High-dose chemotherapy followed by autologous transplantation has been shown to improve response rates and survival in multiple myeloma and other malignancies. However, autografts frequently contain detectable tumor cells. Enrichment for stem cells using anti-CD34 antibodies has been shown to reduce autograft tumor contamination in phase I/II studies. To more definitively assess the safety and efficacy of CD34 selection, a phase III study was completed in 131 multiple myeloma patients randomized to receive an autologous transplant with either CD34-selected or unselected peripheral blood progenitor cells after myeloablative therapy. Tumor contamination in the autografts was assessed by a quantitative polymerase chain reaction detection assay using patient-specific, complementarity-determining region (CDR) Ig gene primers before and after CD34 selection. A median 3.1 log reduction in contaminating tumor cells was achieved in the CD34 selected product using the CEPRATE SC System (CellPro, Inc, Bothell, WA). Successful neutrophil engraftment was achieved in all patients by day 15 and no significant between-arm difference for time to platelet engraftment occurred in patients who received an infused dose of at least 2.0 × 106 CD34+ cells/kg. In conclusion, this phase III trial demonstrates that CD34-selection of peripheral blood progenitor cells significantly reduces tumor cell contamination yet provides safe and rapid hematologic recovery for patients receiving myeloablative therapy.
MULTIPLE MYELOMA is a fatal neoplasm in which malignant plasma cells derived from a single transformed cell (clone) accumulate in the bone marrow and produce an abundance of monoclonal Ig. Although conventional therapy with oral melphalan and prednisone has achieved remissions in approximately 40% of patients, the disease remains incurable, with a median overall survival of 30 to 36 months.1-3 Attempts to improve overall survival have led to the evaluation of more aggressive, multiagent, conventional-dose chemotherapy regimens such as M2, VCMP, VBAP, and ABCM. Unfortunately, despite improved response rates, a meta-analysis of several prospective randomized trials showed no significant improvement in the survival of patients treated with these multidrug regimens compared with standard doses of melphalan and prednisone.4 Because the dose intensity of standard chemotherapy is often limited by hematologic toxicity, higher doses of chemotherapy followed by autologous bone marrow transplantation have been attempted to improve upon myeloma cell kill.5-8 High-dose melphalan followed by autologous bone marrow transplantation was shown to improve response rates, disease-free survival, and overall survival in a randomized study when compared with standard chemotherapy.9 10 However, the majority of myeloma patients treated with autologous bone marrow transplantation develop progressive disease within 3 years.
The use of peripheral blood progenitor cells (PBPCs) as the source of hematopoietic stem cells has been evaluated as an alternative approach to improve upon the efficacy of autologous transplantation in patients with multiple myeloma.11-13 Several studies have demonstrated that PBPCs can be used for autologous transplantation and that restoration of hematopoiesis occurred more rapidly when compared with bone marrow stem cells.12 14-16 Furthermore, PBPCs can be harvested without the use of general anesthesia. As a result, PBPCs have become the preferred source for autografts in support of myeloablative therapy.
Malignant cells have been detected in bone marrow harvests of patients with multiple myeloma17,18 as well as in the peripheral blood and in the leukapheresis products.18-21 A recent prospective study of 33 patients found an inverse correlation between the monoclonal plasma cell concentration in leukapheresis or peripheral blood samples and disease-free survival.18 In this study, the presence of ≥0.2 × 106 malignant plasma cells/L was a significant predictor of early relapse. Because circulating tumor cell numbers may simply reflect overall tumor burden, this finding does not directly demonstrate that the reinfusion of circulating myeloma cells contributed to disease recurrence. However, studies using gene marking techniques and clonogenic tumor cell assays in other malignancies suggest that tumor cells infused in an autologous graft can contribute to relapse and affect overall outcome.22 23
Initial studies in patients with multiple myeloma evaluated ex vivo purging of bone marrow harvests by incubation with cyclophosphamide derivatives or monoclonal B-cell antibodies.24-26 These purging procedures substantially reduced autograft tumor burden yet were associated with significant delays in engraftment. Because the malignant clone in myeloma does not express CD34,27 an alternative approach for purging the autograft uses the positive selection of stem cells using anti-CD34 antibodies. This method of purging has the advantage of more widespread applicability among tumor types not expressing CD34 and eliminates the need to expose the autograft to potentially harmful agents.
Autologous transplantation using CD34-selected products can provide effective hematopoietic support for patients receiving myeloablative therapy.15,28,29 In a phase I/II study of 37 myeloma patients, neutrophil engraftment (absolute neutrophil count [ANC] >500/μL) and platelet (>20,000/μL) engraftment were rapid (median of 12 days for both) provided that a cell dose of at least 2 × 106 CD34+cells/kg was reinfused. Tumor cell contamination was measured before and after CD34 selection using a sensitive polymerase chain reaction (PCR) assay based on the unique Ig heavy-chain variable region (VDJ) sequence expressed by the myeloma cell clone. Tumor cells were detected in 70% of the unmanipulated PBPC products. The CD34 selection using the CEPRATE SC Stem Cell Concentration System (CellPro, Inc, Bothell, WA) reduced the tumor cell contamination between 2.7 and 4.5 logs.15
A phase III, randomized study was conducted to more definitively evaluate the tumor-purging capabilities of the CEPRATE SC System. Tumor cell contamination of the autografts was quantified using a PCR-based tumor detection assay.15 17 To determine the safety of CD34 selection using the CEPRATE SC System, hematologic recovery and toxicity after autologous transplantation with either CD34 selected or unselected PBPCs were compared.
MATERIALS AND METHODS
Study design.
From January 1995 to June 1996, 134 patients with multiple myeloma between the ages of 18 and 70 years were enrolled in a phase III open-label, randomized trial at 15 sites in North America, including the University of California, Los Angeles (UCLA), The Toronto Hospital, the Dana Farber Cancer Institute (DFCI), Washington University, the University of South Florida, Johns Hopkins University, the University of California, San Francisco (UCSF), the University of Texas at San Antonio, the University of Pennsylvania, the Southern California Kaiser Permanente Medical Group, the University of Nebraska, Baylor University, the University of Arizona, and the University of California, San Diego (UCSD). Data were analyzed as of January 1998. The study design was approved by the Institutional Review Boards of all participating institutions and by the Food and Drug Administration under an investigational device exemption (IDE). All patients gave written informed consent to participate.
All patients had a diagnosis of multiple myeloma and were staged based on the criteria developed by Durie and Salmon.30 Patients were eligible for enrollment if they had evidence of one of the following features at diagnosis or any time thereafter: (1) intermediate to high M-component production rates (IgG ≥5 g/dL or IgA ≥3 g/dL or urine M-component ≥4 g/24 h); (2) more than one osteolytic bone lesion, or radiographic evidence of diffuse osteoporosis; (3) β-2 microglobulin ≥3 mg/L; and (4) nonsecretory myeloma if bone marrow plasmacytosis was greater than 30%. Patients were required to have stable or responsive disease after a minimum of three cycles of chemotherapy. Patients who had received more than 3 months of alkylator-based therapy and/or 6 months of any other prior chemotherapy or disease progression at anytime were ineligible for study entry. Patients who had a creatinine level ≥2 mg/dL, Karnofsky performance status less than 70%, or other significant heart, lung, or gastrointestinal dysfunction were ineligible. Randomization was stratified by patient age (<55 or ≥55 years) and site.
Study schema.
Figure 1 shows a schema of the study. Collection of autologous PBPCs occurred within 3 weeks of the patient’s evaluation for eligibility. The mobilization regimen consisted of cyclophosphamide (2.5 g/m2) administered intravenously on the first day of mobilization, prednisone (2 mg/kg/d) administered orally on the first 4 days, and granulocyte colony-stimulating factor (G-CSF; 10 μg/kg/d) administered subcutaneously beginning on the second day and continuing until the last day of leukapheresis.
Schema of study design. Eligible patients were registered and PBPCs were mobilized. Before initiating leukapheresis, patients were randomized to one of the two treatment arms. All patients received high-dose chemotherapy followed by an infusion of either CD34-selected or unselected PBPCs, with GM-CSF administered posttransplant. The primary study period was completed at 6 months posttransplant with follow-up currently at 1 year posttransplant. Additional follow-up is planned on a yearly basis.
Schema of study design. Eligible patients were registered and PBPCs were mobilized. Before initiating leukapheresis, patients were randomized to one of the two treatment arms. All patients received high-dose chemotherapy followed by an infusion of either CD34-selected or unselected PBPCs, with GM-CSF administered posttransplant. The primary study period was completed at 6 months posttransplant with follow-up currently at 1 year posttransplant. Additional follow-up is planned on a yearly basis.
Before leukapheresis, patients were randomized to receive CD34 selected (the selected arm) or unselected PBPC (the unselected arm). After the PBPC collection was completed, patients received a myeloablative high-dose chemotherapy regimen consisting of busulfan and cyclophosphamide. A total dosage of 14 mg/kg of busulfan (0.875 mg/kg administered orally 4 times daily for 4 days on days −7 to −4) and 120 mg/kg of cyclophosphamide (60 mg/kg administered intravenously daily for 2 days on days −3 and −2) was administered to all patients as in the previous phase I/II study.15 Patients received the thawed CD34 selected or unselected products 2 days after the last infusion of cyclophosphamide. To speed hematologic recovery, granulocyte-macrophage colony-stimulating factor (GM-CSF) at 250 μg/m2 (maximum of 500 μg) was administered daily until the patient’s ANC was at least 1,000/μL for 2 consecutive days. Each site used its own standardized supportive care protocols for patients on both arms of the study.
According to the protocol, the first 58 patients (28 selected arm and 30 unselected arm) began leukapheresis no sooner than day 15 of PBPC mobilization and had to have a white blood cell (WBC) count ≥1,000/μL on two occasions more than 24 hours apart and a platelet count ≥30,000/μL. The day 15 start date was chosen for scheduling convenience and was based on successful stem cell collection in the phase II protocol using this schema. Because of concerns that the peak of circulating CD34+ cells was being missed before day 15, possibly because these patients were less heavily pretreated, the remaining 76 patients (39 selected arm, 34 unselected arm, and 3 patients not randomized) began leukapheresis as soon as the hematologic parameters outlined above were met. Leukapheresis was performed daily until at least 5.0 × 108 total nucleated cells/kg were obtained (minimum of 2 days). For patients randomized to receive a CD34 selected autograft, there was an additional stopping criterion of at least 4.0 × 106 nucleated cells/kg in the selected product after processing on the CEPRATE SC System. This criterion was based on the assumption that the selected product would contain at least 50% CD34+ cells; thus, patients would receive an infused CD34+ cell dose of at least 2 × 106 cells/kg.
Study endpoints.
There were two primary study endpoints. The primary efficacy endpoint was a reduction of tumor cell contamination in the PBPC product after processing with the CEPRATE SC System. Success was defined as greater than a 2 log reduction in the number of contaminating tumor cells. The primary safety endpoint was successful neutrophil engraftment (the first day of two consecutive ANCs ≥500/μL after the nadir) on or before day 14 after stem cell infusion. There were other secondary endpoints, including the following: number of tumor cells infused; infusion-related toxicities; time to neutrophil engraftment; time to platelet engraftment (7 consecutive days of platelet transfusion independence) and platelet recovery (platelet count ≥20 × 103/μL and platelet transfusion independence for at least 7 days); the number of patients with bleeding episodes, infections, and adverse events; days of hospitalization; number of red blood cell (RBC) or platelet transfusions; and long-term engraftment and immune reconstitution posttransplant. The rate of progression-free survival was another secondary endpoint. Although the data are immature, progression-free and overall survival at 1 year posttransplant is presented.
Leukapheresis, processing, and cryopreservation.
Autologous PBPCs were procured by continuous flow leukapheresis using a Cobe Spectra (Cobe, Lakewood, CO). Blood volume processed per run was 10 L. Patients randomized to receive unselected PBPCs each had individual leukapheresis products cryopreserved in 10% dimethyl sulfoxide (DMSO) by control-rated freezing and stored in the vapor phase of liquid nitrogen. Patients randomized to receive CD34-selected PBPCs had each individual leukapheresis products selected using the CEPRATE SC System, as previously described15 following the manufacturer’s protocol. The CD34-selected PBPCs were then cryopreserved in 7.5% DMSO by control-rated freezing and stored in the vapor phase of liquid nitrogen. For all patients receiving CD34-selected PBPCs, the CD34-depleted fraction was cryopreserved for backup in the event of graft failure.
Flow cytometry and colony-forming unit (CFU) analysis.
Samples were analyzed by Cytometry Associates, Inc (Brentwood, TN) using fluorochrome-conjugated monoclonal antibodies and a flow cytometer. Briefly, samples from the leukapheresis product, CD34-selected product, and the CD34-depleted product were incubated with anti-CD14 monoclonal antibody conjugated with fluorescein isothiocyanate (CD14-FITC) and with either anti-CD34 monoclonal antibody (CD34-PE) or an isotypic control (MsIgG-PE) conjugated with phycoerythrin. In addition, the cells were stained with 7-Actinomycin D (7-AAD) to assess viability. After acquisition of data from the flow cytometer, the CD34+ cells were identified based on Boolean gating of light scatter, viability, and absence of CD14 expression. The percentage of positive CD34+ cells was calculated by dividing the number of CD34+ events, derived from the above gating strategy, by the total nucleated cells acquired and multiplying this result by 100.
The culture and enumeration of CFU (including granulocyte-macrophage, granulocyte-erythroid-monocyte-macrophage, and burst-forming unit-erythroid colonies) were performed by each site using standard media and procedures.29
Myeloma Ig gene identification.
The Ig heavy chain variable region (VH) or light chain (light chain only secreting tumors) variable region (VL) sequence expressed by the myeloma clone was determined using a bone marrow aspirate obtained from each patient at study entry, not at diagnosis. When present, the predominant, appropriately sized PCR product was excised from an agarose gel impregnated with ethidium bromide, cloned, and sequenced by methods previously described.15 17 A minimum of three identical clones of five was required to assign an Ig sequence to the myeloma cell.
Assessment and quantification of tumor contamination.
Tumor contamination was determined using a PCR assay as previously described in the phase I/II study.15,17 Briefly, DNA was extracted from bone marrow and peripheral blood mononuclear cells, unselected leukapheresis products, and CD34-selected and CD34-depleted PBPCs products using standard techniques and quantitated using a fluorometer (Hoeffer Scientific, San Francisco, CA). The VHand VL myeloma gene sequences were compared (DNAsis; Hitachi, San Bruno, CA) with known germline gene segments.31 32 Patient-specific oligonucleotides (Operon, Alameda, CA) were designed complementary to the most unique sequences in the sense (CDR1 or CDR2) and antisense orientation (CDR3). PCR conditions were optimized using the PCR Optimizer Kit (Invitrogen, San Diego, CA) on bone marrow DNA diluted 100-fold with 0.6 μg of placental DNA. Sixty cycles of amplification were performed with Taq polymerase and Taq antibody.
To quantify the tumor cell contamination, multiple PCR reactions were performed with sample DNA serially diluted in 0.5 log increments with placental DNA. Five reactions were performed at each serial dilution until PCR products were no longer detectable. Tumor contamination was calculated by a Poisson distribution analysis of positive and negative reactions at each serial dilution.15,17 33 Amplification of tubes containing a diluted bone marrow specimen (positive), placental DNA (negative), and water only (negative) using the patient-specific primers served as controls for each assay. To reduce assay-to-assay variability, the leukapheresis product and CD34-selected product collected from the same patient were amplified simultaneously in the same PCR machine. All PCR products were electrophoresed through ethidium bromide-impregnated agarose gels, photographed, and scored in triplicate by technicians blinded to sample identification.
Immune reconstitution.
The process of enriching for stem cells by CD34 selection passively removes lymphocytes from the PBPC collection and may potentially impact immune reconstitution. To determine whether immune function was more significantly impaired in those patients who received a CD34-selected autograft, lymphocyte immunophenotyping and quantitative Ig levels were performed at the premobilization, day-100, 6-month, and 1-year posttransplant visits.
Statistical methods.
An intent-to-treat primary analysis of engraftment (safety) and tumor purging (efficacy) was performed that included all eligible and randomized patients. Patients who did not engraft their neutrophils (ANC ≥500) on or by day 14 after transplant and randomized patients not infused were considered engraftment failures. Comparisons between the treatment arms in terms of the demographic, baseline, efficacy, and safety data were performed using the two-sample t-test or Wilcoxon rank sum test for continuous data and Fisher’s Exact Test (FET) for categorical data. Time to engraftment, survival time, and progression-free survival time were calculated from the date of transplant and summarized using Kaplan-Meier curves.34 The treatment arms were compared using the Cox proportional hazards model. Multiple regression with stepwise variable selection was used in the analyses of factors influencing time to engraftment.
RESULTS
Patient demographics.
Between January 1995 and June 1996, 134 patients were enrolled at 15 sites throughout North America. Three patients were not randomized; one patient elected to receive an allogeneic transplant, another patient died before randomization, and the third developed disease progression before randomization. There were 131 patients randomized in the intent-to-treat population, and 130 patients underwent transplantation. One patient had inadequate mobilization of stem cells and did not proceed to transplant; this patient had been randomized on the trial and is included in the primary analyses. The treatment arms were similar at the time of the premobilization visit with respect to performance and disease status, β-2 microglobulin levels, and the number of previous cycles of chemotherapy. There was a significantly higher proportion of females in the selected arm (45% v 27%,P = .030; Table 1). The only other baseline characteristics that differed significantly between the treatment arms were a lower median CD4/CD8 ratio, platelet, and WBC counts (1.3 v 1.7, P = .032; 110 v152 × 103/μL, P = .002; 15.9 v 26.2 × 103/μL, P = .042, respectively) for patients in the selected arm at the time of randomization (Table 1and see Table 6).
Demographic Characteristics and Hematology Measurements at Randomization
| . | CD34 Selected Arm (n = 67) . | Unselected Arm (n = 64) . | P Value . |
|---|---|---|---|
| Sex | |||
| Male | 37 (55%) | 47 (73%) | .030 |
| Female | 30 (45%) | 17 (27%) | |
| Age (yr) | |||
| Median | 51 | 52 | .852 |
| Range | 37-70 | 34-68 | |
| Karnofsky performance status | |||
| 70-89 | 18 (28%) | 14 (23%) | .577 |
| 90-100 | 47 (72%) | 46 (77%) | |
| Disease status | |||
| Complete remission | 5 (8%) | 10 (16%) | .175 |
| Partial remission | 46 (69%) | 38 (59%) | |
| Stable disease | 16 (24%) | 16 (25%) | |
| Years since first histopathologic diagnosis | |||
| Median | 0.5 | 0.5 | .874 |
| Range | 0.2-3.3 | 0.2-2.9 | |
| β-2 Microglobulin (mg/L) | |||
| Median | 1.7 | 2.0 | .161 |
| Range | 0.3-7.3 | 0.7-5.0 | |
| C-Reactive protein (mg/dL) | |||
| Median | 0.4 | 0.3 | .931 |
| Range | 0.3-2.7 | 0.3-5.4 | |
| No. of cycles of chemotherapy | |||
| Median | 4 | 4 | .958 |
| Range | 2-9 | 2-9 | |
| WBC (×103/μL) | |||
| Mean ± SEM | 24.6 ± 2.6 | 32.5 ± 2.8 | .042 |
| Median | 15.9 | 26.2 | |
| Range | 1.0-89.3 | 3.9-95.1 | |
| Platelets (×103/μL) | |||
| Mean ± SEM | 115.7 ± 7.1 | 148.5 ± 7.7 | .002 |
| Median | 110 | 152 | |
| Range | 17-275 | 28-309 |
| . | CD34 Selected Arm (n = 67) . | Unselected Arm (n = 64) . | P Value . |
|---|---|---|---|
| Sex | |||
| Male | 37 (55%) | 47 (73%) | .030 |
| Female | 30 (45%) | 17 (27%) | |
| Age (yr) | |||
| Median | 51 | 52 | .852 |
| Range | 37-70 | 34-68 | |
| Karnofsky performance status | |||
| 70-89 | 18 (28%) | 14 (23%) | .577 |
| 90-100 | 47 (72%) | 46 (77%) | |
| Disease status | |||
| Complete remission | 5 (8%) | 10 (16%) | .175 |
| Partial remission | 46 (69%) | 38 (59%) | |
| Stable disease | 16 (24%) | 16 (25%) | |
| Years since first histopathologic diagnosis | |||
| Median | 0.5 | 0.5 | .874 |
| Range | 0.2-3.3 | 0.2-2.9 | |
| β-2 Microglobulin (mg/L) | |||
| Median | 1.7 | 2.0 | .161 |
| Range | 0.3-7.3 | 0.7-5.0 | |
| C-Reactive protein (mg/dL) | |||
| Median | 0.4 | 0.3 | .931 |
| Range | 0.3-2.7 | 0.3-5.4 | |
| No. of cycles of chemotherapy | |||
| Median | 4 | 4 | .958 |
| Range | 2-9 | 2-9 | |
| WBC (×103/μL) | |||
| Mean ± SEM | 24.6 ± 2.6 | 32.5 ± 2.8 | .042 |
| Median | 15.9 | 26.2 | |
| Range | 1.0-89.3 | 3.9-95.1 | |
| Platelets (×103/μL) | |||
| Mean ± SEM | 115.7 ± 7.1 | 148.5 ± 7.7 | .002 |
| Median | 110 | 152 | |
| Range | 17-275 | 28-309 |
Demographic characteristics were evaluated for all patients in the intent-to-treat population. Comparability between arms was assessed by χ2 tests for sex, Karnofsky performance status, and disease status at registration and by two-sample t-tests for age, β-2 microglobulin at registration, and time (years) from first histopathologic diagnosis to date of randomization. Comparability between arms was assessed by the Wilcoxon rank sum test for C-reactive protein at registration and the number of cycles of prior chemotherapy. Hematology measurements were evaluated at time of randomization. Two-sample t-tests were used to compare the treatment arms with respect to the hematology measurements. The mean WBC count and mean platelet count at randomization were significantly lower in the CD34 selected arm compared with the unselected arm.
Stem cell collection and enrichment.
A median of two leukaphereses were required for both treatment arms to reach the target of 5 × 108 nucleated cells/kg. However, 18 of the 67 patients in the selected arm required an additional leukapheresis to meet the second criterion of 4 × 106 nucleated cells/kg in the selected product. Consequently, the mean number of leukaphereses required for patients in the selected arm was 3.0 (range, 2 to 8) versus 2.3 for patients in the unselected arm (range, 2 to 6; P = .002).
The median number of CD34+ cells in the leukapheresis product before processing was 10.13 and 8.67 × 106/kg for the selected and the unselected arms, respectively (P = .253). After enrichment, the median number of CD34+ cells was 5.40 × 106/kg in the selected arm for a median CD34+ cell yield of 52%. The median purity of CD34+ cells was 0.9% before CD34 selection and 66.4% after selection. As expected, the patients in the selected arm had significantly fewer nucleated cells/kg (P < 0.001) and CD34+ cells/kg (P = .012) infused than did the patients in the unselected arm (Table 2). The median number of CD34 cells in the cryopreserved backup stem cell product (CD34-depleted product) was 2.78 × 106CD34+ cells/kg.
Processing of PBPCs: Total of All Leukaphereses
| Processing Step . | Processing Variable . | P Value . | |
|---|---|---|---|
| CD34-Selected Arm (n = 67) . | Unselected Arm (n = 64) . | ||
| CD34+ cells/kg | |||
| Product at start (×106) | n = 60 | n = 56 | |
| Median | 10.13 | 8.67 | .253 |
| Range | 0-81.29 | 0.68-76.18 | |
| Selected product (×106) | n = 61 | ||
| Median | 5.40 | NA | .012 |
| Range | 0.03-24.79 | ||
| CFU-GM/kg | |||
| Product at start (×104) | n = 58 | n = 49 | |
| Median | 88.21 | 112.35 | .704 |
| Range | 0-532.28 | 1.97-936.4 | |
| Selected product (×104) | n = 58 | ||
| Median | 59.8 | NA | .287 |
| Range | 0-634.58 | ||
| Processing Step . | Processing Variable . | P Value . | |
|---|---|---|---|
| CD34-Selected Arm (n = 67) . | Unselected Arm (n = 64) . | ||
| CD34+ cells/kg | |||
| Product at start (×106) | n = 60 | n = 56 | |
| Median | 10.13 | 8.67 | .253 |
| Range | 0-81.29 | 0.68-76.18 | |
| Selected product (×106) | n = 61 | ||
| Median | 5.40 | NA | .012 |
| Range | 0.03-24.79 | ||
| CFU-GM/kg | |||
| Product at start (×104) | n = 58 | n = 49 | |
| Median | 88.21 | 112.35 | .704 |
| Range | 0-532.28 | 1.97-936.4 | |
| Selected product (×104) | n = 58 | ||
| Median | 59.8 | NA | .287 |
| Range | 0-634.58 | ||
Processing data were evaluated for CD34+ cells per kilogram and CFU-GM per kilogram on patients who had data available for every leukapheresis and at each specific processing step. For patients in the CD34 selected arm, the selected product was cryopreserved; for patients in the unselected arm, the product at start was cryopreserved. The patients in the CD34-selected arm had significantly fewer CD34+ cells per kilogram infused (P = .012). There was no significant difference between the arms in the number of CFU-GM per kilogram infused (P = .287).
Abbreviation: NA, not applicable.
There were no significant differences between the treatment arms in terms of CFU/kg either before or after CD34 selection (P = .287). The median number of CFU per kilogram infused was 53.5 × 104/kg in the selected arm and 102.4 × 104/kg in the unselected arm.
Tumor purging efficacy of the CEPRATE SC System.
Bone marrow samples were obtained from every patient at the time of study registration. A minimum of 3 months of prior chemotherapy was required, potentially affecting the ability to identify the clonal Ig sequences. A clonal Ig sequence was able to be identified and used in the analysis for 47 patients: 28 (42%) in the selected arm and 19 (30%) in the unselected arm. A clonal Ig sequence was not identified for 81 patients (36 in the selected arm and 45 in the unselected arm) for the following reasons: normal SPEP and UPEP at study entry (22), ineffective (19) or polyclonal (24) Ig gene amplification, and inadequate (12) or unavailable (4) BM specimens. In addition, there were 3 patients that had clonal Ig gene sequences identified who were not included in the analyses: for 2 patients, PCR conditions could not be adjusted to specifically amplify only the myeloma Ig gene product, and 1 patient did not have samples obtained from leukapheresis products.
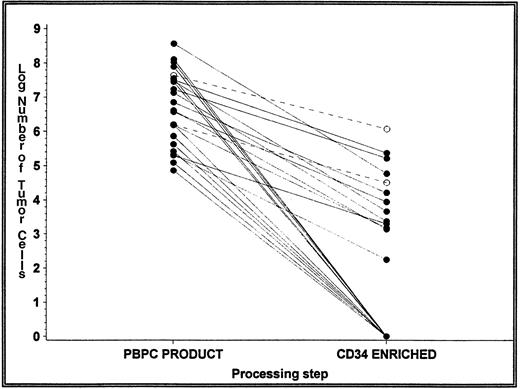
There were no detectable tumor cells in the initial leukapheresis PBPC product of 4 patients in each treatment arm (14% in the selected arm and 21% in the unselected arm; see Table 4). A median of 2.6 × 106 and 2.3 × 106 tumor cells was detected in the initial leukapheresis product of patients in the selected and unselected arms, respectively (Table 3). The primary efficacy endpoint of the study was a greater than 2 log reduction in tumor cell contamination after CD34 selection using the CEPRATE SC System. This was evaluated in 24 patients in the selected arm for whom a clonal Ig sequence could be identified and who had detectable tumor cells in the PBPC product before CD34 selection (Fig 2). Of the 24 patients with detectable tumor cells in this PBPC product, 11 (46%) had no detectable tumor cells in the CD34-selected product. To estimate the log depletion of tumor cells for these patients, the minimal detection limit was used for the percentage of tumor cells, after CD34 selection in the calculations. Thus, the mean and median log depletion of tumor cells may be underestimated. A median 3.10 log (mean, 3.29 log) depletion of tumor cells was achieved by the CEPRATE SC System in the selected arm (range, 1.56 to 6.02 logs). Moreover, the number of tumor cells infused was significantly lower in the selected arm (median, 0; range, 0 to 1.2 × 106) compared with the unselected arm (median, 2.3 × 106; range, 0 to 930 × 106; Table3). Overall, the number of patients who received an autograft with no detectable tumor cells was also significantly greater in the selected arm (54% v 21%, P = .036; Table 4).
Reduction in Number of Tumor Cells
| No. of Tumor Cells . | CD34-Selected Arm (n = 28) . | Unselected Arm (n = 19) . |
|---|---|---|
| PBPC product (×106) | ||
| Mean ± SEM | 31.9 ± 13.8 | 95.6 ± 52.6 |
| Median | 2.6 | 2.3 |
| Range | 0-363.9 | 0-930.3 |
| CD34-selected product (×106) | ||
| Mean ± SEM | 0.07 ± 0.04 | NA |
| Median | 0 | |
| Range | 0-1.2 | |
| CD34-depleted product (×106) | ||
| Mean ± SEM | 39.5 ± 15.2 | NA |
| Median | 9.9 | |
| Range | 0-375.4 | |
| Log depletion of tumor cells | n = 24 | |
| Mean ± SEM | 3.29 ± 0.26 | NA |
| Median | 3.10 | |
| Range | 1.56-6.02 |
| No. of Tumor Cells . | CD34-Selected Arm (n = 28) . | Unselected Arm (n = 19) . |
|---|---|---|
| PBPC product (×106) | ||
| Mean ± SEM | 31.9 ± 13.8 | 95.6 ± 52.6 |
| Median | 2.6 | 2.3 |
| Range | 0-363.9 | 0-930.3 |
| CD34-selected product (×106) | ||
| Mean ± SEM | 0.07 ± 0.04 | NA |
| Median | 0 | |
| Range | 0-1.2 | |
| CD34-depleted product (×106) | ||
| Mean ± SEM | 39.5 ± 15.2 | NA |
| Median | 9.9 | |
| Range | 0-375.4 | |
| Log depletion of tumor cells | n = 24 | |
| Mean ± SEM | 3.29 ± 0.26 | NA |
| Median | 3.10 | |
| Range | 1.56-6.02 |
The number of tumor cells was assessed in the first leukaphereses using the patient-specific VH primers. The clonal Ig sequence was identified for 28 patients and 19 patients in the CD34-selected and unselected arms, respectively. The number of tumor cells before processing (PBPC product) and after processing (CD34-selected and CD34-depleted products) was assessed in the CD34-selected arm. The number of tumor cells in the PBPC product before cryopreservation was assessed in the unselected arm. There was no significant difference between the arms in the number of tumor cells in the PBPC product (P = .957, Wilcoxon rank sum test). For patients in the CD34-selected arm with detectable tumor cells in the PBPC product, the log depletion of tumor cells after processing on the CEPRATE SC System was calculated.
Abbreviation: NA, not applicable.
Depletion of tumor cells from first leukapheresis. Tumor contamination was determined in the PBPC product before and after CD34 selection. The number of tumor cells was calculated as the product of the total number of nucleated cells and the percentage of tumor cells detected. The figure represents the log number of tumor cells detected in the 24 of 28 patients (86%) in the CD34-selected arm with detectable tumor cells in the first leukapheresis. For those products purged below the detection level of the assay, the line intersects 0 on the x-axis (no detectable tumor cells). Only 2 of the 24 patients had their autografts purged of tumor cells by less than 2 logs (dashed lines).
Depletion of tumor cells from first leukapheresis. Tumor contamination was determined in the PBPC product before and after CD34 selection. The number of tumor cells was calculated as the product of the total number of nucleated cells and the percentage of tumor cells detected. The figure represents the log number of tumor cells detected in the 24 of 28 patients (86%) in the CD34-selected arm with detectable tumor cells in the first leukapheresis. For those products purged below the detection level of the assay, the line intersects 0 on the x-axis (no detectable tumor cells). Only 2 of the 24 patients had their autografts purged of tumor cells by less than 2 logs (dashed lines).
Number of Patients With No Detectable Tumor Cells From First Leukapheresis
| . | CD34-Selected Arm (n = 28) . | Unselected Arm (n = 19) . | P Value . |
|---|---|---|---|
| PBPC product | 4 (14%) | 4 (21%) | .545 |
| Infused product | 15 (54%) | 4 (21%) | .036 |
| . | CD34-Selected Arm (n = 28) . | Unselected Arm (n = 19) . | P Value . |
|---|---|---|---|
| PBPC product | 4 (14%) | 4 (21%) | .545 |
| Infused product | 15 (54%) | 4 (21%) | .036 |
Four patients in each arm had no detectable tumor cells in the first leukapheresis. After CD34 selection, 11 of the 24 patients in the CD34-selected arm had no detectable tumor cells. Thus, a total of 15 patients (54%) received an infused product with no detectable tumor cells in the CD34-selected arm compared with 4 patients (21%) in the unselected arm.
Hematologic recovery and toxicity.
Successful neutrophil engraftment (ANC ≥500/μL) by day 14 was achieved by 63 of 67 patients (94%) in the selected arm and all 64 patients in the unselected arm. Three of the 4 patients in the selected arm who did not achieve neutrophil engraftment by day 14 engrafted on day 15. The fourth patient was not transplanted because of an inadequate stem cell collection but was included in the intent-to-treat analysis as an engraftment failure. The median time to neutrophil engraftment was 12 days for both arms (Table 5).
Results of Secondary Safety Endpoints
| Endpoint . | CD34Selected Arm (n = 66) . | Unselected Arm (n = 64) . | P Value . |
|---|---|---|---|
| Median (range) | Median (range) | ||
| Days to neutrophil engraftment | 12 (9-15) | 12 (9-14) | .013 |
| Days to platelet engraftment | 11 (1-56) | 9 (1-42) | .003 |
| Days to platelet recovery | 11 (8-119) | 10 (7-42) | .004 |
| Platelet transfusions (events/patient) | 3 (0-26) | 2 (0-24) | .011 |
| RBC transfusions (units/patient) | 4 (0-30) | 4 (0-18) | .208 |
| Days of initial hospitalization | 17 (4-60) | 16 (12-69) | .123 |
| Days of rehospitalization | 0 (0-32) | 0 (0-19) | .849 |
| No. (%) | No. (%) | ||
| Incidence of infections (day 0 to day 100) | 37 (56%) | 36 (56%) | .492 |
| No. of infections/patient (day 0 to day 100) | |||
| 0 Infections | 29 (44%) | 28 (44%) | .702 |
| 1 Infection | 19 (29%) | 22 (34%) | |
| 2 Infections | 12 (18%) | 10 (16%) | |
| 3-5 Infections | 6 (9%) | 4 (6%) | |
| Incidence of bleeding events (day 0 to day 100) | 8/66 (12%) | 3/64 (5%) | .207 |
| Endpoint . | CD34Selected Arm (n = 66) . | Unselected Arm (n = 64) . | P Value . |
|---|---|---|---|
| Median (range) | Median (range) | ||
| Days to neutrophil engraftment | 12 (9-15) | 12 (9-14) | .013 |
| Days to platelet engraftment | 11 (1-56) | 9 (1-42) | .003 |
| Days to platelet recovery | 11 (8-119) | 10 (7-42) | .004 |
| Platelet transfusions (events/patient) | 3 (0-26) | 2 (0-24) | .011 |
| RBC transfusions (units/patient) | 4 (0-30) | 4 (0-18) | .208 |
| Days of initial hospitalization | 17 (4-60) | 16 (12-69) | .123 |
| Days of rehospitalization | 0 (0-32) | 0 (0-19) | .849 |
| No. (%) | No. (%) | ||
| Incidence of infections (day 0 to day 100) | 37 (56%) | 36 (56%) | .492 |
| No. of infections/patient (day 0 to day 100) | |||
| 0 Infections | 29 (44%) | 28 (44%) | .702 |
| 1 Infection | 19 (29%) | 22 (34%) | |
| 2 Infections | 12 (18%) | 10 (16%) | |
| 3-5 Infections | 6 (9%) | 4 (6%) | |
| Incidence of bleeding events (day 0 to day 100) | 8/66 (12%) | 3/64 (5%) | .207 |
A number of secondary safety endpoints were evaluated for between-arm differences. There were statistically significant differences between the arms for four endpoints: times to neutrophil engraftment, platelet engraftment, and platelet recovery and number of platelet transfusion events. There were no statistically significant differences between the treatment arms for any of the other secondary safety endpoints.
Secondary safety endpoints assessed the times to platelet engraftment and recovery as well as RBC and platelet transfusions. Although all patients achieved platelet engraftment, the times to platelet engraftment and recovery were statistically significantly longer in the selected arm (median of 11 days for both platelet engraftment and recovery) than in the unselected arm (median of 9 and 10 days for platelet engraftment and recovery, respectively; P = .003 andP = .004; Table 5). In addition, the number of platelet transfusion events per patient was significantly greater in the selected arm (median, 3; range, 0 to 26) than in the unselected arm (median, 2; range, 0 to 24; P = .011; Table 5). The median number of RBC transfusions was comparable for both arms (median, 4 U;P = .21; Table 5).
Multiple regression analysis of factors potentially influencing the time to platelet engraftment and recovery showed that the number of CD34+ cells per kilogram in the infused product and the platelet count at the time of randomization had significant (P ≤ .01) effects on both platelet engraftment and recovery. Additionally, the WBC count at the premobilization visit had a significant (P = .048) effect on time to platelet engraftment. Both platelet count and WBC count at randomization were significantly lower in the selected arm compared with the unselected arm. When the 108 patients (55 in the selected arm and 53 in the unselected arm) who received a dose of at least 2.0 × 106 CD34+ cells/kg (target dose suggested by protocol and based on the phase I/II study15) were compared, there were no significant differences between the arms in time to platelet engraftment (Fig 3), time to platelet recovery, or number of platelet transfusion events.
Kaplan-Meier probability of achieving platelet engraftment for the patients who received at least 2 × 106 CD34+ cells/kg in the infused product. Of the 130 patients infused, the number of CD34+ cells per kilogram was not assessed in 5 patients and were less than 2 × 106 CD34+ cells/kg for 17 patients. For the remaining 108 patients with at least 2 × 106CD34+ cells/kg infused, probabilities of the time to achieve platelet engraftment (transfusion independence) were the same for the unselected arm (n = 53) and the selected arm (n = 55). Similar results occurred for the probabilities of the time to achieve platelet recovery.
Kaplan-Meier probability of achieving platelet engraftment for the patients who received at least 2 × 106 CD34+ cells/kg in the infused product. Of the 130 patients infused, the number of CD34+ cells per kilogram was not assessed in 5 patients and were less than 2 × 106 CD34+ cells/kg for 17 patients. For the remaining 108 patients with at least 2 × 106CD34+ cells/kg infused, probabilities of the time to achieve platelet engraftment (transfusion independence) were the same for the unselected arm (n = 53) and the selected arm (n = 55). Similar results occurred for the probabilities of the time to achieve platelet recovery.
Adverse events.
The percentage of patients with at least one episode of hypertension during the 24-hour posttransplant period was significantly higher in the unselected arm (53%) than in the selected arm (33%; P = .023). The percentage of patients with at least one episode of bradycardia during the 24-hour posttransplant period was higher in the unselected arm (9%) compared with the selected arm (2%; P = .060). Other than these two specific events, the incidence of adverse events was similar between the arms. No significant differences were detected between the treatment arms for days of posttransplant hospitalization, percentage of patients rehospitalized, days of rehospitalization, the percentage of patients with at least one bleeding event, or number of bleeding events (Table 5). One patient in the CD34-selected arm received the backup CD34-depleted product on day 119 posttransplant due to a failure to achieve platelet recovery, although the patient was platelet transfusion independent. In retrospect, this patient was ineligible for the study due to progressive disease before mobilization and received an autograft containing only 1.7 × 106 CD34+ cells/kg. The platelet count remained less than 20 × 103/μL after receipt of the CD34-depleted product.
Immune reconstitution/infections/hospital days.
Patients randomized to the selected arm had a significantly lower baseline CD4/CD8 ratio and this difference persisted posttransplant at the day-100, 6-month, and 1-year time points (Table 6). Although the CD4 lymphocyte count was not significantly different at baseline, it was significantly lower in the selected arm at the day-100 and 6-month visits (211v 298 cells/μL, P = .003; and 261 v 314 cells/μL, P = .018, respectively). By the 1-year visit there was no significant difference in the median CD4+ lymphocyte count between the arms. The median CD3+, CD8+, CD56+, and CD19+ lymphocyte counts were not significantly different between the arms at more than one of the evaluation time points. There was no significant difference between the selected arm and the unselected arm with respect to IgA, IgM, or IgG levels by the day-100, 6-month, or 1-year visits.
Differences in Lymphocyte Populations Between the Arms
| Parameter . | CD34Selected Arm . | Unselected Arm . | P Value . |
|---|---|---|---|
| No. of CD4+ cells/μL | |||
| Baseline | |||
| Median | 596 | 510 | .346 |
| Range | 93-1,983 | 138-1,291 | |
| Day 100 | |||
| Median | 211 | 298 | .003 |
| Range | 51-951 | 54-1,052 | |
| 6 mo | |||
| Median | 261 | 314 | .018 |
| Range | 66-679 | 125-1,072 | |
| 1 yr | |||
| Median | 368 | 403 | .121 |
| Range | 77-718 | 140-710 | |
| CD4+/8+ ratio | |||
| Baseline | |||
| Median | 1.3 | 1.7 | .032 |
| Range | 0.4-8.6 | 0.2-4.9 | |
| Day 100 | |||
| Median | 0.3 | 0.6 | <.001 |
| Range | 0.1-1.2 | 0.1-1.6 | |
| 6 mo | |||
| Median | 0.4 | 0.6 | <.001 |
| Range | 0.1-1.2 | 0.2-1.9 | |
| 1 yr | |||
| Median | 0.6 | 0.8 | .014 |
| Range | 0.2-2.5 | 0.2-1.7 |
| Parameter . | CD34Selected Arm . | Unselected Arm . | P Value . |
|---|---|---|---|
| No. of CD4+ cells/μL | |||
| Baseline | |||
| Median | 596 | 510 | .346 |
| Range | 93-1,983 | 138-1,291 | |
| Day 100 | |||
| Median | 211 | 298 | .003 |
| Range | 51-951 | 54-1,052 | |
| 6 mo | |||
| Median | 261 | 314 | .018 |
| Range | 66-679 | 125-1,072 | |
| 1 yr | |||
| Median | 368 | 403 | .121 |
| Range | 77-718 | 140-710 | |
| CD4+/8+ ratio | |||
| Baseline | |||
| Median | 1.3 | 1.7 | .032 |
| Range | 0.4-8.6 | 0.2-4.9 | |
| Day 100 | |||
| Median | 0.3 | 0.6 | <.001 |
| Range | 0.1-1.2 | 0.1-1.6 | |
| 6 mo | |||
| Median | 0.4 | 0.6 | <.001 |
| Range | 0.1-1.2 | 0.2-1.9 | |
| 1 yr | |||
| Median | 0.6 | 0.8 | .014 |
| Range | 0.2-2.5 | 0.2-1.7 |
Cell-mediated immunity posttransplant was assessed by measuring lymphocyte populations using immunophenotyping. There were no significant differences between the treatment arms in median counts for total lymphocytes, CD3+, CD8+, CD19+, or CD56+ lymphocyte subsets that were consistently observed at the day-100, 6-month, or 1-year visits. There were significant differences between the arms at more than one of the posttransplant visits for the number of CD4+ cells and the CD4/CD8 ratio.
There were no significant differences between the treatment arms in the number of patients with infections or the type of infections during the transplant (day 0 to 100) or posttransplant (after day 100) periods and neither were there differences in days of hospitalization (Table 5).
Clinical response, progression-free survival, and overall survival.
Disease status and survival were assessed at the day-100, 6-month, and 1-year follow-up visits. The percentage of patients in complete remission (CR), defined by a lack of detectable monoclonal protein on serum and urine immunoelectrophoresis and less than 5% plasma cells within the bone marrow, was 28% at 1 year. At study enrollment, twice as many patients in CR were randomized to receive an unselected transplant (16% v 8%; P = .17). This ratio persisted throughout the study with CR rates two to three times as high for patients receiving an unselected product (arm B). The CR rates at day 100, 6 months, and 1 year were 11%, 14%, and 18% for patients on arm A, versus 29%, 35%, and 38% for patients on arm B, respectively, with only the first two time points being statistically significant.
There were 2 transplant-related deaths before day 100 in the unselected arm (infection on day 14 and veno-occlusive disease [VOD] on day 68 posttransplant) and none in the selected arm. At 1 year, 19 of 66 patients (29%) in the selected arm and 21 of 64 patients (33%) in the unselected arm had progressed or died. Although these data remain immature, there is no apparent difference between the two treatment arms with respect to progression-free survival (Fig 4) and overall survival at 1 year posttransplant.
Kaplan-Meier probability of progression-free survival based on data as of January 1998. The probabilities of the time to progression as of 1 year posttransplant were the same for patients on the unselected arm and the selected arm.
Kaplan-Meier probability of progression-free survival based on data as of January 1998. The probabilities of the time to progression as of 1 year posttransplant were the same for patients on the unselected arm and the selected arm.
DISCUSSION
This study represents the first controlled, randomized phase III trial comparing the use of CD34-selected versus unselected peripheral blood autografts for transplantation in patients undergoing high-dose chemotherapy. Because autografts collected from patients with multiple myeloma,8,15,17,20 breast cancer35, non-Hodgkin’s lymphoma,27 and other malignancies have been found to contain malignant cells, methods to remove these undesirable cells have been developed in the hope that such a step could improve patient outcome. Although the contribution of these contaminating cells remains to be definitively determined, recent studies suggest that these cells may promote disease relapse. In one study, bone marrow autograft cells collected from patients with acute myeloid leukemia (AML) undergoing high-dose chemotherapy were marked with the neomycin resistance gene. Of the 12 patients assessed, 4 subsequently relapsed and the neomycin resistance gene was detectable within the leukemic cells in 3 of these patients (neomycin gene presence was indeterminate in the fourth patient).22Studies completed by Sharp et al23demonstrated an increased relapse rate for those patients reinfused with morphologically appearing normal bone marrow but containing clonogenic lymphoma cells after in vitro culture.29Finally, relapse rates for non-Hodgkin’s lymphoma patients were significantly higher for those patients with autografts incompletely purged by the use of anti–B-cell antibodies and complement.24 36 Whereas the results obtained from the latter study could be explained by an inherent property of the malignancy that made tumor purging less effective or simply reflect total body tumor burden, the study of AML patients convincingly demonstrates that malignant cells within the autograft can survive and grow within a patient after reinfusion.
Our study of 131 randomized patients with multiple myeloma demonstrated that the CEPRATE SC System effectively purged the autografts without compromising hematopoietic recovery after myeloablative therapy. For patients randomized to the selected arm, the median number of tumor cells in the autograft was 3.3 logs lower than the median for their counterparts in the unselected arm. In addition, the true efficiency of the purging procedure is underestimated, because the CD34-selected products without detectable tumor cells were assumed to have tumor cell contamination at the lower limit of detection for the assay. In fact, in the majority of patients, the autografts had no detectable tumor cells after CD34 selection (Table 4).
The purging efficiency reported in this study is similar to results reported in the published literature of multiple myeloma patients receiving a CD34-selected autograft using the CEPRATE SC System.15,28,37-40 In the studies that quantified the level of tumor cell depletion, a greater than 2.5 log depletion of tumor cells was commonly observed.15,28,40 Similar studies evaluating the results of tumor cell depletion using the CEPRATE SC System for patients with non-Hodgkin’s lymphoma and breast cancer yielded comparable results.35 41-43
Whereas phase II studies evaluating tumor cell purging methods that treated the autografts with either antibody plus complement or cyclophosphamide derivatives have demonstrated equally efficient purging, these procedures appear to substantially prolong engraftment times. In contrast, CD34 selection of the autograft using the CEPRATE SC System appears safe. The median time to neutrophil engraftment was 12 days for patients in both arms of this study and all patients who received a transplant in the trial achieved an ANC of ≥500 by day 15. These results are consistent with other large studies of autologous PBPC transplantation whereby 97% to 100% of patients achieved neutrophil engraftment after treatment with either unselected PBPCs44-46 or CD34-selected PBPCs.37,47-49Furthermore, the median 12-day engraftment is comparable to previous studies using the CEPRATE SC System.47-50
Median times to platelet engraftment and recovery were slightly prolonged for patients receiving a CD34-selected transplant. Although this 1- to 2-day difference was statistically significant, there was no clinical impact as measured by an increased incidence of bleeding events or RBC transfusions in the patients who received a CD34-selected autograft. Additional analyses determined that two factors influenced the posttransplant platelet engraftment and recovery time: the platelet count at the time of randomization (immediately before leukapheresis) and the infused cell dose of CD34+ cells. Patients receiving a cell dose less than 2 × 106CD34+ cells/kg (n = 11 in each arm) were at increased risk for a delay in time to platelet engraftment and recovery, regardless of treatment arm. However, there was no significant delay in the time to platelet engraftment and recovery for patients in the selected arm who received at least 2 × 106 CD34 + cells/kg (Fig 3). These results suggest that CD34+ cell dose, and not processing with the CEPRATE SC System, was the main factor influencing time to platelet engraftment. Furthermore, these results are consistent with other studies reporting threshold doses of CD34+ cells required for rapid platelet engraftment,44-48 including the phase I/II study in multiple myeloma that established a threshold dose of 2 × 106 CD34+ cells/kg.15We were unable to establish a minimum CD34+ cell dose that affected neutrophil engraftment, because all transplanted patients had neutrophil engraftment by day 15 postinfusion. Furthermore, there was no indication that there were significant decreases in time to neutrophil engraftment in patients receiving CD34+ cell doses greater than 2 × 106/kg.
Recently, there has been a concern regarding a potential detrimental effect of CD34 selection on the recovery of immune function. The clinical manifestation of delayed recovery of immune function is an increase in the incidence of infections. Despite the presumptive median 2.6 log elimination of lymphocytes by the CEPRATE SC System, there were no significant differences in the incidence or type of infections between the patients who received a CD34-selected autograft compared with patients who received an unselected graft. Additional clinical parameters evaluated in the study supported the safety of CD34 selection with the CEPRATE SC System. These results are in contrast to a recently published report using a different CD34 selection method in which patients had delayed engraftment and immune reconstitution.51 Furthermore, patients receiving a CD34-selected graft demonstrated a significantly lower incidence of diastolic hypertension in the 24 hours after transplantation that likely resulted from the reduced cell debris and quantity of DMSO contained within the selected autograft and subsequently reinfused to the patient.
In summary, this study showed that CD34 selection effi-ciently purged contaminating tumor cells from peripheral blood autografts in patients with multiple myeloma without adversely affecting the ability of the hematopoietic stem cells to restore hematopoiesis. In particular, when at least 2 × 106 CD34+ cells/kg were infused, times to neutrophil and platelet engraftment were not significantly affected by the selection procedure. Patients receiving a CD34-selected autograft also had a lower incidence of toxicities associated with the autograft infusion and had no greater incidence of infections or bleeding events when compared with patients receiving an unselected autograft.
Further follow-up of the patients enrolled and transplanted on this trial will be required to determine whether tumor purging by CD34 selection will have a significant effect on progression-free and overall survival for multiple myeloma patients, although this study may not be sufficiently powered to detect a small but still clinically relevant difference. In the absence of larger randomized phase III trials, tumor purging, as a component of a combined treatment strategy, may be a more important issue to be addressed in clinical studies. To that end, the CEPRATE SC System can provide efficient tumor removal without compromising hematopoietic recovery.
ACKNOWLEDGMENT
The authors thank Drs Mike White, Monica Krieger, and Amy Sing for their editorial contributions and Donna Speron for her assistance in the preparation of this manuscript.
Supported in part by a grant from CellPro, Inc (Bothell, WA).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Robert Vescio, MD, University of California, Los Angeles, 111-H West LA VAMC, 11301 Wilshire Blvd, Los Angeles, CA 90073.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal