Chronic granulomatous disease (CGD) is a disorder of host defense due to genetic defects of the superoxide (O2-) generating NADPH oxidase in phagocytes. A membrane-bound cytochrome b558, a heterodimer consisting of gp91-phox and p22-phox, is a critical component of the oxidase. The X-linked form of the disease is due to defects in the gp91-phox gene. We report here biochemical and genetic analyses of patients with typical and atypical X-linked CGD. Immunoblots showed that neutrophils from one patient had small amounts of p22-phoxand gp91-phox and a low level of O2-forming oxidase activity, in contrast to the complete absence of both subunits in two patients with typical CGD. Using polymerase chain reactions (PCR) on cDNA and genomic DNA, we found novel missense mutations of gp91-phox in the two typical patients and a point mutation in the variant CGD, a characteristic common to two other patients with similar variant CGD reported previously. Spectrophotometric analysis of the neutrophils from the variant patient provided evidence for the presence of heme of cytochromeb558. Recently, we reported another variant CGD with similar amounts of both subunits, but without oxidase activity or the heme spectrum. A predicted mutation at amino acid 101 in gp91-phox was also confirmed in this variant CGD by PCR of the genomic DNA. These results on four patients, including those with two variant CGD, are discussed with respect to the missense mutated sites and the heme binding ligands in gp91-phox.
CHRONIC GRANULOMATOUS disease (CGD) is a severe clinical syndrome characterized by recurrent bacterial and fungal infections with granuloma formation.1 The disease is caused by failure of the NADPH oxidase in phagocytes (neutrophils, eosinophils, monocytes, and macrophages), a superoxide (O2-) forming enzyme required for microbicidal activity. This enzyme system consists of multiple factors including membrane-associated cytochrome b558 and cytosolic components for oxidase activation.2-6 Cytochromeb558 is a heterodimer formed by a 91-kD glycosylated heavy chain, gp91-phox, and a 22-kD light chain, p22-phox. CGD is now known to be caused by deficiency of one of four proteins; gp91-phox, p22-phox, p47-phox, and p67-phox, respectively.2,7,8 Several mutations of gp91-phoxwith X-linked recessive CGD have been reported: deletional, splicing, missense, nonsense, and duplicational mutations.6 9
Here we report molecular and biochemical studies on three patients with X-linked CGD: one variant and two typical. The variant CGD has small amounts of both p22-phox and gp91-phox with O2- forming NADPH oxidase activity. Sequence analyses of the gp91-phox gene showed novel point mutations in the two typical cases of CGD and a conservative point mutation in the patient with variant CGD. Spectrophotometric studies on neutrophils from these patients were performed using an accurate, sensitive technique for analysis of the heme spectrum of cytochromeb558.10 Our results on the variant CGD are compared with those on another variant X-linked CGD that showed similar amounts of both subunits, but no oxidase activity or the heme spectrum.11 In the latter CGD, the predicted mutation at amino acid 101 replacing His by Tyr was further confirmed by polymerase chain reaction (PCR) of genomic DNA. Taken together with the spectrophotometric data, the results on all four CGDs are discussed with respect to the heme binding histidines and the mutated sites in the gp91-phox protein.
MATERIALS AND METHODS
Case reports.
Three male patients, a 10-year old (T.M.), a 19-year old (T.H.), and a 25-year old (J.O.), all had recurrent episodes of bacterial infection and were diagnosed as having X-linked recessive CGD by functional analysis of their and their mothers’ neutrophils at the age of 6 months, 5 years, and 6 years, respectively. Patients T.H. and J.O. had received operations for liver abscesses before the diagnosis of CGD. Although they often suffered from perianal abscesses, inguinal lymphadenitis, stomatitis, and skin abscesses, they were relieved of severe infection by administration of sulfamethoxazole-trimethoprim.
Materials.
Ficoll-Paque and Dextran T-500 were purchased from Pharmacia Biotech, Uppsala, Sweden; diisopropyl fluorophosphate (DFP) was from Wako Pure Chemicals, Osaka, Japan; Sodium-p-tosyl-L-lysine chloromethyl ketone (TLCK), zymosan, and phorbol 12-myristate 13-acetate (PMA) were from Sigma, St Louis, MO; phenylmethylsulfonyl fluoride (PMSF) was from Nakarai Co, Kyoto, Japan. All other reagents were of analytical grade. Reagents for the analysis of RNA and DNA are described below. Opsonized zymosan (op-zy) (20 mg/mL) was prepared as described previously.12 PMA was dissolved in dimethyl sulfoxide (20 mg/mL) and diluted with Ca2+-free Krebs Ringer phosphate buffer [KRP: 122 mmol/L NaCl, 4.9 mmol/L KCl, 1.2 mmol/L MgCl2, 17 mmol/L sodium phosphate buffer (pH 7.4)] to 20 μg/mL before use.
Isolation of neutrophils.
Neutrophils from the three CGD patients, their parents, and healthy controls were collected from 10 to 20 mL samples of heparinized venous blood by Dextran sedimentation, followed by hypotonic treatment for lysis of red blood cells, and then stepwise separation with Ficoll-Paque according to Böyum’s method13 with some modifications.10 The cells, consisting of 98% to 99% neutrophils and less than 1% eosinophils, were suspended in ice-cold Ca2+-free KRP before use. All donors gave their informed consent.
Assay of cellular O2- formation.
Neutrophils were suspended in a narrow-cuvette at 1.0 to 2.0 × 106 cells/700 μL in KRP containing 5 mmol/L glucose and 0.6 mmol/L CaCl2 and were continuously stirred with a wind-mill cell mixer14 for the assay of O2- release from stimulated cells. The rates of O2- formation by neutrophils from donors in the presence of PMA (0.14 μg/106 cells) or op-zy (0.4 mg/106 cells) were measured at 37°C by recording superoxide dismutase inhibitable-reduction of ferricytochromec12 at the absorbance difference of 550 to 540 nm in a Hitachi dual wavelength spectrophotometer, model 557 (Hitachi, Tokyo, Japan).
Preparation of membrane and cytosol fractions.
Neutrophils from each donor were treated with 2 mmol/L DFP on ice and were suspended in sonication buffer consisting of 1 mmol/L PMSF, 1 mmol/L TLCK, and 20 mmol/L sodium phosphate buffer (pH 7.4) at a final concentration of 2 × 107 cells/mL. Aliquots of the cell suspensions were placed in microtubes and sonicated in a Bioruptor, model UCD-200TM (Cosmo-Bio, Tokyo, Japan) at 0°C, setting its controller at an optimal sonication time (40 to 50 × 1.5 seconds with intervals of 1 second). After sedimentation of cell debris and nuclei at 400xg for 10 minutes, the supernatants were centrifuged at 2.3 × 105gfor 15 minutes at 2°C in a table top ultracentrifuge (Beckman model TL-100; Beckman, Fullerton, CA) to separate the membrane and cytosol fractions as precipitates and supernatants.
Immunoblot analysis.
Rabbit polyclonal antibodies were raised against synthetic polypeptides corresponding to the COOH-terminal peptides (17 residues) of p22-phox and gp91-phox of cytochromeb558 of human neutrophils. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli with 4% stacking gel and 10% or 11% separating gel.15 Membrane fractions (2 × 106 cells equivalent) were mixed with an equal volume of Laemmli buffer and incubated for 3 minutes at 90°C. After electrophoresis, proteins were transferred to a nitrocellulose membrane, which was treated with Ponceau and rabbit antiserum against each peptide.16 The specific bindings of the antibodies to protein bands were detected using goat antirabbit IgG conjugated with horseradish peroxidase. Color was developed with 0.01% H2O2 and 0.03% 4-chloro-1-naphthol, and the color density was measured with an image analyzer (FAS II, TOYOBO, Osaka, Japan), as reported recently.11
Spectrophotometric measurement.
The sonicated neutrophils were exposed to carbon monoxide (CO) as follows.10 A soft rubber ball (diameter, 7 cm), which had been deflated with a needle, was inflated through the needle with CO gas from a small bomb containing 99.95% CO gas. An aliquot of the sonicated sample (200 to 300 μL) was loaded into a sterile syringe, into which CO gas in the ball was then withdrawn through a needle to be a volume ratio of 1:3 (sample, CO). After blocking the needle with a piece of silicon, the syringe was turned slowly upside down several times for 10 minutes to allow binding of contaminating hemoglobin with CO. All of the above procedures were performed in a draft chamber. The CO-treated sample was delivered by syringe into a microcuvette plugged with a rubber septum. Absorption spectra were measured in a Unisoku spectrophotometer model USP-530 (Unisoku Co, Hirakata). Heme proteins in the CO-treated sample were reduced with a few grains of sodium dithionite and the reduced minus oxidized difference spectra were measured in the range of 400 to 700 nm. The spectra obtained were further processed to obtain differential spectra in the range of 540 to 580 nm.10
Preparations of RNA and DNA.
RNA was isolated from Epstein-Barr virus (EBV)-transformed cell lines by first dissolving the cells in 4.0 mol/L guanidine thiocyanate; subsequently, RNA was precipitated through 5.7 mol/L cesium chloride by centrifugation. The mRNA was then isolated using an oligo d(T) column as described by Aviv and Leder.17 First strand cDNA was synthesized using a cDNA synthesis Kit (SuperscriptTMII; GIBCO, Rockville, MD). The protein coding region of the gp91-phox cDNA was amplified by PCR in three overlapping fragments using three pairs of synthetic oligo nucleotide primers as described previously.18 Cycle parameters were: 30 cycles of 1 minute at 94°C, 1 minute at 60°C, and 4 minutes at 72°C. The PCR products were purified with a Geneclean II kit (BIO 101, Inc, Vista, CA) to remove the primers and then subcloned into a plasmid pGEMT (Promega, Madison, WI). Sequence analyses were performed by the dideoxy chain terminating method using an automated DNA sequencer (Model 373A; Applied Biosystems, Foster, CA) according to the manufacturer’s instructions. Multiple clones of each fragment were sequenced in both directions.
Genomic DNA was isolated from circulating blood leukocytes from donors to confirm each mutation. Exons plus intron boundary sequences were amplified by PCR using primers corresponding to sequences on the flanking introns7 as indicated in Table 1. The conditions for PCR were as described above. The PCR product was digested with Taq I (Takara, Kyoto, Japan) for exon 9 of patient T.H. and with MspI for exon 12 of patient T.M. The sizes of the fragments were analyzed in 3% agarose gel. The PCR products for exon 10 of patient J.O. and exon 4 of patient T.K. were sequenced as described above.
RESULTS
Neutrophils from the two patients (T.M. and J.O.) had no O2- generating activity, whereas those from the other patient (T.H.) exhibited slight O2-generation (6% to 7% and 8% to 9% of the control activities on stimulation with PMA and op-zy, respectively).
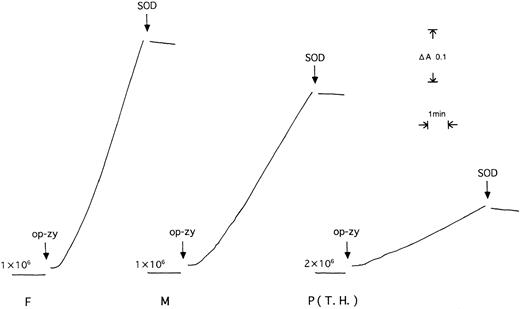
Figure 1 shows traces of O2- generation by phagocytosing cells from the patient (T.H.) and his mother and father. The rates were 3.9 ± 0.7, 25 ± 1 and 44 ± 3 nmol O2-/minute/107 cells (n = 3), which were 8.8%, 55%, and 100% of the control value, respectively.
O2- formation by neutrophils from patient T.H. (P) and his father (F) and mother (M). The rate of O2--dependent reduction of ferricytochromec was recorded by spectrophotometric measurement of the 550 to 540 nm absorption difference. Neutrophils (1.0 to 2.0 × 106 cells/700 μL KRP) were stimulated by addition of op-zy. The numbers on traces are cell numbers added to cuvettes.
O2- formation by neutrophils from patient T.H. (P) and his father (F) and mother (M). The rate of O2--dependent reduction of ferricytochromec was recorded by spectrophotometric measurement of the 550 to 540 nm absorption difference. Neutrophils (1.0 to 2.0 × 106 cells/700 μL KRP) were stimulated by addition of op-zy. The numbers on traces are cell numbers added to cuvettes.
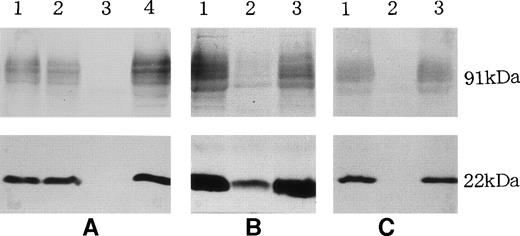
Figure 2 shows immunoblots of the membrane fractions from neutrophils of the CGD patients. Two patients (T.M. and J.O.) had no p22-phox or gp91-phox (Fig 2A and C), characteristic of typical X-linked recessive CGD, whereas the membranes from the variant CGD patient (T.H.) had small amounts of both p22-phox and gp91-phox (Fig 2B). The glycosylated heavy chain, gp91-phox, showed broad electrophoretic migration, and so the stained band appeared faint, but detectable in the gel, in contrast to its complete absence in the two typical cases of CGD. Densitometric analysis of the stained bands of the variant CGD showed the presence of p22-phox and gp91-phox at 14% and 13% of the control, respectively (Table 1).
Immunoblot analyses of neutrophils from CGD patients, T.M. (A), T.H. (B), and J.O. (C). The membrane fractions from neutrophils (1 to 2 × 106 cell equivalents) were electrophoresed and transferred to nitrocellulose membranes, which were treated with antiserum against polypeptides of p22-phox and gp91-phox and stained with Ponceau. (A) Patient T.M. (3), mother (2), and controls (1 and 4). (B) Patient T.H. (2) and controls (1 and 3). (C) Patient J.O. (2) and controls (1 and 3). The protein concentration of the variant CGD (B) loaded was higher than those in other proteins (A and C).
Immunoblot analyses of neutrophils from CGD patients, T.M. (A), T.H. (B), and J.O. (C). The membrane fractions from neutrophils (1 to 2 × 106 cell equivalents) were electrophoresed and transferred to nitrocellulose membranes, which were treated with antiserum against polypeptides of p22-phox and gp91-phox and stained with Ponceau. (A) Patient T.M. (3), mother (2), and controls (1 and 4). (B) Patient T.H. (2) and controls (1 and 3). (C) Patient J.O. (2) and controls (1 and 3). The protein concentration of the variant CGD (B) loaded was higher than those in other proteins (A and C).
Gene Mutations and Heme Spectra of the Patients With CGD
| Patient . | O2-Generating Activity . | Exon . | Nucleotide Change . | Predicted Amino Acid Change . | Immunoblot cyt b . | Heme Spectrum 558 nm . | CGD Type . | |
|---|---|---|---|---|---|---|---|---|
| α . | β . | |||||||
| T.M. | − | 12 | T1558 → C | Trp516 → Arg | − | − | − | X910 |
| T.H. | ± | 9 | G937 → A | Glu309 → Lys | + | ± | ± | X91− |
| J.O. | − | 10 | T1271 → C | Leu420 → Pro | − | − | − | X910 |
| T.K.* | − | 4 | C313 → T | His101 → Tyr | ± | ± | − | X91− |
| Patient . | O2-Generating Activity . | Exon . | Nucleotide Change . | Predicted Amino Acid Change . | Immunoblot cyt b . | Heme Spectrum 558 nm . | CGD Type . | |
|---|---|---|---|---|---|---|---|---|
| α . | β . | |||||||
| T.M. | − | 12 | T1558 → C | Trp516 → Arg | − | − | − | X910 |
| T.H. | ± | 9 | G937 → A | Glu309 → Lys | + | ± | ± | X91− |
| J.O. | − | 10 | T1271 → C | Leu420 → Pro | − | − | − | X910 |
| T.K.* | − | 4 | C313 → T | His101 → Tyr | ± | ± | − | X91− |
Abbreviations: detectable, (+); undetectable, (−); slightly detectable, (±); α (p22-phox) and β (gp91-phox) subunits of cytochrome b558, (cytb).
Tsuda et al.11
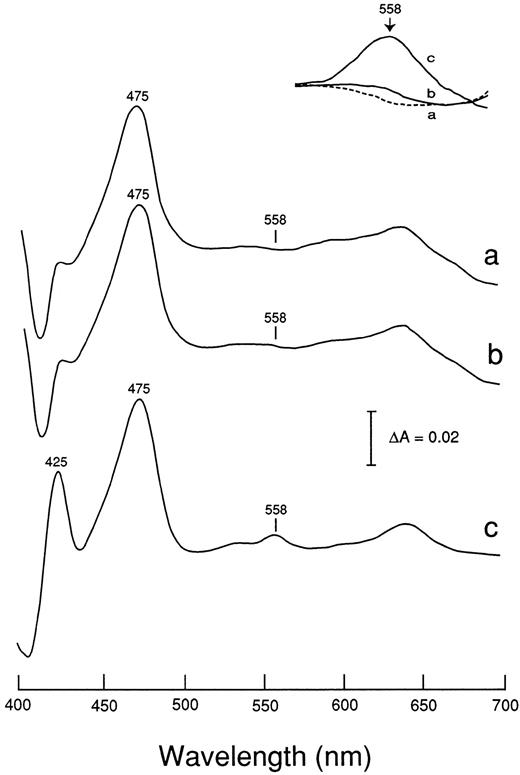
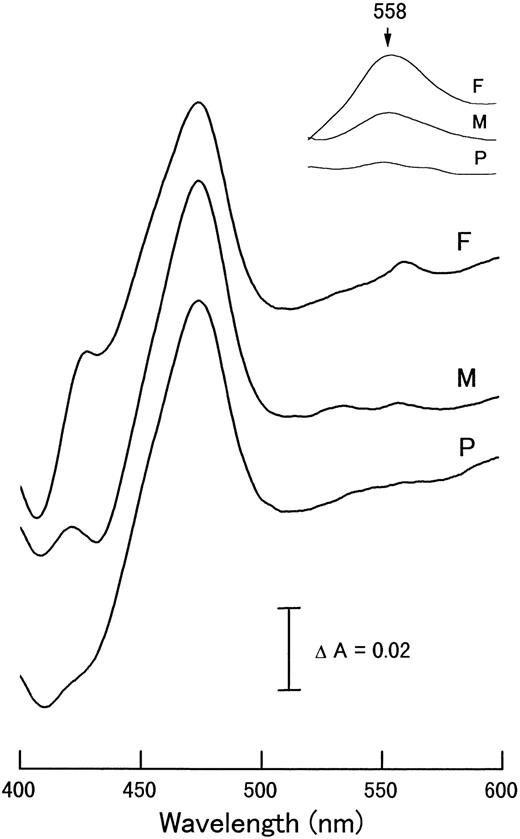
Figure 3 shows the reduced minus oxidized absorption difference spectra of neutrophils from a typical CGD patient; J.O. (Fig 3A), the variant CGD patient; T.H. (Fig 3B), and a healthy donor (Fig 3C). The spectrum of the variant CGD (Fig 3B) showed a small peak at 558 nm of cytochrome b, in contrast to the complete absence of the peak in the typical CGD (Fig 3A). The large absorption band of myeloperoxidase with a peak at 475 nm gave no evidence for the presence of eosinophils, because eosinophil peroxidase gives a distinct peak at 453 nm, causing a shoulder even if the preparation is contaminated with only a few percent of this cell fraction. Indeed, the cell fraction from the variant CGD patient contained 99% neutrophils, but undetectable eosinophils. Figure 4shows the reduced minus oxidized difference spectra of neutrophils from the variant CGD family: father (F), mother (M), and the patient (P). The spectra in the α-band region were five times enlarged (inset of Fig 4). The spectra obtained (Fig 4) were differentiated as d[ΔA]/dλ, where ΔA is the absorption difference and λ is the wavelength, as shown in Fig 5. The differential spectrum of the variant CGD (T.H.) shows a small peak in the range of 550 to 560 nm in contrast to that of typical cases of X-CGD (J.O. or T.M.), which show only a trough, but no peak,10 as shown in the inset. The differential spectra gave further evidence for the indication of the presence of heme in the spectrum of the variant CGD. The heme contents of samples of the variant P, and theMand F were about 12%, 48%, and 100% of the control value, respectively.
Reduced minus oxidized difference spectra of neutrophils from patient J.O. (a), patient T.H. (b) and a healthy control (c). Neutrophil sonicates (6 × 106 cells/250 μL) were treated with CO and then the reduced minus oxidized difference spectra were measured. The inset shows the -band region enlarged 5.4-fold.
Reduced minus oxidized difference spectra of neutrophils from patient J.O. (a), patient T.H. (b) and a healthy control (c). Neutrophil sonicates (6 × 106 cells/250 μL) were treated with CO and then the reduced minus oxidized difference spectra were measured. The inset shows the -band region enlarged 5.4-fold.
Reduced minus oxidized difference spectra of neutrophils from variant CGD patient T.H. (P) and his mother (M) and father (F). The inset shows the -band region enlarged fivefold. The difference spectra were measured as in Fig 3.
Reduced minus oxidized difference spectra of neutrophils from variant CGD patient T.H. (P) and his mother (M) and father (F). The inset shows the -band region enlarged fivefold. The difference spectra were measured as in Fig 3.
Differential spectra. The spectra in Fig 4 were differentiated as d[▵A]/dλ (▵A = absorption difference of the spectra, λ = wavelength) in the region of the -band: patient T.H. (P), mother (M) and father (F). The inset shows differential spectra of the patient with cytochrome b558deficiency; P (-) and of a control (C).
Differential spectra. The spectra in Fig 4 were differentiated as d[▵A]/dλ (▵A = absorption difference of the spectra, λ = wavelength) in the region of the -band: patient T.H. (P), mother (M) and father (F). The inset shows differential spectra of the patient with cytochrome b558deficiency; P (-) and of a control (C).
The protein coding regions of overlapping fragments of gp91-phox cDNA from CGD patients, carriers, and healthy controls were amplified by PCR and the products were subjected to sequence analysis. All three patients showed normal sized fragments on an agarose gel (data not shown). The sequence of patient T.M. showed a point mutation at bp 1558 of thymine to cytosine, predicting an amino acid substitution at residue 516 of Trp to Arg. In the sequence of patient T.H. with variant CGD, a point mutation at bp 937 of guanine to adenine was found, predicting an amino acid substitution at residue 309 of Glu to Lys. The sequence of patient J.O. showed a point mutation at bp 1271 of thymine to cytosine, predicting a Leu to Pro conversion at amino acid position 420.
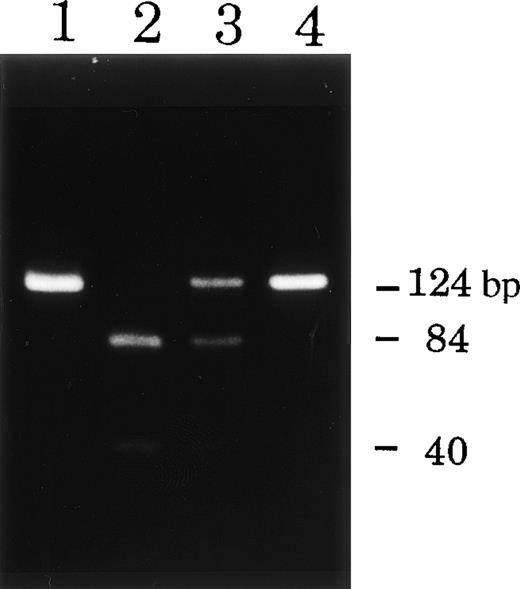
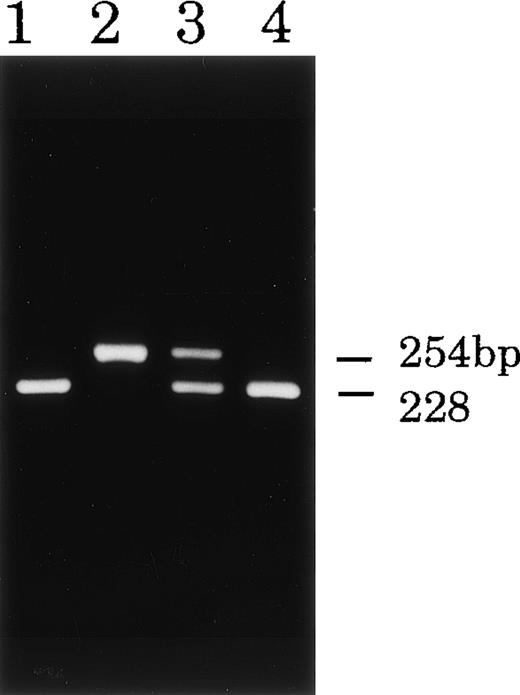
To confirm the mutations found, genomic DNA fragments containing these mutated sites were amplified by PCR and then analyzed by means of restriction digestion or direct sequencing. As expected, the PCR products from the patients and their carriers had the predicted sizes on the gel. Incubation of exon 12 of patient T.M. with MspI resulted in fragmentation into two pieces: distinct 84 bp and weak 40 bp bands (Fig 6, lane 2) in contrast to the two controls, which gave a single band of 124 bp (lanes 1 and 4 in Fig 6). Exon 12 of the mother of T.M. (Fig 6, lane 3) showed partial fragmentation into weak 84 and 40 bp bands, providing evidence for the carrier state. The restriction enzyme TaqI did not digest the PCR product of exon 9 of patient T.H. (Fig 7, lane 2), but completely digested those of exon 9 of his father and a healthy control to yield a 228 bp product (with slight 26 bp) (Fig 7, lanes 1 and 4). The PCR product of his mother showed partly digested products as evidence of the carrier state (Fig 7, lane 3). The PCR products of exon 10 of patient J.O. and his mother were directly sequenced because no available restriction enzyme was found for the mutated site. The T1271 to C substitution of patient J.O. was identified in the read sequence of exon 10, and the mother was heterozygous for the mutation (data not shown).
Restriction digestion analysis of DNA fragments from patient T.M. and his mother. DNA samples (exon 12) from the patient (lane 2) and his mother (lane 3) and healthy controls (lanes 1 and 4) were digested with MspI. DNA from the CGD patient showed abnormal restriction digestion patterns of the gp91-phox gene and that from the mother showed evidence of the carrier state.
Restriction digestion analysis of DNA fragments from patient T.M. and his mother. DNA samples (exon 12) from the patient (lane 2) and his mother (lane 3) and healthy controls (lanes 1 and 4) were digested with MspI. DNA from the CGD patient showed abnormal restriction digestion patterns of the gp91-phox gene and that from the mother showed evidence of the carrier state.
Restriction digestion analysis of DNA fragments from CGD patient T.H. and his parents. DNA samples (exon 9) from patient T.H. (lane 2), the father (lane 1), mother (lane 3), and a control (lane 4). When digested with Taq I, DNA from patient T.H. showed abnormal restriction fragment patterns and that from the mother showed evidence of a carrier state.
Restriction digestion analysis of DNA fragments from CGD patient T.H. and his parents. DNA samples (exon 9) from patient T.H. (lane 2), the father (lane 1), mother (lane 3), and a control (lane 4). When digested with Taq I, DNA from patient T.H. showed abnormal restriction fragment patterns and that from the mother showed evidence of a carrier state.
We identified missense mutations in the gene encoding gp91-phoxin the three patients with X-linked CGD, as summarized in Table 1. In addition to the three families, we recently reported another variant CGD (patient T.K.) in which neutrophils have similar amounts of both p22-phox and gp91-phox, but show no O2- forming activity or the heme spectrum of cytochrome b558,11 in contrast to the present variant CGD (T.H.). The mutated site in patient T.K. was found at residue 101 causing replacement of His by Tyr, probably one of the heme binding residues for bis-histidine coordination of cytochromeb558. In the present study, the predicted mutated site, H101Y, was confirmed at a position in exon 4 with the PCR product of genomic DNA, as shown in Table 1.
DISCUSSION
All four missense mutations are illustrated in Fig 8 with the predicted domains. Sequence analyses of the two typical cases of X-linked CGD (T.M. and J.O.) showed novel missense mutations; W516R and L420P, respectively. The absence of the heme spectrum (X910) in the two patients suggests that a replacement at residue 420 or 516 causes a very unstable gp91-phox protein, leading to the complete absence of cytochrome b558 in the membranes. Residue 420, where Leu is replaced by Pro in patient J.O. is involved in the predicted NADPH-binding domain.19-21 The substitution of a positively charged Arg for the nonpolar hydrophobic residue Trp at residue 516 might alter the folding properties of the peptide backbone. In the other variant CGD patient (T.H.), the missense mutation at residue 309 is interesting, having common characteristics with two other patients with variant CGD reported previously, showing the same replacement of Glu by Lys.22 This conservative substitution of a positively charged residue for a negatively charged one might alter the ionic properties of the peptide, whereas all E309K cases have a small amount of cytochrome b558 and a low level of O2- generating oxidase activity. This fact suggests that residue Glu 309 and its adjacent residues in gp91-phox are neither involved in the domain forming a stable complex with p22-phox nor in the domain holding flavin adenine dinucleotide (FAD) because the conversion of charged residues is critical for electron and proton transfer reactions.
A topological scheme of the heme binding histidines and the mutated residues in gp91-phox. Mutations in the gene encoding gp91-phox were detected in the four patients; H101Y (patient T.K.), E309K (patient T.H.), L420P (patient J.O.), and W516R (patient T.M.). A cytochrome P450-like alignment: residues 78-85 FLRGSSAC85.27 A cytosolic loop: residues 87-94: STRVRRQL.28 Taken together with previous studies on ESR and MCD spectra,29 the spectrophotometric studies on the CGD neutrophils provided a topological scheme for the locations of the hemes in gp91-phox. For details, see text.
A topological scheme of the heme binding histidines and the mutated residues in gp91-phox. Mutations in the gene encoding gp91-phox were detected in the four patients; H101Y (patient T.K.), E309K (patient T.H.), L420P (patient J.O.), and W516R (patient T.M.). A cytochrome P450-like alignment: residues 78-85 FLRGSSAC85.27 A cytosolic loop: residues 87-94: STRVRRQL.28 Taken together with previous studies on ESR and MCD spectra,29 the spectrophotometric studies on the CGD neutrophils provided a topological scheme for the locations of the hemes in gp91-phox. For details, see text.
Finegold et al23 suggested from the similarity in residues between the yeast iron reductase (FRE1) and gp91-phox that the corresponding residues coordinating heme may be two pairs of histidines (101 and 115; 209 and 222) buried in the separate hydrophobic domains. However, there is no direct evidence for the heme binding-ligands in gp91-phox except our results on the heme spectra of phagocyte cytochrome b558 from the two variant CGD patients. From the differential spectra, it is possible to distinguish between the presence and absence of heme, even in the presence of a minute amount of cytochrome b558 from CGD patients. Neutrophils from the variant CGD with a novel mutated site at His101 showed a similar differential spectrum11to those of typical X-linked CGD patients whose phagocytes have no subunits and show no heme spectrum (Fig 5, inset). In contrast, the present variant CGD showed a distinct differential spectrum with a peak in the left of the α-band (550 to 560 nm) (Fig 5), indicating the presence of the heme of cytochrome b558, which may account for the O2- forming oxidase activity. Comparative studies on these heme spectra confirmed that one of the heme binding histidines is His101. Bolscher et al24 reported a typical X-linked CGD patient with a point mutation at the same His101 to arginine in gp91-phox, suggesting that this part is important in binding of the heme or for formation of a stable complex with p22-phox. Although patients with many other mutations of histidine residues at the N-terminal have been reported,6 9 most of these patients had typical X-linked CGD and their phagocytes had neither gp91-phox nor p22-phox, so the heme binding ligands could not be identified by spectrophotometry.
In addition to immunoblot analyses, we detected the gp91-phoxprotein in neutrophils from the two variant CGD patients (T.H. and T.K.) by flow cytometry of the cells using monoclonal antiboby 7D525 (data not shown), which has recently been identified as an anti-gp91–phox antibody (M. Nakamura, personal communication, March 1998). Yu et al26 have recently been investigating the biosynthesis of cytochrome b558. Their results suggest that heme incorporation is important in the assembly of cytochromeb558 to form a stable complex of gp91-phoxand p22-phox. Consistent with their report, in the present variant CGD (T.H.), the proteins of both subunits and the heme in the cytochrome b558 were closely associated, as shown by immunoblots and spectra. In contrast, the other variant CGD (T.K.) showed similar amounts of the two subunits, but no heme.11The point mutation at residue 101 with replacement of His by Tyr is probably similar to FRE1 mutants that express FRE1 protein, but do not show a heme spectrum.23
Previous electron spin resonance (ESR) studies on neutrophil cytochrome b558 provided indirect evidence that His101, located in the vicinity of Cys85 in a cytochrome P450-like alignment (residues 78-85: FLRGSSAC85), is the fifth (proximal) heme binding ligand, and the sixth (distal) ligand may be His209, because pyridine treatment of cytochromeb558 changed the ESR signal of the bis-histidine coordination to a cytochrome P450-like signal, possibly through the replacement of His101 by the nearby residue Cys85.27 As shown in Fig 8, Cys85may be adjacent to His101 in the two transmembrane regions because a cytosolic loop of gp91-phox (residues 87-94: STRVRRQL) is located between Cys85 and His101.28 The results of comparative studies on the heme spectra of the two variant CGDs support the prediction from previous ESR studies. Because near-infrared magnetic circular dichroism (NIR-MCD) spectra demonstrated the presence of two distinct forms of the heme with bis-histidine coordination,29 another pair of heme-binding histidines may be present, probably within a short distance (4 to 5 Å) from the outer surface,30 close to the glycosylation sites31 (Fig 8).
ACKNOWLEDGMENT
We thank Dr M. Nakamura (Institute of Tropical Medicine, Nagasaki University, Nagasaki) for supplying monoclonal antibody 7D5.
Supported in part by grants from the Ministry of Education, Science, and Culture of Japan and the Tokyo Metropolitan Institute of Medical Science.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Katsuko Kakinuma PhD, Department of Inflammation Research, The Tokyo Metropolitan Institute of Medical Science, Honkomagome 3-18-22, Bunkyo-ku, Tokyo, 113-8613 Japan.

![Fig. 5. Differential spectra. The spectra in Fig 4 were differentiated as d[▵A]/dλ (▵A = absorption difference of the spectra, λ = wavelength) in the region of the -band: patient T.H. (P), mother (M) and father (F). The inset shows differential spectra of the patient with cytochrome b558deficiency; P (-) and of a control (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.2098.406k09_2098_2104/5/m_blod40609005x.jpeg?Expires=1765905078&Signature=VQk6zWsxwFHfzfckG99iaLJm9LiQGQkeU0ehkx5sgekHvOfq9bNQZyhv8z88~JvTLUPfLztiYVDpdI56izpHem~mAXOq8DYtgEOwHFHg54VUiRrBBgMj-~-AMfiSMysHuO8JgH5jxZagGsGYs8yE3ws2eUXxVfCDXSPQPKYf6zPBjuaAhxNesu09VhCkuNXS763wiaq8WD1JF5Z6NpgaPfsDcyzGcCQ1LW47besTUvC5U1I9CbbXJwHJ8uTVGxuSzAieY8p3LW~UlfCB6uKJwYIz~uvB-u7ZaD9KuPfYJ6r4XmclVN5hWBaHwhVLYFJn7wgjBouIUlPFL4X4o0Y93w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal