We have recently described a significant correlation between human immunodeficiency virus-1 (HIV-1) RNA replication and monocyte chemotactic protein-1 (MCP-1) levels in the cerebrospinal fluid (CSF) of individuals with the acquired immunodeficiency syndrome (AIDS) with HIV encephalitis (E). Because local macrophages (microglia) are the cells predominantly infected in the brain, we investigated whether in vitro HIV infection affects MCP-1 production in mononuclear phagocytes (MP). MCP-1 secretion and expression were consinstently upregulated over constitutive levels in human monocyte-derived macrophages (MDM) infected with the M-tropic R5 BaL strain of HIV-1. HIV replication was required for this effect, as demonstrated by the absence of chemokine upregulation after infection in the presence of 3’-azido-3’-deoxythimidine (AZT) or cell-exposure to heat-inactivated (▵°) virus. MCP-1 induction was not restricted to HIV-1 BaL, but was also observed during productive infection of MDM with two primary isolates differing for entry coreceptor usage and of U937 cells with the X4 HIV-1 MN strain. Based on the observation that exogenous HIV-1 Tat induced MCP-1 expression in astrocytes, we also investigated its role in MDM and U937 cells. Exogenous Tat induced MCP-1 production from MDM in a concentration-dependent manner, however, it was not effective on uninfected U937 cells or on the chronically infected U937-derived cell line U1. Transfection of Tat-expressing plasmids moderately activated HIV expression in U1 cells, but failed to induce MCP-1 expression in this cell line or in uninfected U937 cells. HIV replication-dependent expression of MCP-1 in MP may be of particular relevance for the pathogenesis of HIV infection in nonlymphoid organs such as the brain.
IN ADDITION TO CD4+ T lymphocytes, mononuclear phagocytes (MP) have been earlier identified as targets for human immunodeficiency virus (HIV) infection both in vitro1,2 and in vivo.3,4 Because of the limited HIV-induced cytopathic effects and their ability to accumulate high levels of HIV particles in intracellular compartments,2,4-6HIV-infected macrophages may serve as “Trojan horses” and disseminate the virus in different organs, including the lungs, skin, lymph nodes, and the brain (reviewed in Poli and Fauci7). Of note, brain macrophages (microglia) are the main cells sustaining HIV replication in this organ and represent the major cellular determinant of HIV encephalitis (HIV-E), a pathological condition often associated with dementia and other opportunistic infections.3,4,8 Secretory products, including cytokines, released by HIV-infected macrophages have been implicated in the neuronal damage observed in acquired immunodeficiency syndrome (AIDS) patients with dementia,9,10 although the mechanisms underlying monocyte recruitment to the brain are poorly understood. In this regard, elevated levels of transforming growth factor-β (TGF-β), a cytokine with a potent chemotactic activity for monocytes,11 have been described in brain tissue of AIDS patients.12 In vitro, HIV infection upregulated production of TGF-β in monocyte-derived macrophages (MDM).12Recently, in vitro HIV infection of MDM has been linked to increased secretion of monocyte inflammatory protein-1α (MIP-1α) and MIP-1β.13,14 Of note is the fact that MIP-1α mRNA was detected in brain tissue of patients with HIV-E.13 These findings suggest a potentially important contribution of these CC chemokines in the recruitment of monocytes to the brain occurring in some HIV-infected individuals.
Monocyte chemotactic protein-1 (MCP-1), a CC chemokine produced by MP, is a potent activator of monocyte function.15,16 MCP-1 selectively binds to CCR2, a chemokine receptor shared with MCP-2, -3, and -4,15,16 although binding to CCR10 has also been recently reported.17 MCP-1 has a crucial role in monocyte recruitment in vivo in several organs and tissues, including the brain, an observation supported by studies in transgenic mice overexpressing MCP-118-20 and in CCR2-deficient mice.21-23 In addition, the role of MCP-1 in monocyte infiltration in several inflammatory diseases has been described.15 16
Regarding HIV infection, unlike MIP-1α, MIP-1β and regulated upon activation normal T-cell expressed and secreted (RANTES), MCP-1 has not been previously associated with inhibition of HIV infection,24 although CCR2 may act as coreceptor for some HIV strains.25 However, inhibition of virus replication by MCP-1 has been recently reported,26 and we have observed modulation of virus replication by MCP-1 in ex vivo cultures established from infected individuals.27 Of interest, a selective accumulation of MCP-1, but not of other chemokines, in the cerebrospinal fluid (CSF) of AIDS patients with cytomegalovirus encephalitis (CMV-E)28 and, more recently, also in patients with HIV-E29 and AIDS-associated dementia30 has been described, suggesting an important role of this chemokine in the pathogenesis of these HIV-associated diseases of the central nervous system (CNS). In one of these studies,30 exogenous addition of Tat induced MCP-1 expression in cultures of human fetal brain astrocytes. Here we have investigated whether HIV replication or cell stimulation by exogenously added or endogenously expressed Tat could affect MCP-1 production in primary human MDM and in the U937 promonocytic cell line.
MATERIALS AND METHODS
MDM.
Monocytes were isolated from buffy coats of healthy HIV seronegative blood donors by Ficoll-Hypaque and Percoll (Pharmacia, Uppsala, Sweden) gradients, as described.31 Purity was ≥ 90%, as determined by flow cytometry analysis for CD14 expression, using mouse antihuman CD14 monoclonal antibody (MoAb) (IgG2a; Caltag Labs, Burlingame, CA) followed by fluorescein isothiocyanate (FITC)-conjugated goat antimouse MoAb (Jackson Immunoresearch Labs, Inc, West Grove, PA). Monocytes were seeded in 48-well plates (Falcon, Becton-Dickinson Labware, Lincoln Park, NJ) at 0.2 × 106 cells/mL in Dulbecco’s modified Eagle’s medium (DMEM) (BioWhittaker, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS) (Hyclone Europe, Oud-Beijerland, The Netherlands) and 5% pooled HS. All media and sera were monitored for low content of endotoxin by the lymulus amoebocyte lysate assay (BioWhittaker).
HIV infection of MDM.
Five to 7-day old MDM were infected with the M-tropic R5 BaL strain of HIV-132 at the multiplicity of infection (MOI) of 0.1 or with primary HIV isolates (4 × 103 cpm of reverse transcriptase (RT) activity per 0.2 × 106 cells). Fifty percent of culture media were replaced with fresh media twice a week. Aliquots of culture supernatants were harvested at the indicated time points and stored at −80°C. Primary HIV isolates were established by cocultivation of HIV-infected patients’ peripheral blood mononuclear cells (PBMC) with phytohemagglutinin (PHA-P, 5 μg/mL, Sigma Chemical Corp, St Louis, MO) activated PBMC from uninfected individuals. The fusogenic ability of the viruses was tested on the standard human T-lymphotropic virus-1 (HTLV-1)–transformed MT-2 cell line, as previously described.33 Chemokine coreceptor usage was tested on U87 astrocytic cells stably transfected with human CD4 alone or together with one of the following human chemokine receptors: CXCR4, CCR2B, CCR3, or CCR5, as reported.34
HIV infection of the promonocytic U937 cell line.
Promonocytic U937 cells35 were resuspended in RPMI 1640 (BioWhittaker), supplemented with 10% FCS (Hyclone Europe), at the concentration of 1 × 106 cells/mL and infected with the X4 MN strain of HIV-1 (4 × 104 cpm RT activity per 1 × 106 cells). After 1 hour of incubation, the excess of free virus was removed by dilution with RPMI 1640 and centrifugation; the cells were then seeded in duplicate cultures in 48-well plastic plates (Falcon, Becton Dickinson Labware) at the concentration of 2 × 105 cells/mL/well. The cultures were fed every 4 to 5 days with fresh medium. Aliquots of culture supernatants were harvested and stored at −80°C.
U1 cell line.
HIV-RT assay.
HIV replication, monitored as Mg++-dependent RT activity released into supernatants, was measured as previously described.37
Cytokine assays.
A homemade enzyme-linked immunosorbent assay (ELISA)28 29was used to measure MCP-1 levels in culture supernatants, whereas a commercially available ELISA kit for tumor necrosis factor (TNF)-α was purchased from R&D Systems (Minneapolis, MN). Comparable concentrations of MCP-1 were measured in parallel tests conducted with this homemade and a commercially available ELISA kit (R&D Systems).
RT-polymerase chain reaction (PCR) detection of MCP-1 mRNA.
Total cellular RNA was isolated from MDM by RNAzol B (Duotech S.r.l., Milan, Italy) at different times after the beginning of the cultures in the presence or absence of HIV. RNA (0.5 μg) was reverse transcribed, and aliquots corresponding to 1/40 of the cDNA obtained were amplified in a 50-μL volume of the reaction mixture in the presence of 1.25 U AmpliTaq Gold DNA polymerase (Perkin-Elmer Corp, Norwalk, CT) and 1 μL antisense primer end-labeled with γ-32P–adenosine triphosphate (Amersham, Arlington Heights, IL), corresponding to approximately 200,000 to 600,000 cpm. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified as internal control. The sequences of the primers used and amplification conditions were previously reported.38 To test for the linearity of amplification, twofold serial dilutions (seven dilutions starting from 1:20) of a standard cDNA obtained by retrotranscribing 1 μg of total RNA extracted from PBMC isolated from a healthy donor and stimulated for 6 hours with PHA39 were amplified in each PCR assay. The PCR products were analyzed by electrophoresis in 5% polyacrylamide gels and visualized by autoradiography.
Experiments with HIV-1 Tat.
Lipopolysaccharide (LPS)-free (by the lymulus amoebocyte lysate assay) full-length (1-86) synthetic Tat was purchased from Tecnogen (Piana di Monte Verna, CS, Italy) and added at different concentrations to both MDM and U937 or U1 cells, as further specified. Plasmids expressing either Tat or Y26ATat, in which a tyrosine residue at position 26 was replaced by alanine causing the loss of HIV-1 long terminal repeats (LTR) transactivating capacity40 (kind gift of Ben Berkhout, University of Amsterdam, Amsterdam, The Netherlands), were transiently transfected into uninfected U937 cells or in chronically infected U1 cells by diethyl aminoethyl (DEAE) dextran.40
RESULTS
HIV infection superinduces MCP-1 secretion in human MDM.
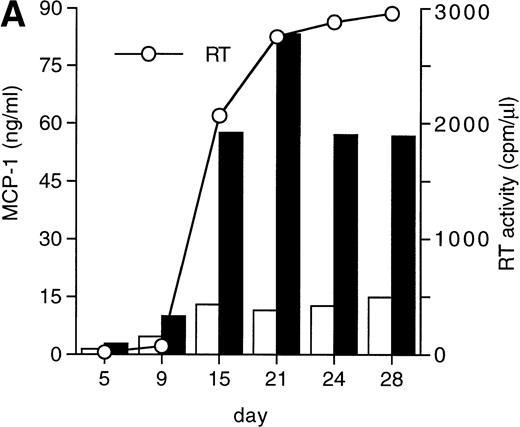
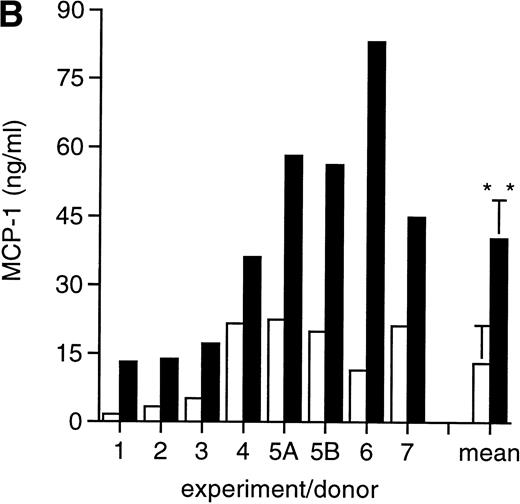
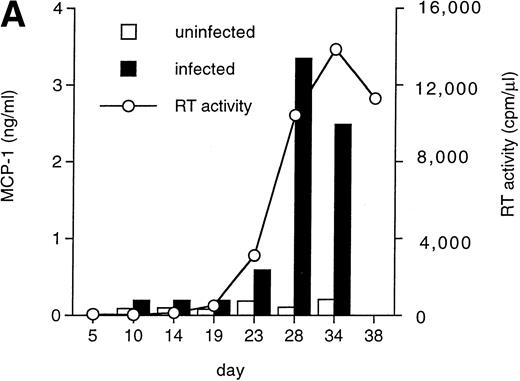
MCP-1 was constitutively secreted by uninfected MDM, as previously reported.41 However, a clear-cut upregulation of chemokine secretion was consistently observed in HIV-infected MDM (Fig 1A), ranging from twofold to fivefold increase over constitutive levels in seven individual experiments performed with cells obtained from eight independent donors (Fig 1B). Of interest, the peak of MCP-1 secretion paralleled the peak of virus replication. TNF-α, previously shown not to be induced by HIV replication in MDM,13 42 was measured as a control, and it was undetectable in our HIV-infected cultures (data not shown).
HIV infection of MDM superinduces MCP-1 secretion. Human MDM were infected with HIV-1 Bal (MOI: 0.1). Culture supernatants were tested for MCP-1 and RT activity contents. (A) One experiment representative of seven independently performed is shown. (B) MCP-1 levels measured at the peak of RT activity in seven independent experiments with cells obtained from eight donors; the mean ± standard deviation (SD) is shown. ** P < .01 versus uninfected by paired Student’s t test. (□) Uninfected; (▪) infected.
HIV infection of MDM superinduces MCP-1 secretion. Human MDM were infected with HIV-1 Bal (MOI: 0.1). Culture supernatants were tested for MCP-1 and RT activity contents. (A) One experiment representative of seven independently performed is shown. (B) MCP-1 levels measured at the peak of RT activity in seven independent experiments with cells obtained from eight donors; the mean ± standard deviation (SD) is shown. ** P < .01 versus uninfected by paired Student’s t test. (□) Uninfected; (▪) infected.
HIV replication is required for MCP-1 induction in MDM.
Binding of HIV to CD4 has been reported to induce cytokine expression in MDM.43,44 In other studies, viral replication was required for cytokine induction.12-14 To rule out the possibility that exposure to the virus could account for HIV-induced MCP-1 secretion in our infected cultures, MDM were infected with HIV-1 BaL at the MOI of 0.1 in the presence or absence of 10 μmol/L 3’-azido-3’-deoxythymidine (AZT). In addition, MDM were exposed to virus inactivated by heating at 56°C for 1 hour (Δ°-HIV). Both AZT treatment and heat inactivation abolished virus replication and diminished MCP-1 secretion to the levels of uninfected MDM (Table 1). These findings support the hypothesis that MCP-1 superinduction is dependent on virus replication.
HIV Replication Is Required for MCP-1 Induction in MDM
| . | MCP-1 (ng/mL) . | |||
|---|---|---|---|---|
| Donor A . | Donor B . | Donor C . | Mean ± SD . | |
| Uninfected | 21.5 | 22.4 | 18.1 | 20.7 ± 1.8 |
| HIV | 36.0 (1290) | 58.2 (441) | 56.3 (2403) | 50.2 ± 10.0 |
| HIV + AZT | 15.4 (18) | 40.3 (29) | 17.6 (17) | 24.4 ± 11.3 |
| Δ°-HIV | 13.5 (61) | 20.2 (12) | 21.8 (19) | 18.5 ± 3.6 |
| . | MCP-1 (ng/mL) . | |||
|---|---|---|---|---|
| Donor A . | Donor B . | Donor C . | Mean ± SD . | |
| Uninfected | 21.5 | 22.4 | 18.1 | 20.7 ± 1.8 |
| HIV | 36.0 (1290) | 58.2 (441) | 56.3 (2403) | 50.2 ± 10.0 |
| HIV + AZT | 15.4 (18) | 40.3 (29) | 17.6 (17) | 24.4 ± 11.3 |
| Δ°-HIV | 13.5 (61) | 20.2 (12) | 21.8 (19) | 18.5 ± 3.6 |
Human MDM from three healthy donors were infected with HIV-1 BaL (MOI: 0.1) in the presence or absence of AZT (10 μmol/L), or they were exposed to heat-inactivated (Δ°)-HIV-1 BaL. MCP-1 was measured in the supernatants at peak RT activity (cpm/μL), which occurred at day 17 for donor A, and at day 20 for donors B and C, respectively.
HIV infection induces MCP-1 mRNA expression in human MDM.
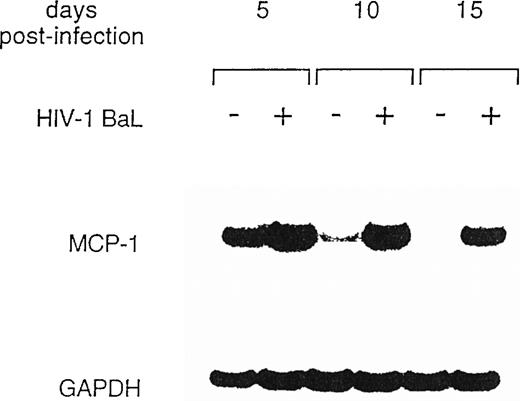
To further explore MCP-1 regulation by HIV, we analyzed MCP-1 expression in MDM at different days postinfection and in parallel uninfected cultures by RT-PCR. In preliminary experiments, we observed that MCP-1 mRNA was undetectable in freshly isolated monocytes, but was induced at high levels after 20 hours of culture (data not shown), as previously reported.41 High levels of MCP-1 expression were found at the time of infection, after 5 to 7 days of culture (data not shown), which remained elevated until days 10 to 12, before declining (Fig 2). Higher levels of MCP-1 mRNA were consinstently observed in HIV-infected MDM. Noteworthy, MCP-1 mRNA was no longer detectable in uninfected MDM 15 days after infection, but it was still clearly expressed in the parallel-infected cultures (Fig 2).
Expression of MCP-1 mRNA in MDM after HIV infection. MDM were infected with HIV-1 BaL (MOI: 0.1). Total RNA was extracted and MCP-1 expression was measured by RT-PCR at the indicated days postinfection. On normalization against GAPDH and given the arbitrary value of 1 to the MCP-1 mRNA of uninfected cultures after densitometric scanning (Molecular Dynamics, Sunnyvale, CA), RNA induction levels in HIV-infected MDM were 1.4 at day 5 and 12.4 at day 10, respectively. The results are representative of three independent experiments.
Expression of MCP-1 mRNA in MDM after HIV infection. MDM were infected with HIV-1 BaL (MOI: 0.1). Total RNA was extracted and MCP-1 expression was measured by RT-PCR at the indicated days postinfection. On normalization against GAPDH and given the arbitrary value of 1 to the MCP-1 mRNA of uninfected cultures after densitometric scanning (Molecular Dynamics, Sunnyvale, CA), RNA induction levels in HIV-infected MDM were 1.4 at day 5 and 12.4 at day 10, respectively. The results are representative of three independent experiments.
MCP-1 secretion is upregulated in MDM after infection with two primary isolates differing in coreceptor usage.
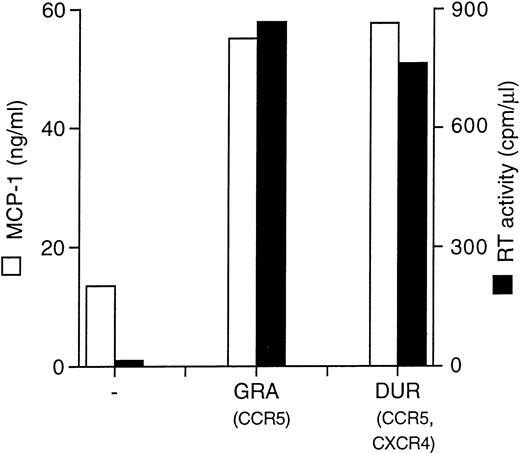
Two different primary HIV isolates (GRA and DUR, R5 monotropic and X4/R5 dualtropic, consistent with their nonsyncytium inducing, NSI, and SI phenotypes in MT-2 cells, respectively), were normalized for RT activity counts (4 × 103 cpm/well/2 × 105 cells) and tested for MCP-1 induction in MDM. Both viruses gave rise to a productive infection in MDM and superinduced MCP-1 secretion (Fig 3).
Primary HIV isolates (GRA and DUR) superinduce MCP-1 secretion in MDM. MCP-1 levels present in uninfected and infected cultures at the peak of RT activity (day 28) are shown.
Primary HIV isolates (GRA and DUR) superinduce MCP-1 secretion in MDM. MCP-1 levels present in uninfected and infected cultures at the peak of RT activity (day 28) are shown.
HIV infection of U937 cells induces MCP-1 production.
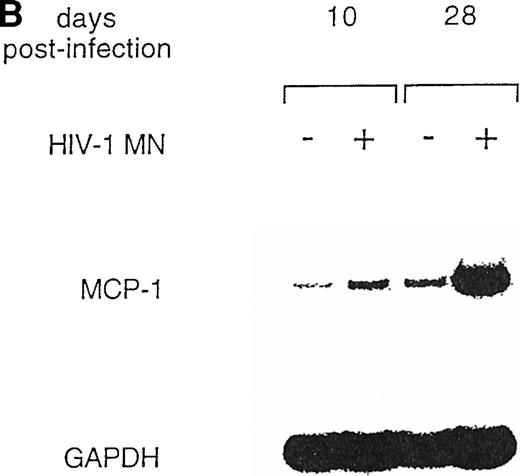
U937 is a well-known promonocytic cell line35 that can be productively infected with X4 strains of HIV-1,36,45characterized by low constitutive secretion of MCP-1 inducible by several agents, including phorbol esters, interleukin-6 (IL-6), interferon-γ (IFN-γ), and TNF-α.38 Therefore, we tested whether HIV infection could modulate MCP-1 expression in this cell line. MCP-1 was detected at very low levels in the supernatants of uninfected cells, whereas HIV-1 MN-infected cells showed increased MCP-1 secretion (Fig 4A). As observed with MDM, the peak of MCP-1 production coincided with or slightly preceded that of viral replication. In addition, the upregulation of MCP-1 secretion in HIV-infected cells was correlated to increased levels of mRNA expression, as detected by RT-PCR (Fig 4B).
HIV-1 MN infection of U937 promonocytic cells upregulates MCP-1. (A) MCP-1 protein levels and RT activity were measured at the indicated days postinfection. (B) MCP-1 expression measured by RT-PCR in uninfected and HIV-infected U937 cell cultures from which chemokine secretion was determined (A). RNA induction levels, calculated as described in the legend to Fig 2, were 1.5 at day 10 and 4.7 at day 28 postinfection, respectively.
HIV-1 MN infection of U937 promonocytic cells upregulates MCP-1. (A) MCP-1 protein levels and RT activity were measured at the indicated days postinfection. (B) MCP-1 expression measured by RT-PCR in uninfected and HIV-infected U937 cell cultures from which chemokine secretion was determined (A). RNA induction levels, calculated as described in the legend to Fig 2, were 1.5 at day 10 and 4.7 at day 28 postinfection, respectively.
Thus, upregulation of MCP-1 expression appears to be a general property of replication-competent HIV, regardless of its ability to use different chemokine receptors as ports of entry and/or its T-lymphocytic versus macrophage in vitro cell tropisms.
Exogenous Tat stimulates MCP-1 secretion from MDM, but not from U937 or U1 cell lines.
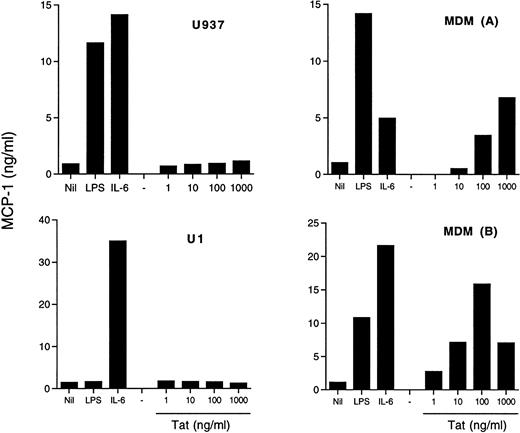
In addition to its transactivating capacity consequent to binding to the TAR RNA sequence of HIV,46,47 Tat has been shown to modulate several cell functions, including cytokine production.48,49 In particular, addition of exogenous Tat to cultures of fetal astrocytes has resulted in the induction of MCP-1 expression.30 We, therefore, investigated whether a similar mechanism was operational in our model systems. Of concern is the possibility that LPS contamination may influence the results of these experiments. For this reason, we tested our Tat preparation for the presence of LPS contamination by the lymulus amoebocyte lysate assay, and it was found to be negative. Both MDM from different donors and two U937 cell lines, one sensitive (U937) and the other one (U1) known to be insensitive50 51 to LPS stimulation, were incubated with Tat at concentrations between 1 ng/mL and 1 μg/mL, and MCP-1 was measured in the supernatants. Both cell lines secreted substantial amounts of MCP-1 under stimulation by IL-6 or, in the case of U937 cells, by LPS, but they were totally unresponsive to exogenous Tat (Fig 5). In contrast, MDM were induced to secrete MCP-1 by Tat stimulation in a concentration-dependent manner (Fig 5).
Effect of exogenous Tat on MCP-1 secretion in U937 and U1 cell lines and in MDM. Both U937 and U1 cells (4 × 105cells/mL) and 5-day old MDM of two donors were incubated with LPS (10 ng/mL), IL-6 (10 ng/mL), or Tat at the indicated concentrations. MCP-1 was measured in U937 and U1 culture supernatants after 48 hours and in MDM culture supernatants after 72 hours of incubation.
Effect of exogenous Tat on MCP-1 secretion in U937 and U1 cell lines and in MDM. Both U937 and U1 cells (4 × 105cells/mL) and 5-day old MDM of two donors were incubated with LPS (10 ng/mL), IL-6 (10 ng/mL), or Tat at the indicated concentrations. MCP-1 was measured in U937 and U1 culture supernatants after 48 hours and in MDM culture supernatants after 72 hours of incubation.
The potential role of endogenously expressed Tat was investigated by transient transfection experiments in the U937/U1 cell systems. A plasmid containing a functional mutation in tat (Y26ATat), known to abolish its HIV transactivating activity,40 was used as a control. Of note, endogenous Tat produced by U1 cells has been characterized as nonfunctional.52 Transfection of Tat-expressing plasmids, but not of control plasmids, resulted in a modest, but clearly detectable, induction of HIV expression from U1 cells 96 hours after transfection (data not shown), consistent with previous reports.47,48,52 53 In contrast, no evidence of MCP-1 secretion was obtained either from U1 or uninfected U937 cells by Tat transfection (data not shown).
Thus, Tat expression or secretion appears to be dispensable for HIV-mediated induction of MCP-1 in monocytic cells.
DISCUSSION
In the present study, we have observed a superinduction of MCP-1 expression and secretion in MDM by productive HIV-1 infection. HIV-induced MCP-1 was not restricted to HIV-1 BaL, but was observed both in MDM infected with two primary isolates differing for entry coreceptor usage, and in U937 cells infected by the X4 laboratory adapted strain MN. Tat induced MCP-1 secretion in MDM, but not in the U937 cell line and in chronically infected U937-derived U1 cells in which activation of HIV expression by Tat was demonstrated.
The superimposable kinetics of MCP-1 secretion and virus replication in HIV-infected MP together with the absence of MCP-1 superinduction after infection in the presence of AZT or with Δ°-HIV strongly indicate a close linkage between HIV replication and MCP-1 induction. In this regard, transcription of the human MCP-1 gene requires synergy between two NF-kB/Rel dimers and Sp1, which is essential for the basal transcription of this gene.54 A similar synergy between NF-kB/Rel and Sp1 is crucial for HIV-1 gene expression.55Of interest, several proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 have been reported to upregulate both HIV expression (reviewed in Vicenzi et al56) and MCP-1 production.31,38,57 Recently, the HIV-1–encoded Tat gene product has been shown to upregulate MCP-1 expression in astrocytes.30 In consideration of the fact that Tat can functionally interact with both NF-kB and Sp-1 cellular transcription factors,58,59 this observation on the one hand supports the hypothesis that MCP-1 and HIV expression could be transcriptionally coregulated. On the other hand, our results indicate that Tat, either endogenously expressed after transfection or exogenously added, is not sufficient to induce MCP-1 secretion, at least in U937 cells, although it stimulated production of this chemokine in MDM. The reasons for this functional dichotomy are unclear, but they may be related to the different stages of differentiation of these monocytic cells. In this regard, Tat was shown to induce migration of monocytes by interaction with the vascular endothelial growth factor receptor (VEGFR)-1/Flt-1 receptor.60 Of note, in similar experiments, Tat failed to induce MCP-1 in moncytes (S.S. and S.M., unpublished). In addition, VEGFR-1/Flt-1–dependent effects of Tat were shown to peak sharply at 10 ng/mL and rapidly declining at higher or lower concentrations,59 whereas Tat-dependent induction of MCP-1 peaked at 100 ng/mL in MDM cultures established from one donor and did not achieve plateau levels in those of a second individual (Fig 5). The effects described here with exogenous Tat were independent from detectable levels of LPS, as demonstrated by the fact that this viral protein failed to induce MCP-1 production from LPS-responsive U937 cells. Similar results were obtained when U937 and U1 cells were transfected by a Tat-expressing plasmid. Although activation of HIV expression was observed in U1 cells (but not in control cultures transfected with a mutagenized Tat-expressing vector40), this did not lead to MCP-1 expression. Similar negative results were observed after parallel transfections of U937 cells (data not shown). The definition of the pathway involved in Tat-mediated activation of MCP-1 in MDM, involving the dissection of which domains of the viral protein are involved,61 deserves further investigation.
At the clinical level, a correlation between plasma levels of MCP-1 and HIV viremia has been recently reported in advanced AIDS patients.62 A coregulation of MCP-1 expression and HIV replication would account for the significant and selective correlation existing between the elevated levels of HIV RNA and those of MCP-1 in the CSF of AIDS patients with HIV-E, but not with other opportunistic diseases (with the exception of CMV-E) or without infections of the CNS.28-30 Of note is the fact that HIV-E is a pathological condition mainly sustained by virus replication in brain macrophages (microglia) and astrocytes.3,4 8 Increased MCP-1 production by HIV replicating microglial cells may play an important role in the recruitment of circulating monocytes to the brain, therefore feeding the pathological inflammatory process sustaining HIV-E.
ACKNOWLEDGMENT
The authors thank Dr Ben Berkhout of the Department of Human Retrovirology of the University of Amsterdam for provision of the Tat expressing plasmids and Dr Chiara Bovolenta for helpful discussions.
Supported by grants from the National Project for Research Against AIDS of the Istituto Superiore di Sanità (ISS), Rome, Italy. M.M. is the recipient of a fellowship from ANLAIDS, Rome, Italy. S.G. is a fellow of ISS.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Manuela Mengozzi, PhD, P2/P3 Laboratories, DIBIT, Via Olgettina n. 58, 20132 Milano, Italy; e-mail: poli.guido@hsr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal