Abstract
Progress toward gene therapy of β-chain hemoglobinopathies has been limited in part by poor expression of globin genes in virus vectors. To derive an optimal expression cassette, we systematically analyzed the sequence requirements and relative strengths of theAγ- and β-globin promoters, the activities of various erythroid-specific enhancers, and the importance of flanking and intronic sequences. Expression was analyzed by RNase protection after stable plasmid transfection of the murine erythroleukemia cell line, MEL585. Promoter truncation studies showed that theAγ-globin promoter could be deleted to −159 without affecting expression, while deleting the β-globin promoter to −127 actually increased expression compared with longer fragments. Expression from the optimal β-globin gene promoter was consistently higher than that from the optimal Aγ-globin promoter, regardless of the enhancer used. Enhancers tested included a 2.5-kb composite of the β-globin locus control region (termed a μLCR), a combination of the HS2 and HS3 core elements of the LCR, and the HS-40 core element of the -globin locus. All three enhancers increased expression from the β-globin gene to roughly the same extent, while the HS-40 element was notably less effective with theAγ-globin gene. However, the HS-40 element was able to efficiently enhance expression of a Aγ-globin gene linked to the β-globin promoter. Inclusion of extended 3′ sequences from either the β-globin or the Aγ-globin genes had no significant effect on expression. A 714-bp internal deletion ofAγ-globin intron 2 unexpectedly increased expression more than twofold. With the combination of a −127 β-globin promoter, anAγ-globin gene with the internal deletion of intron 2, and a single copy of the HS-40 enhancer, γ-globin expression averaged 166% of murine -globin mRNA per copy in six pools and 105% in nine clones. When placed in a retrovirus vector, this cassette was also expressed at high levels in MEL585 cells (averaging 75% of murine -globin mRNA per copy) without reducing virus titers. However, recombined provirus or aberrant splicing was observed in 5 of 12 clones, indicating a significant degree of genetic instability. Taken together, these data demonstrate the development of an optimal expression cassette for γ-globin capable of efficient expression in a retrovirus vector and form the basis for further refinement of vectors containing this cassette.
THE USE OF retrovirus vectors for the gene therapy of β chain hemoglobinopathies has been limited, in part, by the restricted size of these vectors1 and the effect of globin gene and enhancer sequences on vector titer and stability. In the case of retrovirus vectors for human β-globin and γ-globin, these problems have been addressed to some degree by introducing several genetic alterations to the coding sequence and including enhancer elements from the β-globin locus control region (μLCR)2,3 or the α globin locus enhancer.4Initial studies with retrovirus vectors for γ-globin have shown that the γ-globin gene introns are required for maximal expression, but the presence of intron sequences greatly diminished vector titers.5 Because of the efficient antisickling properties of fetal hemoglobin (HbF) and the therapeutic impact of even moderate HbF levels in homozygous β thalassemia, we sought to develop an optimal expression cassette for γ-globin for inclusion in retrovirus vectors. Such a cassette should have the following properties: (1) a combination of promoter and enhancer, which can direct therapeutic levels of globin gene expression; (2) all unnecessary sequences removed to provide an acceptable size; and (3) lack of sequences, which significantly reduce vector titers or contribute to vector instability. To meet the goals, we studied the sequence requirements and strengths of the Aγ- and β-globin promoters, compared activities of various erythroid-specific enhancer elements, and assessed the influence of 3′ flanking sequences and the internal portion of the second intron of the Aγ-globin gene. Expression was measured by plasmid transfection of the murine erythroleukemia cell line MEL585 and quantitative RNase protection analysis of stable pools. An optimal expression cassette was also introduced into a murine leukemia virus (MLV)-based vector, and vector titer, stability, and expression was assessed.
MATERIALS AND METHODS
Plasmid constructs.
All constructs were made in the plasmid vector pBluescript (Stratagene, La Jolla, CA) using standard molecular cloning procedure.6The contents of the various constructs are shown in Table 1 and are as follows: the 2.3-kbStuI/HindIII fragment (coordinates 39050-41382, GenBank humhbb): Aγ-globin gene with −382 promoter. The 2.2-kbApaI/HindIII fragment (39230-41382): theAγ-globin gene with −201 promoter. The 2.1-kbAvaII/HindIII fragment (39271-41382): the humanAγ-globin gene with −159 promoter. The 2.1-kbNcoI/HindIII fragment (39290-41382): theAγ-globin gene with −141 promoter. The 753-bpHindIII fragment (41382-42135): the Aγ-globin gene 3′ enhancer. The 5.0-kb BglII fragment (60629-65610): the β-globin gene with −1560 promoter and 3′ enhancer. The 4.2-kb HpaI/BglII fragment (61372-65610): the β-globin gene with −614 promoter and 3′ enhancer. The 3.7-kb SnaBI/BglII fragment (61921-65610): the β-globin gene with −267 promoter and 3′ enhancer. The 3.6-kb RsaI/BglII fragment (62060-65610): the β-globin gene with −127 promoter and 3′ enhancer. The 3.5-kb AvrII/BglII fragment (62101-65610): the β-globin gene with −86 promoter and 3′ enhancer. The 1.5-kb BspHI/BglII fragment (64081-65610) was deleted from the above β-globin gene constructs to remove the β-globin 3′ enhancer. The βpr.-Aγ-globin hybrid genes were made by linking either the −127 or −267 β-globin promoters (RsaI 62060 or SnaBI 61921 to NcoI 62238) to the Aγ-globin gene coding sequence (NcoI toHindIII, 39483-41382) using the NcoI sites as a junction point. In the constructs with the internal deletion ofAγ-globin intron 2, the 714-bpXhoI(blunt)/HpaI fragment (39960-40674) was removed. The 2.5-kb μLCR was described previously.7 The α-globin HS-40 core is contained in the 356-bp TaqI/XmnI fragment (a gift from Douglas R. Higgs, University of Oxford, Oxford, UK). The HS3 core is contained in a 784-bp PstI fragment (4348-5132). The 298-bp HS2 core is contained in aHindIII/AluI (8486-8784) fragment. The enhancers were oriented in the same direction as transcription, except where noted.
DNA Fragments Used in the Constructs
| Fragment . | GenBank humhbb Coordinate . | Content of Fragment . |
|---|---|---|
| StuI/HindIII | 39050-41382 | Aγ-globin gene with −382 promoter |
| ApaI/HindIII | 39230-41382 | Aγ-globin gene with −201 promoter |
| AvaII/HindIII | 39271-41382 | Aγ-globin gene with −159 promoter |
| NcoI/HindIII | 39290-41382 | Aγ-globin gene with −141 promoter |
| HindIII/HindIII | 41382-42135 | Aγ-globin gene 3′ enhancer |
| Bgl II/Bgl II | 60629-65610 | β-globin gene with −1560 promoter |
| HpaI/Bgl II | 61372-65610 | β-globin gene with −614 promoter |
| SnaBI/Bgl II | 61921-65610 | β-globin gene with −267 promoter |
| RsaI/Bgl II | 62060-65610 | β-globin gene with −127 promoter |
| AvrII/Bgl II | 62101-65610 | β-globin gene with −86 promoter |
| BspHI/Bgl II | 64081-65610 | β-globin gene 3′ enhancer |
| PstI/PstI | 4348-5132 | HS3 |
| HindIII/AluI | 8486-8784 | HS2 |
| TaqI/XmnI | αHS-40 |
| Fragment . | GenBank humhbb Coordinate . | Content of Fragment . |
|---|---|---|
| StuI/HindIII | 39050-41382 | Aγ-globin gene with −382 promoter |
| ApaI/HindIII | 39230-41382 | Aγ-globin gene with −201 promoter |
| AvaII/HindIII | 39271-41382 | Aγ-globin gene with −159 promoter |
| NcoI/HindIII | 39290-41382 | Aγ-globin gene with −141 promoter |
| HindIII/HindIII | 41382-42135 | Aγ-globin gene 3′ enhancer |
| Bgl II/Bgl II | 60629-65610 | β-globin gene with −1560 promoter |
| HpaI/Bgl II | 61372-65610 | β-globin gene with −614 promoter |
| SnaBI/Bgl II | 61921-65610 | β-globin gene with −267 promoter |
| RsaI/Bgl II | 62060-65610 | β-globin gene with −127 promoter |
| AvrII/Bgl II | 62101-65610 | β-globin gene with −86 promoter |
| BspHI/Bgl II | 64081-65610 | β-globin gene 3′ enhancer |
| PstI/PstI | 4348-5132 | HS3 |
| HindIII/AluI | 8486-8784 | HS2 |
| TaqI/XmnI | αHS-40 |
Plasmid transfection.
The murine erythroleukemia cell line MEL585 was grown in RPMI-1640 medium (GIBCO-BRL, Grand Island, NY) supplemented with 10% heat-inactivated defined fetal bovine serum (FBS; Gibco BRL). A total of 107 cells in log-phase growth were resuspended in 0.5 mL HEPES-buffered sucrose (272 mmol/L sucrose, 8 mmol/L HEPES, pH 7.4), chilled in an electroporation cuvette with a 0.4 cm electrode gap, mixed with DNA, and transfected with a Gene Pulse electroporator (Bio-Rad, Hercules, CA) at 500 V, 1 μF. Cotransfection was achieved using 10 μg of linearized experimental plasmid DNA plus 1 μg of PGK-Neo8 as a selectable marker. Cells were then transferred to 70-cm2 tissue culture flasks with 20 mL of growth medium and after 48 hours, G418 was added at a concentration of 0.7 mg/mL active component. G418-resistant pools were used for analysis after 10 to 14 days. The MEL cell pools contained a large number of clones so that the variation in expression due to position effects was minimized.9
Retrovirus vector.
The retrovirus vector construct was generated using the MLV vector LNSX,1 which expresses Neo using the viral 5′ long terminal repeat promoter. The expression cassette consists of the 356-bp HS-40 enhancer, −127 β-globin promoter (RsaI-NcoI, 62060-62238), and Aγ-globin gene (NcoI/HindIII, 39483-41382) with the 714-bp internal deletion of intron 2 described above. This cassette was inserted in the unique BamHI (blunt) and StuI sites of LNSX in the opposite orientation with respect to virus transcription. Retrovirus vector producer lines were generated essentially as described.1 In short, vector plasmid was used to transfect the ecotropic packaging line PE5011 by CaPO4precipitation and after 48 hours, virus supernatant was used to transduce the amphotropic packaging line PA317.10 After an additional 24 hours, the transduced PA317 cells were plated at low dilution with 0.5 mg/mL active G418, and individual drug-resistant colonies were isolated. Virus titer was assayed by serial dilution and transfer of G418 resistance to naive NIH3T3 cells as previously described.11 Cells were maintained at 37°C, 7.5% CO2 in Dulbecco’s Modified Eagle’s medium (DMEM; GIBCO-BRL) supplemented with 10% FBS, 2 mmol/L L-glutamine (GIBCO-BRL), 1 mmol/L sodium pyruvate (GIBCO-BRL), and 0.1 mmol/L nonessential amino acids (GIBCO-BRL). Vector-containing supernatant was collected from semiconfluent plates after a 48-hour culture at 33°C, passed through a 0.44-μm filter, and stored at −70°C. MEL585 cells were transduced by 24-hour culture in this vector-containing supernatant plus 8 μg/mL polybrene (hexadimethrine bromide; Sigma Chemical Co, St Louis, MO) at 1 to 2 × 105 cells/mL. The cells were then washed and plated at limiting dilution in 96-well flat-bottom dishes with 0.6 mg/mL active G418, and individual clones were expanded after 10 to 14 days for analysis.
Quantification of globin mRNA.
Transfected or transduced MEL585 cells were induced to differentiate by culture in 3 mmol/L N,N1-hexamethylene bisacetamide (HMBA; Aldrich, Milwaukee, WI) and 10 μmol/L hemin (Sigma) starting at 2 to 3 × 105 cells/mL as previously described. Cells were collected after 3 days, and total RNA was isolated using guanidine thiocyanate-acid-phenol as described.12 Globin gene transcripts were quantified by RNase protection as previously described13 using the following probes: pT7βmlinearized with BsaI to give a 206-bp protected fragment within exon 2 of the human β-globin gene; pT7Aγ(170) linearized with BstEII to give a 170-bp protected fragment within exon 2 of the human Aγ-globin gene; and pT7Mα (128) linearized with HindIII to give a 128-bp protected fragment within exon 1 of the murine α-globin gene. A total of 500 ng RNA was hybridized overnight at 48°C with 106 cpm of each radiolabeled probe. A pilot experiment confirmed that probe was in excess under these conditions. After digestion with RNase A and T1, the protected fragments were separated on 6% polyacrylamide-8 mol/L urea gels, and autoradiography was performed without intensifying screens. Signal intensities were quantified by Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Southern analysis.
Genomic DNA was isolated by standard methods.6 For copy number determinations, DNA from transfected cells was digested withEcoRI, which cuts twice in each plasmid construct. Fluorometry was then used to accurately determine the DNA concentration, and 10 μg of the digested DNA was separated on 1.0% agarose gels and blotted onto nylon filters. The blots were probed with radiolabeledBamHI/EcoRI fragments for exon 2 of eitherAγ-globin or β-globin, and a murine α-globin probe from pUCmuα (a gift from Margaret H. Baron, Mt Sinai School of Medicine, New York, NY) as a loading control. Normal human genomic DNA digested with EcoRI and run in parallel served as a copy number standard. Signals were quantified on a Phosphorimager. For the studies with the retrovirus vectors, 10 μg DNA was digested withKpnI, which cuts once in each of the virus LTRs, separated on 0.8% agarose gels, and blotted onto nylon filters. The blots were probed with a radiolabeled 923-bp PstI fragment for Neo.
RESULTS
Truncations of the Aγ-globin gene promoter.
Our previous studies in transgenic mice carrying μLCRAγ gene recombinants showed that truncation of the Aγ-globin promoter to position −201 relative to the mRNA cap site allows high-level expression in adult blood (15% to 20% of murine α-globin mRNA per copy), while truncation to −141 (which deletes the CACCC box at −145 and the GATA-1/Oct-1 motifs around −175) completely abolishes γ gene expression.14 We have also shown that the sequence between −382 and −730 of theAγ gene promoter contains a position-dependent silencer of Aγ gene expression.14 These results suggest that sequences upstream of −382 Aγ-globin promoter should be omitted from virus vectors to prevent silencing.
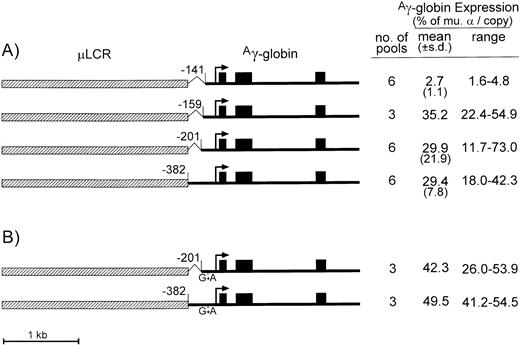
To test if the Aγ-globin gene promoter could be further truncated without impairing the promoter strength, μLCRAγ-globin expression plasmids containing the −201 Aγ or the −141 Aγ promoter truncations, a −382 Aγ promoter truncation, and a −159 Aγ promoter truncation (which leaves the CACCC box intact, but removes the GATA-1 and Oct-1 motifs) were used (Fig 1A). These constructs were cotransfected into MEL585 cells with a neomycin resistance plasmid, and the indicated numbers of pools were selected under G418. After induction with HMBA and hemin, total RNA and genomic DNA were isolated, and the levels of Aγ-globin mRNA and transgene copy numbers were measured by quantitative RNase protection and Southern analysis. As summarized to the right of Fig 1A, the construct with the −159 truncation expressed Aγ-globin at an average 35% of endogenous murine α-globin on a per copy basis, with a range of 22.4% to 54.9%. This expression level is similar to that of constructs containing the −382 or the −201Aγ-globin promoter truncations (averaging about 29% each). These results suggest that the CACCC box and more proximal regulatory elements are required for μLCR-enhanced expression of theAγ-globin gene, and that all other distal elements of theAγ promoter can be omitted from the constructs of retrovirus vectors.
Diagrams and expression of constructs containing the μLCR and Aγ-globin gene with promoter truncations. (A) Is for constructs containing the normal Aγ-globin promoter sequence, while (B) is for constructs containing the indicated G → A point mutation at position −117 of theAγ-globin promoter associated with hereditary persistence of fetal hemoglobin. In the diagrams to the left, the μLCR is indicated by the thick hatched bar, while the Ag-globin gene is indicated by the thin filled bar. Exons are indicated by the filled boxes, the site of transcription initiation (cap site) by the arrow, while the positions of the individual promoter truncations are relative to the cap site. To the right of each panel is shown the number of transfected MEL585 pools included in the analysis, along with the mean, standard deviation (for data sets containing more than three pools), and range of expression for each construct. Expression was determined by quantitative RNase protection and is expressed as a percentage of the level of endogenous murine -globin mRNA on a per copy basis.
Diagrams and expression of constructs containing the μLCR and Aγ-globin gene with promoter truncations. (A) Is for constructs containing the normal Aγ-globin promoter sequence, while (B) is for constructs containing the indicated G → A point mutation at position −117 of theAγ-globin promoter associated with hereditary persistence of fetal hemoglobin. In the diagrams to the left, the μLCR is indicated by the thick hatched bar, while the Ag-globin gene is indicated by the thin filled bar. Exons are indicated by the filled boxes, the site of transcription initiation (cap site) by the arrow, while the positions of the individual promoter truncations are relative to the cap site. To the right of each panel is shown the number of transfected MEL585 pools included in the analysis, along with the mean, standard deviation (for data sets containing more than three pools), and range of expression for each construct. Expression was determined by quantitative RNase protection and is expressed as a percentage of the level of endogenous murine -globin mRNA on a per copy basis.
Introduction of HPFH −117 point mutation.
Several naturally occurring point mutations in theAγ-globin promoter cause hereditary persistence of fetal hemoglobin, possibly through different mechanisms.15Previously reported transient and stable transfection assays suggested that the −117 G → A mutation in theAγ-globin promoter increases Aγ-globin expression even in the absence of enhancers.16 17 To determine whether this point mutation could further elevateAγ-globin expression in the presence of the μLCR, the −117 G → A transistion was introduced into the μLCRAγ-globin constructs containing the −201, or the −382 Aγ-globin promoter truncations described above, and expression was analyzed in pools of stably transfected MEL585 cells (Fig 1B).
In constructs carrying the −201 or the −382 truncations, expression increased about 50% by addition of the −117 G → A mutation (average of 42.3% v 29.9% for the −201 truncation [P = .041] and 49.5%v 29.4% for the −382 truncation [P = .01]) per copy of murine α-globin indicating that this point mutation only modestly increases Aγ-globin expression in μLCR-containing constructs.
Truncations of the β-globin promoter.
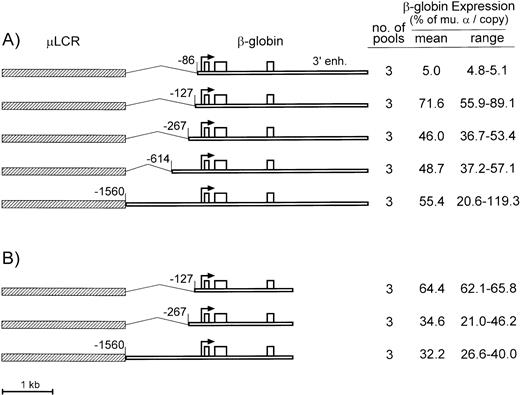
A similar truncation approach was used to define the minimal promoter for human β-globin gene. As diagrammed in Fig 2A, the control construct consisted of the μLCR enhancer linked to a 5-kb BglII fragment containing a −1560-bp promoter and the 3′ β enhancer. This construct contains all of the necessary cis-regulatory elements defined by transgenic mouse studies.18 Four truncations were tested, including a deletion to −614, a deletion to −267, which has been used before in retrovirus vectors for β-globin,2 3 a deletion to −127, which includes both proximal and distal CACCC boxes, and a deletion to −86, which removes both CACCC boxes.
Diagrams and expression of constructs containing the μLCR and β-globin gene with promoter truncations. (A) Is for constructs containing the β-globin gene extended to include the 3′ enhancer sequence, while (B) is for constructs containing the β-globin gene without the 3′ enhancer. In the diagrams to the left, the μLCR is indicated by the thick hatched bar, while the β-globin gene is indicated by the thin open bar. Exons are indicated by the open boxes, the site of transcription initiation (cap site) by the arrow, and the positions of the individual promoter truncations are relative to the cap site. To the right of each panel is indicated the number of transfected MEL585 pools included in the analysis along with the mean and range of expression for each construct.
Diagrams and expression of constructs containing the μLCR and β-globin gene with promoter truncations. (A) Is for constructs containing the β-globin gene extended to include the 3′ enhancer sequence, while (B) is for constructs containing the β-globin gene without the 3′ enhancer. In the diagrams to the left, the μLCR is indicated by the thick hatched bar, while the β-globin gene is indicated by the thin open bar. Exons are indicated by the open boxes, the site of transcription initiation (cap site) by the arrow, and the positions of the individual promoter truncations are relative to the cap site. To the right of each panel is indicated the number of transfected MEL585 pools included in the analysis along with the mean and range of expression for each construct.
As summarized to the right of Fig 2A, the β-globin mRNA levels for the −1560 control averaged 55.4% per copy of murine α-globin, with a range of 20.6% to 119.3%. The β-globin gene with the −614 and −267 truncations were expressed at similar levels, averaging 48.7% and 46.0% per copy of murine α-globin, respectively, indicating the absence of either positive or negative elements within the −267 to −614 region. Expression of the β-globin gene with the −127 truncation was higher, averaging 71.6% (±16.7) per copy of murine α-globin. The −86 truncation decreased β-globin mRNA expression to 5.0% per copy of murine α-globin gene, suggesting that one or both β-globin gene CACCC boxes participate in the interaction between the β-globin promoter and the μLCR.
Deletion of the β-globin 3′ enhancer.
All of the β-globin gene constructs tested above contain 3′ sequences identified in erythroid cell lines and transgenic mice to have enhancer activity.19-22 To determine whether the 3′ β-globin enhancer had any discernible activity in the presence of the μLCR, this element was deleted in three of the β-globin gene constructs described above (the −1560 control and the −267 and −127 promoter truncations). As seen in Fig 2B, the β-globin gene having a −127 truncated promoter and deleted 3′ enhancer was expressed at 64.4% of murine α-globin mRNA per copy. This level is not statistically different (P = .5) from the level of the corresponding β-globin gene construct with the 3′ enhancer (71.6%). Similar results were obtained for the −267 truncation (34.6% v 48.7%) and the −1560 promoter (32.2% v 55.4%). It is noteworthy that the −127 truncated promoter was also better than the longer promoters in the absence of the β-globin 3′ enhancer.
Enhancing activity of HS3/HS2 combination.
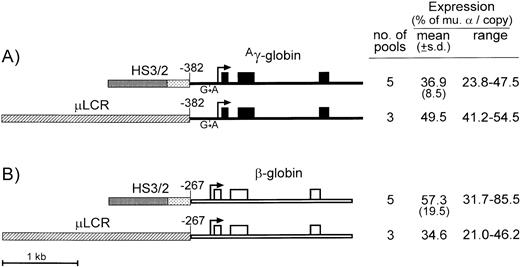
In the studies described above, the μLCR originally described by Forrester et al,7 was used as an erythroid-specific enhancer. This element is an abbreviated version of the natural 22-kb β-globin LCR23 and contains sequences from four of the erythroid-specific DNase I hypersensitive sites (HS1-4) thought to be critical for specific enhancer function.23-26 Although the μLCR retains most of the enhancing activity of the whole LCR,7 at 2.5 kb, this cassette is too large to include in conventional retrovirus vectors, which contain a full-length globin gene and selectable marker. Previous studies of individual HS core elements suggested that pair-wise combinations may also potentially provide nearly full LCR activity.18 To test this possibility, the most evolutionarily conserved sequences from HS2 and HS3 were combined and tested for enhancer activity as described above using both Aγ-globin and β-globin genes. The 299-bp HS2 fragment contains most of the sequences identified by DNase footprinting,27 and it is almost half the size of the 732-bp HS2 fragment used in the μLCR. The 784-bp HS3 fragment contains all of the sequences identified by DNase footprinting28 and is slightly larger than the 564-bp HS3 fragment used in the μLCR.
As diagrammed in Fig 3, theAγ-globin gene used in these studies contained the −382 promoter and −117 G → A mutation, while the β-globin gene contained the −267 promoter and no 3′ enhancer. The Aγ-globin cassette linked to the HS2/HS3 enhancer was expressed at 36.9% ± 8.5% per copy of murine α-globin, compared with an average 49.5% for the same cassette linked to the μLCR (Fig 3A). In the case of the β-globin gene, linkage to the HS2/HS3 enhancer led to an average expression of 57.3% ± 19.5% per copy of murine α-globin, compared with an average 34.6% for the same cassette linked to the μLCR (Fig 3B). In both cases, the differences were not statistically significant (P = .07 for Aγ-globin and P = .13 for β-globin), indicating that the 1.0-kb HS2/HS3 composite retains most of the enhancing activity of the larger 2.5-kb μLCR, regardless of whether it is linked to genes for Aγ- or β-globin.
Diagrams and expression of constructs designed to compare enhancing activity of the μLCR and HS3/HS2. (A) Is for constructs containing the Ag-globin gene (thin filled bars) with the −382 promoter and G → A transition at position −117, while (B) is for constructs containing the β-globin gene with the −267 promoter and no 3′ enhancer (thin open bars). Exons are indicated by the boxes, and the sites of transcription initiation (cap sites) are shown by the arrows. The μLCR is indicated by the thick hatched bar, while the HS3/HS2 enhancer core fragments are indicated by heavy and light stippling, respectively. To the right of each panel, the number of transfected MEL585 pools included in the analysis is shown along with the mean, standard deviation, and range of expression for each construct as described in the legend to Fig 1.
Diagrams and expression of constructs designed to compare enhancing activity of the μLCR and HS3/HS2. (A) Is for constructs containing the Ag-globin gene (thin filled bars) with the −382 promoter and G → A transition at position −117, while (B) is for constructs containing the β-globin gene with the −267 promoter and no 3′ enhancer (thin open bars). Exons are indicated by the boxes, and the sites of transcription initiation (cap sites) are shown by the arrows. The μLCR is indicated by the thick hatched bar, while the HS3/HS2 enhancer core fragments are indicated by heavy and light stippling, respectively. To the right of each panel, the number of transfected MEL585 pools included in the analysis is shown along with the mean, standard deviation, and range of expression for each construct as described in the legend to Fig 1.
Enhancing activity of α-globin HS-40.
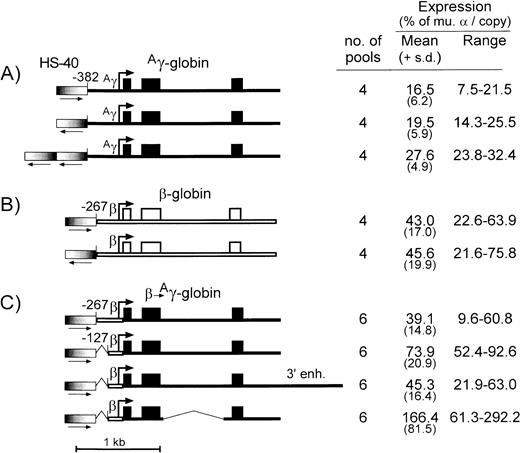
The HS-40 element from the α-globin locus functions as a classical enhancer, but it is unable to confer copy number-dependent expression to a linked gene in a stable transfection assay and in transgenic mice.29-32 The 356-bp HS-40 fragment used here contains the core DNase I hypersensitive site and multiple binding motifs for erythroid and ubiquitous trans-acting factors.30 32To further define the specific function of this enhancer fragment, the series of plasmid constructs diagrammed in Fig 4 were generated and tested for expression in stably transfected MEL585 pools. Placing the HS-40 fragment in the same orientation with an Aγ-globin gene containing a −382 promoter (Fig 4A) enhanced expression to 16.5% ± 6.2% per copy of murine α-globin, compared with 19.5% ± 5.9% for the same construct containing the HS-40 fragment in the opposite orientation. Similar experiments were performed with the HS-40 fragment linked to the β-globin gene cassette with the −267 promoter and no 3′ enhancer (Fig 4B); when positioned in the same orientation as transcription, the HS-40 fragment enhanced expression to 43.0% ± 17.0% per copy of murine α-globin, compared with 45.6% ± 19.9% for the same construct containing the HS-40 fragment in the opposite orientation. These results suggest that the HS-40 enhancer is orientation independent. Comparison of the findings of Figs 4A and B with Fig 1A and B also show that the enhancing activity of HS-40 is statistically indistinguishable from that of the μLCR. When two copies of the HS-40 fragment were linked to the −382Aγ-globin gene, expression increased to 27.6% ± 4.9% per copy of murine α-globin. Although higher than the equivalent vector with one copy of the HS-40 fragment (19.5% ± 5.9%; P = .038), it is not clear whether the enhancing activity is additive.
Diagram and expression of constructs designed to test the enhancing activity of HS-40 and novel cassettes forAγ-globin. (A) Is for constructs containing theAγ-globin gene (thin filled bar) with the −382 promoter and various arrangements of the HS-40 enhancer, (B) is for constructs containing the β-globin gene with the −267 promoter (thin open bar) and various arrangements of the HS-40 enhancer, and (C) is for constructs containing hybrid expression cassettes with the β-globin promoter, various versions of the Aγ-globin gene, and a single copy of the HS-40 enhancer in the same orientation. Exons are indicated by the boxes, the sites of transcription initiation (cap sites) by the arrows, and the segments of the β-globin promoter andAγ-globin intron 2, which were deleted, are indicated by a thin line. The HS-40 enhancer is indicated by the thick bars with graded fill, and the orientation is shown underneath by an arrow. To the right of each panel is indicated the number of transfected MEL585 pools included in the analysis, along with the mean, standard deviation, and range of expression for each construct as described in the legend to Fig 1.
Diagram and expression of constructs designed to test the enhancing activity of HS-40 and novel cassettes forAγ-globin. (A) Is for constructs containing theAγ-globin gene (thin filled bar) with the −382 promoter and various arrangements of the HS-40 enhancer, (B) is for constructs containing the β-globin gene with the −267 promoter (thin open bar) and various arrangements of the HS-40 enhancer, and (C) is for constructs containing hybrid expression cassettes with the β-globin promoter, various versions of the Aγ-globin gene, and a single copy of the HS-40 enhancer in the same orientation. Exons are indicated by the boxes, the sites of transcription initiation (cap sites) by the arrows, and the segments of the β-globin promoter andAγ-globin intron 2, which were deleted, are indicated by a thin line. The HS-40 enhancer is indicated by the thick bars with graded fill, and the orientation is shown underneath by an arrow. To the right of each panel is indicated the number of transfected MEL585 pools included in the analysis, along with the mean, standard deviation, and range of expression for each construct as described in the legend to Fig 1.
Further modifications of the Aγ-globin gene.
As described above, the strength of the Aγ-globin gene promoter is about half of that from the β-globin gene, regardless of the enhancer used. To increase Aγ-globin expression, we generated the series of hybrid genes diagrammed in Fig 4C in which the promoter for β-globin was linked to 3′ transcription cassette for Aγ-globin. This was done using a naturally occurringNcoI restriction site present at the translational start codon of both the Aγ- and β-globin genes.
Using the α-globin HS-40 enhancer, −267 β-globin promoter, and conventional Aγ-globin cassette, expression levels were 39.1% ± 14.8% per copy of murine α-globin. This expression level was increased to 73.9% ± 20.9% per copy of murine α-globin when the β-globin promoter was truncated to −127. This is statistically higher than the activity of a recombinant containing the HS-40 and a −267 β-globin promoter (P = .008). These data indicate that the HS-40 can efficiently enhance γ-globin gene expression through the β-globin promoter, and that truncation of the β-globin promoter to −127 can increase expression of a linked γ-globin gene in a fashion similar to that observed with the intact β-globin genes (Fig 2A).
We also used this system to investigate the enhancing ability of a 3′ Aγ-globin regulatory element, which was initially identified through a transient transfection assay.33 Although this element was unable to enhanceAγ-globin gene expression in transgenic mice, it did confer copy number-dependent expression, suggesting that it may help stabilize the interaction between LCR and Aγ gene promoter.34 When the 3′ Aγ-globin regulatory element was added to the recombinant containing the HS-40 enhancer, −127 β-globin promoter, and Aγ-globin transcription cassette, expression averaged 45.3% ± 16.4% per copy of murine α-globin, significantly less (P = .007) than the 73.9% ± 20.9% observed for the same vector without the 3′ Aγ-globin regulatory element.
HS-40/βpr.-Aγ construct with intron deletion.
Because of the size limits inherent in conventional retrovirus vectors, we also used the HS-40/βpr.-Aγ construct to test the effect of a large internal deletion in intron 2 of theAγ-globin gene (Fig 4C). This deletion removes 714 bp from the center of the intron, but leaves intact splice donor and acceptor sites. When this deletion was introduced into a hybrid construct containing the HS-40 enhancer, −127 β-globin promoter, and the Aγ-globin gene, expression levels averaged 166% ± 81.5% per copy of murine α-globin. This was statistically greater (P = .02) than the 73.9% ± 20.9% observed for the same vector with an intact second intron.
To confirm this result, an additional nine MEL585 clones transfected with this construct were isolated. Expression ofAγ-globin mRNA in these clones averaged 104.5% per copy of murine α-globin, with a range of 43.0% to 233.1% and a standard deviation of ± 53.4%. Previous studies9 have shown that such large variations in expression are characteristic of MEL cell clones.
Testing the HS-40/βpr.-Aγ(Δ2) expression cassette in a retrovirus vector.
To determine whether similar expression levels could be achieved via retrovirus-vector mediated transduction, the cassette described above was incorporated into the MLV vector LNSX. As seen in Fig 5A, this cassette was inserted in the opposite orientation with respect to transcription to prevent splicing of the genomic transcript and consisted of a single copy of the HS-40 enhancer, the β-globin gene promoter truncated to position −127, and the genomic elements of the Aγ-globin gene starting with the first exon and containing the large internal deletion of intron 2. Producer lines were generated using the amphotropic packaging line PA317 and screened for functional titer by serial dilution of virus supernatant and transfer of G418 resistance to NIH3T3 cells. The best of 12 clones screened gave a titer of 3 × 106colony-forming units (CFU) per mL, which is essentially equivalent to the of 5 × 106 CFU/mL achieved with the parental vector in parallel. These data indicate that the various elements of the hybrid expression cassette do not adversely effect virus titers, a major prerequisite for the generation of therapeutic vectors.
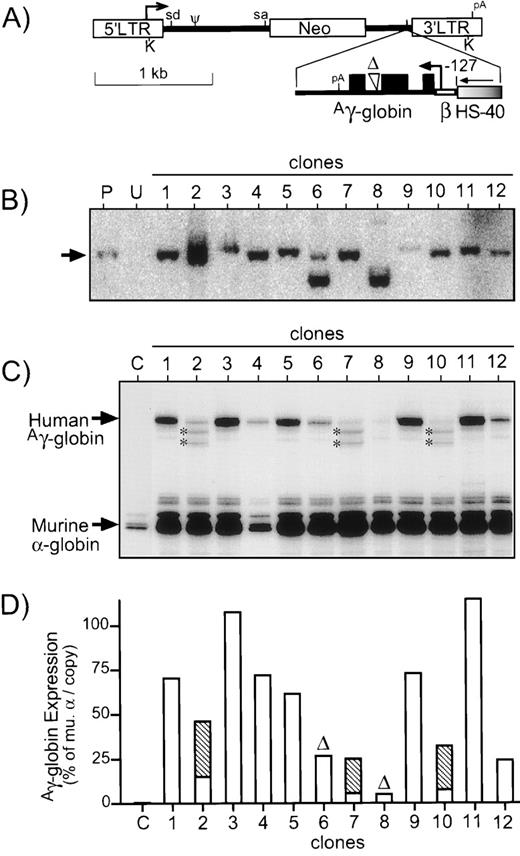
Diagram and analysis of retrovirus vector. (A) The retrovirus vector HS40-6 was generated using the MLV-based vector LNSX1 indicated at the top, which expresses Neo from the promoter in the 5′ LTR. The optimal expression cassette was inserted in the opposite orientation with respect to viral transcription. This cassette consists of a single copy of the HS-40 enhancer (graded fill), the β-globin gene promoter (open thin bar) truncated to position −127, and the genomic elements of theAγ-globin gene (closed thin bar) starting with the first exon (filled boxes) and containing the large internal deletion (▵) of intron 2. Heavy arrows, sites of transcription initiation; sd/sa, vector splice donor/acceptor sites; Ψ, packaging signal; pA, polyadenylation sites; K, KpnI restriction sites used for Southern analysis. (B) DNA was prepared from clones of vector-transduced MEL585 cells and analyzed for intact provirus by digestion with KpnI (which cuts once in each LTR) and probing for Neo. Controls include DNA from untransduced MEL585 cells (U) and the producer clone (P) used to generate virus supernatant. The expected position of intact provirus is indicated to the left of the panel with an arrow. The limited signal for clone no. 9 was due to a loading error. (C) RNase protection analysis for Aγ-globin expression in the 12 MEL585 clones transduced with the retrovirus vector. The positions of the protected fragments forAγ-globin (170 bp, exon 2) and murine -globin (128 bp, exon 1) are indicated to the left of the panel. Two novel protected fragments in samples no. 2, 7, and 10 are indicated by asterisks. (D) The protected fragments in (C) were quantified by Phosphorimager, and expression of the transduced Aγ-globin cassette is reported as a percentage per copy of endogenous murine -globin. For clones no. 2, 7, and 10, the contribution from the secondary bands are indicated by the hatched portion of the bar. Clones no. 6 and 8 are marked with (▵) to indicate they contain deleted provirus.
Diagram and analysis of retrovirus vector. (A) The retrovirus vector HS40-6 was generated using the MLV-based vector LNSX1 indicated at the top, which expresses Neo from the promoter in the 5′ LTR. The optimal expression cassette was inserted in the opposite orientation with respect to viral transcription. This cassette consists of a single copy of the HS-40 enhancer (graded fill), the β-globin gene promoter (open thin bar) truncated to position −127, and the genomic elements of theAγ-globin gene (closed thin bar) starting with the first exon (filled boxes) and containing the large internal deletion (▵) of intron 2. Heavy arrows, sites of transcription initiation; sd/sa, vector splice donor/acceptor sites; Ψ, packaging signal; pA, polyadenylation sites; K, KpnI restriction sites used for Southern analysis. (B) DNA was prepared from clones of vector-transduced MEL585 cells and analyzed for intact provirus by digestion with KpnI (which cuts once in each LTR) and probing for Neo. Controls include DNA from untransduced MEL585 cells (U) and the producer clone (P) used to generate virus supernatant. The expected position of intact provirus is indicated to the left of the panel with an arrow. The limited signal for clone no. 9 was due to a loading error. (C) RNase protection analysis for Aγ-globin expression in the 12 MEL585 clones transduced with the retrovirus vector. The positions of the protected fragments forAγ-globin (170 bp, exon 2) and murine -globin (128 bp, exon 1) are indicated to the left of the panel. Two novel protected fragments in samples no. 2, 7, and 10 are indicated by asterisks. (D) The protected fragments in (C) were quantified by Phosphorimager, and expression of the transduced Aγ-globin cassette is reported as a percentage per copy of endogenous murine -globin. For clones no. 2, 7, and 10, the contribution from the secondary bands are indicated by the hatched portion of the bar. Clones no. 6 and 8 are marked with (▵) to indicate they contain deleted provirus.
Supernatant from the best producer clone was used to transduce MEL585 cells, and individual clones were isolated by limiting dilution and selection with G418. These clones were then analyzed for intact provirus by Southern blotting and for expression ofAγ-globin by RNase protection. As seen in Fig 5B, 10 of 12 clones contained fully intact provirus. In clone no. 6, there was some intact provirus, but most of the provirus appeared to contain an internal deletion estimated to be about 0.5 kb. In the case of clone no. 8, most of the provirus appeared to have a deletion of about the same size as in clone no. 6, as well as a faint band about 1 kb larger than the intact vector.
After induction, RNase protection analysis was performed on 12 MEL585 clones (Fig 5C). Excluding the clones with obvious provirus deletions,Aγ-globin mRNA expression averaged 62.7% ± 31.7% per copy of murine α-globin, with a range of 24.2% to 114.7%. Two extra bands were observed in the RNase protection analysis of three clones (nos. 2, 7, and 10) indicating abnormal splicing. The average Aγ-globin mRNA expression in the remaining seven clones was 74.8% ± 30.1%. This level of expression is statistically indistinguishable from the 104.5% ± 53.4% observed for the plasmid construct containing the same cassette in the set of nine transfected MEL585 clones (P = .20), suggesting that expression of this cassette is not affected by elements from the retrovirus vector. High level expression of Aγ-globin protein was confirmed in the virus vector-transduced MEL585 clones described in Fig 5 by immunofluorescent staining and flow cytometry (data not shown). These data show the ability of this vector to generate high functional titers and express Aγ-globin at high levels in the erythroleukemia cell line MEL585.
DISCUSSION
Effective gene therapy of β-chain hemoglobinopathies will require vectors, which are capable of expressing the transferred globin gene at near physiologic levels at the appropriate stage of erythroid differentiation. Initial studies with retrovirus vectors for human γ-globin showed that an extended γ-globin promoter and coding sequences alone were not sufficient to achieve high levels of expression.5 Although the promoters from the γ- and β-globin genes have been intensively studied for many years, their relative strengths in isolation remain unclear, due in part to differences in experimental systems and construct components. We made a series of truncations in both the Aγ- and β-globin promoters and coupled them to the same μLCR enhancer. The natural globin coding sequences, rather then heterologous reporter genes, were used to assure the presence of any critical cis-regulatory elements within the transcribed regions, and to allow for the direct assessment of authentic globin gene transcripts. Maximal expression was achieved in this system even after deleting several upstream motifs, including the binding sites for GATA-1 and Oct-1 present around position −175 of the Aγ-globin promoter. The high level of expression from the truncated promoters may be due, in part, to the relative proximity of the μLCR enhancer in these constructs. In the case of the plasmid constructs used here, the μLCR is already located directly adjacent to the promoters. This possibility is supported by studies in mice, where expression of anAγ-globin transgene linked to LCR elements in a plasmid was found to persist into adulthood,13 compared with the normal developmental silencing observed when theAγ-globin gene and LCR were separated on cosmids or yeast artificial chromosomes.35 36
Globin gene expression in vivo is absolutely dependent on locus-specific enhancers such as the β-globin LCR or the α-globin HS-40 element. The β-globin LCR consists of five DNase I hypersensitive sites. Three of these sites, HS2, HS3, and HS4 have shown enhancing activity, while the enhancing activity of HS1 has only been observed in combination with other HS elements.37 The core elements of these sites have been mapped to about 200 to 300 bp each using a combination of DNase footprinting, evolutionary conservation, and functional assays (reviewed in Stamatoyannopoulos and Nienhuis38). The core sequence of the α-globin HS-40 enhancer has likewise been mapped to about 300 bp.30 These cores share several common features, including binding sites for erythroid-specific transcription factors such as GATA-1 and NF-E2, as well as for ubiquitous factors, such as the GT motifs. This similarity is mirrored in the relative enhancing ability of these elements. As shown in the studies reported here, there is no significant difference between the enhancing activity of the μLCR, the combination of HS2 and HS3, or the HS-40 core when MEL cells are used as target cells. However, studies in transgenic animals have shown important differences between the functions of the β-globin LCR and the α-globin HS-40. The LCR is capable of opening and maintaining the chromosome structure, as evidenced by the copy number-dependent expression of genes linked to this element.23 The HS-40, on the other hand, is unable to confer copy number-dependent expression to a linked gene.31Moreover, the expression levels of genes linked to the HS-40 in transgenic mice decrease with age,39 implying that HS-40 element cannot resist heterochromatin spreading. It is critical to determine whether globin gene expression cassettes incorporating the HS-40 element are capable of efficient, long-term expression in vivo.
An unexpected finding in this study was the effect of internal sequences from Aγ-globin intron 2 on gene expression. Aside from its role in splicing, no other enhancing or suppressing activity has previously been ascribed to sequences from this intron. Initial studies with reporter genes in the absence of the LCR suggested that an enhancing activity may be present in the second intron of the β-globin gene.20,21 However, such activity was not detected in subsequent studies where the LCR was included in the constructs.18 In retrovirus vectors for β-globin, a full deletion of intron abolished expression even in the presence of LCR sequences,40,41 while expression could be restored by only deleting an internal portion of this intron.2 In the case of retrovirus vectors for γ-globin, a full deletion of intron 2 (needed to achieve high titers) resulted in a twofold decrease in expression.5 The 714-bp internal deletion ofAγ-globin intron 2 reported here resulted in 2.3-fold higher expression compared with the same construct with a full-length intron 2. Whether the increased expression is due to increased transcript stability, increased rate of transcription, or a facilitated interaction between the enhancer and the promoter remains to be determined.
We have previously found a very large variation in globin gene expression among MEL cell clones tranfected with plasmids9or YACs containing the β-globin locus,42 and we have concluded that this line cannot be used for studying the function of the LCR or the sequences, which protect the globin genes from the effects of the position of integration. In contrast to single clones, pools composed of more than 50 clones are useful for expression studies because the variation between individual clones is normalized when a large number of clones are contained in a pool.9 Ideally, studies of globin gene expression cassettes should be performed using primary cells in transgenic mice, but this approach is impractical when a large number of constructs have to be functionally characterized. As shown here, when MEL cell pools containing a large number of clones are used, meaningful data can be obtained.
We felt it was important to determine whether retrovirus vector sequences have any intrinsic properties, which may function to suppress expression of the globin gene in the HS-40/βpr-Aγ(Δ intron 2) cassette. We found no statistically significant difference in expression between cells transfected with the plasmid construct and cells transduced with a retrovirus vector containing the HS-40/βpr-Aγ(Δ intron 2) cassette. This observation suggests that the retrovirus vector sequences need not necessarily impair globin gene expression, and that the HS-40 enhancer and −127 β-globin promoter may function independently of the promoters and enhancers of the virus LTR.
ACKNOWLEDGMENT
We thank Betty Mastropaolo, XiaoChun Wang, Mike Mikiska, and Mike Knibbe for technical support. We would also like to thank Kenneth R. Peterson for providing the −117 G → AAγ-globin promoter, A. Dusty Miller for providing the retrovirus vector LNSX and the packaging cell lines PA317 and PE501, Douglas R. Higgs for providing the α-globin HS-40 enhancer fragment, and Margaret H. Baron for providing the mouse α-globin plasmid.
Supported by Grant No. HL 53750 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to George Stamatoyannopoulos, MD, Professor of Medicine, Head, Division of Medical Genetics, University of Washington, Box 357720, Seattle, WA 98195; e-mail:gstam@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal