Bruton’s tyrosine kinase (Btk) has been shown to play a role in normal B-lymphocyte development. Defective expression of Btk leads to human and murine immunodeficiencies. However, the exact role of Btk in the cytoplasmic signal transduction in B cells is still unclear. This study represents a search for the substrate for Btk in vivo. We identified one of the major phosphoproteins associated with Btk in the preB cell line NALM6 as the Wiskott-Aldrich syndrome protein (WASP), the gene product responsible for Wiskott-Aldrich syndrome, which is another hereditary immunodeficiency with distinct abnormalities in hematopoietic cells. We demonstrated that WASP was transiently tyrosine-phosphorylated after B-cell antigen receptor cross-linking on B cells, suggesting that WASP is located downstream of cytoplasmic tyrosine kinases. An in vivo reconstitution system demonstrated that WASP is physically associated with Btk and can serve as the substrate for Btk. A protein binding assay suggested that the tyrosine-phosphorylation of WASP alters the association between WASP and a cellular protein. Furthermore, identification of the phosphorylation site of WASP in reconstituted cells allowed us to evaluate the catalytic specificity of Btk, the exact nature of which is still unknown.
BRUTON’S TYROSINE kinase (Btk) is a cytoplasmic tyrosine kinase that is involved in the pathogenesis of human and murine B-cell deficiencies (human X-linked agammaglobulinemia [XLA]1,2 and murine X-linked immunodeficiency [XID]3,4). Since the identification of this tyrosine kinase, several reports have demonstrated the involvement of Btk in cytoplasmic signal transductions through the B-cell antigen receptor (BCR),5-9 the high-affinity IgE receptor (FcεRI),10 the interleukin-5 (IL-5) receptor,11 and CD38.12 Btk, a member of the Btk/Tec family (Itk, Tec, Bmx, Txk), is composed of several domains, including a PH (pleckstrin homology), TH (tec homology), SH (src homology) 3, SH2, and SH1 (=kinase) domain from the N to C termini.13 Several molecules that interact with these domains of Btk have been described,14-19 although the unique role of Btk in B-cell development has remained unclear.
The Wiskott-Aldrich syndrome (WAS)20,21 is another X-linked hereditary immunodeficiency, characterized by thrombocytopenia, eczema, and abnormal humoral and cell-mediated immunity. The gene responsible for WAS has been identified and termed WAS protein (WASP).22 Recent studies have shown that WASP specifically associates with the activated form of CDC42, suggesting that WASP is involved in regulating cytoskeletal architecture.23,24 This finding may explain the abnormalities seen in the cytoskeleton of hematopoietic cells in WAS patients, although arguments can be put forward against such a role for WASP.25,26 X-chromosome inactivation studies in obligate carriers for WAS have demonstrated a nonrandom inactivation pattern in most hematopoietic cell lineages, including T cells, B cells and CD34+ cells, 27suggesting the requirement of WASP for the normal differentiation and growth of hematopoietic cells. A variety of morphologic and functional abnormalities affecting T and B lymphocytes, neutrophils, and platelets have been identified. WAS patients consistently fail to mount an antibody response to polysaccharides and often respond poorly to protein antigens.28,29 In addition, transmembrane signaling in B cells of WAS patients has been reported to be defective.30 Exon 10 of WASP contains several polyproline stretches, which represent several potential SH3 domain binding motifs. Binding studies using glutathione S-transferase (GST)-SH3 fusion proteins have suggested that the proline-rich region of WASP binds, at least in vitro, a variety (>10) of SH3 containing proteins, which include those of the Btk/Tec family kinases.31-35 However, it seems doubtful that WASP is involved in the signaling pathways of all these SH3 domain containing molecules and, in fact, only a few of them (Nck,31 Fyn, Fgr,34 Grb2,35and PSTPIP, the recently described cytoskeletal association protein36) have been demonstrated to bind WASP in vivo. Although it was reported that PSTPIP is involved in the control of the cytoskeleton together with WASP and that tyrosine-phosphorylation of PSTPIP regulates the SH3-mediated binding of WASP to PSTPIP, the significance of the potential binding of WASP to the tyrosine kinases has not been demonstrated. In addition, no studies have evaluated WASP as a possible substrate of these tyrosine kinases.
We report in this study that WASP can serve as a participant in the tyrosine kinase pathway in B-lineage cells. WASP was found to be constitutively tyrosine-phosphorylated in the pre-B–cell line NALM6; furthermore, we showed that WASP is transiently tyrosine-phoshphorylated after BCR cross-linking on B cells. A reconstitution cell system allowed us to observe the association of WASP and Btk in vivo and to identify WASP as the substrate for Btk. Protein binding assay demonstrated that the phosphorylation of WASP dramatically alters the association between WASP and an unidentified 220-kD cellular protein. The phosphotyrosine motif of WASP by Btk was determined, which may give a new insight to the still-unknown substrate specificity of Btk.
MATERIALS AND METHODS
Cell lines and antibodies.
RAMOS cells,37 an Epstein-Barr virus (EBV)-negative Burkitt’s lymphoma cell line, were obtained from the Health Science Research Resources Bank (HSRRB, Osaka, Japan). NALM6 cells, a human pre-B–cell line that has been described earlier,38,39 and NALM16 cells,40 a human pro-B–cell line, were obtained from Fujisaki Cell Center Hayashibara (FCCH) Biochemical Laboratories, Inc (Okayama, Japan). These cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 500 μmol/L 2-mercaptoethanol. 293T cells41 were provided by Dr Takashi Fujita (The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). Anti-WASP polyclonal antibody 503, generated against amino acid residues 209 to 226 of human WASP, has been described previously.42 Anti-Btk monoclonal antibody 43-3B was generated by immunizing mice with a fusion protein consisting of GST and the unique region (amino acid residues 1 to 186) of the human Btk. Hybridomas were generated as previously described43 and were screened by enzyme-linked immunosorbent assay using the GST-Btk unique region fusion protein or GST alone. The antibody from clone 43-3B binds the GST-Btk unique region, but not GST alone. This antibody could detect a 77-kD immunoblot band in the lysate of PA317 cells that stably expressed Btk protein by transfection of Btk cDNA,1but not in the lysate of nontransfected PA317 cells. The same 77-kD band was detected in the lysate of B cells, but not in that of T cells (data not shown). Antibody 43-3B was shown to recognize human as well as mouse Btk, whereas another anti-Btk monoclonal antibody, 48-2H, which was generated against the SH3 domain of Btk and previously described by us,43 44 only recognizes human Btk. Mouse antiphosphotyrosine monoclonal antibody 4G10, purified mouse myeloma IgG2b (isotype-matched control for 43-3B), the anti-T7 tag monoclonal antibody, and F(ab′)2 fragment of goat antihuman IgM (μ chain specific) were purchased from Upstate Biotechnology Inc (Lake Placid, NY), Zymed Laboratories Inc (SanFrancisco, CA), Novagen, Inc (Madison, WI), and Cappel ICN Pharmaceuticals Inc (Aurora, OH), respectively.
Constructs and mutagenesis.
The structure of human Btk cDNA was previously described 1and the cDNA was inserted into the pEF-BOS mammalian expression vector.45 Human WASP cDNA (nucleotides 2-1708 of the WASP gene)46 was generated by reverse transcriptase-polymerase chain reaction (RT-PCR) from normal peripheral blood mononuclear cells with the aid of synthetic oligonucleotide primers and inserted into the pcDNA3 (Invitrogen Co, San Diego, CA) mammalian expression vector or the pRc/CMV mammalian expression vector (Invitrogen) in which two T7 sequences (MASMTGGQQMG) had been inserted in tandem.47 Point mutations were introduced by a T7 DNA polymerase-based site directed mutagenesis system (Stratagene, La Jolla, CA), and confirmed by limited nucleotide sequencing.
Cell stimulation.
For BCR stimulation, 1 × 108 RAMOS cells were preincubated for 30 minutes in 1 mL of serum-free RPMI 1640 medium at 37°C and subsequently incubated for the indicated time at 37°C with 100 μg of a F(ab′)2 fragment of goat antihuman IgM (μ chain specific). Stimulation was terminated by cell lysis with the ice-cold Triton X-100 lysis buffer described below.
Transfection.
The Btk or WASP expression vectors (total, 10 μg) were transfected into 293T cells with Lipofectamine (GIBCO/BRL, Rockville, MD) and the cells were harvested 48 hours later. For the phosphorylation assay of WASP, cells were lysed in a Triton X-100 lysis buffer described below. For the coimmunoprecipitation experiments, cells were lysed in the digitonin lysis buffer described below.
Cell lysis, immunoprecipitation, and GST-SH3 binding assay.
For the coimmunoprecipitation experiments, we used a digitonin lysis buffer containing 1% digitonin, 10 mmol/L triethanolamine (pH 7.5), 150 mmol/L NaCl, 10 mmol/L iodoacetoamide, 1 mmol/L EDTA, and protease inhibitors (1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, and 10 μg/mL aprotinin). The GST-SH3 binding assay used a NP-40 lysis buffer containing 0.2% NP-40, 10 mmol/L HEPES (pH 7.0), 143 mmol/L KCl, 5 mmol/L MgCl2, the same protease inhibitors as for the digitonin lysis buffer, and 1 mmol/L sodium orthovanadate. To perform the transfections and the cell stimulation experiments, we selected a Triton X-100 lysis buffer containing 1% Triton X-100, 0.05% sodium dodecyl sulfate (SDS), 10 mmol/L NaH2PO4/Na2HPO4 (pH 7.0), 150 mmol/L NaCl, the same protease inhibitors as for the other lysis buffer, and 1 mmol/L sodium orthovanadate. To detect the tyrosine-phosphorylation of WASP after BCR cross-linking of RAMOS cells, we used 0.5 mmol/L pervanadate instead of 1 mmol/L sodium orthovanadate as the phosphotyrosine phosphatase (PTPase) inhibitor by the reason described in the Discussion. Pervanadate stock solution (10 mmol/L) was prepared by mixing equal volumes of 20 mmol/L sodium orthovanadate (pH 10) and 20 mmol/L of H2O2. The mixture was allowed to stand at room temperature for 15 minutes and then added to the Triton X-100 lysis buffer for a final concentration of 0.5 mmol/L.
For the immunoprecipitation experiments, the cell lysate was centrifuged for 15 minutes at 14,000 rpm at 4°C and the supernatant was precleared for 30 minutes at 4°C with an excess amount of protein-A Sepharose CL4B beads (Pharmacia, Uppsala, Sweden). The precleared lysate was incubated for 60 minutes at 4°C with the appropriate antibodies, followed by conjugation with protein-A Sepharose CL4B beads for 60 minutes at 4°C, and then washed four times with a suitable buffer. For the GST-SH3 binding assay, the cell lysate was centrifuged for 15 minutes at 14,000 rpm at 4°C and the supernatant was precleared for 30 minutes at 4°C with glutathione-Sepharose 4B beads (Pharmacia). The precleared lysate was incubated for 120 minutes at 4°C with 8 μg of GST or GST-SH3 (corresponding to amino acid residues 212 to 275 of human Btk) bound to glutathione-Sepharose 4B beads. The beads were then washed four times with NP-40 lysis buffer and boiled for 5 minutes with a 2× SDS loading buffer. Proteins were fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 5% to 15% gradient).
Protein analysis.
Western analysis was performed as described previously.43As primary antibodies, the antiphosphotyrosine antibody 4G10 was used at 1 μg/mL and the anti-Btk monoclonal antibody 43-3B was used at 3 μg/mL. The anti-WASP polyclonal antibody 503 was used at 1:3,000 dilution. Immunoreactive proteins were detected by the Enhanced Chemiluminescence System (Amersham, Bucks, UK). The in vitro kinase assay was performed as described previously,43,48 except for the addition of denatured enolase (5 μg) as a transphosphorylation substrate. 35S-metabolic labeling was performed as also described previously.1 48
RESULTS
WASP is a major phosphoprotein associated with the Btk-SH3 domain in the pre-B–cell line NALM6.
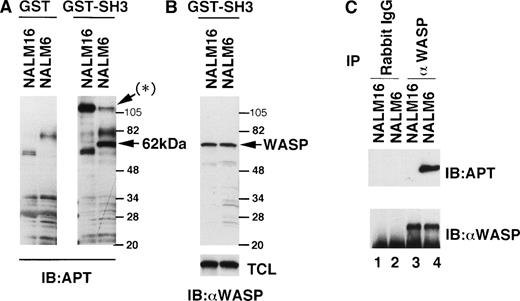
To identify the possible substrate(s) for Btk, we searched for molecules that could physically associate with Btk and, in addition, could be tyrosine-phosphorylated in B-lineage cells. Lysates of NALM16 or NALM6 cells were incubated with a GST-SH3 (Btk) fusion protein conjugated to beads and the precipitated phosphoproteins were detected by immunoblotting with the antiphosphotyrosine antibody 4G10. A phosphorylated 62-kD protein was detected in the precipitate from the NALM6 cell lysate but not in that of the NALM16 cells or in the precipitate by GST alone (Fig 1A). In addition to the 62-kD protein, a 120-kD tyrosine-phosphorylated protein associated with GST-SH3 (Btk) was identified (the asterisk in Fig 1A) in the cell lysates of both cell lines, although the band representing the phosphorylation of this protein in NALM6 cells was weak compared with that of the NALM16 cells. The 120-kD phosphoprotein was subsequently identified to be Cbl (data not shown), which was previously described as a SH3 domain binding protein.49
Constitutive tyrosine-phosphorylation of WASP in NALM6 cells. (A) Serum-starved 5 × 107 cells (NALM16 and NALM6) were lysed with the NP-40 lysis buffer and, after preclearance, lysates were incubated with GST alone or BtkSH3-GST fusion protein bound to glutathione-Sepharose 4B beads at 4°C for 2 hours. After washing, the beads were boiled with 2× SDS loading buffer and samples were fractionated by SDS-PAGE (5% to 15% gradient gel) and immunoblotted (IB) with the antiphosphotyrosine (APT) antibody 4G10. The asterisk indicates Cbl (120 kD). Molecular mass standards are shown in kilodaltons. (B) The same membrane was reprobed with the anti-WASP antibody 503 (top). Total cell lysates (TCL) of NALM16 and NALM6 cells were loaded and immunoblotted with the anti-WASP antibody 503 (bottom). (C) NALM16 and NALM6 cells (1 × 108) were lysed with the NP-40 lysis buffer and, after preclearance, lysates were immunoprecipitated (IP) with the anti-WASP antibody 503 (lanes 3 and 4) or preimmune rabbit IgG as a control (lanes 1 and 2). Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top), followed by reprobing with the anti-WASP antibody 503 (bottom).
Constitutive tyrosine-phosphorylation of WASP in NALM6 cells. (A) Serum-starved 5 × 107 cells (NALM16 and NALM6) were lysed with the NP-40 lysis buffer and, after preclearance, lysates were incubated with GST alone or BtkSH3-GST fusion protein bound to glutathione-Sepharose 4B beads at 4°C for 2 hours. After washing, the beads were boiled with 2× SDS loading buffer and samples were fractionated by SDS-PAGE (5% to 15% gradient gel) and immunoblotted (IB) with the antiphosphotyrosine (APT) antibody 4G10. The asterisk indicates Cbl (120 kD). Molecular mass standards are shown in kilodaltons. (B) The same membrane was reprobed with the anti-WASP antibody 503 (top). Total cell lysates (TCL) of NALM16 and NALM6 cells were loaded and immunoblotted with the anti-WASP antibody 503 (bottom). (C) NALM16 and NALM6 cells (1 × 108) were lysed with the NP-40 lysis buffer and, after preclearance, lysates were immunoprecipitated (IP) with the anti-WASP antibody 503 (lanes 3 and 4) or preimmune rabbit IgG as a control (lanes 1 and 2). Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top), followed by reprobing with the anti-WASP antibody 503 (bottom).
Because of a recent report suggesting that the GST-SH3 (Btk) fusion protein binds to the polyproline region of WASP33 and because the reported molecular size of WASP on SDS-PAGE42was similar to that of the 62-kD protein that was constitutively tyrosine-phosphorylated in NALM6 cells and bound to the Btk-SH3 domain, we investigated whether this phosphoprotein could be WASP. The reprobing of the membrane with the anti-WASP antibody 503 (Fig 1B, top panel) showed that the two bands completely coincided, strongly suggesting that this 62-kD phosphoprotein is indeed WASP. The amounts of WASP recovered from the GST-SH3 (Btk) fusion protein coated beads, as well as the total amounts of WASP in the cell lysates (Fig 1B, bottom panel), were similar between these cell lines, suggesting that the binding affinity of WASP with the Btk-SH3 domain is independent on the phosphorylation of WASP. To confirm the phosphorylation of WASP, the lysate of NALM6 cells was immunoprecipitated with the anti-WASP antibody 503 and immunoblotted with the antiphosphotyrosine antibody 4G10. As shown in Fig 1C, the tyrosine-phosphorylation of WASP was readily detectable in this cells, demonstrating that WASP is constitutively tyrosine-phosphorylated in NALM6 cells. This observation suggests that WASP, one of the Btk-SH3 domain binding proteins, could serve as the substrate for tyrosine kinase(s) in B-lineage cells at least under certain intracellular conditions.
WASP is tyrosine-phosphorylated after BCR cross-linking on B cells.
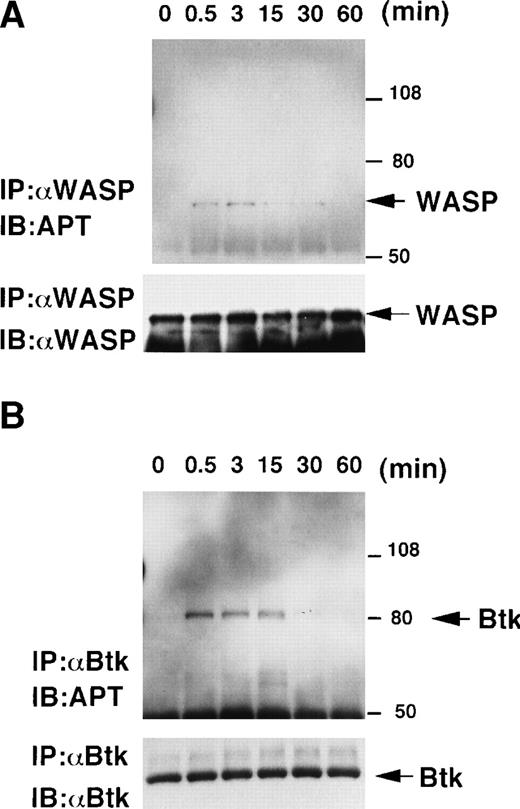
The identification of WASP as the tyrosine-phosphorylated protein in a B-lineage cell line prompted us to examine the possibility that WASP would also become tyrosine-phosphorylated in B cells after some physiological stimulation such as BCR cross-linking. After exposure to the anti-μ antibody, RAMOS cells were lysed in Trition X-100 lysis buffer containing pervanadate at different time points (Fig 2). WASP was immunoprecipitated from the lysates using the anti-WASP antibody 503, and the tyrosine-phosphorylation of WASP was evaluated by immunoblotting with the antiphosphotyrosine antibody 4G10 (Fig 2A, top panel). At the same time points, the tyrosine-phosphorylation of Btk was assessed as a control experiment by immunoprecipitating the lysates using the anti-Btk antibody 48-2H. As previously reported,5 6 rapid tyrosine-phosphorylation of Btk became apparent after the addition of the anti-μ antibody, reached a maximum at 0.5 minutes, and decreased within 30 minutes in our experiment (Fig 2B, top panel). As shown in Fig 2A, WASP was also transiently tyrosine-phosphorylated, reaching a peak at 3 minutes after stimulation and having decreased to an undetectable level (no band visible even after long exposure of the immunoblot) by 60 minutes. This increase in tyrosine-phosphorylation after BCR cross-linking was not due to a change in the amount of WASP proteins in the cell lysates (Fig 2A, bottom panel). The observation that WASP is tyrosine-phosphorylated after BCR cross-linking suggests that WASP is an active participant in the cytoplasmic tyrosine kinase pathway triggered by BCR cross-linking on B cells.
BCR cross-linking induces tyrosine-phosphorylations of Btk and WASP. Serum-starved 1 × 108 RAMOS cells were stimulated with 100 μg/mL F(ab′)2 fragment of goat antihuman IgM antibody for the indicated times (0, 0.5, 3, 15, 30, and 60 minutes). Cells were lysed with Triton X-100 lysis buffer and, after preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 (A) or the anti-Btk antibody 48-2H (B). Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (A and B; top). These membranes were reprobed with the anti-WASP antibody 503 (A; bottom) or the anti-Btk antibody 43-3B (B; bottom).
BCR cross-linking induces tyrosine-phosphorylations of Btk and WASP. Serum-starved 1 × 108 RAMOS cells were stimulated with 100 μg/mL F(ab′)2 fragment of goat antihuman IgM antibody for the indicated times (0, 0.5, 3, 15, 30, and 60 minutes). Cells were lysed with Triton X-100 lysis buffer and, after preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 (A) or the anti-Btk antibody 48-2H (B). Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (A and B; top). These membranes were reprobed with the anti-WASP antibody 503 (A; bottom) or the anti-Btk antibody 43-3B (B; bottom).
Tyrosine-phosphorylation of WASP by Btk in an in vivo reconstitution system.
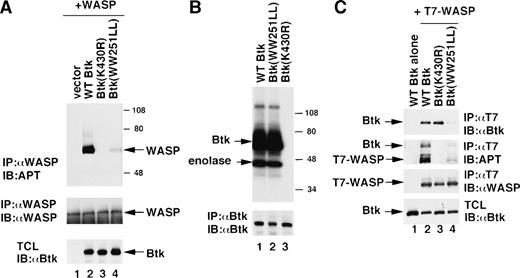
Because of the findings that WASP can be tyrosine-phosphorylated in vivo and associates with the SH3 domain of Btk, we decided to assess the possibility of WASP being actually tyrosine-phosphorylated by Btk in vivo. Human WASP cDNA inserted into the pcDNA3 expression vector was transfected with or without Btk cDNA into 293T cells. After lysis with a Triton X-100 lysis buffer, the lysates were immunoprecipitated by using the anti-WASP antibody 503. Tyrosine-phosphorylation of the precipitated WASP was evaluated by immunoblotting with the antiphosphotyrosine antibody 4G10. As shown in Fig 3A, tyrosine-phosphorylation of WASP was not observed in 293T cells when WASP alone was expressed (lane 1; a faint band was detected by very long exposure of the immunoblot; data not shown). In contrast, prominent tyrosine-phosphorylation of WASP was detected when WASP was coexpressed with Btk (lane 2) despite the approximately equal amounts of precipitated WASP from cell lysates (Fig3A, middle panel), indicating that the presence of Btk was requirement for the phosphorylation of WASP on its tyrosine. To further investigate the role of Btk in the tyrosine-phosphorylation of WASP, we selected two mutant Btk proteins for coexpression with WASP in 293T cells. It has been previously shown that the substitution of lysine by arginine at amino acid residue 430 (K430R mutant) generates a kinase-inactive Btk.50 This mutation completely abolished the kinase activity of Btk in the 293T cell reconstitution system (Fig 3B, lane 3). In the second mutant Btk protein, the two conserved tryptophans at codons 251 and 252 were replaced by two leucines (WW252LL mutant); this substitution has been shown to greatly diminish the interaction of SH3 domains with their ligands (Fig 3C, lane 4 of top panel and previous studies19 51-55). However, the kinase activities of both wild and WW252LL mutant Btk, when transfected into 293T cells, were at a similar level (Fig 3B, lanes 1 and 2). No significant tyrosine-phosphorylation of WASP was observed when WASP and K430R mutant Btk were coexpressed in 293T cells (Fig 3A, lane 3), whereas cotransfection of WASP and WW251LL mutant Btk resulted in a much lower level of tyrosine-phosphorylation (Fig 3A, lane 4) when compared with that induced by wild-type Btk (lane 2), despite the fact that the protein levels expressed by the transfections were identical (Fig 3A, middle panel for WASP and bottom panel for Btk). These results demonstrate that WASP, under this experimental condition, can be tyrosine-phosphorylated by the kinase activity of Btk and that the direct interaction between WASP and the SH3 domain of Btk is required for this phosphorylation to occur.
(A) Phosphorylation of WASP by Btk in a reconstitution system. Cotransfection of pcDNA3/WASP with pEF-BOS vector alone (lane 1), pEF-BOS/wild-type (WT) Btk (lane 2), pEF-BOS/Btk (K430R) (lane 3) or pEF-BOS/Btk (WW251LL) (lane 4) into 293T cells was performed with lipofectamine. Cells were harvested after 48 hours and lysed with Triton X-100 lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top). The same membrane was reprobed with the anti-WASP antibody 503 (middle). To detect the expression of Btk constructs, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom). (B) In vitro kinase assays of wild and mutant Btk. The expression vectors of Btk (WT) (lane 1), Btk (WW251LL) (lane 2), or Btk (K430R) (lane 3) were transfected into 293T cells. Cells were harvested after 48 hours, and the lysates were immunoprecipitated with the anti-Btk antibody 48-2H. The in vitro kinase assay was performed with denatured enolase as a substrate. The bottom panel demonstrates the expression of Btk protein in the lysates by immunoblotting with the anti-Btk antibody 43-3B. (C) Association of WASP with wild-type or mutant Btk. Transfection of pEF-BOS/wild type (WT) Btk (lane 1) or cotransfection of T7 epitope-tagged WASP with pEF-BOS/wild-type (WT) Btk (lane 2), pEF-BOS/Btk (K430R) (lane 3), or pEF-BOS/Btk (WW251LL) (lane 4) into 293T cells was performed with lipofectamine. Cells were harvested after 48 hours and lysed with digitonin lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-T7 tag antibody and immunoblotted with the anti-Btk antibody 43-3B (top) or with the antiphosphotyrosine (APT) antibody 4G10 (second), followed by reprobing with the anti-WASP antibody 503 (third). To detect the expression of Btk proteins, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom).
(A) Phosphorylation of WASP by Btk in a reconstitution system. Cotransfection of pcDNA3/WASP with pEF-BOS vector alone (lane 1), pEF-BOS/wild-type (WT) Btk (lane 2), pEF-BOS/Btk (K430R) (lane 3) or pEF-BOS/Btk (WW251LL) (lane 4) into 293T cells was performed with lipofectamine. Cells were harvested after 48 hours and lysed with Triton X-100 lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top). The same membrane was reprobed with the anti-WASP antibody 503 (middle). To detect the expression of Btk constructs, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom). (B) In vitro kinase assays of wild and mutant Btk. The expression vectors of Btk (WT) (lane 1), Btk (WW251LL) (lane 2), or Btk (K430R) (lane 3) were transfected into 293T cells. Cells were harvested after 48 hours, and the lysates were immunoprecipitated with the anti-Btk antibody 48-2H. The in vitro kinase assay was performed with denatured enolase as a substrate. The bottom panel demonstrates the expression of Btk protein in the lysates by immunoblotting with the anti-Btk antibody 43-3B. (C) Association of WASP with wild-type or mutant Btk. Transfection of pEF-BOS/wild type (WT) Btk (lane 1) or cotransfection of T7 epitope-tagged WASP with pEF-BOS/wild-type (WT) Btk (lane 2), pEF-BOS/Btk (K430R) (lane 3), or pEF-BOS/Btk (WW251LL) (lane 4) into 293T cells was performed with lipofectamine. Cells were harvested after 48 hours and lysed with digitonin lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-T7 tag antibody and immunoblotted with the anti-Btk antibody 43-3B (top) or with the antiphosphotyrosine (APT) antibody 4G10 (second), followed by reprobing with the anti-WASP antibody 503 (third). To detect the expression of Btk proteins, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom).
To further examine the interaction of these molecules, we next compared the association of WASP with wild-type Btk with that with mutant Btks. To evaluate the association of these molecules more precisely, T7 epitope-tagged WASP was transiently coexpressed with wild-type or mutant Btk in 293T cells. The cell lysates (1% digitonin) were immunoprecipitated by the anti-T7 tag antibody, and the coprecipitated Btk was detected by immunoblotting with the anti-Btk antibody 43-3B (Fig 3C, top panel). Simultaneously, the tyrosine-phosphorylation of the precipitated T7-WASP and Btk was evaluated by immunoblotting with the antiphosphotyrosine antibody 4G10 (Fig 3C, second panel). As a result of using the digitonin lysis buffer, which is less stringent than the Triton X-100 lysis buffer (1% Triton X-100 plus 0.05% SDS), the coprecipitation of T7-WASP and wild-type Btk could be readily observed (Fig 3C, lane 2 of top and second panels). The amount of Btk coprecipitated with T7-WASP was almost identical regardless of the activation of Btk or the phosphorylation of WASP (lanes 2 and 3 of top and second panels), suggesting that the association of Btk with WASP is constitutive and does not depend on the activation or phosphorylation of Btk or WASP.
Tyrosine-phosphorylation of WASP alters the association of a 220-kD cellular protein with WASP.
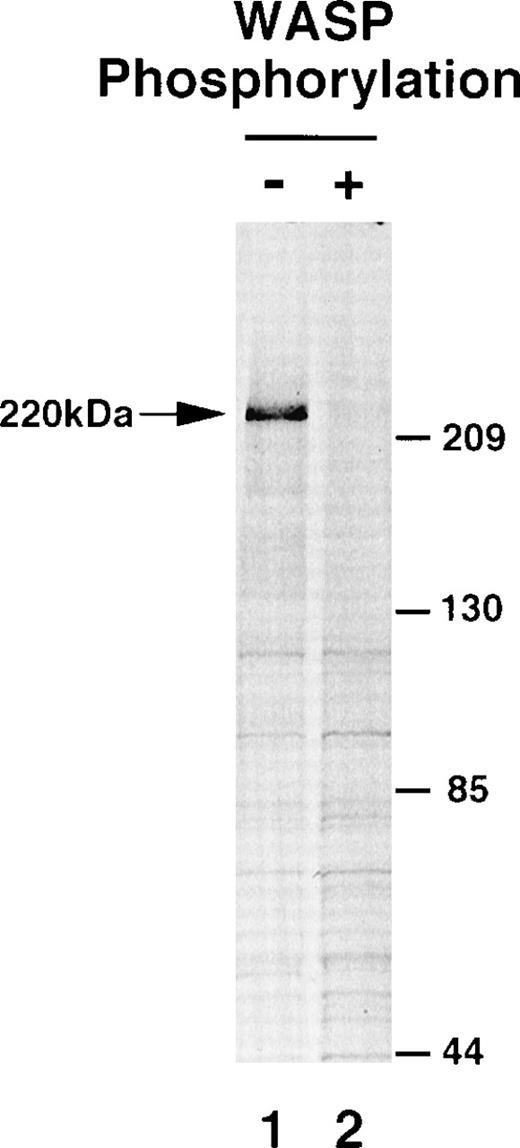
To investigate the functional significance of the tyrosine-phosphorylation of WASP, we examined possible changes in the association between WASP and cellular proteins. T7-WASP was transiently coexpressed in 293T cells either with wild-type Btk or K430R mutant Btk (phosphorylated or unphosphorylated WASP, respectively). Expressed WASP proteins were then immunopurified by incubation with the anti-T7 tag antibody followed by conjugation with protein A beads and washing with the Triton X-100 lysis buffer. Silver staining of the immunoprecipitates demonstrated that purified WASP proteins were essentially free of proteins other than Ig used for purification (data not shown). Purified WASP proteins were then incubated with the RAMOS cell lysate that was metabolically labeled with35S-methionine (see Materials and Methods). After washing, labeled proteins bound to the beads were visualized by autoradiograpy. As shown in Fig 4, binding of a 220-kD cellular protein was detected in the presence of unphosphorylated WASP (lane 1), whereas such binding of this 220-kD protein was greatly reduced in the presence of phosphorylated WASP (lane 2). There were no other significant differences between the binding of the proteins to phosphorylated and unphosphorylated WASP. This result suggests that WASP bound to an unidentified 220-kD cellular protein under these experimental conditions and that this association was greatly reduced by the tyrosine-phosphorylation of WASP.
Tyrosine-phosphorylation of WASP alters the association of a 220-kD cellular protein with WASP. WASP was expressed by cotransfection with Btk (K430R) (lane 1) or wild-type Btk (lane 2). Unphosphorylated (−) or phosphorylated (+) WASP proteins were immunopurified and then incubated with 35S-metabolically labeled RAMOS cell lysate. Labeled proteins bound to the beads were detected by autoradiography.
Tyrosine-phosphorylation of WASP alters the association of a 220-kD cellular protein with WASP. WASP was expressed by cotransfection with Btk (K430R) (lane 1) or wild-type Btk (lane 2). Unphosphorylated (−) or phosphorylated (+) WASP proteins were immunopurified and then incubated with 35S-metabolically labeled RAMOS cell lysate. Labeled proteins bound to the beads were detected by autoradiography.
The phosphotyrosine motif of WASP is similar to the autophosphorylation site of Btk.
As demonstrated above, WASP can serve as the substrate for Btk at least in the in vivo reconstitution system used by us. Because only a few molecules have been reported to date to serve as Btk substrate, the observed in vivo phosphorylation of WASP by Btk provided a unique opportunity to study the in vivo substrate specificity of Btk. WASP has seven tyrosines located at amino acid residues 51, 83, 88, 102, 107, 212, and 291 (Fig 5A). The flanking amino acid residues of each of the tyrosines are shown in Fig5B. To identify the phosphorylation site of WASP by Btk, we generated seven constructs in which each of the seven tyrosines of WASP was replaced by a phenylalanine (designated as Y51F, Y83F, Y88F, Y102F, Y107F, Y212F, and Y291F mutant). Each of these mutant constructs was then cotransfected with Btk cDNA into 293T cells and the tyrosine-phosphorylation of the mutant WASP was evaluated. As shown in Fig 5C, Y51F, Y83F, Y88F, Y102F, Y107F, and Y212F mutant proteins were phosphorylated at a level similar to that of wild-type WASP (lanes 1 through 7). However, in the case of Y291F mutant protein, the major 62-kD band representing tyrosine-phosphorylation of WASP by Btk was absent and only a very weak band in the high molecular range was observed. As shown in Fig 5D, the introduction of Y291F mutation into WASP did not alter the association of WASP with Btk, meaning that the absence of phosphorylation does not imply a weaker association of Btk with the Y291F mutant than that with wild-type WASP. These results indicate that Btk phosphorylates WASP on its tyrosine 291 in the reconstituted cells and, furthermore, indicate that the phosphorylation of WASP does not alter the association with Btk. The very weak band seen in Y291F mutant transfected 293T cells (Fig 5C, lane 8 of top panel) may represent a weak phosphorylation of other tyrosine residues of WASP, possibly due to the hyperexpressions of Btk and WASP in the reconstitution system. As will be discussed later, the flanking amino acid residues of tyrosine 291 of WASP exhibit a distinct similarity to those of tyrosine 223, the autophosphorylation site of Btk55 (Fig 5B).
Identification of the phosphorylation site of WASP by Btk. (A) A schematic representation of the structure of WASP and its functional domains (pleckstrin homology domain [PH], the GTPase binding domain [GBD], and proline rich regions [Poly-Pro]) and the distribution of the tyrosine residues. The positions and amino acid numbers of tyrosine residues are indicated (arrows). (B) The flanking amino acid sequences of each tyrosine residue in WASP and of tyrosine 223 (the autophosphorylation site) of Btk are listed. (C) Coexpression of Btk and mutant WASP constructs. Cotransfection of pEF-BOS/wild-type Btk with pcDNA3/wild-type WASP (lane 1), pcDNA3/Y51F (lane 2), pcDNA3/Y83F (lane 3), pcDNA3/Y88F (lane 4), pcDNA3/Y102F (lane 5), pcDNA3/Y107F (lane 6), pcDNA3/Y212F (lane 7), or pcDNA3/Y291F mutant WASP (lane 8) was performed with lipofectamine. Cells were harvested after 48 hours and lysed with Triton X-100 lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top). The same membrane was reprobed with the anti-WASP antibody 503 (middle). To detect the expression of Btk proteins, the total cell lysates (TCL) were loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom). (D) Association of wild-type or Y291F mutant WASP with Btk. Transfection of pEF-BOS/wild-type Btk as a control (lane 1) or cotransfection of pEF-BOS/wild-type Btk with T7 epitope-tagged WASP (lane 2) or T7 epitope-tagged Y291F (lane 3) into 293T cells was performed to transiently express the proteins. Cells were harvested after 48 hours and lysed with digitonin lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-T7 tag antibody and immunoblotted with the anti-Btk antibody 43-3B (top), followed by reprobing with the anti-WASP antibody 503 (middle). To detect the expression of Btk proteins, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom).
Identification of the phosphorylation site of WASP by Btk. (A) A schematic representation of the structure of WASP and its functional domains (pleckstrin homology domain [PH], the GTPase binding domain [GBD], and proline rich regions [Poly-Pro]) and the distribution of the tyrosine residues. The positions and amino acid numbers of tyrosine residues are indicated (arrows). (B) The flanking amino acid sequences of each tyrosine residue in WASP and of tyrosine 223 (the autophosphorylation site) of Btk are listed. (C) Coexpression of Btk and mutant WASP constructs. Cotransfection of pEF-BOS/wild-type Btk with pcDNA3/wild-type WASP (lane 1), pcDNA3/Y51F (lane 2), pcDNA3/Y83F (lane 3), pcDNA3/Y88F (lane 4), pcDNA3/Y102F (lane 5), pcDNA3/Y107F (lane 6), pcDNA3/Y212F (lane 7), or pcDNA3/Y291F mutant WASP (lane 8) was performed with lipofectamine. Cells were harvested after 48 hours and lysed with Triton X-100 lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top). The same membrane was reprobed with the anti-WASP antibody 503 (middle). To detect the expression of Btk proteins, the total cell lysates (TCL) were loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom). (D) Association of wild-type or Y291F mutant WASP with Btk. Transfection of pEF-BOS/wild-type Btk as a control (lane 1) or cotransfection of pEF-BOS/wild-type Btk with T7 epitope-tagged WASP (lane 2) or T7 epitope-tagged Y291F (lane 3) into 293T cells was performed to transiently express the proteins. Cells were harvested after 48 hours and lysed with digitonin lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-T7 tag antibody and immunoblotted with the anti-Btk antibody 43-3B (top), followed by reprobing with the anti-WASP antibody 503 (middle). To detect the expression of Btk proteins, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom).
DISCUSSION
The recent observation that mutations within Btk and WASP cause characteristic immunodeficiency disorders clearly demonstrates the importance of these proteins for normal lymphocyte function. In the present study, we demonstrated that WASP, which has been reported to be one of the Btk-SH3 domain binding proteins, is a participant in the cytoplasmic tyrosine kinase pathway in B-lineage cells and may actually serve as the substrate of Btk in vivo.
In contrast to our finding presented here, one report claimed that the tyrosine-phosphorylation of WASP was not observed after BCR cross-linking.33 This discrepancy may be due to the addition of pervanadate to the cell lysis buffer that we used in all of our procedures. We initially observed that the tyrosine-phosphorylation of WASP was barely visible when a lysis buffer was used that contained orthovanadate only. Because of residual PTPase activities observed even at high concentrations of orthovanadate and the fact that its inhibitory effect is reversible,56 we decided to add pervanadate to our buffer. Pervanadate, consisting of a variety of complexes formed between orthovanadate and hydrogen peroxide, is much more potent than orthovanadate as a PTPase inhibitor and its inhibitory effect is irreversible.56 The observation that the addition of pervanadate to the lysis buffer was a prerequisite for the detection of tyrosine-phosphorylation of WASP suggests that phosphorylated WASP is very sensitive to cellular PTPases. Although exposure of cells to pervanadate has been shown to mimic limited receptor signalings under certain conditions,57-59 the time course of phosphorylation after BCR cross-linking (Fig 2A) demonstrates that the increased tyrosine-phosphorylation of WASP we observed depends on the signaling through the BCR and was not simply an artifact of pervanadate. Several cytoplasmic tyrosine kinases, including Btk, Syk, and such Src-family kinases as Lyn, have been reported to be involved in the cytoplasmic signal transduction via BCR. The rapid tyrosine-phosphorylation of WASP after BCR cross-linking thus suggests an involvement of WASP in the cytoplasmic tyrosine kinase pathway in B-lineage cells. Simultaneously, in another report, we have recently demonstrated that WASP is transiently tyrosine-phosphorylated in normal human platelets after stimulation with thrombopoietin (Imai et al, manuscript submitted). Putting these observations together suggests the involvement of WASP in tyrosine kinase pathways in a variety of hematopoietic cell lineages.
The in vivo reconstitution experiments used in this study have provided evidence that WASP associates with Btk and can serve as its substrate in vivo. These observations raise the possibility that Btk may play a significant role in the phosphorylation of WASP in B cells as well, although the data presented here do not allow us to determine if under physiologic conditons, eg, BCR cross-linking, Btk is the dominant kinase that contributes to the tyrosine phosphorylation of WASP. Several molecules have been reported to associate with the SH3 domain of Btk at least in vitro.33,49,60 However, no evaluations of these molecules as possible substrates of Btk have been reported. Recently, we reported a novel protein Sab, which also binds the SH3 domain of Btk.19 Although in vivo association of Btk and Sab was observed,19 the tyrosine-phosphorylation of Sab by Btk could not be detected when using a reconstitution system similar to the one described in this report (our unpublished data). Therefore, the observed tyrosine-phosphorylation of WASP by Btk cannot be extrapolated to other Btk-SH3 domain binding molecules and they may play different roles in the Btk signaling pathway.
The precise biological significance of the phosphorylation of WASP is currently unknown. However, in this study, we demonstrated that tyrosine-phosphorylation of WASP regulates the association of a 220-kD protein with WASP. It seems likely that some conformational change is induced in WASP by the tyrosine-phosphorylation. The significance of this association is currently being investigated. Our observation also suggests the possibility that the tyrosine-phosphorylation may also alter the interaction between WASP and its other binding partners. Furthermore, one must certainly include the possibility that the tyrosine-phosphorylation of WASP may create a docking site for SH2 or PTB-domain containing protein(s), although the results of our experiment shown in Fig 4 failed to detect any increase in the binding of cellular proteins to the phosphorylated WASP.
With the aid of mutant constructs in which one of the seven tyrosines was systematically replaced with phenylalanine, the major phosphorylation site of WASP by Btk was determined as tyrosine 291. By using a chemical peptide library61 or a phage display approach,62 it has been shown that each of the tyrosine kinases or its family displays distinct substrate specificities that are mainly determined by the residues immediately flanking the phosphorylated tyrosine residue. Several cytoplasmic tyrosine kinases (Fps, Abl, Src family kinases) seem to have a preference for acidic residues (glutamic acid or aspartic acid) at the −4 to −2 positions (especially the −3 position) from the targeted tyrosine, hydrophobic residues (isoleucine, leucine or valine) at the −1 position, acidic residues at the +1 and +2 positions, and hydrophobic residues that tend to dominate at the +3 position. In contrast, the cytoplasmic tyrosine kinase Syk commands acidic residues at the −1 position. Although the general substrate specificity of Btk has not yet been determined, it is of interest that the flanking amino acid residues of tyrosine 291 (SKLIYDFI) of WASP have a distinct similarity to those of tyrosine 223, the autophosphorylation site of Btk, which is located in its SH3 domain55 and has the flanking sequence VVALYDYM (Fig 5B). Comparing the flanking sequences of these two tyrosines, the following common characteristics were noted: −4 to −2 (nonacidic), −1 (hydrophobic), +1 (acidic), +2 (aromatic), and +3 (hydrophobic). These considerations may also explain why tyrosine 551 of Btk, which is located in its catalytic domain and corresponds to the autophosphorylation sites of other cytoplasmic tyrosine kinases,63 serves as a transphosphorylation site for Src family kinases (mainly Lyn)55 rather than as a site for autophosphorylation by Btk itself. The −3 and −2 positions from tyrosine 551 of Btk are occupied by acidic residues (the flanking sequence of tyrosine 551 is LDDEYTSS). It appears, therefore, that the flanking sequence of tyrosine 551 is preferred by Src family kinases rather than Btk itself.
As mentioned in the introduction, several observations have suggested that the B cells of WAS patients contain some intrinsic defects. However, in contrast to the clearly identified defect in the B cells of XLA patients or XID mice, these developmental or functional defects in WAS-B cells have still not been precisely defined. Clarification of the significance of the tyrsoine-phosphorylation of WASP, which is mediated by cytoplasmic tyrosine kinase as described in this report, may further clarify the exact roles of the immunodeficiency-causing molecules in cytoplasmic signal transduction as well as the exact pathogenesis of these diseases.
ACKNOWLEDGMENT
The authors thank Dr Hajime Karasuyama (The Tokyo Metropolitan Institute of Medical Science) and Drs Nobuo Sakaguchi and Kazuhiko Kuwahara (Kumamoto University, Kumamoto, Japan) for their valuable comments and thank Dr Shigekazu Nagata (Osaka University Medical School, Osaka, Japan) and Dr Yoshihiro Takemoto (Nippon Glaxo Limited, Tsukuba Research Laboratory, Ibaraki, Japan) for providing materials.
Supported by grants from the Ministry of Education, Science and Culture of Japan (to T.K. and S.T.), from the Ministry of Health and Welfare of Japan (to S.T.), from the National Institutes of Health (HD17427), and from the March of Dimes Birth Defects Foundation (6-FY96-0330; to H.D.O.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Satoshi Tsukada, MD, PhD, Department of Medicine III, Osaka University Medical School, 2-2, Yamada-oka Suita City, Osaka 565, Japan; e-mail:tsukada@imed3.med.osaka-u.ac.jp.

![Fig. 5. Identification of the phosphorylation site of WASP by Btk. (A) A schematic representation of the structure of WASP and its functional domains (pleckstrin homology domain [PH], the GTPase binding domain [GBD], and proline rich regions [Poly-Pro]) and the distribution of the tyrosine residues. The positions and amino acid numbers of tyrosine residues are indicated (arrows). (B) The flanking amino acid sequences of each tyrosine residue in WASP and of tyrosine 223 (the autophosphorylation site) of Btk are listed. (C) Coexpression of Btk and mutant WASP constructs. Cotransfection of pEF-BOS/wild-type Btk with pcDNA3/wild-type WASP (lane 1), pcDNA3/Y51F (lane 2), pcDNA3/Y83F (lane 3), pcDNA3/Y88F (lane 4), pcDNA3/Y102F (lane 5), pcDNA3/Y107F (lane 6), pcDNA3/Y212F (lane 7), or pcDNA3/Y291F mutant WASP (lane 8) was performed with lipofectamine. Cells were harvested after 48 hours and lysed with Triton X-100 lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-WASP antibody 503 and immunoblotted with the antiphosphotyrosine (APT) antibody 4G10 (top). The same membrane was reprobed with the anti-WASP antibody 503 (middle). To detect the expression of Btk proteins, the total cell lysates (TCL) were loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom). (D) Association of wild-type or Y291F mutant WASP with Btk. Transfection of pEF-BOS/wild-type Btk as a control (lane 1) or cotransfection of pEF-BOS/wild-type Btk with T7 epitope-tagged WASP (lane 2) or T7 epitope-tagged Y291F (lane 3) into 293T cells was performed to transiently express the proteins. Cells were harvested after 48 hours and lysed with digitonin lysis buffer. After preclearance, lysates were immunoprecipitated with the anti-T7 tag antibody and immunoblotted with the anti-Btk antibody 43-3B (top), followed by reprobing with the anti-WASP antibody 503 (middle). To detect the expression of Btk proteins, the total cell lysates (TCL) were also loaded and immunoblotted with the anti-Btk antibody 43-3B (bottom).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.2003.406k13_2003_2012/5/m_blod40613005w.jpeg?Expires=1769140426&Signature=UrOPDvwmf-GgsjdikmJKmd7tQEwvXmu~aE4faKj45npAg4xv4LPCGGKUxvd8o64AAZNivN7wgUDJxcE7FLabcnIG1lymLcBkv1Sutj0rga0CPYywwgH7-yn05EWwOA6OwVlmdKV-ROvJmioyvz32HePXySLmZAw9KAat3sStlJeSLvZgnl22h8ATOUCsyilNAsLM7yKhbw9~d19U0wmUu5rnLdK5yf1wuR5sFn5yeizDn3fOyalkPt2NRLKcXj8LelazaYUH7Xqr-Kxhgh9WExlniiYycOG01GmVN1xS6xSCol1TDJh73OlhaTHctA~vWKmYiV6U6XuvpLdJMTOJnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal