Nitric oxide (NO) plays an important role in normal neural cell function. Dysregulated or overexpression of NO contributes to neurologic damage associated with various pathologies, including human immunodeficiency virus (HIV)-associated neurological disease. Previous studies suggest that HIV-infected monocyte-derived macrophages (MDM) produce low levels of NO in vitro and that inducible nitric oxide synthase (iNOS) is expressed in the brain of patients with neurologic disease. However, the levels of NO could not account for the degree of neural toxicity observed. In this study, we found that induction of iNOS with concomitant production of NO occurred in primary human astrocytes, but not in MDM, when astrocytes were cocultured with HIV-1–infected MDM. This coincided with decreased HIV replication in infected MDM. Supernatants from cocultures of infected MDM and astrocytes also stimulated iNOS/NO expression in astrocytes, but cytokines known to induce iNOS expression (interferon-γ, interleukin-1β, and tumor necrosis factor-) were not detected. In addition, the recombinant HIV-1 envelope protein gp41, but not rgp120, induced iNOS in cocultures of uninfected MDM and astrocytes. This suggests that astrocytes may be an important source of NO production due to dysregulated iNOS expression and may constitute one arm of the host response resulting in suppression of HIV-1 replication in the brain. It also leads us to speculate that neurologic damage observed in HIV disease may ensue from prolonged, high level production of NO.
HUMAN IMMUNODEFICIENCY virus-1 (HIV-1) infection of the brain can cause neurologic deficits in the absence of opportunistic infections or associated malignancies. HIV-1–associated cognitive/motor complex is a severe form of these neurologic impairments, which is observed in 20% to 30% of patients with acquired immunodeficiency syndrome (AIDS).1-3 The characteristic neuropathology of HIV-1–associated cognitive/motor complex consists of infiltrating macrophages, multinucleated giant cells, astrogliosis, myelin pallor, and neuronal loss.4Productive HIV-1 infection in the brain occurs predominantly in macrophages, microglia, and multinucleated giant cells.5,6More recent observations using highly sensitive methods suggest that infection of astrocytes may also occur with restricted virus replication, affirming that the effects of HIV on astrocytes may be indirect.7-11
The pathogenesis of HIV-1–associated cognitive/motor complex is not well understood. In the majority of patients with HIV disease, virus enters the brain early in the course of systemic infection, but not all patients develop neurologic disease.12,13 The correlation between the disease severity and viral load is unconvincing,3,14-17 and the neurotoxicity of the virus itself is controversial.18,19 In AIDS-related vacuolar myelopathy and sensory neuropathy, little or no virus is found.20 21 These findings suggest that indirect mechanisms are most likely responsible for the development of HIV-related neuronal disease.
One means by which indirect effects may be exerted upon neural cells is via nitric oxide (NO) production. NO is a highly reactive gaseous molecule produced during the conversion of L-arginine to L-citrulline, catalyzed by a family of NO synthases (NOS),22 which exerts diverse effects.23 It is known that small amounts of NO produced by neuronal NO synthase (nNOS) act as a neurotransmitter, and excess NO is directly or indirectly neurotoxic via stimulation ofN-methyl-D-aspartate (NMDA) receptors, leading to necrosis and apoptosis.24-28 Inducible NOS (iNOS) is an attractive candidate for mediating NO-associated neurotoxicities, because it functions independently of signals that modulate other forms of the enzymes (eNOS and nNOS). After induction of iNOS protein, NO production continues until cellular processes remove the protein or the enzymatic substrates are depleted. Thus, a large amount of NO may be produced by iNOS, as is observed when rodent macrophages are stimulated during inflammatory and immune responses. Furthermore, NO has been shown to exhibit antiviral activity against a wide range of viruses in rodents (eg, herpes simplex, vaccinia, ectromelia, vesicular stomatitis, Friend leukemia, Coxsackie, Japanese encephalitis viruses, and Coronavirus).29-36 The existence of NO-mediated antiviral activity against HIV-1 could account for the observed low levels of virus detectable in vivo in the presence of significant neurologic damage.
When modeling possible mechanisms of HIV pathogenesis, it is apparent that activation of the iNOS pathway in humans differs from that in rodents. In fact, the effect of NO in the human host defense has been difficult to study because much lower levels of NO are present in human macrophages than in rodents.37,38 However, antiviral activity of NO against Epstein-Barr and hepatitis C viruses in the human system has recently been reported.39,40 It has also been shown that HIV-1 infection41 and HIV envelope proteins induce iNOS expression42-45 and that NO is involved in IgE-induced HIV-1 expression in chronically infected monocytic cells,46 but data show that human macrophages may not be a potent source of iNOS/NO. Studies have demonstrated in vivo iNOS expression in the brains of patients with HIV-1–associated cognitive/motor complex,41,45,47 although it is unclear which cell types are responsible for iNOS/NO production. Human astrocytes, as well as human macrophages, are documented to produce iNOS under certain conditions.48-52 Thus, it is conceivable that either cell type could indirectly contribute to the pathogenesis of HIV-1–associated cognitive/motor complex via induction of iNOS and subsequent production of NO in response to the virus. This prompted us to examine which cells produce iNOS during HIV-1 infection and to determine whether NO has anti–HIV-1 activity.
In this study, we show that HIV-1–infected monocyte-derived macrophages (MDM) induce iNOS production in primary human astrocytes and that astrocyte-derived NO inhibits HIV-1 replication in MDM. These results suggest that astrocyte-derived NO may contribute to HIV-1 replication as well as brain cell injury in AIDS patients and may provide new insight into the pathogenesis of HIV-1–associated cognitive/motor complex.
MATERIALS AND METHODS
Reagents.
Recombinant HIV-1IIIB gp41 (amino acids 1 through 241; rgp41) was purchased from Intracel Corp (Cambridge, MA). Recombinant full-length HIV-1IIIB gp120 (rgp120) was purchased from Celltech, Inc (Slough, Berks, UK) and was provided to us by the Developmental Therapeutics Branch, Division of AIDS, NIAID, NIH (Bethesda, MD). HIV-1ADA and neutralizing anti-gp41 antibody (Ab) were supplied by the NIH AIDS Research and Reference Reagent Program (Rockville, MD). Virus stock was expanded in human macrophages; culture supernatants were then harvested, virus was pelleted by ultracentrifugation, and the virions were resuspended in fresh medium at a 1,000× concentration (Advanced Biotechnologies, Columbia, MD). NG-monomethylL-arginine (L-NMMA) andS-nitroso-N-acethylpenicillamine (SNAP) were purchased from Calbiochem-Novabiochem Co (La Jolla, CA) and Sigma Chemical Co (St Louis, MO), respectively.
Preparation and HIV-1 infection of primary cells.
Human fetal astrocytes were kindly provided by Drs Eugene Major and Katherine Conant (NINDS, NIH) and grown in minimal essential medium (MEM; Life Technologies, Inc, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, and gentamicin (5 μg/mL). Human adult astrocytes were kindly provided by Dr Kathryn Carbone and Steven Rubin (CBER, FDA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) supplemented with 10% FCS, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 50 U/mL penicillin/streptomycin. The purity of the astrocyte cultures was greater than 95% by immunohistochemical staining for glial fibrillary acidic protein (GFAP).
Monocytes were isolated and infected with HIV-1ADA as described previously.53 Briefly, monocytes were isolated from peripheral blood mononuclear cells (PBMC) of HIV-seronegative donors by countercurrent centrifugal elutriation using a Beckman system (Beckman Instruments, Fullerton, CA). These cells were greater than 99% viable and greater than 95% pure as determined by Giemsa stain of representative cytocentrifuge preparations. To generate MDM, elutriated monocytes were incubated in DMEM supplemented with 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 50 U/mL penicillin/streptomycin, and 10% pooled human serum (DMEM medium) for 5 to 8 days before infection.
MDM were then exposed to HIV-1ADA (1 × 106 cpm RT activity/1 × 106 MDM) for 4 hours, washed, and then cultured in fresh DMEM medium. In coculture experiments, infected MDM were harvested by cell scraping at day 1 postinfection and then replated on astrocytes. At 3-day intervals, 80% of the total volume of medium was replaced. Aliquots of harvested culture supernatant were stored at −70°C until use.
Reverse transcriptase (RT) assay.
The RT assay was performed as described previously.54Briefly, harvested culture supernatants were diluted with an equal volume of Tris buffer (pH 7.8)/0.05% Triton X-100, and then duplicate diluted samples were incubated with a solution containing poly (rA) (Pharmacia LKB, Piscataway, NJ), oligo (dT) (Pharmacia LKB), MgCl2, and 3H-labeled dTTP (NEN, Boston, MA) for 2 hours at 37°C. Finally, 10% trichloracetic acid (TCA) was added to each sample, and samples were transferred to glass-fiber filters (Wallac, Turku, Finland) and counted on a beta scintillation counter (Pharmacia LKB).
RNA isolation and RT-polymerase chain reaction (PCR).
Total cellular RNA was isolated with Ultraspec RNA (Biotecx, Houston, TX) according to the manufacturer’s instructions. Levels of iNOS mRNA were determined using a semiquantitative RT-PCR, as described previously.55 After reverse transcription of 4 μg of each RNA sample using random primers, each sample was subjected to PCR using primers for β-actin mRNA. On the basis of the amount of β-actin PCR product, aliquots of reverse transcriptase product representing equivalent amounts of β-actin cDNA were amplified using primers for iNOS and again for β-actin. PCR was performed in a reaction mixture (50 μL) containing cDNA, 200 mmol/L each dNTP, 1 μCi α-[32P]dCTP (3,000 Ci/mmol; NEN), 1 μmol/L each primer, 5% dimethyl sulfoxide (DMSO; Sigma Chemical Co), and 2.5 U AmpliTaq DNA polymerase (Perkin Elmer Cetus, Norwalk, CT) in reaction buffer supplied by the manufacturer. Primers were designed to amplify a 459-bp product of human iNOS and a 577-bp product of human β-actin. The sense strand primer for iNOS was TGTGCCACCTCCAGTCCAGTGACA and the antisense strand primer was GCTCATCTCCCGTCAGTTGGTAGG; the β-actin sense strand primer was ATCTGGCACCACACCTTCTACA and GTTTCGTGGATGCCACAGGACT was the antisense strand primer. Amplifications were performed in a thermocycler (GeneAmp PCR System 9600; Perkin Elmer Cetus). After an initial denaturation step at 94°C for 3 minutes, PCR proceeded with 25 or 35 amplification cycles (94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds), followed by an extension step at 72°C for 5 minutes. Aliquots of each amplification were analyzed by electrophoresis on 7% acrylamide (Long Ranger; AT Biochem, Malvern, PA) Tris-borate EDTA gels, followed by autoradiography and quantitation by Phosphorimager (Molecular Dynamics, Sunnyvale, CA). DNA sizes were determined using mobility standards derived by T4 DNA polymerase end-labeling of Gel Marker DNA (Research Genetics, Huntsville, AL). The cytokine-stimulated A172 cell line (American Type Culture Collection, Rockville, MD) was used as a positive control (PC) for human iNOS.50
Analysis of NO production.
NO production was assessed via measurement of nitrite in culture supernatants by the Griess reaction.56 Fifty microliters of culture supernatant was mixed with 100 μL of Griess reagent (Sigma Chemical Co) containing 1% sulfanilamide and 0.1% D-naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid. After 10 minutes, samples were read at 570 nm on an enzyme-linked immunosorbent assay (ELISA) reader (ICN Biomedicals Inc, Costa Mesa, CA). The sample concentration was determined by comparison against a standard curve using sodium nitrite (Sigma Chemical Co).
Flow cytometric analysis.
For two-color staining of cell surface antigen and intracellular antigen, a suspension of 1 × 106 cells was incubated for 30 minutes at 4°C with a 1:5 dilution of phycoerythrin (PE)-labeled anti-CD14 Ab (Becton Dickinson, San Jose, CA) or PE-labeled mouse IgG and then washed two times with phosphate-buffered saline (PBS). After fixation and permeabilization of cells using Cytofix/Cytoperm (Pharmingen, San Diego, CA) for 20 minutes at 4°C, cells were blocked with Perm/Wash solution (Pharmingen) containing normal mouse serum, normal rabbit serum, and AlbuMax I (Life Technologies, Inc) and then incubated with a 1:1,000 dilution of anti-iNOS Ab (Calbiochem-Novabiochem Co) or normal rabbit serum in Perm/Wash solution containing AlbuMax I for 60 minutes at room temperature. After washing with Perm/Wash solution, anti-iNOS Ab was detected with a goat F(ab′) antirabbit IgG conjugated to fluorescein isothiocyanate (FITC; Boehringer Mannheim Co, Indianapolis, IN) at a 1:50 dilution. For two-color staining of intracellular antigens, cells were fixed and permealized by Cytofix/Cytoperm, blocked, and then incubated with a 1:20 dilution of anti-GFAP Ab (Boehringer Mannheim Co) or normal mouse IgG in Perm/Wash solution containing AlbuMax I for 60 minutes at room temperature. Anti-GFAP Ab was followed by detection with a goat F(ab′) antimouse IgG conjugated to PE (Immunotech International, Westbrook, ME). After washing three times with Perm/Wash solution, cells were incubated with anti-iNOS Ab or normal rabbit serum for 60 minutes at room temperature, followed by detection with a goat F(ab′) antirabbit IgG conjugated to FITC. Finally, stained cells were resuspended in PBS and analyzed in a flow cytometer (Becton Dickinson).
Cytokine assays.
All cytokines were quantitated using ELISA kits purchased from Immunotech International. Sensitivities of the kits are 0.08 IU/mL for interferon-γ (IFN-γ), 5 pg/mL for interleukin-1β (IL-1β), and 5 pg/mL for tumor necrosis factor-α (TNF-α).
RESULTS
Production of NO and expression of iNOS occurs in cocultures of HIV-1–infected MDM and human astrocytes.
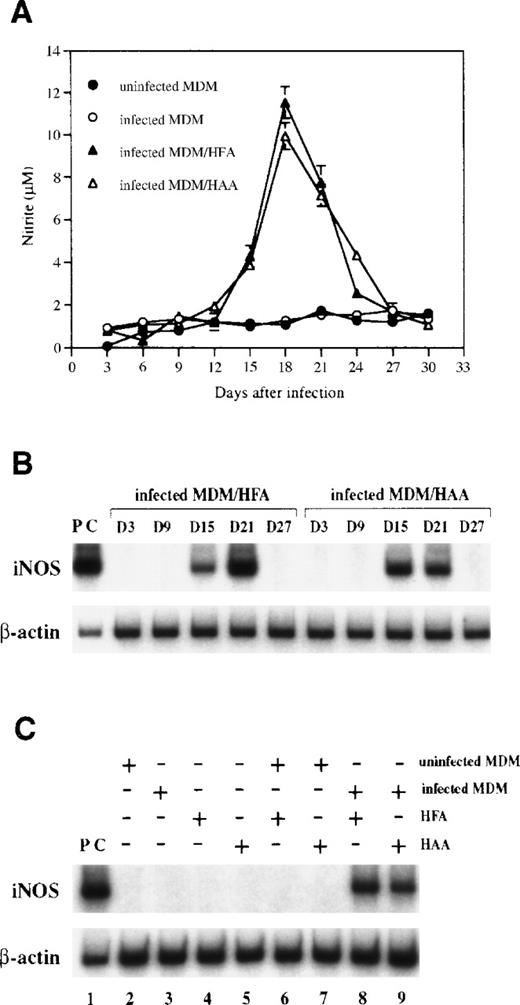
It has previously been reported that HIV-1 infection induces iNOS expression with subsequent low level NO production by human macrophages.41 However, we did not detect NO in HIV-1–infected macrophages at levels greater than those present in uninfected control MDM when MDM were infected with HIV-1 and monitored in culture for 30 days (Fig 1A). Because NO production is reported to be enhanced when infected macrophages are cocultured with astrocytes,41 we also examined levels of NO production in cocultures of HIV-1 infected MDM and primary human fetal (HFA) or adult (HAA) astrocytes. As shown in Fig 1A, production of NO in cocultures, measured as the concentration of nitrite in culture supernatants, occurred at levels approximately 10-fold in excess of control levels. RT-PCR analysis showed that iNOS is expressed in cocultures between days 15 and 21 postinfection (Fig 1B). However, expression was not observed in either MDM or astrocytes cultured alone or in cocultures of uninfected MDM and astrocytes (Fig 1C), suggesting that the presence of both infected MDM and astrocytes is required for induction of iNOS.
NO is produced and iNOS is expressed in cocultures of HIV-1–infected MDM and human astrocytes. (A) MDM precultured for 7 days in DMEM medium were infected with HIV-1ADA. At day 1 postinfection, infected MDM were cocultured with HFA and HAA at a 2:3 ratio and were fed with fresh DMEM medium every 3 days. Nitrite was measured in culture supernatants by the Griess reaction. Each bar represents the mean ± SD in triplicate samples of supernatants. (B) Levels of iNOS mRNA in cocultures with infected MDM and astrocytes were analyzed by RT-PCR. Amplification cycles used were 35 cycles for iNOS and 25 cycles for β-actin. The cytokine-stimulated A172 cell line was used as a positive control. Days after infection are shown above the lanes. (C) Results of the RT-PCR analysis of different cultures at day 18 postinfection are presented. Lane 1, positive control (PC); lane 2, uninfected MDM; lane 3, infected MDM; lane 4, HFA; lane 5, HAA; lane 6, HFA cocultured with uninfected MDM; lane 7, HAA cocultured with uninfected MDM; lane 8, HFA cocultured with infected MDM; lane 9, HAA cocultured with infected MDM.
NO is produced and iNOS is expressed in cocultures of HIV-1–infected MDM and human astrocytes. (A) MDM precultured for 7 days in DMEM medium were infected with HIV-1ADA. At day 1 postinfection, infected MDM were cocultured with HFA and HAA at a 2:3 ratio and were fed with fresh DMEM medium every 3 days. Nitrite was measured in culture supernatants by the Griess reaction. Each bar represents the mean ± SD in triplicate samples of supernatants. (B) Levels of iNOS mRNA in cocultures with infected MDM and astrocytes were analyzed by RT-PCR. Amplification cycles used were 35 cycles for iNOS and 25 cycles for β-actin. The cytokine-stimulated A172 cell line was used as a positive control. Days after infection are shown above the lanes. (C) Results of the RT-PCR analysis of different cultures at day 18 postinfection are presented. Lane 1, positive control (PC); lane 2, uninfected MDM; lane 3, infected MDM; lane 4, HFA; lane 5, HAA; lane 6, HFA cocultured with uninfected MDM; lane 7, HAA cocultured with uninfected MDM; lane 8, HFA cocultured with infected MDM; lane 9, HAA cocultured with infected MDM.
Induction of iNOS occurs in astrocytes, but not in MDM.
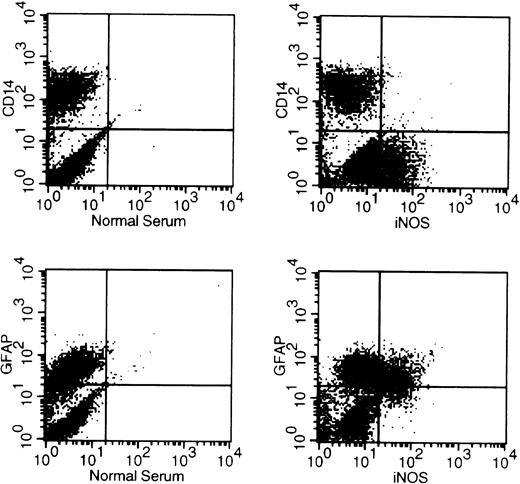
The expression of iNOS has been previously demonstrated in both human macrophages and astrocytes.41,44 48-52 Therefore, we wanted to clarify whether infected MDM, astrocytes, or both cells produce iNOS in our coculture system. Cocultured cells were harvested at day 18 postinfection and then stained with antibodies for CD14, GFAP, and iNOS using an intracellular antigen detection method. We found that 85% to 95% of MDM stained positive for CD14 and 70% to 80% of primary astrocytes were GFAP positive by flow cytometric analysis, whereas more than 95% of the astrocytes were positive for GFAP by immunohistochemistry. As shown in Fig 2, iNOS was detected in cells that were CD14− and GFAP+, suggesting that astrocytes, but not MDM, expressed iNOS.
Cells producing iNOS in cocultures are astrocytes but not MDM. MDM precultured for 7 days in DMEM medium were infected with HIV-1ADA. At day 1 postinfection, infected MDM were cocultured with HFA and were fed with fresh DMEM medium every 3 days. At day 21 postinfection, cells were stained with antibodies for CD14, GFAP, and iNOS using an intracellular antigen detection method and analyzed using a flow cytometer.
Cells producing iNOS in cocultures are astrocytes but not MDM. MDM precultured for 7 days in DMEM medium were infected with HIV-1ADA. At day 1 postinfection, infected MDM were cocultured with HFA and were fed with fresh DMEM medium every 3 days. At day 21 postinfection, cells were stained with antibodies for CD14, GFAP, and iNOS using an intracellular antigen detection method and analyzed using a flow cytometer.
Coculture supernatants induce iNOS in astrocytes.
Because coculture of HIV-infected MDM with human astrocytes induced iNOS expression in astrocytes, we next determined whether culture supernatants could mediate this effect. Human fetal astrocytes were incubated with or without various culture supernatants for 7 days, and iNOS expression was determined by RT-PCR. As shown in Fig 3, only supernatants obtained from cocultures of infected MDM and astrocytes harvested 18 to 21 days postinfection induced iNOS expression in astrocytes; iNOS expression was not observed in astrocytes incubated with coculture supernatants harvested 3 to 6 days postinfection, when little RT activity could be detected. Furthermore, elevations of RT activity or iNOS expression could not be detected when astrocytes were directly exposed to HIV-1ADA in the same manner as MDM. Thus, HIV-1 does not directly induce iNOS expression in primary astrocytes. These data therefore suggest that HIV-infected MDM, and not HIV alone, are responsible for induction of iNOS in astrocytes; that soluble cell- or virus-derived factors are capable of mediating this effect; and that threshold levels of these factors are required for iNOS induction.
Coculture supernatant induces iNOS in astrocytes. Total RNA was extracted from MDM/HFA cocultures at day 18 postinfection and from HFA cultures incubated for 7 days with different supernatants. Levels of iNOS mRNA in HFA were analyzed by RT-PCR. The cytokine-stimulated A172 cell line was used as a positive control. Lane 1, positive control; lane 2, HFA; lane 3, HFA cocultured with uninfected MDM; lane 4, HFA cocultured with infected MDM; lane 5, HFA incubated with HIV-1; lane 6, HFA incubated with day 18 to 21 supernatant of uninfected MDM culture; lane 7, HFA incubated with day 18 to 21 supernatant of infected MDM culture; lane 8, HFA incubated with day 18 to 21 supernatant of coculture with uninfected MDM and HFA; lane 9, HFA incubated with day 3 to 6 supernatant of coculture with infected MDM and HFA; lane 10, HFA incubated with day 18 to 21 supernatant of coculture with infected MDM and HFA.
Coculture supernatant induces iNOS in astrocytes. Total RNA was extracted from MDM/HFA cocultures at day 18 postinfection and from HFA cultures incubated for 7 days with different supernatants. Levels of iNOS mRNA in HFA were analyzed by RT-PCR. The cytokine-stimulated A172 cell line was used as a positive control. Lane 1, positive control; lane 2, HFA; lane 3, HFA cocultured with uninfected MDM; lane 4, HFA cocultured with infected MDM; lane 5, HFA incubated with HIV-1; lane 6, HFA incubated with day 18 to 21 supernatant of uninfected MDM culture; lane 7, HFA incubated with day 18 to 21 supernatant of infected MDM culture; lane 8, HFA incubated with day 18 to 21 supernatant of coculture with uninfected MDM and HFA; lane 9, HFA incubated with day 3 to 6 supernatant of coculture with infected MDM and HFA; lane 10, HFA incubated with day 18 to 21 supernatant of coculture with infected MDM and HFA.
HIV gp41 induces iNOS in astrocytes when MDM are present.
Because the HIV-1 envelope proteins gp120 and gp41 have been reported to induce NO production in macrophage and mixed glial cell cultures,43-45 we tested the ability of rgp120 and rgp41 to induce iNOS expression in human astrocytes. The recombinant protein preparations used had previously been shown to have biological activity in similar in vitro cell culture systems.45 57 As shown in Fig 4A, neither rgp120 nor rgp41 induced iNOS in cultures of astrocytes or MDM alone (lanes 3, 7, and 15). However, when uninfected MDM were present in culture with astrocytes, rgp41 induced iNOS expression, whereas rgp120 did not (Fig 4A, lanes 11 and 12). NO was also detected when rgp41 was added to cocultures of uninfected MDM and astrocytes (Fig 4B). When tested using aLimulus Amebocyte Lysate assay, endotoxin was not detected in the rgp41 preparation. Furthermore, anti-gp41 antibody or a competitive inhibitor of NOS, L-NMMA, reversed NO production mediated by rgp41. Taken together, these data suggest that gp41 is required, but not sufficient, for induction of iNOS in astrocytes (Fig 4B).
iNOS is induced by gp41 in cocultures of uninfected MDM and astrocytes. (A) Levels of iNOS mRNA were analyzed by RT-PCR. Total RNA was extracted from MDM, HFA, and HAA cultures or cocultures incubated for 7 days with rgp41 (10 ng/mL) and rgp120 (10 ng/mL). Total RNA from cells stimulated by cytokine mixture containing IFNγ (10 ng/mL), IL-1β (100 ng/mL), and TNF- (100 ng/mL) was extracted after 8 hours of treatment. The cytokine-stimulated A172 cell line was used as a positive control. Cells are shown above the lanes. Lane 1, positive control; lanes 2, 6, 10, 14, and 18, no treatment; lanes 3, 7, 11, 15, and 19, treatment with rgp41; lanes 4, 8, 12, 16, and 20, treatment with rgp120; lanes 5, 9, 13, 17, and 21, treatment with cytokine mixture. (B) HFA were cocultured with uninfected MDM in the presence and absence of rgp41 (10 ng/mL), anti-gp41 Ab (1 mg/mL), and L-NMMA (500 μmol/L) for 9 days. At 3-day intervals, cultures were fed with fresh DMEM medium, and rgp41, L-NMMA and anti-gp41 Ab were replenished. Nitrite in culture supernatants at day 9 was measured by the Griess reaction. Each bar represents the mean ± SD in triplicate samples of supernatants.
iNOS is induced by gp41 in cocultures of uninfected MDM and astrocytes. (A) Levels of iNOS mRNA were analyzed by RT-PCR. Total RNA was extracted from MDM, HFA, and HAA cultures or cocultures incubated for 7 days with rgp41 (10 ng/mL) and rgp120 (10 ng/mL). Total RNA from cells stimulated by cytokine mixture containing IFNγ (10 ng/mL), IL-1β (100 ng/mL), and TNF- (100 ng/mL) was extracted after 8 hours of treatment. The cytokine-stimulated A172 cell line was used as a positive control. Cells are shown above the lanes. Lane 1, positive control; lanes 2, 6, 10, 14, and 18, no treatment; lanes 3, 7, 11, 15, and 19, treatment with rgp41; lanes 4, 8, 12, 16, and 20, treatment with rgp120; lanes 5, 9, 13, 17, and 21, treatment with cytokine mixture. (B) HFA were cocultured with uninfected MDM in the presence and absence of rgp41 (10 ng/mL), anti-gp41 Ab (1 mg/mL), and L-NMMA (500 μmol/L) for 9 days. At 3-day intervals, cultures were fed with fresh DMEM medium, and rgp41, L-NMMA and anti-gp41 Ab were replenished. Nitrite in culture supernatants at day 9 was measured by the Griess reaction. Each bar represents the mean ± SD in triplicate samples of supernatants.
Stimulation of astrocytes with IFNγ, IL-1β, and TNF-α can also induce iNOS expression (Fig 4 and previous studies48-52). Therefore, we tested supernatants from cocultures of HIV-1–infected MDM and astrocytes for the presence of these cytokines by ELISA. As shown in Table 1, IFNγ, IL-1β, and TNF-α were not detected, suggesting that these cytokines are not involved in the induction of iNOS in our coculture system.
Cytokine and Nitrite Production in Cocultures of HIV-1–Infected MDM and Human Fetal Astrocytes
| Days After Infection . | RT Activity (cpm) . | IFN-γ (IU/mL) . | IL-1β (pg/mL) . | TNF-α (pg/mL) . | Nitrite (μmol/L) . |
|---|---|---|---|---|---|
| 3 | 423 | <0.08 | <5.0 | <5.0 | 1.1 |
| 9 | 1,412 | <0.08 | <5.0 | <5.0 | 1.5 |
| 15 | 7,120 | <0.08 | <5.0 | <5.0 | 1.2 |
| 21 | 6,307 | <0.08 | <5.0 | <5.0 | 10.1 |
| 27 | 4,938 | <0.08 | <5.0 | <5.0 | 7.6 |
| Days After Infection . | RT Activity (cpm) . | IFN-γ (IU/mL) . | IL-1β (pg/mL) . | TNF-α (pg/mL) . | Nitrite (μmol/L) . |
|---|---|---|---|---|---|
| 3 | 423 | <0.08 | <5.0 | <5.0 | 1.1 |
| 9 | 1,412 | <0.08 | <5.0 | <5.0 | 1.5 |
| 15 | 7,120 | <0.08 | <5.0 | <5.0 | 1.2 |
| 21 | 6,307 | <0.08 | <5.0 | <5.0 | 10.1 |
| 27 | 4,938 | <0.08 | <5.0 | <5.0 | 7.6 |
Human monocytes were precultured for 8 days, infected with HIV-1ADA, and cocultured with human fetal astrocytes. Cells were fed every 3 days. Harvested supernatants were analyzed for cytokine and nitrite production as well as RT activity.
NO inhibits HIV-1 replication in MDM. Our data thus far demonstrated that HIV-1 induces iNOS expression and NO production in human astrocytes through a mechanism that involves the gp41 envelope protein and requires the presence of MDM. Because NO exhibits antiviral activity in rodent and other human systems,29-36,39 40 we studied the anti–HIV-1 activity of NO using the NO-donor compound, SNAP. HIV-1ADA was incubated with or without SNAP for 3 hours and then MDM were infected with non–SNAP-treated and SNAP-treated viruses. MDM infected with nontreated virus were cultured in the presence and absence of SNAP. As shown in Fig 5, SNAP inhibited virus replication in MDM in a dose-dependent manner when added after adsorption. In addition, the pretreatment of virus with 1 mmol/L SNAP had no effect on the ability of virus to infect MDM (Fig 5), and this reagent had no effect on RT activity at any dose when purified virus was incubated directly with SNAP for 3 days (data not shown). MDM viabilities were greater than 98% after 4 weeks of culture in the presence and absence of SNAP as determined by the MTT (3-[4,5-dimethylthiozol-2-yl]-2,5-diphenyltetrazolium bromide) cell viability assay. These findings suggest that NO generated by SNAP is neither virucidal nor cytotoxic for MDM.
NO inhibits HIV-1 replication in MDM. HIV-1ADA was incubated with or without SNAP for 3 hours and then MDM was precultured for 7 days in DMEM medium were infected with non–SNAP-treated and SNAP-treated viruses. MDM infected with nontreated virus were maintained in the presence and absence of SNAP. Cultures were fed with fresh DMEM medium and SNAP was replenished every 3 days. Data are representative of three experiments and reflect the average levels of RT activity present in duplicate samples of supernatants, which differed by not more than 15%.
NO inhibits HIV-1 replication in MDM. HIV-1ADA was incubated with or without SNAP for 3 hours and then MDM was precultured for 7 days in DMEM medium were infected with non–SNAP-treated and SNAP-treated viruses. MDM infected with nontreated virus were maintained in the presence and absence of SNAP. Cultures were fed with fresh DMEM medium and SNAP was replenished every 3 days. Data are representative of three experiments and reflect the average levels of RT activity present in duplicate samples of supernatants, which differed by not more than 15%.
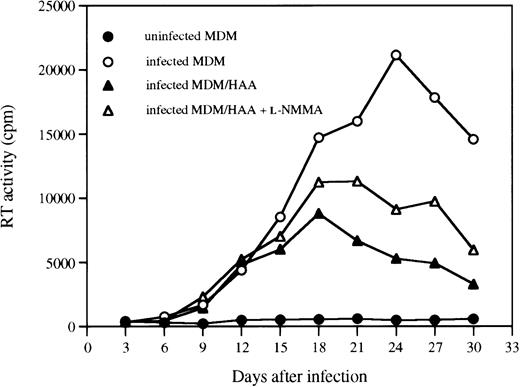
Our recent studies show that human astrocytes can also suppress HIV-1 replication in MDM via production of a soluble factor(s) (Hori et al, manuscript submitted). Therefore, we examined the effect of the NO inhibitor, L-NMMA, on cocultures of infected MDM and astrocytes to confirm that NO plays a role in astrocyte-mediated inhibition of HIV replication. As shown in Fig 6, virus replication, as measured by RT activity, was substantially decreased when infected MDM were cocultured with astrocytes. Inclusion of L-NMMA in the cocultures partially reversed the suppressive effect of astrocytes, suggesting that NO is, at least in part, responsible for the suppressive effect of astrocytes on HIV-1 replication in MDM.
A NOS inhibitor partially reverses the HIV-1 suppressive effect of astrocytes. MDM precultured for 7 days in DMEM medium were infected with HIV-1ADA. At day 1 postinfection, infected MDM were cocultured with HAA at a 2:3 ratio. Cocultures were fed with fresh DMEM medium with or without L-NMMA (500 μmol/L) every 3 days. Data are representative of two experiments and reflect the average levels of RT activity present in duplicate samples of supernatants, which differed by not more than 15%.
A NOS inhibitor partially reverses the HIV-1 suppressive effect of astrocytes. MDM precultured for 7 days in DMEM medium were infected with HIV-1ADA. At day 1 postinfection, infected MDM were cocultured with HAA at a 2:3 ratio. Cocultures were fed with fresh DMEM medium with or without L-NMMA (500 μmol/L) every 3 days. Data are representative of two experiments and reflect the average levels of RT activity present in duplicate samples of supernatants, which differed by not more than 15%.
DISCUSSION
In this study, we have shown that HIV-1 induces iNOS/NO expression in human astrocytes when cocultured with MDM by a mechanism involving gp41 and perhaps other soluble factors. We also showed that astrocyte-derived NO exerts antiviral activity through inhibition of virus replication in infected MDM. Thus, human astrocytes can regulate HIV-1 replication through the effects of NO in vitro and astrocyte-derived NO may influence the viral burden in the brain in vivo. Our study also suggests that the astrocyte may be an important, potent source of high level, unregulated NO production during HIV infection in the brain, which, in turn, raises the possibility that astrocyte-derived NO may mediate neural toxicity during HIV infection.
It was previously reported that HIV-1 infection induced low-level iNOS expression and NO production in human macrophages from 4 of 6 donors tested.41 We detected neither iNOS expression nor NO production after HIV-1 infection of MDM obtained from 7 normal donors. However, we found induction of iNOS in human fetal and adult astrocytes, but not macrophages, upon coculture of these primary astrocytes with HIV-1–infected human MDM. The issue regarding iNOS expression in human macrophages has been controversial for many years. Although we do not deny the ability of human macrophages to produce iNOS, its induction in human MDM appears to be highly restricted and further studies are necessary to determine the optimal conditions required for induction. The fact that RNA encoding iNOS could be detected in brain tissues from AIDS patients with severe neurological disease41 45 indicates that NO may be involved in neurologic AIDS and stresses the importance of identifying cells expressing iNOS in vivo.
Consistent with a previous report that gp41 induces iNOS in primary cultures of mixed rat neuronal and glial cells,45 we found that rgp41 also induces iNOS expression in cocultures of human MDM and astrocytes. However, rgp41 could not directly stimulate astrocytes to produce iNOS in the absence of uninfected MDM, but could when supplemented with the MDM. NO, as measured by nitrite production, was detected 6 days after rgp41 stimulation, which indicates that some intermediate event is required. Although IFNγ, IL-1β, and TNF-α are known to induce iNOS, production of these cytokines could not be detected in coculture supernatants and could therefore not account for iNOS induction. This suggests that an additional unidentified factor(s) may be involved in the induction of iNOS in astrocytes and that macrophages may regulate their expression after interaction with the HIV-1 gp41 envelope protein. Efforts are underway to identify the factor(s) resulting from the interaction between MDM and astrocytes and to clarify the molecular mechanisms by which gp41 induces iNOS in astrocytes.
Our studies thus far have demonstrated that NO inhibits HIV-1 replication in MDM without directly killing the virus. The diverse biological effects of NO are known to result from its interactions with target proteins that contain metals or thiols strategically located at either allosteric or active sites.23 These target proteins include ion channels, transporters, enzymes, G proteins, and transcription factors. Among them, NF-κB is the one most frequently associated with HIV-1 replication.58 NO can directly inhibit DNA binding to NF-κB by S-nitrosylation of the cysteine-62 residue of the p50 subunit.59 However, the effect of NO on NF-κB activation differs with the system under investigation. Lander et al60,61 found that NO-donor compounds induce NF-κB translocation via G protein activation in PBMC. In contrast, others found that NO-donor compounds inhibit cytokine-induced NF-κB activation through stabilization and increased expression of IkBα.62,63 Recently, Togashi et al64 have shown that NF-κB activation is inhibited in Jurkat cells by pyrrolidine dithiocarbamate, an inhibitor of NF-κB and a scavenger of NO, but is induced in astrocytes that have higher levels of constitutive NOS and p50. Intriguingly, Ouaaz et al46 have reported that CD23/FcεRII-mediated NO production induces HIV-1 expression in U1 cells with low-level constitutive production of p24, whereas virus replication is decreased in another variant of U1 with high constitutive production of p24 levels. Thus, the paradoxical actions of NO are likely to be dependent on a given biological model.
The pathogenesis of HIV-1–associated cognitive/motor complex is complicated and not well understood. In this study, we have shown that HIV-1–infected MDM stimulate human astrocytes to express iNOS in vitro via a mechanism involving gp41 and another as yet unidentified factor(s). Because gp41, unlike gp120, is a membrane-anchored protein, it is conceivable that free virions with exposed gp41 could stimulate astrocytes in vivo. In addition, our observation that NO inhibits HIV-1 replication in MDM, coupled with the fact that neurons and oligodendrocytes are generally more susceptible to the effects of NO than astrocytes and microglia,65 66 may explain the discrepancy between disease severity and viral burden in the brain. Furthermore, it is possible that the manifestations of neurologic HIV-disease attributable to NO are actually a side effect of the host attempt to inhibit virus replication.
ACKNOWLEDGMENT
The authors thank Drs Eugene Major and Katherine Conant for providing human fetal astrocytes and Dr Kathryn Carbone and Steven Rubin for providing human adult astrocytes. We also thank Dr Finbarr Murphy for endotoxin testing of rgp41 and Drs Kathryn Carbone and Eda Bloom for critical reading of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
This is a US government work. There are no restrictions on its use.
REFERENCES
Author notes
Address reprint requests to Kathleen A. Clouse, PhD, FDA/CBER/Division of Cytokine Biology (HFM-508), 1401 Rockville Pike, Rockville, MD 20852; e-mail: clouse@cber.fda.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal