity against peptide-pulsed and mHag-positive target cells by ex vivo–induced HA-1– and HA-2–specific CTLs. HA-1 CTLs no. 1, 2, 3, and 4 and HA-2 CTLs no. 1 and 2 were generated from different individuals using PBDC (HA-1 CTLs no. 1, 4 and HA-2 CTLs no. 1, 2) or BMDC (HA-1 CTLs no. 2, 3). The cytotoxic activities of in vivo–induced HA-1– and HA-2–specific CTLs against the same target cells are shown as comparison. Target cells: autologous PHA-blasts (◊); autologous PHA-blasts pulsed with peptide (⧫; EBV-LCL positive for HA-1 (n = 4) or HA-2 (n = 3) (▧); EBV-LCL negative for HA-1 (n = 3) or HA-2 (n = 3) (◍); HA-1– or HA-2–negative EBV-LCL pulsed with HA-1 or HA-2 peptide (◍).
The ex vivo–induced HA-1– and HA-2–specific CTLs were tested for their hematopoietic cell restricted reactivity and compared with the in vivo–induced HA-1– and HA-2–specific CTLs (Fig 3). PHA-blasts, but not fibroblasts (neither after IFN-γ or after TNF-α stimulation) were recognized by ex vivo (and in vivo)–induced HA-1– and HA-2–specific CTLs. Fibroblasts were only lysed after pulsing with the mHag peptides, demonstrating their susceptibility to CTL-mediated lysis (Fig 3).
Hematopoietic cell–restricted cytolysis mediated by ex vivo–induced HA-1– and HA-2–specific CTLs. Data are representative for all ex vivo–induced CTLs tested. The cytotoxic activities of in vivo–induced HA-1– and HA-2–specific CTLs against the same target cell panel are shown for comparison. All target cells used in these specificity experiments were derived from the same HLA-A*0201+, HA-1+, HA-2+healthy volunteer. Target cells: PHA-blasts (▧); fibroblasts (⧫); fibroblasts cultured with IFN-γ + TNF- (both 300 U/mL) (◍); fibroblasts cultured with IFN-γ + TNF- and pulsed with 10 μg/mL peptide (◍).
Hematopoietic cell–restricted cytolysis mediated by ex vivo–induced HA-1– and HA-2–specific CTLs. Data are representative for all ex vivo–induced CTLs tested. The cytotoxic activities of in vivo–induced HA-1– and HA-2–specific CTLs against the same target cell panel are shown for comparison. All target cells used in these specificity experiments were derived from the same HLA-A*0201+, HA-1+, HA-2+healthy volunteer. Target cells: PHA-blasts (▧); fibroblasts (⧫); fibroblasts cultured with IFN-γ + TNF- (both 300 U/mL) (◍); fibroblasts cultured with IFN-γ + TNF- and pulsed with 10 μg/mL peptide (◍).
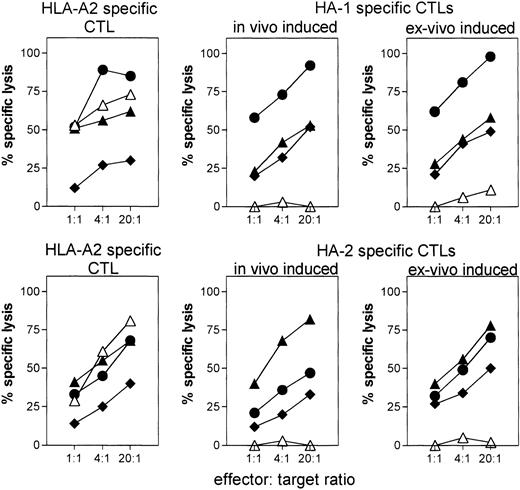
The ex vivo–induced HA-1– and HA-2–specific CTLs were subsequently analyzed for cytolytic activity against, for this study the most relevant target cells, leukemic cells from AML and ALL patients. In vivo–induced HA-1– and HA-2–specific CTLs and an HLA-A*0201–specific alloreactive CTL were used as control effector cells. As shown in Fig 4, AML and ALL cells were lysed by the HLA-A*0201–specific alloreactive CTL, and by the in vivo–induced HA-1– and HA-2–specific CTLs, showing that the leukemic cells were HLA-A*0201–positive and expressed HA-1 or HA-2 antigens. Importantly, the ex vivo–induced CTLs lysed the leukemic cells efficiently and comparable to the control effector cells (Fig 4). The level of cytotoxicity could be significantly enhanced following IFN-γ and TNF-α treatment of the leukemic cells, indicating that cytokines upregulate HLA class I expression on the leukemic cells (Fig 4).
Lysis of HA-1+ or HA-2+leukemic cells by ex vivo–induced HA-1– and HA-2–specific CTLs. Data are representative for all ex vivo–induced CTLs. The lysis of same target cells by in vivo–induced HA-1– and HA-2–specific CTLs, and by control HLA-A*0201–specific CTL clone are shown for comparison. Target cells: HA-1− or HA-2− EBV-LCL (△); HA-1+ or HA-2+ EBV-LCL (▧); leukemic cells positive for HA-1 (n = 4) or HA-2 (n = 3) (⧫); HA-1+ or HA-2+ leukemic cells cultured with IFN-γ + TNF- (◍).
Lysis of HA-1+ or HA-2+leukemic cells by ex vivo–induced HA-1– and HA-2–specific CTLs. Data are representative for all ex vivo–induced CTLs. The lysis of same target cells by in vivo–induced HA-1– and HA-2–specific CTLs, and by control HLA-A*0201–specific CTL clone are shown for comparison. Target cells: HA-1− or HA-2− EBV-LCL (△); HA-1+ or HA-2+ EBV-LCL (▧); leukemic cells positive for HA-1 (n = 4) or HA-2 (n = 3) (⧫); HA-1+ or HA-2+ leukemic cells cultured with IFN-γ + TNF- (◍).
The feasibility of adoptive immunotherapy with ex vivo–generated CTLs depends also on their expandability to sufficient numbers. We scored the expansion rates of HA-1– and HA-2–specific CTLs generated by DC and calculated the total yield based on the expansion without cryopreservation (Fig 5). Sufficient numbers of CTLs for adoptive immunotherapy can be obtained by initiating the cell cultures with 5 × 107 responder cells. Figure 5 shows that two HA-2–specific CTLs induced by PBDC showed expansion rates of greater than 9-, 25-, and 8-fold at the second, third, and fourth week, respectively, yielding a total of 3 × 109 to 1010 CTLs at the end of the fourth week. The expansion kinetics of the HA-1–specific CTLs were slower, but the cells expanded consistently with doubling times of 2 to 3 days during each restimulation. It is estimated that 109HA-1–specific CTLs can be obtained after 5 weeks of culture (Fig 5).
Expansion of HA-1– and HA-2–specific CTLs. Data represent a theoretical calculation based on the actual expansion rates of the CTLs. The expansion rates of the CTLs were calculated as follows: expansion rate = Total number of the cells after restimulation/no. of restimulated cells. The initial number of responder cells was 5 × 107 and the total yield of CTLs before each restimulation was calculated by multiplying the expansion rate per week with the number of theoretically restimulated cells. (◍,◊) Two HA-2–specific CTLs induced by PBDC; (△) HA-1–specific CTL induced by PBDC; (⧫) HA-1–specific CTL induced by BMDC.
Expansion of HA-1– and HA-2–specific CTLs. Data represent a theoretical calculation based on the actual expansion rates of the CTLs. The expansion rates of the CTLs were calculated as follows: expansion rate = Total number of the cells after restimulation/no. of restimulated cells. The initial number of responder cells was 5 × 107 and the total yield of CTLs before each restimulation was calculated by multiplying the expansion rate per week with the number of theoretically restimulated cells. (◍,◊) Two HA-2–specific CTLs induced by PBDC; (△) HA-1–specific CTL induced by PBDC; (⧫) HA-1–specific CTL induced by BMDC.
DISCUSSION
We show that mHag HA-1– and HA-2–specific CTLs can reproducibly be generated ex vivo from HLA-A*0201–positive, mHag HA-1– and/or HA-2–negative healthy blood donors using DC pulsed with synthetic peptides. These CTLs display specific cytotoxic activity against mHag-positive target cells, including leukemic cells from AML and ALL patients, but not against nonhematopoietic cells. Sufficient numbers of CTLs can be generated for adoptive immunotherapy purposes.
To establish the optimal protocol for the ex vivo generation of HA-1– and HA-2–specific CTLs, we have compared DC, PBMC, and monocytes as APCs. Only DC induced potent HA-1– and HA-2–specific CTLs. These results underscore the superior capacity of DC to induce T-cell responses from naive precursors and confirm the current opinion on the DC characteristics.16 We observed that monocytes or poorly enriched DC can also induce mHag peptide-specific CTLs, but these CTLs fail to recognize the naturally expressed mHag. This finding may have implications for studies in which the immunogenicity of putative tumor-associated peptides is tested by loading on PBMC, monocytes, or on partially maturated monocyte-derived DC. In various studies, CTLs generated against these putative tumor-associated peptides does not recognize the tumor cells.22 23 This may suggest that these putative peptides are not present on the tumor cells, or according to our findings, only appropriately generated DC are capable of inducing CTLs recognizing the naturally expressing ligand.
The functional analysis of the HA-1– and HA-2–specific CTLs generated ex vivo shows (Figs 2 to 4) no autoreactivity, or reactivity to third-party alloantigens. Thus, these CTLs can be safely transferred to the patients. Furthermore, the results clearly show that ex vivo–generated CTLs lyse hematopoietic cells effectively, but not the nonhematopoietic system cells, such as fibroblasts. Adoptive transfer of HA-1– or HA-2–specific CTLs to HA-1– or HA-2–positive patients will spare the patient’s nonhematopoietic tissues. Thus, upon adoptive transfer of HA-1– and HA-2–specific CTLs, a low risk of GVHD is expected. Some precaution may be necessary, since we have previously demonstrated that HA-1 disparity between patient and donor is associated with the development of GVHD in adults.24Therefore, in the clinical trials we will transfer the CTLs not before 50 to 60 days post BMT. It is expected that most recipient hematopoietic cells will then be replaced by HA-1– or HA-2–negative donor cells. Alternatively, one may consider to transduce the HA-1– and HA-2–specific CTLs with a suicide gene that will make the in vivo elimination of cells possible if adverse effects occur.25Most importantly, our study shows that the ex vivo–generated HA-1– and HA-2–specific CTLs effectively lyse leukemic cells derived from AML and ALL patients, both type of leukemias being resistant to DLI treatment. The level of cytotoxicity could be significantly enhanced following IFN-γ and TNF-α treatment of the leukemic cells, indicating that HLA class I antigens are upregulated. HA-1– and HA-2–specific CTL clones produce IFN-γ and TNF-α in vitro (manuscript in preparation). It is possible that cytokine production by HA-1– and HA-2–specific CTLs occurs in-vivo as well. Alternatively, the efficacy of adoptive immunotherapy with HA-1– and HA-2–specific CTLs may be enhanced by coadministration of IFN-α in resistant cases. After the successful application of EBV-specific CTLs as specific adoptive immunotherapy of EBV-related malignancies,26 our results now open a new possibility for the treatment of relapsed, HA-1– and/or HA-2–positive leukemia patients with HA-1– or HA-2–specific CTLs induced ex vivo from their HLA-identical, mHag-negative bone marrow donors.
ACKNOWLEDGMENT
We thank Dr M. Oudshoorn for critically reading the manuscript.
Supported in part by grants from the Dutch Cancer Foundation (Koningin Wilhelmina Fonds), Leukemia Society of America, Leiden University Medical Center, and the J.A. Cohen Institute for Radiopathology and Radiation Protection. B.E. is a Leukemia Society of America Translational Research Awardee.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tuna Mutis, MD, PhD, Department of Immunohematology and Blood Bank, Leiden University Medical Center, Bldg 1, E3-Q, Box 9600, 2300 RC Leiden, The Netherlands; e-mail: Mutis@rullf2.leidenuniv.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal