Abstract
The Janus kinase, JAK3 plays an important role in interleukin-2 (IL-2)–dependent signal transduction and proliferation of T lymphocytes. Our findings show that prostaglandin E2(PGE2) can inhibit upregulation of JAK3 protein in naive T cells and can downregulate its expression in primed cells. Reduction in JAK3 was selective because expression of other tyrosine kinases (JAK1, p56lck, and p59fyn) and signal transducer and activator of transcription (STAT)5, which are linked to IL-2 receptor (IL-2R) signaling pathway, were not affected. Inhibition of JAK3 may be controlled by intracellular cyclic adenosine monophosphate (cAMP) levels, as forskolin, a direct activator of adenylate cyclase and dibutyryl cAMP (dbcAMP), a membrane permeable analogue of cAMP suppressed JAK3 expression. Moreover, 3-isobutyl-1-methylxanthine (IBMX), an inhibitor of cAMP phosphodiesterase, potentiated PGE2-induced suppression of JAK3. In naive T cells, but not primed T cells, PGE2 and other cAMP elevating agents also caused a modest reduction in surface expression of the common gamma chain (γc) that associates with JAK3. The absence of JAK3, but not IL-2R in T cells correlated with impaired IL-2–dependent signal transduction and proliferation. The alteration in IL-2 signaling included decreased tyrosine phosphorylation and DNA binding activity of STAT5 and poor induction of the c-Myc and c-Jun pathways. In contrast, IL-2–dependent induction of Bcl-2 was unaffected. These findings suggest that suppression of JAK3 levels may represent one mechanism by which PGE2 and other cAMP elevating agents can inhibit T-cell proliferation.
T LYMPHOCYTE ACTIVATION through antigen presentation to the T-cell receptor-CD3 complex (TCR-CD3) results in the passage from G0 to G1 of the cell cycle and in the development of T-cell competency.1-3 This process is prerequisite for further cell cycle progression1-3 and involves activation of genes that encode cytokines and receptors such as interleukin-2 (IL-2) and the IL-2 receptor (IL-2R), respectively.3,4T-cell activation results in expression of IL-2Rα (55 kD), common γ chain (γc) (64 kD), and an increase in the level of IL-2Rβ (75 kD).4,5 The high-affinity IL-2R contains all three chains, while expression and heterodimerization of the β and γ chains alone is sufficient to mediate IL-2–dependent signaling.4-7 IL-2 binding to its receptor controls movement from G1 into S phase of the cell cycle and therefore plays an important role in T-cell proliferation.3 4
Signal transduction through the IL-2R is initiated by activation of tyrosine kinases associated with IL-2Rβ and γc.3,4,8-15This includes Janus family protein tyrosine kinase (PTK) JAK3 that is associated with the carboxy-terminal region of γc and JAK1 that is associated with the serine-rich region of the β chain.3,4,12-15 The src family of kinases (Lck, Fyn, and Lyn) are activated through their interaction with the acidic region of the IL-2Rβ chain, whereas the Syk-Zap-70 family of PTK interacts with the serine-rich region of the β chain.11,16 Very early events in IL-2–dependent signaling involves tyrosine phosphorylation and activation of the JAK kinases that may precede activation of the Src and Syk-Zap-70 kinases and the phosphorylation of a number of substrates.17 These early events ultimately result in the induction of three distinct signaling pathways linked to the IL-2R that are defined by the expression of c-myc, c-fos/jun, and Bcl-2, respectively.3,4 Activation of these pathways leads to movement of T cells into the S phase of the cell cycle.4
Prostaglandin E2 (PGE2) is known to inhibit T-cell activation.18-20 The mechanism of suppression appears to be through increasing levels of the intracellular second messenger, cyclic adenosine monophosphate (cAMP), that binds to its receptor, protein kinase A (PKA).21 IL-2 and IL-2Rα gene expression are both targets of PGE2-induced suppression, possibly through the inhibition of early events in T-cell signaling that include calcium influx and phosphatidylinositol breakdown.22,23 PGE2 is also known to inhibit the DNA binding activity of the transcription factor, NFκB to the IL-2 transcriptional start site thereby blocking formation of IL-2.24 Increasing cAMP levels and activation of PKA also alters PKC-induced transcriptional regulation of members of thejun and fos family of genes.25 More recent studies indicate that PGE2-induced inhibition of T-cell proliferation is mediated through blocking IL-2–dependent G1-S transition.26 27 However, little is known about the effect PGE2 has on signal transduction elements that are linked to the IL-2R.
Here we show that PGE2 inhibited induction of JAK3 expression in naive T cells and downregulated its expression in primed cells. The suppression of JAK3 was selective, as PGE2 had minimal effect on expression of other tyrosine kinases linked to the IL-2R. Moreover, JAK3 expression may be regulated by an increase in cAMP, as forskolin and dibutyryl cAMP (dbcAMP), which increase cAMP levels, induced a similar defect. This reduction in JAK3 resulted in impaired phosphorylation and DNA binding activity of signal transducer and activator of transcription (STAT)5 and decreased induction of c-Myc and c-Jun, but not Bcl-2. The block in IL-2–dependent proliferation and signaling in both naive and primed T cells coincided with suppression in JAK3 rather than with a reduction in IL-2R expression. Because JAK3 is critical to IL-2–dependent signaling and proliferation, its sensitivity to PGE2 and other agents that elevate cAMP may make it a prime target for suppressing IL-2–dependent cell cycle progression in lymphocytes.
MATERIALS AND METHODS
Antibodies and reagents.
PGE2, 3-isobutyl-1-methylxanthine (IBMX), dbcAMP, isoquinolinesulfonamide dihydrochloride (H-89), and myristoylated protein kinase inhibitor (PKI) amide were purchased from Biomol (Plymouth Meeting, PA). Forskolin, phorbol 12,13-dibutirate (PDB), and ionomycin were obtained from Sigma (St Louis, MO). Recombinant human IL-2 was provided by Chiron Therapeutics (Emeryville, CA). Anti-CD3 antibody (OKT3) was purchased from Ortho (Raritan, NJ). Phytohemagglutinin (PHA) was purchased from Difco (Detroit, MI). Antibody to Jak3 was prepared as previously described.28Protein kinase assay kit was obtained from Calbiochem (San Diego, CA) and the cAMP EIA kit was purchased from Cayman Chemical Co (Ann Arbor, MI). Antibody to JAK1 and STAT5 was purchased from Transduction Laboratories (Lexington, KY), whereas antibody to p59fynwas obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Antibody to p56lck was purchased from UBI (Lake Placid, NY). Antibodies to α (CD25) and β (CD122) chains of the IL-2R were obtained from Becton Dickinson (Mountain View, CA) and the anti-γc chain antibody (3B5) was prepared as previously described.29 Antibodies to c-Myc (9E10), c-Fos, and Bcl-2 were purchased from Santa Cruz Biotechnology Inc. Mouse monoclonal antiphosphotyrosine Ab, 4G10 was purchased from UBI. Horseradish peroxidase-conjugated sheep antimouse Ig (Cat. No. NA9310) and donkey antirabbit (NA934) were purchased from Amersham (Arlington Heights, IL). The following antibody-coated beads were purchased from Miltenyi Biotech (Sunnyvale, CA): anti-CD14, anti-CD16, and anti-CD19. Fluorescein isothiocyanate (FITC)-conjugated anti-CD45RB and phycoerythrin (PE)-conjugated anti-CD5 antibodies were purchased from Becton Dickinson (Mountain View, CA). Medium used for the culture of T cells was RPMI 1640 (Bio-Whittaker, Walkersville, MD) supplemented with 10% fetal calf serum (FCS) (Hyclone, Logan, UT), 200 mmol/L L-glutamine, gentamycin (50 mg/L), 100 mmol/L sodium pyruvate, and 10 mmol/L nonessential amino acids. Cell lines used in these studies included the transformed T-cell lymphoma, Jurkat and a primary CD4+ human T-cell line (CD4K) that recognizes autologous renal cell cancer.
Isolation of peripheral blood–derived T lymphocytes.
Peripheral blood T lymphocytes (T-PBL) from healthy volunteers were isolated and purified as previously described.30,31 PBL were isolated using Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation. Thereafter, T cells were depleted of macrophages, B cells, and natural killer (NK) cells by incubating with microbeads coated with antibodies to CD14, CD19, and CD16, respectively. Negative selection by magnetic separation was performed as previously reported30 31 (Miltenyi Biotech Inc). The T-cell isolation procedure yielded cells that were more than 92% positive for CD3.
To obtain primed T cells, highly enriched populations of CD3+ cells purified from the peripheral blood of healthy volunteers were cultured at a density of 1 × 106/mL for 3 days in complete RPMI medium supplemented with PHA (10 μg/mL).
Immunocytometry.
Surface expression of IL-2Rβ (CD122), IL-2Rα (CD25), and γc on peripheral blood–derived T cells was detected using immunofluorescence based flow cytometry before and at various times after stimulation with anti-CD3 and IL-2 in the presence and absence of cAMP enhancing agents.32 The following monoclonal antibody (MoAb) conjugates were used to phenotypically identify and quantitate lymphocytic subsets: anti-CD3-PE and anti-CD25-PE from Becton Dickinson; anti-IL-2Rβ-FITC from Endogen (Cambridge, MA) and anti-γc (3B5). All analysis was performed for 3,000 or 10,000 event list mode files acquired through a forward versus orthogonal scatter gate predefined for CD45+CD14− cells. Matched isotypic controls were used for each particular subclass of Ig and system used.
Analyses on the FACScan (Becton Dickinson) were performed using an argon ion laser (Spectra Physics Laser, Mountain View, CA) with 15 mW of 488 nm excitation. Live gating of the forward and orthogonal scatter channels was used to exclude debris and to selectively acquire lymphocytes events. All values presented are based on percent lymphocytes as determined by light scatter. Individual fluorescence populations were determined through the use of acquisition and contouring/quadrant analysis software (Cell Quest; Becton Dickinson). Results were reported as a percentage of CD3+IL-2Rβ, CD3+ CD25+, and CD3+γc+ cells in suspension corrected for nonspecific binding of isotypic controls. The individual contour plots were derived by joining dots of the same density.
Measurement of cAMP.
Freshly isolated T cells (1 × 106 cells/mL) were incubated in RPMI 1640 at 37°C in the presence of 100 μmol/L of the phosphodiesterase inhibitor IBMX for 30 minutes followed by incubation for 5 minutes with PGE2 (10 μmol/L). Intracelluler cAMP levels were measured according to a protocol provided with cAMP EIA kit (Cayman Chemical Co).
Assessment of T-cell viability.
After T cells were cocultured in media, PGE2 (0.1, 1, and 10 μmol/L), dbcAMP (1 mmol/L) or forskolin (100 μmol/L), the number of viable cells was determined by trypan blue dye exclusion. We also determined if PGE2, dbcAMP, and forskolin induced apoptosis in T cells using a DNA fragmentation detection kit from Oncogene Research Products (Cambridge, MA).
Assay for T-cell proliferation.
Proliferation of T cells was determined by the uptake of [3H] thymidine.30 Lymphocytes were cultured at a density of 5 × 104 cells/well in U bottom 96-well plates in triplicate for each culture condition. Cells were stimulated with one of the following in the presence and absence of different concentrations of PGE2, with and without IBMX; (1) medium, (2) 1,000 IU/mL of recombinant IL-2 in the absence and (3) presence of cross-linked anti-CD3 (OKT3). Two days after initiation of culture, cells were pulsed with 1 μCi of [3H] thymidine (6.7 Ci/mmol; NEN, Dupont, Wilmington, DE) and harvested 24 hours later using a PHD harvester (Cambridge Technology Inc, Cambridge, MA). Filters were placed in scintillation fluid (Ecoscint, National Diagnostic, Manville, NJ) and counted in a β-counter (Beckman, Fullerton, CA). Results are expressed as mean counts per minute (cpm) (±standard error of mean [SEM] of triplicate samples).
Stimulation of T cells with cross-linked anti-CD3 antibody (OKT3) was performed by preincubating flasks with 1 mol/L Tris buffer (pH 8.0) containing 10 μg/mL of antibody for 30 minutes. Thereafter, the flasks were washed twice with RPMI to remove unbound antibody and then cells were added at a density of 1 × 106/mL for culturing.
Western blot analysis and immunoprecipitation.
Cells were lysed as previously described33 directly in buffer (50 mmol/L Tris [pH 7.6], 150 mmol/L NaCl, 1% Triton X-100) containing protease and phosphatase inhibitors; aprotinin (5 μg/mL), leupeptin (2 μg/mL), sodium fluoride (50 mmol/L), phenylmethylsulfonyl fluoride (PMSF) (1 mmol/L), and sodium ortho-vanadate (1 mmol/L). Samples were placed on ice for 20 minutes with occasional vortexing. Protein concentration was measured with a commercial kit (Bio-Rad, Richmond, CA).
Equivalent amounts of proteins from whole cell lysates (10 μg) were mixed with an equal volume of 2X Laemmli sample buffer, boiled, and resolved by electrophoresis in 7.5%, 10%, and 12% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE). The proteins were transferred from the gel to a nitrocellulose membrane using an electroblotting apparatus (Bio-Rad) (15 V, 3 mA/cm2 for 24 minutes). Membranes were incubated in blocking solution containing 5% nonfat dry milk in TRIS-buffered saline overnight to inhibit nonspecific binding. The membranes were then incubated with specific antibody (1 μg/mL) for 2 hours. After washing in TRIS with 0.1% Tween 20 for 30 minutes, membranes were incubated for another 30 minutes with horseradish peroxidase-conjugated secondary antibody. The membranes were then washed and developed with enhanced chemiluminescence (ECL Western Blotting Kit; Amersham).
Immunoprecipitation of STAT5 was performed by adding 4 μg of the anti-STAT5 Ab to 100 μg of cell lysate protein for 2 hours at 4°C. Thirty microliters of protein G-Sepharose-conjugated beads was added for 1 hour. Immunoprecipitates were washed three times with lysis buffer (50 mmol/L Tris [pH 7.4], 150 mmol/L NaCl, 1% Triton X-100) containing protease and phosphatase inhibitors; aprotinin (5 μg/mL), leupeptin (2 μg/mL), pepstatin (5 μg/mL), sodium pyrophosphate (5 mmol/L), sodium fluoride (50 mmol/L), PMSF (1mmol/L), and sodium ortho-vanadate (1 mmol/L). Proteins were eluted by boiling for 7 minutes in Laemmli sample buffer. Equivalent amounts of protein were resolved on 7.5% SDS-PAGE and transferred to nitrocellulose membranes for Western blot analysis.
In some experiments, densitometry scanning of immunoblots was performed as follows. The developed X-omat AR film was placed on a white light box by Fotodyn and its image captured by a High Resolution CCD camera (Sierra Scientific, Sunnyvale, CA). NIH Image 1.57 (National Institutes of Health, Bethesda, MD) was the program used to analyze the density of each band by graphically plotting the images and calculating the area under each peak. The percent reduction in expression of a given protein by PGE2and other agents that increase cAMP was calculated using T cells cultured in medium as the control.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts isolated from T cells were incubated in 25 μL reaction mixture containing 20 mmol/L HEPES, 80 mmol/L NaCl, 0.1 mmol/L EDTA, 1 mmol/L dithiorthreitol (DTT), 8% glycerol, and 2 μL of poly (dI-dC) (Pharmacia, Piscataway, NJ) for 15 minutes at 4°C. For antibody-mediated supershift studies, 5 μL of the anti-STAT5 antibody was added during the preincubation for 30 minutes at room temperature before the addition of labeled probe. The probe used for the EMSA was the radiolabled IRF1-GAS (Interferon Regulatory Factor 1-Gamma Activating Site) that has affinity for STAT 1-6. The probe was prepared by annealing the coding strand template to a 10-base complementary primer and a nick enzyme was used to complete the overhang in the presence of [α-32P] deoxycytidine triphosphate (dCTP). After the probe was added to the nuclear extracts, the samples were incubated at room temperature for 20 minutes and then run on a 6% nondenaturing polyacrylamide gel. The gels were dried and analyzed by autoradiography.
Statistical analysis.
The paired t-test was used to determine if results (ie, JAK3 protein levels) obtained from T cells cultured in media (with and without stimulus) are statistically different from those where T cells were cultured with cAMP elevating agents. The results are presented as percent reduction in mean pixel number [(1-mean pixel number of treated T cells/mean pixel number of media control T cells) × 100]. All statistical tests were performed using a 5% level of significance.
RESULTS
PGE2 inhibits the induction of JAK3 in resting human peripheral blood T lymphocytes and downregulates its expression in primed cells.
Recent studies have shown that JAK3 is required to activate IL-2R pathway for T-cell proliferation.4,14,17,34-36 Loss of JAK3 expression or kinase activity results in impaired activation of the IL-2R signaling pathway.17 JAK3 protein expression is known to be regulated in T lymphocytes.8 28 In resting lymphocytes, JAK3 is constitutively expressed at low levels and stimulation with either PMA/ionomycin or signaling through the TCR/CD3 complex is known to upregulate its expression (Fig 1). The findings reported here show that increased JAK3 expression induced by stimulation of naive peripheral blood T cells with anti-CD3 plus IL-2 was blocked by PGE2 when added at the beginning of culture (Fig 1). PGE2 at 10 μmol/L consistently had the greatest effect on JAK3 expression (n = 3 experiments). Western blotting experiments showed that the inhibition of JAK3 expression was selective, as PGE2 had no effect on the levels of other IL-2R–linked kinases, JAK1, p56lck, and p59fyn. Densitometry scanning of the Western blots confirmed that after 48 hours of stimulation JAK3 protein levels increased and that the presence of PGE2 reduced its expression in three separate experiments (mean 56.6 ± 8.6 standard deviation [SD] % reduction, P< .001).
PGE2 inhibits upregulation of JAK3 in naive T cells. (A) Highly enriched naive T cells were cultured for 48 hours under the following conditions; medium alone; cross-linked anti-CD3 and IL-2 (1,000 U/mL) alone and anti-CD3/IL-2 plus PGE2 with and without IBMX. Cell lysates were then subjected to immunoblotting using antibodies to JAK1, JAK3, p56lck, and p59fyn. In this and all experiments, equal amounts of protein were added to each lane. Densitometry readings of the immunoblot showed the following reduction of JAK3 by PGE2alone and with IBMX: 0% 0.1 μmol/L PGE2, 4% 1 μmol/L PGE2, 40% 10 μmol/L PGE2, 63% 0.1 μmol/L PGE2/IBMX, 68% 1 μmol/L PGE2/IBMX, and 76% 10 μmol/L PGE2/IBMX. (B) Proliferation was assessed by the uptake of [3H] thymidine as described in Materials and Methods. (C) Effect of PGE2 on cAMP production in resting T cells. Freshly isolated T cells were incubated in RPMI 1640 at 37°C in the presence of 100 μmol/L of the phosphodiesterase inhibitor IBMX for 30 minutes followed by incubation for 5 minutes with PGE2 (10 μmol/L) before measuring cAMP levels. A significant reduction in JAK3 expression was observed in three experiments (P < .001).
PGE2 inhibits upregulation of JAK3 in naive T cells. (A) Highly enriched naive T cells were cultured for 48 hours under the following conditions; medium alone; cross-linked anti-CD3 and IL-2 (1,000 U/mL) alone and anti-CD3/IL-2 plus PGE2 with and without IBMX. Cell lysates were then subjected to immunoblotting using antibodies to JAK1, JAK3, p56lck, and p59fyn. In this and all experiments, equal amounts of protein were added to each lane. Densitometry readings of the immunoblot showed the following reduction of JAK3 by PGE2alone and with IBMX: 0% 0.1 μmol/L PGE2, 4% 1 μmol/L PGE2, 40% 10 μmol/L PGE2, 63% 0.1 μmol/L PGE2/IBMX, 68% 1 μmol/L PGE2/IBMX, and 76% 10 μmol/L PGE2/IBMX. (B) Proliferation was assessed by the uptake of [3H] thymidine as described in Materials and Methods. (C) Effect of PGE2 on cAMP production in resting T cells. Freshly isolated T cells were incubated in RPMI 1640 at 37°C in the presence of 100 μmol/L of the phosphodiesterase inhibitor IBMX for 30 minutes followed by incubation for 5 minutes with PGE2 (10 μmol/L) before measuring cAMP levels. A significant reduction in JAK3 expression was observed in three experiments (P < .001).
It is known that exposure of naive and activated peripheral blood T cells to PGE2 results in increased production of intracellular second messenger cAMP.37-39 In our experiments, the cAMP production in T lymphocytes induced by 10 μmol/L PGE2/IBMX was up sixfold to eightfold higher than in cells not exposed to PGE2/IBMX (Fig 1). Because the suppressive activity of PGE2 appears to be mediated by cAMP, we determined if other agents that increase cAMP would suppress JAK3 expression. Here we determined if IBMX would potentiate the suppression of JAK3 by stabilizing the levels of cAMP increased by PGE2. Cyclic nucleotides are regulated by cAMP phosphodiesterase and IBMX is known to block the action of this enzyme resulting in an increase in intracellular levels of cAMP.21At the concentration used in these experiments, IBMX (10 μmol/L) had little effect on JAK3 levels however, it did increase the suppression of JAK3 mediated by a suboptimal concentration of PGE2 (0.1 μmol/L PGE2) (Fig 1). The difference in JAK3 expression in naive T cells cultured with or without PGE2/IBMX was statistically significant (P = .004 for naive T cells [n = 3]). In the same experiments, it was noted that the suppression of T-cell proliferation by PGE2 alone and when combined with IBMX paralleled the inhibition in JAK3 expression (Fig 1).
We also determined whether PGE2 with and without IBMX would downregulate JAK3 that is expressed in activated T cells. Western blotting was performed on T cells that had been stimulated with PHA for 72 hours and then cultured in the presence and absence of PGE2 for 24 hours. Representative data are presented in Fig 2 and shows that primed T cells express significant levels of JAK3; however, coculture with 10 μmol/L PGE2 reduced expression of JAK3 by 58% without having any affect on the other kinases. In addition, IMBX (10 μmol/L) and PGE2 (0.1 μmol/L) at concentrations not effective alone did suppress JAK3 expression when combined (P < .001 for primed T cells [n = 8]).
PGE2 downregulated JAK3 expression in primed T cells. CD3+ peripheral blood lymphocytes were cocultured with PHA for 3 days. Primed cells were then cultured for 24 hours with medium IBMX, PGE2, or PGE2 plus IBMX before performing a Western blot to detect JAK1, JAK3, p59fyn, or p56lck. Representative data are presented.
PGE2 downregulated JAK3 expression in primed T cells. CD3+ peripheral blood lymphocytes were cocultured with PHA for 3 days. Primed cells were then cultured for 24 hours with medium IBMX, PGE2, or PGE2 plus IBMX before performing a Western blot to detect JAK1, JAK3, p59fyn, or p56lck. Representative data are presented.
The inhibition of JAK3 expression by PGE2 was induced by dbcAMP and forskolin in T cells.
Additional experiments examined whether dbcAMP, an analog of cAMP, and forskolin, a diterpin that activates adenylate cyclase, could suppress JAK3 levels (Fig 3).21 Western blotting was performed after naive T cells were stimulated with anti-CD3/IL-2 for 24 and 48 hours in the presence and absence of cAMP elevating agents. Both forskolin and dbcAMP inhibited the stimulus-dependent expression of JAK3, whereas these agents had little effect on the expression of p56lck, p59fyn, or JAK1. The expression of STAT5 was also examined, as it is involved in the IL-2–dependent signaling pathway.4,17,40 41 In contrast to JAK3, the levels of STAT 5 were not affected by either dbcAMP or forskolin.
Forskolin and dbcAMP can mimic the effect of PGE2 and selectively suppress JAK3 expression in naive and primed T cells. (A) CD3+ lymphocytes were stimulated with anti-CD3/IL-2 for 24 and 48 hours in the presence of PGE2(10 μmol/L), Forskolin (100 μmol/L), or dbcAMP (1 mmol/L). Western blotting was performed for JAK1, JAK3, p59fyn, p56lck, and STAT5. (B) Primed T cells were cultured with medium, Forskolin (100 μmol/L) or dbcAMP (1 mmol/L) for 48 hours before Western blotting. Representative data from 1 of 4 experiments. (C) Primary CD4+ T-cell line (CD4K) and Jurkat T cells were cultured with dbcAMP (1 mmol/L) for 48 hours before Western blotting. Representative data from 1 of 4 experiments are presented.
Forskolin and dbcAMP can mimic the effect of PGE2 and selectively suppress JAK3 expression in naive and primed T cells. (A) CD3+ lymphocytes were stimulated with anti-CD3/IL-2 for 24 and 48 hours in the presence of PGE2(10 μmol/L), Forskolin (100 μmol/L), or dbcAMP (1 mmol/L). Western blotting was performed for JAK1, JAK3, p59fyn, p56lck, and STAT5. (B) Primed T cells were cultured with medium, Forskolin (100 μmol/L) or dbcAMP (1 mmol/L) for 48 hours before Western blotting. Representative data from 1 of 4 experiments. (C) Primary CD4+ T-cell line (CD4K) and Jurkat T cells were cultured with dbcAMP (1 mmol/L) for 48 hours before Western blotting. Representative data from 1 of 4 experiments are presented.
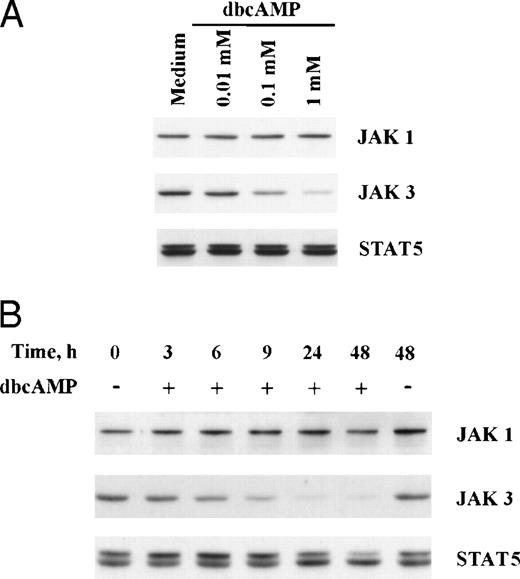
Similar findings were observed when primed T cells were cocultured with forskolin or dbcAMP (Figs 3 and 4). Concentration-dependent suppression of JAK3 by dbcAMP is presented in Fig 4 and illustrates that inhibition is seen with 0.1 mmol/L dbcAMP (54% reduction), although 1 mmol/L is most active (84% reduction). The time frame required for suppression of JAK 3 in primed T cells by dbcAMP was also examined. Cells were stimulated with PHA for 3 days and then washed and cultured with dbcAMP for various lengths of time. As seen in Fig 4, the reduction of JAK3 protein by dbcAMP was noticeable after 6 hours of incubation (31% reduction) and was near completion by 24 hours (92% reduction). An analysis of all experiments showed a significant reduction in JAK3 levels for both naive T cells (n = 5,P < .001) and for primed T cells (n = 8, P < .001) cocultured with dbcAMP. The fact that both forskolin and dbcAMP could mimic the suppression induced by PGE2 is consistent with the idea that Jak3 expression is regulated by elevated cAMP levels. The decreased expression of JAK3 induced by dbcAMP is not unique to normal human T cells because this suppression was also noted with an untransformed T-cell line (CD4K) and the transformed line, Jurkat (Fig 3).
Suppression of JAK3; concentration and time dependent studies with dbcAMP. (A) Primed T cells were cultured for 24 hours with various concentrations of dbcAMP before Western blotting. (B) Primed T cells were cultured with 1 mmol/L dbcAMP for various lengths of time before Western blotting. An analysis of all experiments showed a significant reduction in JAK3 levels for both naive T cells (n = 5,P < .001) and for primed T cells (n = 8, P < .001) cocultured with dbcAMP.
Suppression of JAK3; concentration and time dependent studies with dbcAMP. (A) Primed T cells were cultured for 24 hours with various concentrations of dbcAMP before Western blotting. (B) Primed T cells were cultured with 1 mmol/L dbcAMP for various lengths of time before Western blotting. An analysis of all experiments showed a significant reduction in JAK3 levels for both naive T cells (n = 5,P < .001) and for primed T cells (n = 8, P < .001) cocultured with dbcAMP.
It is also important to note that the reduction of JAK3 expression in naive and primed T cells was not due to reduced viability after coculture with agents that increase cAMP. After incubation with PGE2 (0.1, 1, and 10 mmol/L), dbcAMP (1 mmol/L), or forskolin (100 μmol/L), the number of viable cells as determined by trypan blue staining was comparable to that of T cells cultured in medium alone (>90% trypan blue negative). We also showed that PGE2 (10 mmol/L), dbcAMP (1 mmol/L), and forskolin (100 μmol/L) did not induce any significant apoptosis in T cells as determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The level of apoptotic T cells cultured for 48 hours in PGE2 (6.9%), dbcAMP (10.1%), and forskolin (7.4%) was low and not different from that of T cells cultured in medium (7%).
In naive, but not primed T cells, cAMP elevating agents selectively suppressed surface expression of the JAK3-associated γc chain without affecting IL-2Rβ.
Because γc and IL-2Rβ interact with JAK3 and JAK1, respectively, and both chains are required for IL-2R signaling and proliferation, the impact that PGE2/IBMX had on their surface expression was examined along with the IL-2Rα chain. As previously reported, the percentage of resting T cells that express IL-2R chains is low, but is highly inducible after 48 hours of stimulation with anti-CD3/IL-2. In agreement with previous studies, PGE2/IBMX caused a partial reduction in surface expression of the α chain (21% reduction, Fig 5).22,39PGE2/IBMX also modestly suppressed induction of γc. After 48 hours of stimulation, there is a significant increase in the percentage of T cells that express γc and the presence of PGE2/IBMX reduced the number of cells expressing γc by 28% percent (Fig 5).4 5 In contrast, PGE2/IBMX has no effect on the expression of IL-2Rβ.
In naive T cells PGE2/IBMX caused a partial reduction in IL-2R and γc surface expression, but had no effect on IL-2Rβ. Surface expression of IL-2R, IL-2Rβ, and the common γ chain were examined on CD3+ peripheral blood T cells before and 48 hours after stimulation with anti-CD3 plus IL-2 in the presence and absence of PGE2/IBMX. γ chain expression was determined by staining cells with 3B5. IL-2R and IL-2Rβ were detected by staining the anti-p55–PE and anti-p75–PE in combination with CD3-FITC. The percent reduction in the number of T cells expressing IL-2R chains was: 21% IL-2R, 0% IL-2Rβ, and 28% γc. The percentage of cells in each quadrant is presented adjacent to the histogram. Similar results were obtained in two additional experiments.
In naive T cells PGE2/IBMX caused a partial reduction in IL-2R and γc surface expression, but had no effect on IL-2Rβ. Surface expression of IL-2R, IL-2Rβ, and the common γ chain were examined on CD3+ peripheral blood T cells before and 48 hours after stimulation with anti-CD3 plus IL-2 in the presence and absence of PGE2/IBMX. γ chain expression was determined by staining cells with 3B5. IL-2R and IL-2Rβ were detected by staining the anti-p55–PE and anti-p75–PE in combination with CD3-FITC. The percent reduction in the number of T cells expressing IL-2R chains was: 21% IL-2R, 0% IL-2Rβ, and 28% γc. The percentage of cells in each quadrant is presented adjacent to the histogram. Similar results were obtained in two additional experiments.
The impact PGE2/IBMX and other agents that increase cAMP would have on downregulating IL-2R expression on primed T cells was also examined. After 3 days of stimulation, β chain expression is detectable, but at low levels, whereas the levels of the α and γc remain elevated (Fig 6). In contrast to results with naive cells, none of the agents that increase cAMP downregulated IL-2R αβγ expression on primed T cells after 48 hours of coculture. At this time, JAK3 was reduced and representative data with dbcAMP is presented (Fig 6).
In primed T cells, dbcAMP did not downregulate IL-2R expression, but did suppress JAK3 expression. (A) Highly purified T cells were activated for 3 days with PHA and then cultured for an additional 48 hours in medium alone or medium supplemented with 1 mmol/L dbcAMP. Surface levels of IL-2R, IL-2Rβ, and the γ chain were then determined by staining cells as described in the legend to Fig 5. The percentage of cells in each quadrant is presented adjacent to the histogram. (B) Aliquots of the same cells were subjected to Western blotting to determine if dbcAMP suppressed JAK3. Representative data from one of four experiments are presented.
In primed T cells, dbcAMP did not downregulate IL-2R expression, but did suppress JAK3 expression. (A) Highly purified T cells were activated for 3 days with PHA and then cultured for an additional 48 hours in medium alone or medium supplemented with 1 mmol/L dbcAMP. Surface levels of IL-2R, IL-2Rβ, and the γ chain were then determined by staining cells as described in the legend to Fig 5. The percentage of cells in each quadrant is presented adjacent to the histogram. (B) Aliquots of the same cells were subjected to Western blotting to determine if dbcAMP suppressed JAK3. Representative data from one of four experiments are presented.
Suppression of JAK3 expression by PGE2 correlates with the inhibition of IL-2–dependent signaling pathways for induction of c-Myc and c-Jun.
IL-2R is linked to at least three distinct signaling pathways to activate the protooncogenes critical for IL-2–mediated cell proliferation.4,35 JAK3 appears to play a role in activation of c-Myc and c-Jun, but not the Bcl-2 pathway, as shown by overexpression of a dominant negative mutant of JAK3.4,34 35 Suppression of JAK3 by PGE2/IBMX and other agents that increase cAMP levels raised the issue of whether IL-2–dependent signaling was impaired. To address this issue, we used primed T cells because they provide a way to define the contribution that a loss of JAK3 has on IL-2 signaling in cells that are insensitive to suppression of IL-2R by cAMP elevating agents. After 3 days of priming, T cells were cultured with PGE2/IBMX for 24 hours and then stimulated with IL-2 for various times before performing Western blot. Results of this study are shown in Fig 7. In control cells, the increase in expression of all the protooncogenes was detectable within 6 hours after stimulation with IL-2 and remained elevated for at least 24 hours. As expected, JAK3 expression was significantly reduced by PGE2/IBMX. In the absence of JAK3, the induction of c-Jun by IL-2 was completely inhibited (96% reduction, 24 hours), whereas the induction of c-Myc was only partially suppressed (54%, 24 hours). On the other hand, the IL-2–dependent induction of Bcl-2 was minimally affected (6% reduction) by the loss of JAK3. As seen in Fig 7, the deficiency in JAK3 expression and in IL-2–dependent activation of c-Jun/c-Myc correlated with impaired proliferation to exogenous IL-2. Similar findings were observed when T cells were cocultured with forskolin or dbcAMP (Fig 8).
Suppression of JAK3 expression by PGE2/IBMX correlates with the inhibition of IL-2–dependent signaling pathways for induction of c-Myc and c-Jun. (A) After 3 days of priming, T cells were cultured with PGE2/IBMX for 24 hours and then stimulated with IL-2 for various times before performing Western blot using antibodies to JAK1, JAK3, c-Myc, c-Jun, and Bcl-2. (B) Densitometry scan of immunoblot in (A) showed that after 24 hours of coculture with 10 μmol/L PGE2/10 μmol/L IBMX JAK3 was reduced by 57%, which remained suppressed during IL-2 stimulation (81% reduction). In PGE2/IBMX-treated cells, the IL-2–dependent induction of c-Myc was partially suppressed (54% reduction), whereas the induction of c-Jun was suppressed by 96%. In contrast, PGE2/IBMX had little effect on the increase in Bcl-2 induced by IL-2 stimulation (6% reduction, 24 hours). (C) Primed cells were stimulated with IL-2 (1,000 U/mL) in medium alone or medium supplemented with PGE2/IBMX. Proliferation was assessed after 3 days of culture by measuring the uptake of [3H] thymidine. Representative data are presented (n = 3).
Suppression of JAK3 expression by PGE2/IBMX correlates with the inhibition of IL-2–dependent signaling pathways for induction of c-Myc and c-Jun. (A) After 3 days of priming, T cells were cultured with PGE2/IBMX for 24 hours and then stimulated with IL-2 for various times before performing Western blot using antibodies to JAK1, JAK3, c-Myc, c-Jun, and Bcl-2. (B) Densitometry scan of immunoblot in (A) showed that after 24 hours of coculture with 10 μmol/L PGE2/10 μmol/L IBMX JAK3 was reduced by 57%, which remained suppressed during IL-2 stimulation (81% reduction). In PGE2/IBMX-treated cells, the IL-2–dependent induction of c-Myc was partially suppressed (54% reduction), whereas the induction of c-Jun was suppressed by 96%. In contrast, PGE2/IBMX had little effect on the increase in Bcl-2 induced by IL-2 stimulation (6% reduction, 24 hours). (C) Primed cells were stimulated with IL-2 (1,000 U/mL) in medium alone or medium supplemented with PGE2/IBMX. Proliferation was assessed after 3 days of culture by measuring the uptake of [3H] thymidine. Representative data are presented (n = 3).
Suppression of JAK3 expression by forskolin and dbcAMP correlates with the inhibition of IL-2–dependent signaling pathways for induction of c-Myc and c-Jun. (A) After 3 days of priming, T cells were cultured with forskolin (100 μmol/L) or dbcAMP (1 mmol/L) for 24 hours and then stimulated with IL-2 for various times before performing Western blot using antibodies to JAK1, JAK3, c-Myc, and c-Jun. (B) Densitometry scan of immunoblot in (A). After 24 hours of coculture with forskolin (100 μmol/L) or dbcAMP (1 mmol/L), JAK3 was reduced (43% and 49% correspondingly) and remained suppressed during IL-2 stimulation (79% and 94% reduction correspondingly). IL-2–dependent induction of c-Myc was suppressed by 67% (forskolin) and 61% (dbcAMP). The induction of c-Jun was suppressed by 77% (forskolin) and 69% (dbcAMP).
Suppression of JAK3 expression by forskolin and dbcAMP correlates with the inhibition of IL-2–dependent signaling pathways for induction of c-Myc and c-Jun. (A) After 3 days of priming, T cells were cultured with forskolin (100 μmol/L) or dbcAMP (1 mmol/L) for 24 hours and then stimulated with IL-2 for various times before performing Western blot using antibodies to JAK1, JAK3, c-Myc, and c-Jun. (B) Densitometry scan of immunoblot in (A). After 24 hours of coculture with forskolin (100 μmol/L) or dbcAMP (1 mmol/L), JAK3 was reduced (43% and 49% correspondingly) and remained suppressed during IL-2 stimulation (79% and 94% reduction correspondingly). IL-2–dependent induction of c-Myc was suppressed by 67% (forskolin) and 61% (dbcAMP). The induction of c-Jun was suppressed by 77% (forskolin) and 69% (dbcAMP).
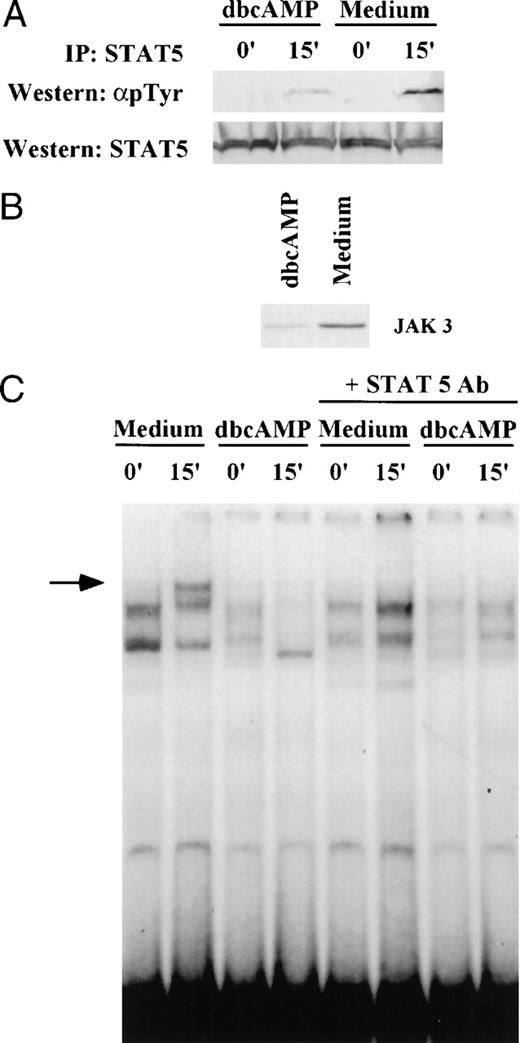
Because IL-2–mediated JAK3 activation results in subsequent tyrosine phosphorylation of STAT5 protein, we examined the impact that JAK3 suppression induced by cAMP elevating agents had on STAT5 activation. Primed T lymphocytes were cultured in the presence of 1 mmol/L dbcAMP for 24 hours followed by IL-2 stimulation for 15 minutes (Fig 9). The suppression of tyrosine phosphorylation of STAT5 protein in dbcAMP-treated cells (68% reduction) paralleled the downregulation of JAK3 expression (71% reduction). These findings show that an early signaling event, STAT5 phosphorylation, linked to JAK3 activation is impaired by dbcAMP. The loss of STAT5 phosphorylation resulted in impaired DNA binding activity. As seen in Fig 9, IL-2 stimulation induced the binding of STAT5 to IRF1-GAS probe in control T cells. The inducible band represents STAT5 binding, as antibody to STAT5 eliminated the expression of this complex in the EMSA. Pretreatment with dbcAMP eliminated the IL-2 inducible STAT5 DNA binding complex. It also reduced the constitutive complexes that bind IRF1-GAS.
dbcAMP inhibits tyrosine phosphorylation of STAT5. (A). Primed T cells were cultured for 24 hours in either medium alone or in the presence of 1 mmol/L dbcAMP and then stimulated with IL-2 (1,000 IU/mL) for 15 minutes. Cell lysates were then prepared and immunoprecipitated with anti-STAT5 antibody. Western blotting was performed on aliquots of the immunoprecipitate to determine whether STAT5 was tyrosine phosphorylated (4G10 Ab) and to assess the level of STAT5 in the immunoprecipitates (anti-STAT5 Ab). (B) Aliquots of the same cells were subjected to Western blotting to determine if dbcAMP suppressed JAK3. (C) EMSA and supershift analyses were performed as indicated in Materials and Methods using nuclear extracts from samples shown in (A). The arrow indicates the migration of the IL-2 inducible complex binding to IRF1-GAS sequence. The loss of this inducible band in the presence of anti-STAT5 antibody shows that this band contains STAT5.
dbcAMP inhibits tyrosine phosphorylation of STAT5. (A). Primed T cells were cultured for 24 hours in either medium alone or in the presence of 1 mmol/L dbcAMP and then stimulated with IL-2 (1,000 IU/mL) for 15 minutes. Cell lysates were then prepared and immunoprecipitated with anti-STAT5 antibody. Western blotting was performed on aliquots of the immunoprecipitate to determine whether STAT5 was tyrosine phosphorylated (4G10 Ab) and to assess the level of STAT5 in the immunoprecipitates (anti-STAT5 Ab). (B) Aliquots of the same cells were subjected to Western blotting to determine if dbcAMP suppressed JAK3. (C) EMSA and supershift analyses were performed as indicated in Materials and Methods using nuclear extracts from samples shown in (A). The arrow indicates the migration of the IL-2 inducible complex binding to IRF1-GAS sequence. The loss of this inducible band in the presence of anti-STAT5 antibody shows that this band contains STAT5.
It is well documented that elevation of cAMP results in activation of cAMP-dependent protein kinase (PKA). To explore the involvement of PKA in the inhibition of JAK3 expression, we preincubated lymphocytes with PKA inhibitors H-89 (100 to 600 nmol/L) or myristoylated PKI amide (75 μmol/L) followed by PGE2 or dbcAMP treatment (n = 4). Surprisingly, both PKA inhibitors failed to restore JAK3 expression in T cells exposed to PGE2, although PKA activity was substantially inhibited (80% reduction by H-89 and 65% reduction by myristoylated PKI amide). These results show that cAMP-mediated inhibition of JAK3 expression is not perturbed when PKA is substantially inhibited, thus suggesting that this cAMP-dependent event is either PKA-independent or requires extremely low levels of PKA activity.
DISCUSSION
The Janus kinases represent a family of nonreceptor tyrosine kinases involved in cytokine receptor induced signal transduction.42 JAK3 is a unique family member because it is present only in lymphoid and myeloid cells and is involved in signal transduction linked to γc, a component of receptors for IL-2, IL-4, IL-7, IL-9, and IL-15.12,28,42-45 The importance of JAK3 and its interaction with γc in lymphoid maturation and activation has been recently documented in murine and human studies.46,47B-cell development and T-cell maturation are impaired in homozygous mutant mice where the JAK3 gene was disrupted by gene targeting.46,47 Moreover, T cells present in these JAK3-deficient mice are defective in proliferation and IL-2 production.46,47 In humans, mutation in γc is known to result in X-linked severe combined immunodeficiency (SCID), a defect in immunity characterized by T-cell lymphopenia and the presence of nonfunctional B cells.48 Genetic defects of JAK3 in patients have also been identified that display immunodeficiencies similar to those seen in X-linked SCID.49 Furthermore, B-cell lines established from JAK3-deficient patients display impaired signaling through the IL-2R and to a lesser degree through IL-4R.17 These studies point to a critical role for JAK3 and γc in the maturation and activation of lymphoid cells. The findings presented here suggest that PGE2 and other agents that increase cAMP may suppress T-cell function by inhibiting JAK3 and to a lesser extent γc expression. This suppression had negative consequences for IL-2R signal transduction and cellular proliferation. It is also possible that the reduction in JAK3 expression may affect signaling via other receptors that are known to share γc.42-45
PGE2 and other cAMP elevating agents can regulate immune responses by inhibiting T-cell activation events including IL-2 and IL-2R expression.18-27 This suppression may involve PKA-mediated inhibition of phospholipase C-γ (PLCγ) enzymatic activity, which in turn, blocks phosphatidyinositol-turnover.22,23 Additional studies have shown that cAMP elevation can alter expression and function of transcription factors involved in IL-2 and IL-2R gene expression. dbcAMP is known to inhibit the PKC-mediated induction of the jun and fos family of genes.25 Moreover, c-Jun transcriptional activity is altered by dbcAMP through PKA antagonizing c-Jun N-terminal kinase activity.50 There are also reports that activation of PKA can block DNA binding activity of NFκB to κB sequences in the IL-2 promoter.24 These studies illustrate that cAMP elevating agents may act at multiple levels to alter gene expression after TCR/CD3 signaling. It also appears that the induction of JAK3 in naive cells after ligation of TCR/CD3 is sensitive to PGE2. Whether the defect in JAK3 expression is the result of transcriptional or posttranscriptional modification is currently under investigation.
In agreement with previous reports, our studies show that PGE2 and other cAMP elevating agents can cause some reduction in surface expression of IL-2Rα after stimulation via TCR/CD3.22,39 It was also noted that PGE2 can cause modest suppression of γc expression after stimulation. In contrast, we did not detect any significant reduction in IL-2Rβ by cAMP elevating agents, yet in another study, IL-2 binding to IL-2Rβ was decreased by forskolin.51 It is not clear what accounts for the differences in results, however, measurement of IL-2 binding versus surface expression and our use of peripheral T lymphocytes versus cultured T-cell lines may be contributing factors. In our studies, however, PGE2/IBMX treatment induced only modest reduction of IL-2Rα and γc in naive T cells and none in primed T cells even though suppression of proliferation was complete. Therefore, our findings are more consistent with the IL-2 unresponsiveness induced by PGE2/IBMX treatment resulting from suppression of JAK3 function rather than from reduced IL-2R expression.
In our experiments, PKA inhibitors had no effect on JAK3 expression in peripheral T lymphocytes exposed to PGE2. These results suggest that cAMP-modulated JAK3 suppression either requires very low levels of PKA activity or is PKA-independent. It is well-documented that PKA-independent pathways are involved in cAMP signaling mechanism in addition to PKA-dependent pathways.52-54
Signaling through the IL-2R is dependent on the activation of several PTKs; JAK3, JAK1, p56lck, p59fyn, and Syk.8-11,13,15,16 Tyrosine phosphorylation of multiple substrates occurs after IL-2 binding to its receptor, and these include the IL-2R chains (β and γ) themselves, various kinases (JAK3, JAK1, p56lck, and p59fyn), the adapter molecule Shc3,4,8-11,13,16,55 along with STAT3 and STAT5.40,41 Recent studies suggest that JAK3 plays an important role in the phosphorylation of JAK1, IL-2Rβ, and STAT5.17,35 IL-2–dependent signaling also results in activation of phosphatidylinositol 3-kinase that regulates the kinase activity of the 70-kD S6 protein kinase (pp70s6k).56 Although the role each kinase plays in IL-2–dependent signaling is not clearly defined, it appears that the activation of these early events ultimately results in the induction of three distinct signaling pathways that are defined by the expression of c-Myc, c-Fos/Jun, and Bcl-2.3,4,34 Recent studies suggest that IL-2–dependent G1-S transition is affected by PGE2, and this may involve altering IL-2R linked signal transduction pathways.27,51 Another study has shown that forskolin can activate PKA and antagonize IL-2–dependent activation of pp70s6k, as well as phosphatidylinositol 3-kinase.56 In contrast, PKA had no effect on IL-2–mediated activation of MAP kinase showing that the inhibitory effect was not global.56
The results presented here suggest that cAMP elevating agents can also alter IL-2–dependent signaling by selectively downregulating the expression of JAK3. In dbcAMP-treated lymphocytes, decrease in JAK3 protein expression was accompanied by proportional reduction of STAT5 tyrosine phosphorylation and DNA binding activity. T cells deficient in JAK3 after treatment with cAMP elevating agents also display the same alterations in IL-2–dependent induction of protooncogenes as lymphoid cells expressing a dominant negative mutant of JAK3. In both cases, there is no induction of c-Jun, reduced expression of c-Myc, and normal upregulation of Bcl-2.34 35 Additional studies are clearly needed to fully define the effect that cAMP elevating agents have on IL-2R signaling and to delineate the mechanism responsible for the selective suppression of JAK3 protein. The downregulation of JAK3 expression in T cells may represent a unique mechanism by which PGE2 and other agents that elevate cAMP can suppress IL-2 responses.
ACKNOWLEDGMENT
We thank Jan Kodish for assisting in the preparation of this manuscript. We also thank Dr Jerome Ritz for providing us with antibody to the common γ chain.
Supported by United States Public Health Service Grant No. CA56937.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Vladimir Kolenko, MD, PhD, The Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195.

![Fig. 1. PGE2 inhibits upregulation of JAK3 in naive T cells. (A) Highly enriched naive T cells were cultured for 48 hours under the following conditions; medium alone; cross-linked anti-CD3 and IL-2 (1,000 U/mL) alone and anti-CD3/IL-2 plus PGE2 with and without IBMX. Cell lysates were then subjected to immunoblotting using antibodies to JAK1, JAK3, p56lck, and p59fyn. In this and all experiments, equal amounts of protein were added to each lane. Densitometry readings of the immunoblot showed the following reduction of JAK3 by PGE2alone and with IBMX: 0% 0.1 μmol/L PGE2, 4% 1 μmol/L PGE2, 40% 10 μmol/L PGE2, 63% 0.1 μmol/L PGE2/IBMX, 68% 1 μmol/L PGE2/IBMX, and 76% 10 μmol/L PGE2/IBMX. (B) Proliferation was assessed by the uptake of [3H] thymidine as described in Materials and Methods. (C) Effect of PGE2 on cAMP production in resting T cells. Freshly isolated T cells were incubated in RPMI 1640 at 37°C in the presence of 100 μmol/L of the phosphodiesterase inhibitor IBMX for 30 minutes followed by incubation for 5 minutes with PGE2 (10 μmol/L) before measuring cAMP levels. A significant reduction in JAK3 expression was observed in three experiments (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2308/5/m_blod40716001w.jpeg?Expires=1765979887&Signature=R6qZrVKe8o4kdZMYBbkpb-uuox7AAxsvEZFYovPpa-IpJ4nnyZqSQ4aTnDNH9DK5VnGY7KC-0fc1FgG0oL--t3LcF3qTMSTF7Qm81Ftm42AgzOpci8wtP3wQIZyEvmskMga2k5MabDwClJWeq7ThJDbYStV59m~wP1vtketvjZUMMZjR5VH74ThfDKzug5vjLdi4ShUatKhyldYYTGDaHfIiakxuB5j4lhHzFRYfWbTaPD9C-caBiVsxA1CpkJ~30cYATEnphS75GH71znk-6TrbFr8wi4HqYs93dnJNrwjVLvOFjINqjPTiBJfC85I1SxgRfcOZ7bTPstWVbfEQhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 7. Suppression of JAK3 expression by PGE2/IBMX correlates with the inhibition of IL-2–dependent signaling pathways for induction of c-Myc and c-Jun. (A) After 3 days of priming, T cells were cultured with PGE2/IBMX for 24 hours and then stimulated with IL-2 for various times before performing Western blot using antibodies to JAK1, JAK3, c-Myc, c-Jun, and Bcl-2. (B) Densitometry scan of immunoblot in (A) showed that after 24 hours of coculture with 10 μmol/L PGE2/10 μmol/L IBMX JAK3 was reduced by 57%, which remained suppressed during IL-2 stimulation (81% reduction). In PGE2/IBMX-treated cells, the IL-2–dependent induction of c-Myc was partially suppressed (54% reduction), whereas the induction of c-Jun was suppressed by 96%. In contrast, PGE2/IBMX had little effect on the increase in Bcl-2 induced by IL-2 stimulation (6% reduction, 24 hours). (C) Primed cells were stimulated with IL-2 (1,000 U/mL) in medium alone or medium supplemented with PGE2/IBMX. Proliferation was assessed after 3 days of culture by measuring the uptake of [3H] thymidine. Representative data are presented (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2308/5/m_blod40716007w.jpeg?Expires=1765979887&Signature=FlfB2FnRhK2ELkAuA4jmTVlfnCxccRpPmNwTs1nPF2l6bLRBHQY3wexWZWpvNS62cLgwmbaTgwFvRkvVuuJ0TXg2x4mIsEmlT5VPjWK0xha0ju5FQQ7jYg~DDYFkedrsDDkDGJ6roUW6vkjeKaSHrVXLOmGYrAyZ37~by-nY38d5xZJUye51pB2blJ7b~gXR5HLAv6cx-fnhBnw2xK5sVZHTyxxdaIX0cl8qvpC8nqFAmbyOlp0KuZIAzdtyKqvdvIorxssoV8fyzqdjwWVtBK6rEjLKOC30HZqtSVodmgRAVoS06bchaf9AFSQD3IrKifQOvrgSH2oTtQUxTCwghg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal