Abstract
It is debated whether follicular large cell lymphoma (FLCL) has a clinical behavior that is distinct from indolent follicular lymphomas, and whether there is a subset of patients who can be potentially cured. We report here our experience with 100 FLCL patients treated at our institution since 1984 with three successive programs. We evaluated the predictive value of pretreatment clinical features, including two risk models, the Tumor Score System and the International Prognostic Index (IPI). With a median follow-up of 67 months, the 5-year survival is 72% and the failure-free survival (FFS) is 67%, with a possible plateau in the FFS curve, particularly for patients with stage I-III disease. Features associated with shorter survival included age ≥60, elevated lactic dehydrogenase (LDH) or beta-2-microglobulin (β2M), advanced stage, and bone marrow involvement. Stage III patients had significantly better survival than stage IV patients (P < .05). By the IPI and Tumor Score System, 80% of the patients were in the lower risk groups; both systems stratified patients into prognostic groups. Patients with FLCL have clinical features and response to treatment similar to that reported for diffuse large cell lymphoma. Prognostic risk systems for aggressive lymphomas are useful for FLCL. A meaningful fraction of patients may possibly be cured when treated as aggressive lymphomas.
FOLLICULAR LARGE cell lymphoma (FLCL) is an uncommon disease, representing 3% to 7% of non-Hodgkin’s lymphomas.1 FLCL is distinguished from other follicular lymphomas both in the Working Formulation, in which it is classified as intermediate grade, and in the Revised European-American Lymphoma (REAL) classification, in which it is designated as follicle center, follicular, grade 3.2 But controversy exists concerning the number of large cells and extent of follicular formation that are required to establish the diagnosis; even the REAL classification provides no precise criteria to determine the grade. Thus, the difficulty in subcategorizing the follicular lymphomas is acknowledged in the literature.3,4 Investigators in many recent series5-7 required that 50% of the cells present be large cells, with a predominance of large noncleaved cells to confirm the diagnosis. Martin et al5 have recently assessed the utility of the Berard method of grading follicular lymphomas in an analysis of 106 patients with follicular lymphomas, and found that the histologic classification according to the Berard criteria was the only independent predictor of survival.
A coherent view of FLCL has been limited not only by the disparate diagnostic criteria, but also by the relative infrequency of FLCL and the small and retrospective nature of most FLCL reports in the literature. In the last 10 to 15 years, FLCL has usually been treated as an intermediate-grade lymphoma, with the same therapeutic strategy as for diffuse large cell lymphoma (DLCL).5-14 Reported responses, failure-free survival (FFS), and survival have been similar to DLCL in many series, but some investigators have observed a pattern of late relapse that parallels the natural history of other follicular lymphomas.9,11 14 Thus, some issues are unresolved, especially whether there is a true plateau in the failure-free survival curve, and consequently whether there is a subset of FLCL patients who are potentially cured.
Analysis of clinical factors of proven prognostic value in the aggressive lymphomas has been insufficiently studied in FLCL because of the low number of FLCL patients in reported series. We report herein our experience with 100 patients with FLCL, uniformly treated on three protocols for intermediate-grade lymphoma at M.D. Anderson from 1984 until the present. We analyze the response, FFS, and survival of these patients. We aim to identify important prognostic factors and to test the accuracy, in FLCL, of current prognostic models developed for aggressive lymphomas. With this large number of patients with long-term follow-up, we also identify patients who may possibly be cured of their disease with current therapy.
MATERIALS AND METHODS
Patients.
One hundred patients with FLCL were treated with three successive programs designed for intermediate-grade lymphoma by the Working Formulation. These 100 patients represent 17% of the 605 patients with follicular lymphoma seen in our institution since 1984. The histologic criteria required the presence of follicular formation and greater than 15 cells per high-power field being of the large cell variety.4 We used the Ann Arbor classification to stage the disease: staging studies included documentation of clinical disease, complete blood cell count, chemistries, serum beta-2-microglobulin (β2M) for most patients, chest x-ray, bone marrow biopsy and aspirate, computed tomography (CT) scan of the abdomen and pelvis, and CT scan of the chest or other tests as indicated.
Treatment programs.
Patients were treated according to three successive risk-adapted strategies. From 1984 to 1988, 47 patients were treated on a protocol that called for CHOP-Bleo (cyclophosphamide, doxorubicin, vincristine, prednisone, bleomycin) and radiotherapy (XRT) for stage I-II patients, and CHOP-Bleo alternating with CMED (cyclophosphamide, methotrexate, etoposide, dexamethasone) for patients with more advanced stage.15 From 1988 on, good-risk patients received CHOP-Bleo alternating with OPEN (vincristine, prednisone, etoposide, mitoxantrone). High-risk patients (N = 22) from 1988 on received an alternating triple therapy (ATT) program of 3 non–cross-resistant regimens in rotation, 1 based on an anthracycline plus alkylator, 1 based on ara-C and cisplatin, and 1 based on ifosfamide and etoposide.16 The model for risk evolved during this time period from a “tumor burden” model to a “tumor score” system.17 18 Human immunodeficiency virus (HIV)-positive patients and patients who had an antecedent malignancy whose prognosis was poor or who had received previous chemotherapy were not eligible. A cardiac ejection fraction greater than 50% measured by echocardiography or cardiac multigated angiogram (MUGA) scan was required. Patients signed the appropriate consent forms according to institutional guidelines.
Follow-up.
The follow-up of the patients was every 3 months during the first year, every 4 months during the next 2 years, every 6 months in the fourth and fifth years, and yearly afterwards. Patients were evaluated by physical examination, blood chemistries including lactic dehydrogenase (LDH) and β2M, periodic bone marrow biopsy, and radiologic studies including chest x-ray and CT scans of the abdomen and pelvis, and chest, when appropriate.
Variables.
Variables tested for their importance as predictors of the outcome were: age, sex, presence of B symptoms, Ann Arbor Stage, Zubrod performance status, LDH, β2M, bone marrow involvement, platelets, white blood cell count (WBC), hemoglobin (Hgb), presence of bulky disease, and number of extranodal sites. Cut-offs were as previously described.18,19 The two risk systems tested were the International Prognostic Index (IPI)19 and Tumor Score.18 Briefly, the Tumor Score system divides the population into two risk groups by assigning one point for the presence of each of five variables: Ann Arbor Stage III-IV, B symptoms, LDH > 10% above the upper limit of normal, β2M ≥3 μg/dL, and each site of bulky disease. As previously reported, patients with scores of 0 to 2 are low risk, and ≥3 defines high risk.
Endpoints and statistical analysis.
The evaluation of response was performed according to standard criteria. CR was defined as the disappearance of all signs and symptoms of disease both clinically and radiographically. Partial remission (PR) was defined as greater than 50% reduction in the sum of the products of the two greatest perpendicular diameters of all measurable lesions.20 Survival and FFS were measured from the time of initial therapy to death or failure, respectively. Failures included patients who relapsed after CR, progressed after PR or less, had treatment stopped because of toxicity, or who died from the lymphoma or treatment-related toxicity.21
Survival and FFS curves were calculated by the Kaplan-Meier method.22 Seven patients who died of tumor-unrelated deaths and were without any sign of relapse of their disease, as well as three patients who were lost to follow up at 2, 6, and 8 years after the diagnosis were censored at that time. Differences among the curves in each tested variable were performed by the log-rank test.20Differences in response according to prognostic variables were tested by the chi-square test of independence or trend as appropriate.20
RESULTS
Patient characteristics.
The median age of the patients in this series was 59 years (range, 24 to 84). There were 53 women and 47 men. Sixty-five patients had a homogeneous pattern of FLCL. Thirty patients had areas of DLCL along with unequivocal FLCL, and 5 patients had areas of FLCL and follicular small cleaved cell lymphoma (FSC) in the same lymph node. Eighteen patients had bone marrow involvement; 8 patients with only large cell lymphoma, 4 with a predominant large cell component mixed with a small cleaved cell component, and 6 with small cleaved cell involvement. By the Ann Arbor system, there were 26 stage I patients, 22 stage II, 29 stage III, and 23 stage IV. Thirteen had constitutional symptoms. Elevated LDH or β2M were present in 30% and 12%, respectively (Table 1).
Outcome According to Clinical Features: 100 Patients With FLCL
| Feature . | No. of Patients . | CR (%) . | 5-yr FFS (%) . | 5-yr Survival (%) . | Median Survival (mo) . |
|---|---|---|---|---|---|
| All patients | 100 | 85 | 67 | 72 | 141 |
| Age | |||||
| <60 | 54 | 91 | 65 | 85 | 120 |
| ≥60 | 46 | 78 | 69 | 57* | 64* |
| Ann Arbor Stage | |||||
| I | 26 | 96 | 85 | 90 | NR |
| II | 22 | 91 | 86 | 73 | 77 |
| III | 29 | 93 | 62 | 71 | 135 |
| IV | 23 | 57* | 37* | 51* | 60* |
| No. E sites | |||||
| 0, 1 | 93 | 87 | 69 | 78 | 124 |
| ≥2 | 7 | 57 | 43 | 29† | 27† |
| LDH | |||||
| Low | 70 | 89 | 73 | 82 | 120 |
| High | 30 | 77 | 53 | 50† | 43† |
| Marrow | |||||
| Negative | 82 | 94 | 77 | 80 | 141 |
| Positive | 18 | 44† | 19† | 41† | 52† |
| β2M‡ | |||||
| <3 | 70 | 91 | 77 | 76 | 113 |
| ≥3 | 10 | 50* | 54* | 12† | 13† |
| IPI | |||||
| Low | 59 | 92 | 75 | 85 | 160 |
| Low-Int | 26 | 81 | 64 | 69 | 108 |
| High-Int | 13 | 77 | 38 | 28 | 35 |
| High | 2 | 0† | 0† | 0† | 12† |
| Tumor score‡ | |||||
| <3 | 67 | 91 | 81 | 77 | 117 |
| ≥3 | 13 | 66* | 44* | 26† | 28† |
| Feature . | No. of Patients . | CR (%) . | 5-yr FFS (%) . | 5-yr Survival (%) . | Median Survival (mo) . |
|---|---|---|---|---|---|
| All patients | 100 | 85 | 67 | 72 | 141 |
| Age | |||||
| <60 | 54 | 91 | 65 | 85 | 120 |
| ≥60 | 46 | 78 | 69 | 57* | 64* |
| Ann Arbor Stage | |||||
| I | 26 | 96 | 85 | 90 | NR |
| II | 22 | 91 | 86 | 73 | 77 |
| III | 29 | 93 | 62 | 71 | 135 |
| IV | 23 | 57* | 37* | 51* | 60* |
| No. E sites | |||||
| 0, 1 | 93 | 87 | 69 | 78 | 124 |
| ≥2 | 7 | 57 | 43 | 29† | 27† |
| LDH | |||||
| Low | 70 | 89 | 73 | 82 | 120 |
| High | 30 | 77 | 53 | 50† | 43† |
| Marrow | |||||
| Negative | 82 | 94 | 77 | 80 | 141 |
| Positive | 18 | 44† | 19† | 41† | 52† |
| β2M‡ | |||||
| <3 | 70 | 91 | 77 | 76 | 113 |
| ≥3 | 10 | 50* | 54* | 12† | 13† |
| IPI | |||||
| Low | 59 | 92 | 75 | 85 | 160 |
| Low-Int | 26 | 81 | 64 | 69 | 108 |
| High-Int | 13 | 77 | 38 | 28 | 35 |
| High | 2 | 0† | 0† | 0† | 12† |
| Tumor score‡ | |||||
| <3 | 67 | 91 | 81 | 77 | 117 |
| ≥3 | 13 | 66* | 44* | 26† | 28† |
Statistically significant adverse features (P < .05).
Statistically significant adverse features (P < .01).
β2M data, and therefore Tumor Score assignment, unavailable for 20 patients.
Response.
Eighty-five patients (85%) achieved a CR, 10 patients (10%) achieved a PR, and 5 patients did not respond to initial therapy. Bone marrow involvement, Ann Arbor stage IV, high β2M, high Tumor Score, and high IPI score were significantly correlated with lower response (Table 1). All nonresponders had a high Tumor Score or high/high-intermediate IPI score. Four were women. All 5 nonresponders were over 65 years old, with a median of 72 years old; other adverse pretreatment prognostic factors included bone marrow involvement in 4, high LDH in 2, and high β2M in 3 (of 4 with β2M data).
Failure-free survival.
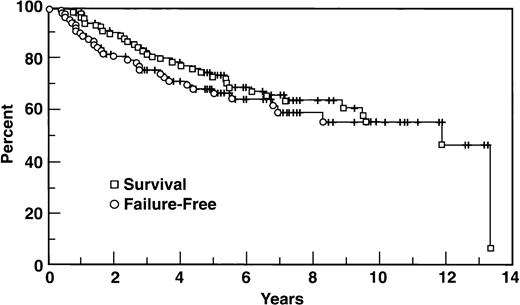
Thirty-two patients eventually progressed, including 22 of the 85 CR patients, 5 of the 10 PR patients, and all 5 nonresponders. At 5 years the FFS is 67%; the median FFS has not been reached (Fig1). For those 32 patients who progressed, the median time to progression was 19 months. Several variables were important to predict FFS (Table 1). The Ann Arbor stage IV group of 23 patients had a FFS at 5 years of 37%, significantly worse than other stages (Fig 2). β2M and marrow involvement were significant adverse factors, and both the Tumor Score and IPI were valuable systems to divide the population into different prognostic groups.
Survival and predictors of survival.
With a median follow-up of 67 months, the median survival is 141 months with a 72% survival at 5 years (95% CI 62% to 82%). There are currently 65 patients alive, and 35 patients who have died, including 28 who died of disease. Seven died of unrelated causes, including 2 patients who died of unknown reasons, 2 who died of a stroke, 2 of lung cancer, and 1 patient who died of a heart attack; all 7 of these patients had achieved CR and had no evidence of disease at the time of their death. All 5 patients with primary refractory disease have died, compared to 6 of the 10 PR patients, and 24 of the 85 CR patients.
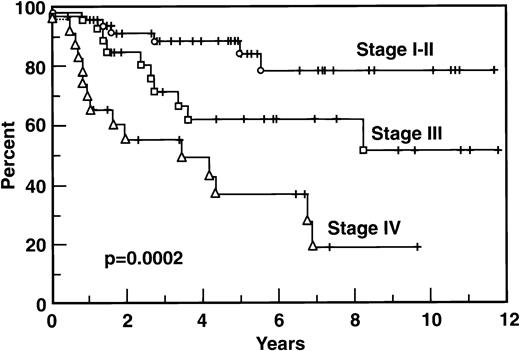
Survival according to clinical features is indicated in Table 1. Eighty-five percent of patients younger than 60 years old survived 5 years versus 57% of those ≥60 years old (P = .011). Stage IV patients had significantly shorter survival than others; survival of the 29 stage III patients was equivalent to the survival of the 48 stage I and stage II patients (Fig 3). High serum LDH correlated with a worse survival: 50% of patients with high LDH survived 5 years versus 82% of these patients with a normal LDH (P = .001). Serum β2M was also strongly correlated with survival: only 12.5% of patients with a β2M level ≥3.0 mg % are alive at 5 years versus 76% of those with β2M lower than 3 (P < .001). The extent of extranodal disease predicted the survival as well, with 66%, 33%, and 0% survival at 5 years for 1, 2, and 3 extranodal sites, respectively, versus 78% for those without extranodal disease (P = .0042). Bone marrow was the most frequent single site of extranodal disease, and it was of prognostic importance: of the 18 patients who had marrow involvement, 13 are dead, and the 5-year survival is 41% versus 80% for those without bone marrow involvement (P = .0019). Only 5 patients had stage IV disease based on sites other than the bone marrow. Of these 5 patients, 4 are alive and 1 patient has died, but the numbers of patients and events are too few to characterize the prognostic importance of specific extranodal sites other than the marrow. The extent of the follicular versus diffuse pattern of the node was not prognostically important: the 30 patients with a follicular plus diffuse pattern had a similar survival to the 65 patients with a homogeneous follicular large cell pattern, as did those 5 patients with a small cleaved cell component (P = .78). Other variables that were analyzed, including sex, platelets, and the presence of bulky disease, were not important as predictors in our analysis.
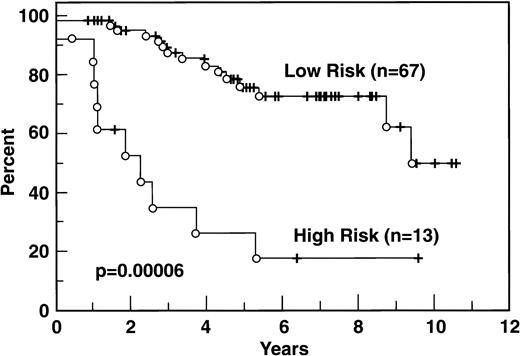
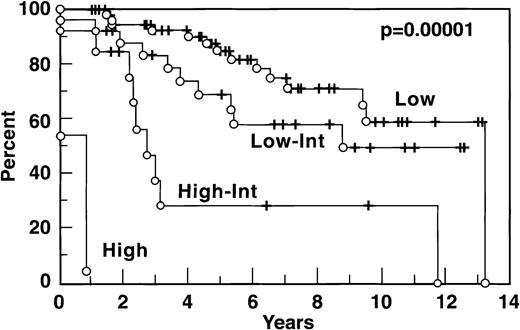
The distribution of patients according to the IPI and the Tumor Score is indicated in Table 1: both models show, for FLCL, a significantly worse prognosis for patients belonging to the higher risk groups (Figs4 and 5).
Survival according to Tumor Score. Adverse features in the Tumor Score are Ann Arbor stage III-IV, β2M ≥3 mg%, high LDH, B symptoms, and the number of bulky masses. Each adverse factor has a score of 1. Low risk is defined as a tumor score of 0 or 1, high risk is a score of ≥2.
Survival according to Tumor Score. Adverse features in the Tumor Score are Ann Arbor stage III-IV, β2M ≥3 mg%, high LDH, B symptoms, and the number of bulky masses. Each adverse factor has a score of 1. Low risk is defined as a tumor score of 0 or 1, high risk is a score of ≥2.
Stage III and IV.
Because in our population the ratio of stages I-II versus III-IV was higher than most published series, we analyzed specifically the 52 patients with stage III-IV disease to have more accurate data to compare with other series. The median survival of the combined stage III-IV group was 103.4 months with a 5-year survival of 63%. However, the 29 stage III patients had a survival at 5 years of 71% versus 51% for the 23 patients with stage IV (P < .05; Fig 3). Relevant prognostic variables for the stage III-IV group are shown in Table 2. LDH and β2M proved useful to stratify stage III-IV patients according to risk for survival. Unexpectedly, age did not predict for FFS, but it was important for survival. Other variables, including gender, performance status, constitutional symptoms, bulky masses, WBC count, and platelets were not predictive of outcome.
Results in Stages III and IV According to Clinical Characteristics
| Feature . | No. of Patients . | 5-yr FFS (%) . | 5-yr Survival (%) . | Median Survival (mo) . |
|---|---|---|---|---|
| Age | ||||
| <60 | 30 | 57 | 82 | 118 |
| ≥60 | 22 | 37 | 31* | 36* |
| Ann Arbor Stage | ||||
| III | 29 | 62 | 71 | 135 |
| IV | 23 | 37† | 51† | 60† |
| LDH | ||||
| Low | 29 | 56 | 71 | 103 |
| High | 23 | 44 | 49† | 45† |
| Marrow | ||||
| Negative | 34 | 66 | 71 | 140 |
| Positive | 18 | 19* | 41† | 55† |
| β2M‡ | ||||
| <3 | 32 | 63 | 64 | 90 |
| ≥3 | 8 | 13* | 19* | 13* |
| IPI | ||||
| Low | 17 | 75 | 85 | 115 |
| Low-Int | 20 | 64 | 68 | 105 |
| High-Int | 13 | 38 | 28 | 35 |
| High | 2 | 0* | 0* | 12* |
| Tumor score‡ | ||||
| <3 | 29 | 64 | 64 | 99 |
| ≥3 | 11 | 39† | 33† | 19† |
| Feature . | No. of Patients . | 5-yr FFS (%) . | 5-yr Survival (%) . | Median Survival (mo) . |
|---|---|---|---|---|
| Age | ||||
| <60 | 30 | 57 | 82 | 118 |
| ≥60 | 22 | 37 | 31* | 36* |
| Ann Arbor Stage | ||||
| III | 29 | 62 | 71 | 135 |
| IV | 23 | 37† | 51† | 60† |
| LDH | ||||
| Low | 29 | 56 | 71 | 103 |
| High | 23 | 44 | 49† | 45† |
| Marrow | ||||
| Negative | 34 | 66 | 71 | 140 |
| Positive | 18 | 19* | 41† | 55† |
| β2M‡ | ||||
| <3 | 32 | 63 | 64 | 90 |
| ≥3 | 8 | 13* | 19* | 13* |
| IPI | ||||
| Low | 17 | 75 | 85 | 115 |
| Low-Int | 20 | 64 | 68 | 105 |
| High-Int | 13 | 38 | 28 | 35 |
| High | 2 | 0* | 0* | 12* |
| Tumor score‡ | ||||
| <3 | 29 | 64 | 64 | 99 |
| ≥3 | 11 | 39† | 33† | 19† |
Statistically significant adverse features (P < .01).
Statistically significant adverse features (P < .05).
β2M data, and therefore Tumor Score assignment, unavailable for 12 patients.
Stages III and IV are grouped together as one adverse feature in both the IPI and the Tumor Score prognostic models. We examined, in our stage III-IV patients, the remaining features of the Tumor Score model for their prognostic relevance. By the Tumor Score system, of 11 patients with two or more additional adverse features besides stage, the survival at 5 years was 33% versus 64% for those 29 patients with only 0 to 1 additional adverse features (P = .018). Thus, even in this group, the Tumor Score was able to capture a group with poor prognosis. Likewise, the IPI was useful to stratify stage III-IV patients into prognostic groups (Table 2).
DISCUSSION
In our current report on 100 patients with FLCL, the survival was 72% at 5 years (median, 141 months). Another recent large series, by Wendum et al,6 reported a 5-year survival of 58% and a median survival of 73 months in their analysis of 89 patients with FLCL. When compared with our population, there were a few differences: our population was somewhat older, with 46% of patients older than 60 versus 35% in their report; conversely, only 52% of our patients presented with Ann Arbor stage III-IV versus 74% in their study. To facilitate comparisons with other series, we performed a separate analysis of our subset of patients with stage III-IV disease: in these 52 patients, the median survival was 103 months with 63% alive at 5 years.
One interesting observation in the current series was the significantly different outcome of stage III and stage IV patients. In our series, patients with Ann Arbor stage III had a better survival than those with stage IV (71% v 51% at 5 years, respectively), as well as a better FFS (62% v 37%, respectively). Both the Tumor Score and IPI prognostic models incorporate stage III-IV as one (equivalent) adverse feature. Our data suggest that to group stage III and IV FLCL patients together may not be totally correct. If this finding is confirmed in other series, this observation might warrant different therapeutic approaches for stage III and stage IV patients.
Besides stage, other important prognostic factors identified in the current series included older age, multiple sites of extranodal involvement, and elevation of serum LDH and β2M. These findings are similar to what has been described by Wendum et al, and, of course, these features are key components of two prognostic models for aggressive lymphomas, the IPI and the Tumor Score. This observation highlights the similarities of FLCL and DLCL, although, notably, the distribution of patients by risk group showed a lower fraction with high risk than is seen in most DLCL series. Specifically, 13 patients on the current series had high-intermediate risk with a 5-year survival of 28%, and 2 had high risk with a 5-year survival of 0%. This distribution among risk groups is similar to the FLCL series of Bartlett et al.7
Among extranodal sites of disease, bone marrow was the most common in our series, and it was a significant adverse prognostic factor. We had previously noted, in another series from our institution,10that marrow involvement itself did not confer a worse prognosis, and we had suggested that in this respect FLCL resembled other follicular lymphomas and contrasted to DLCL where bone marrow involvement predicts a poor prognosis. Our current finding is thus different, and may be explained in part by the paucity of patients with discordant disease in the marrow in the prospective trials from which the current report derives. Of the 18 patients with marrow disease, the majority had large cell lymphoma in the marrow, which is more ominous than having marrow involvement with only small cleaved cell lymphoma.23
Our observation of a possible plateau in the FFS curve in these FLCL patients is in contrast to previous suggestions by some of a behavior more typical of the low-grade follicular lymphomas, with their pattern of constant relapses over time.9,11,14 However, our results are consistent with those of others, who have noted a plateau in the FFS curve in patients treated with anthracycline-based chemotherapy.5-7 The criteria for classifying FLCL may be important in identifying those with potentially curable disease: the Nebraska Lymphoma Study Group, in an earlier report,9 did not observe a convincing FFS plateau, but more recently5the same group did observe an apparent plateau in the FFS curve, when using Berard’s criteria. The occurrence of four late relapses observed in our series is similar to the small number of late relapses seen in the intermediate-grade lymphomas as reported by Cabanillas et al.24 The presence of a small cleaved cell component either in the lymph node or in the marrow may be a risk factor for late relapse, as previously observed.23 Of the 32 patients who relapsed, the median time to relapse of 19 months in this series does not differ from the DLCL population. Another suggestion of the similar biology of FLCL and DLCL is provided by the recent observation that expression of the Bcl-2 protein correlates with a poor prognosis in FLCL,6 as has previously been reported for DLCL.25
It is controversial whether the natural history of FLCL is more akin to indolent follicular lymphomas or to DLCL.5-7,9,11,14Perhaps it is best regarded as a transitional phase, as postulated by Gall and Mallory26 57 years ago and Rappaport et al27 43 years ago. Our data suggest that FLCL has a natural history and response to treatment similar to DLCL. The distribution of variables that predict the outcome suggests that FLCL patients often present with better prognostic features than DLCL patients. Risk systems for DLCL18 19 are useful in FLCL: both the IPI and the Tumor Score stratify patients well, allowing for innovative therapies to be considered to improve the outcome in the poorer outcome groups, while for better prognosis patients, diminished toxicity with standard effective therapy can be the focus. A meaningful fraction of FLCL patients may possibly be cured with the same treatment regimens used for DLCL.
ACKNOWLEDGMENT
The authors thank Joyce Palmer for assistance in preparation of the manuscript, and also collaborators from the Division of Radiation Oncology, and past and present members of the Department of Lymphoma/Myeloma, notably Drs W.S. Velasquez and F. Swan.
Supported in part by National Cancer Institute Core Grant CA16672 awarded to the University of Texas M.D. Anderson Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to P. McLaughlin, MD, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 68, Houston, TX 77030.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal