Abstract
Marrow cells from mice lacking high-affinity receptors for granulocyte-macrophage colony-stimulating factor (GM-CSF; βc−/− mice) were shown to bind and internalize much less GM-CSF than cells from normal (βc+/+) mice. βc−/− mice were used to determine the effect of negligible receptor-mediated clearance on detectible GM-CSF responses to the intravenous injection of endotoxin or the intraperitoneal injection of casein plus microorganisms. Unlike the minor serum GM-CSF responses to endotoxin seen in βc+/+ mice, serum GM-CSF levels rose 30-fold to 9 ng/mL in βc−/− mice even though loss of GM-CSF in the urine was greater than in βc+/+ mice. Organs from βc−/− and βc+/+ mice had a similar capacity to produce GM-CSF in vitro, as did peritoneal cells from both types of mice when challenged in vitro by casein. However, when casein was injected intraperitoneally, βc−/− mice developed higher and more sustained levels of GM-CSF than did βc+/+ mice. The data indicated that receptor-dependent removal of GM-CSF masks the magnitude of GM-CSF responses to endotoxin and local infections. Because of this phenomenon, serum GM-CSF concentrations can be a misleading index of the occurrence or nonoccurrence of GM-CSF responses to infections.
THE GLYCOPROTEIN COLONY-stimulating factors were first detected because of their proliferative action in vitro on hematopoietic cells.1 Subsequent studies have shown that these regulators have a variety of additional actions, including stimulation of the functional activity of mature cells in responding lineages.2
Serum and tissue concentrations of colony-stimulating factors are low under basal conditions3 but are elevated in infections or in response to the injection of bacterial lipopolysaccharide (LPS).2,4 5 The presumption is that these responses are designed to elicit prompt antimicrobial responses by activating existing mature cells and, if necessary, to sustain and amplify such responses by stimulating the production of additional cells.
Gene inactivation studies have indicated that granulocyte colony-stimulating factor (G-CSF) is a major regulator of granulocyte production.6 Despite its stronger proliferative action in vitro, loss of granulocyte-macrophage colony-stimulating factor (GM-CSF) or its high-affinity receptor has no apparent effect on the numbers of granulocytes, monocytes, or eosinophils.7,8However, mice lacking GM-CSF develop alveolar proteinosis often with secondary lung infections, diseases that are likely to be based on loss of an essential stimulating action of GM-CSF on macrophage function. Mice with inactivation of the genes encoding G-CSF and/or GM-CSF die prematurely with a miscellany of infections.9
Although serum levels of G-CSF can be high in spontaneous and experimental infections, serum GM-CSF levels are typically low or undetectible in these situations.10-13 This pattern has led many to conclude that GM-CSF is likely to be of only minor importance as a mediator of responses to microbial infections.
As with other glycoprotein regulators, the serum half-life of agents such as thrombopoietin or GM-CSF is strongly influenced by receptor-mediated endocytosis and degradation of the regulator by responding cells.14 15 In principle, observed serum levels of a regulator may be low as a consequence of this process and then be a misleading index in monitoring regulator responses elicited by an inducing stimulus.
In the present studies, mice lacking high-affinity receptors for GM-CSF because of inactivation of the gene encoding the β-common chain of the GM-CSF receptor (βc−/− mice)8were used to reexamine GM-CSF responses to the injection of LPS and to a simple model bacterial infection. Changes in G-CSF levels were monitored as a control for the general responsiveness of the mice. The data revealed that βc−/− mice do respond to LPS by sharply elevating serum GM-CSF levels and to local infections by clear elevations of local GM-CSF concentrations, suggesting that these responses are largely obscured in normal animals because of receptor-mediated clearance by cells that include the hematopoietic cells responding to GM-CSF.
MATERIALS AND METHODS
Mice.
The generation of C57BL6x129Sv mice lacking high-affinity β-common chain (βc) receptors for GM-CSF has been described previously.8 This knockout line is maintained by interbreeding of mice with homozygous inactivation of the gene encoding the β-common chain (βc−/− mice). Control (βc+/+) mice used were C57BL6x129Sv mice interbred in parallel. All mice were raised and housed under pathogen-free conditions and were used when aged between 2 and 3 months.
Binding and internalization studies.
125I-labeling of recombinant murine GM-CSF (Pepro Tech, Rocky Hill, NJ) was performed as described previously.16Internalization and degradation of 125I-murine GM-CSF by βc−/− and control bone marrow cells were performed and analyzed using an acid elution method validated by extensive previous studies and described in full previously.16 17 Briefly, bone marrow cells were resuspended in RPMI medium containing 10% (vol/vol) fetal calf serum (FCS) and 10 mmol/L HEPES buffer pH 7.4 at 8.5 × 107cells/mL and equilibrated at 37°C. At time zero,125I–GM-CSF was added at 100 ng/mL (5 × 106 cpm/mL) with or without unlabeled GM-CSF at 2 μg/mL. At indicated timepoints 100 μL aliquots were removed (in duplicate), the cells centrifuged through a cushion of FCS, and resuspended in 1 mL of 3% acetic acid in phosphate-buffered (10 mmol/L, pH 7.4) saline (0.15 mol/L NaCl). The cells were centrifuged, and the radioactivity eluted by acetic acid (cell surface) or retained within the cell (internal) was measured in a gamma counter. Specific counts were determined in each case by subtracting counts present in equivalent incubations in which unlabeled GM-CSF was present.
Endotoxin.
Endotoxin (lipopolysaccharide, Difco, Detroit, MI) was dissolved in 0.9% sodium chloride solution, and 5 μg was injected intravenously in an injection volume of 0.2 mL. Urine was collected at intervals after endotoxin injection and low-molecular-weight inhibitors removed by passage through an NAP-5 column (Pharmacia, Uppsala, Sweden). At intervals after injection, mice were anesthetized using penthrane and bled from the axilla to collect serum.
Local peritoneal cavity inflammation and infection.
Mice were injected interperitoneally with 2 mL of an 0.2% (wt/vol) solution of casein (Glaxo Laboratories, Melbourne, Australia) or casein C5890 (Sigma Chemical Co, St Louis, MO) in mouse tonicity phosphate-buffered saline (MTPBS). The Glaxo preparation contains a contaminating population of nonviable bacteria, and the Sigma preparation is contaminated by viable saphrophytic Bacillus organisms.18 In both cases, the organisms are cleared by local neutrophil phagocytosis during the following 2 hours.18 At intervals up to 3 hours after injection, the mice were anesthetized then their blood collected from the axilla. The abdominal cavity was then injected with 2 mL MTPBS and, after massage to mix peritoneal cells with injected harvesting fluid, the cell suspension was removed using a soft plastic pipette. The collected volume was usually 3 mL.
Production of peritoneal cell conditioned media.
Resident peritoneal cells were collected using MTPBS and, after washing, 1 × 106 cells were incubated at 37°C for 3 hours in 1 mL Dulbecco’s modified Eagle’s medium (DMEM) containing 10% newborn calf serum and 0.1 mL MTPBS or 0.1 mL of a 2% solution of casein in MTPBS. Media were then obtained, cells removed by centrifugation then millipore filtration, and the media stored at 4°C before assay.
Production of organ-conditioned media.
Minced organs from 2- to 3-month-old female βc−/− and βc+/+ mice were incubated for 4 days in 1-mL volumes of serum-free DMEM as described previously.19 The media were obtained then millipore filtered and stored at 4°C before assay for CSF content.
Agar cultures.
Agar cultures were performed in 1-mL volumes containing 50,000 bone marrow cells from C57BL6x129Sv mice in 35-mm plastic petri dishes.1 The medium used was DMEM containing a final concentration of 20% newborn calf serum and 0.3% agar. Colony formation was stimulated in replicate cultures by addition of serial dilutions of 0.1 mL of recombinant murine GM-CSF or human G-CSF. Cultures were incubated for 7 days in a fully humidified atmosphere of 10% CO2 in air. After initial scoring at ×35, cultures were fixed by the addition of 1 mL of 2.5% glutaraldehyde. Four hours later, the cultures were floated intact onto glass slides and, after drying, were stained in sequence for acetylcholinesterase, then with Luxol Fast Blue (BDH Laboratory, Poole, UK) and hematoxylin.1 After mounting under coverslips, the cultures were analyzed at ×200 and ×100 to determine the number and composition of colonies in the entire cultures.
GM-CSF and G-CSFassays.
Concentrations of GM-CSF in serum, urine, peritoneal fluid, and cell- or organ-conditioned media were determined by microwell assays using Lux 60 well microtiter trays (Nunc, Naperville, IL) containing 200 FDC-P1 cells in 100 μL DMEM with 10% FCS.19 Serial twofold dilutions of 5 μL of the test material were added to duplicate wells and, after incubation for 48 hours at 37°C in a fully humidifed atmosphere of 10% CO2 in air, viable cells were counted using an inverted microscope. GM-CSF concentrations were calibrated using a parallel titration of 1 ng/ mL of purified recombinant murine GM-CSF (Pepro Tech).
FDC-P1 cells respond to stimulation either by GM-CSF or interleukin-3 (IL-3). The validity of the present assays as specific measurements of GM-CSF was verified by showing that all FDC-P1 stimulating activity in the samples tested was able to be inhibited by 1 μg/mL of a rat anti-mouse GM-CSF monoclonal antibody (Genzyme, Cambridge, MA), an antibody having no inhibitory action on the stimulation of FDC-P1 cells by IL-3. All serum samples were also assayed in parallel using Ba/F3 cells, a cell line responding only to stimulation by IL-3. No stimulation was observed with any sample, indicating the absence of detectible IL-3.
The assay of serum and urine concentrations of G-CSF was performed using a similar general procedure but using 200 Ba/F3 cells engineered to express G-CSF receptors.20 G-CSF concentrations were calibrated using parallel titrations of purified recombinant human G-CSF (Amgen, Thousand Oaks, CA).
The lower detection limit of both CSF assays was 50 pg/mL. Titrations of serum GM-CSF and G-CSF were commenced using a 1:4 serum dilution to avoid the inhibiting effects of serum lipoprotein, and the lower detection limits for sera were therefore 200 pg/mL.
RESULTS
Colony formation stimulated by GM-CSF or G-CSF.
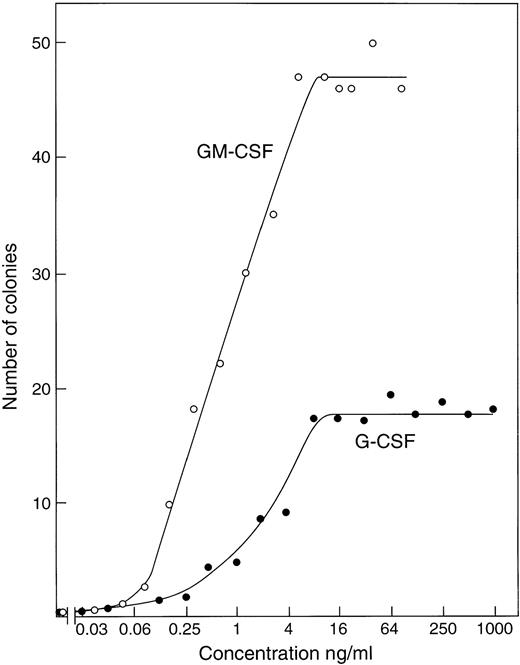
Titration of recombinant GM-CSF or G-CSF using cultures of 50,000 control C57BL6x129Sv (βc+/+) marrow cells showed (Fig 1) that maximal numbers of granulocytic and/or macrophage colonies were stimulated to develop by concentrations of 5 to 10 ng/mL of either CSF. Use of higher G-CSF concentrations, up to 1 μg/mL, did not further increase the number of colonies developing and had only a moderate effect on mean colony cell numbers, which remained relatively low.
Stimulation of colony formation by recombinant GM-CSF or G-CSF in agar cultures of 50,000 control C57BL6x129Sv bone marrow cells. Each point represents the mean value from duplicate cultures.
Stimulation of colony formation by recombinant GM-CSF or G-CSF in agar cultures of 50,000 control C57BL6x129Sv bone marrow cells. Each point represents the mean value from duplicate cultures.
Internalization of GM-CSF by βc−/− and βc+/+ cells.
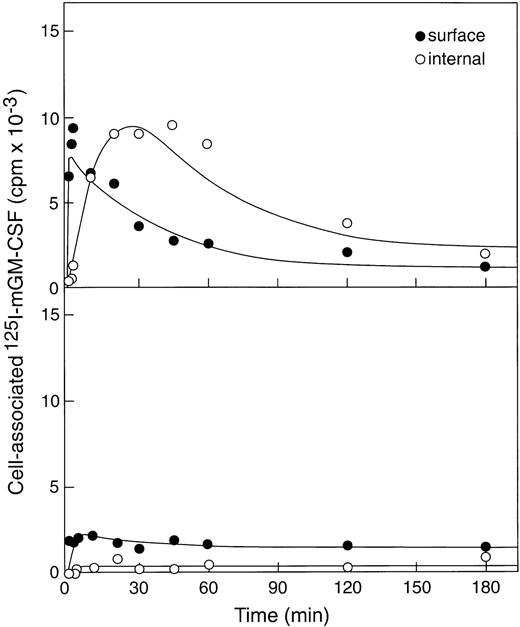
The ability of βc−/− or βc+/+bone marrow cells to use up GM-CSF was evaluated by incubating cells with 125I-labeled murine GM-CSF (100 ng/mL) at 37°C and measuring cell surface–associated (acid elutable) and internalized (nonelutable by acid) radioactivity (Fig2). As expected, given the presence of only low-affinity receptors on βc−/− bone marrow cells,8significantly less GM-CSF was bound by βc−/−cells (∼2,000cpm) than bound by an equivalent number of βc+/+ cells (∼14,000cpm). In addition, GM-CSF was rapidly internalized in βc+/+ cells (ke = 0.12/min) and after 30 minutes showed clear evidence of degradation of GM-CSF because total cell-associated counts decreased to about 3,000 cpm (80% degradation by 3 hours). In contrast, very little if any of the cell-associated GM-CSF was internalized by βc−/− cells, and this situation did not change with time. This very weak internalization precluded any accurate estimate of internalization or degradation rates but allowed the conclusion that little internalization occurred.
Binding and internalization of 125I-labeled rmGM-CSF to βc+/+ bone marrow cells (upper panel) and the much lower binding and insignificant internalization by βc−/− marrow cells (lower panel).
Binding and internalization of 125I-labeled rmGM-CSF to βc+/+ bone marrow cells (upper panel) and the much lower binding and insignificant internalization by βc−/− marrow cells (lower panel).
Serum GM-CSF and G-CSF responses to intravenous endotoxin.
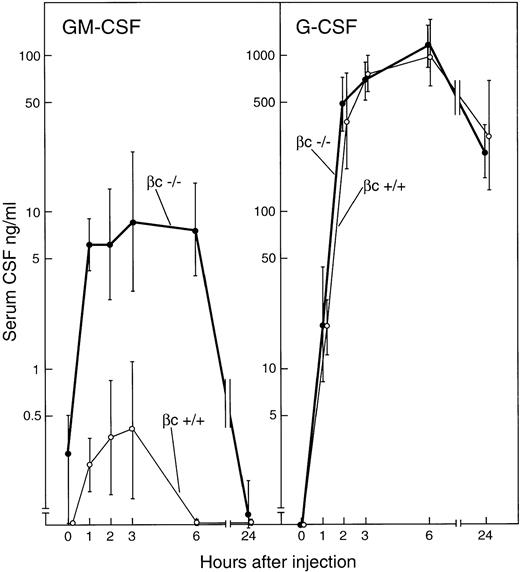
In agreement with previous studies, the intravenous injection of 5 μg endotoxin induced major rises in serum CSF levels peaking 3 to 6 hours after injection.19,21 In control βc+/+ mice, G-CSF was not detectible in the serum of uninjected mice but became detectible 1 hour after injection, then rose sharply and attained a peak at 6 hours of 1,000 ng/mL (Fig 3). Serum G-CSF levels were still elevated at 24 hours after injection. GM-CSF also was not detectible in the serum of uninjected βc+/+ mice and, in agreement with previous studies,21 barely detectible levels of GM-CSF were present in serum from 1 to 3 hours after endotoxin injection, thereafter again becoming undetectible.
Serum concentrations of GM-CSF and G-CSF after the intravenous injection of 5 μg endotoxin to βc+/+ or βc−/− mice. Each point represents mean CSF values ± SD from three separate mice of each genotype at each timepoint.
Serum concentrations of GM-CSF and G-CSF after the intravenous injection of 5 μg endotoxin to βc+/+ or βc−/− mice. Each point represents mean CSF values ± SD from three separate mice of each genotype at each timepoint.
βc−/− mice injected with 5 ug endotoxin showed identical G-CSF responses to those seen in βc+/+mice. In the serum of uninjected βc−/− mice, GM-CSF was present, but in barely detectible concentrations. However, in sharp contrast to the situation with control mice, GM-CSF was readily detectible in the serum of βc−/− mice 1 hour after the injection of endotoxin, with concentrations rising gradually to a peak of 9 ng/mL 3 hours after injection. After 6 hours, GM-CSF concentrations declined and again were barely detectible at 24 hours.
Although serum GM-CSF levels have been noted to be higher in male than in female βc−/−xGM-CSF transgenic mice,15it was of interest that after endotoxin injection, GM-CSF concentrations in female βc−/− mice were 2- to 16-fold higher than in corresponding male βc−/− mice.
Validation of the bioassays.
Bioassays were used in preference to immunoassays because they certified that the material being assayed was biologically active. Previous studies on βc−/− mice showed a major difference between the half-life of injected GM-CSF when comparing bioassays with half-lives established using radiolabeled GM-CSF, the difference being due to inactivation of much of the GM-CSF, although it remained macromolecular.8 15
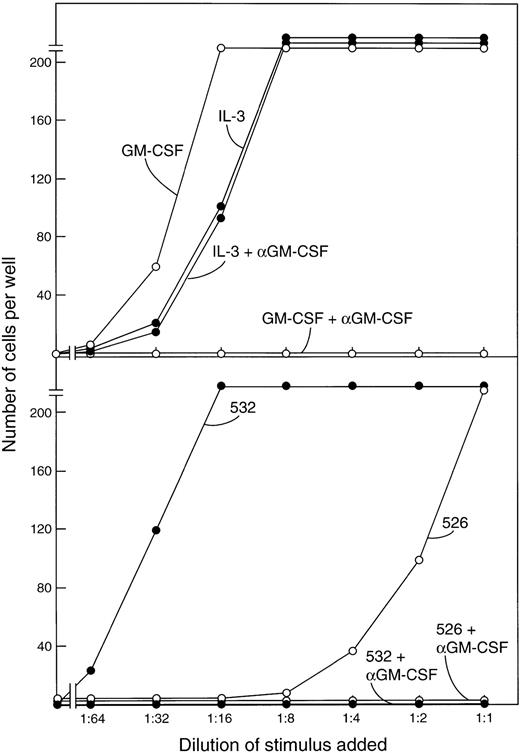
The FDC-P1 cells used in the bioassays also respond to proliferative stimulation by IL-3. Previous studies showed that no detectible IL-3 appears in mouse serum after the injection of endotoxin.19 21 That the active molecule being assayed in the FDC-P1 assays was indeed GM-CSF was verified in the present experiments by showing that all FDC-P1–stimulating activity in the sera was neutralized by a monoclonal GM-CSF antibody as shown in the examples in Fig 4. This antibody had no effect in parallel FDC-P1 cultures stimulated by IL-3.
(Upper panel) Stimulation of the proliferation of FDC-P1 cells by serial dilutions of 1 ng/mL GM-CSF or IL-3 and the selective inhibition of GM-CSF but not IL-3 by a monoclonal GM-CSF antibody. (Lower panel) The stimulating activity of postendotoxin sera for FDC-P1 cells is completely inhibited by the same GM-CSF antibody.
(Upper panel) Stimulation of the proliferation of FDC-P1 cells by serial dilutions of 1 ng/mL GM-CSF or IL-3 and the selective inhibition of GM-CSF but not IL-3 by a monoclonal GM-CSF antibody. (Lower panel) The stimulating activity of postendotoxin sera for FDC-P1 cells is completely inhibited by the same GM-CSF antibody.
The presence of IL-3 in the serum samples was further excluded by assaying all sera in parallel in cultures of Ba/F3 cells, a cell line responding only to stimulation by IL-3.20 No stimulating activity was detected in any sample. This permitted the use of Ba/F3 cells, engineered to express receptors for G-CSF, to be used as a specific assay for G-CSF.
Urine GM-CSF and G-CSF levels after intravenous endotoxin.
Previous studies have shown that intravenously injected GM-CSF appears in the urine, although less than 1% of injected GM-CSF is cleared from the body by this route.15,22 It was also shown that more injected GM-CSF appeared in the urine of βc−/− mice than of βc+/+mice.15 Despite these data, it was possible that in the special circumstances following the injection of endotoxin, this situation might change and that an unusually high loss of GM-CSF in the urine might be responsible for the low serum levels of GM-CSF in βc+/+ mice.
To explore this possibility, urine was collected 3, 6, and 24 hours after the intravenous injection of endotoxin. As shown in Table 1, GM-CSF was detectible in the urine of βc+/+ mice at all timepoints analyzed in concentrations that were higher than in the serum. With βc−/− mice, GM-CSF was also detected in the urine at all timepoints but in concentrations that were much higher than in the urine of βc+/+ mice and again in higher concentrations than in the serum of the βc−/−mice.
Urine Content of GM-CSF and G-CSF After the Intravenous Injection of Endotoxin
| Hours After Injection . | βc−/− Mice . | βc+/+ Mice . | ||
|---|---|---|---|---|
| GM-CSF . | G-CSF . | GM-CSF . | G-CSF . | |
| 3 | 27 ± 12 | 3 ± 2 | 3 ± 2 | 6 ± 10 |
| 6 | 23 ± 3 | 3 ± 3 | 3 ± 1 | 4 ± 6 |
| 24 | 13 ± 6 | 8 ± 7 | 0.3 ± 0.4 | 4 ± 2 |
| Hours After Injection . | βc−/− Mice . | βc+/+ Mice . | ||
|---|---|---|---|---|
| GM-CSF . | G-CSF . | GM-CSF . | G-CSF . | |
| 3 | 27 ± 12 | 3 ± 2 | 3 ± 2 | 6 ± 10 |
| 6 | 23 ± 3 | 3 ± 3 | 3 ± 1 | 4 ± 6 |
| 24 | 13 ± 6 | 8 ± 7 | 0.3 ± 0.4 | 4 ± 2 |
GM-CSF and G-CSF concentrations are expressed as nanograms per milliliter urine. Mean data from three mice per group at each timepoint ± SD.
Urinary loss of GM-CSF did not therefore provide an explanation of the higher serum GM-CSF concentrations in βc−/−versus βc+/+ mice after the injection of endotoxin, because urinary loss of GM-CSF was in fact much higher in the βc−/− mice than in the βc+/+mice.
G-CSF was not detectible in normal mouse urine but was readily detectible following the injection of endotoxin. However in contrast to the GM-CSF data, levels of G-CSF were similar in both types of mice at the timepoints examined.
GM-CSF and G-CSF concentrations in peritoneal exudate fluid.
The injection interperitoneally to mice of casein containing nonviable or saphrophytic bacteria induces a marked accumulation of neutrophils within 3 hours and a rise in peritoneal fluid concentrations of GM-CSF and G-CSF.18 In these earlier studies quantitative cellular responses in the peritoneal cavity to the injection of casein were found to be similar in βc+/+ and βc−/− mice.
As shown in Fig 5, a typical local CSF response was observed in βc+/+ mice following the injection of casein. As noted in previous studies on normal mice,18 GM-CSF responses occurred earlier than G-CSF responses but declined after reaching peak values 2 hours after injection.
GM-CSF and G-CSF concentrations in the peritoneal cavity fluid of βc+/+ and βc−/− mice at intervals after the intraperitoneal injection of casein. Figures are expressed as total nanograms of CSF in the 3-mL fluid volume collected after harvesting. Each point is the mean value ± SD from two female and one male mice of each genotype per timepoint.
GM-CSF and G-CSF concentrations in the peritoneal cavity fluid of βc+/+ and βc−/− mice at intervals after the intraperitoneal injection of casein. Figures are expressed as total nanograms of CSF in the 3-mL fluid volume collected after harvesting. Each point is the mean value ± SD from two female and one male mice of each genotype per timepoint.
βc−/− mice showed similar G-CSF responses to the injection of casein but, in contrast to βc+/+ mice, GM-CSF responses were significantly higher and rose progressively during the 3-hour observation period, reaching a mean level of 20 ng at 3 hours, a level fivefold higher than in casein-injected βc+/+ mice.
In Fig 5, the values are expressed as total nanograms CSF per peritoneal cavity. In normal mice, the resident fluid volume has been estimated as 70 μL,23 and in a real-life peritoneal infection with the accumulation of smaller fluid volumes than used in the present model, the observed CSF levels would represent very high local concentrations of CSF per milliliter fluid.
Production rates of GM-CSF by βc−/− tissues.
The higher GM-CSF responses in βc−/− mice than in βc+/+ mice to the injection of endotoxin or casein might have been based on a constitutively elevated capacity of βc−/− tissues to produce GM-CSF.
This possibility was explored by examining the capacity of peritoneal cells to produce CSF in short-term in vitro cultures after exposure to casein and by determining the capacity of various organs to produce GM-CSF and G-CSF in longer tissue cultures.
In the first approach, peritoneal cells from βc−/− and βc+/+ mice were incubated for 3 hours with or without added casein, the concentrations of cells being 1 × 106 cells/mL to reduce the risk of receptor-mediated removal of CSF. As shown in Table 2, GM-CSF concentrations after 3 hours of incubation were elevated by addition of casein but the concentrations were not significantly higher in cultures of βc−/− cells than in cultures of βc+/+ cells. In cultures prepared containing 5 × 106 cells/mL, only a minor twofold higher concentration of GM-CSF was observed in βc−/− versus βc+/+ cultures (data not shown), the minor difference possibly reflecting the occurrence of some receptor-based clearance in the βc+/+ cultures.
Production of GM-CSF and G-CSF In Vitro by Peritoneal Cells From βc+/+ and βc−/− Mice
| Stimulus . | βc−/− . | βc+/+ . | ||
|---|---|---|---|---|
| GM-CSF . | G-CSF . | GM-CSF . | G-CSF . | |
| Casein (Glaxo) | 21.3 ± 9.2 | 1.5 ± 0 | 13.3 ± 4.6 | 1.8 ± 1.1 |
| Casein (Sigma) | 16.0 ± 0.0 | 1.3 ± 0.4 | 13.3 ± 4.6 | 1.1 ± 0.6 |
| MTPBS | 0.9 ± 1.0 | 0.1 ± 0.06 | 1.1 ± 0.9 | 0.2 ± 0.06 |
| Stimulus . | βc−/− . | βc+/+ . | ||
|---|---|---|---|---|
| GM-CSF . | G-CSF . | GM-CSF . | G-CSF . | |
| Casein (Glaxo) | 21.3 ± 9.2 | 1.5 ± 0 | 13.3 ± 4.6 | 1.8 ± 1.1 |
| Casein (Sigma) | 16.0 ± 0.0 | 1.3 ± 0.4 | 13.3 ± 4.6 | 1.1 ± 0.6 |
| MTPBS | 0.9 ± 1.0 | 0.1 ± 0.06 | 1.1 ± 0.9 | 0.2 ± 0.06 |
A total of 1 × 106 peritoneal cells were incubated for 3 hours in 1 mL DMEM containing 10% newborn calf serum. To replicate cultures, 0.1 mL of 2% casein or 0.1 mL MTPBS was added. Values shown are mean values of CSF concentrations in nanograms per milliliter ± SD from assays on three replicate cultures using both male and female mice.
An analysis of the capacity of various organs from βc−/− and βc+/+ mice to produce GM-CSF after 4 days of incubation in vitro is shown in Table 3. In agreement with previous studies,19 individual organs varied widely in their capacity to produce GM-CSF, but levels produced by βc−/− organs did not significantly differ from those produced by βc+/+ organs.
Production of GM-CSF In Vitro by Organs From βc+/+ and βc−/− Mice
| Organ . | GM-CSF ng/mL . | |
|---|---|---|
| βc+/+ . | βc−/− . | |
| Salivary gland | 0,0 | 0.3,0 |
| Thymus | 16,16,8 | 16,32,16 |
| Lungs | 32,1024,256 | 32,512,256 |
| Heart | 16,32,2 | 4,8,1 |
| Liver | 0,0,0 | 0,0,0 |
| Spleen | 1,4,0.5 | 2,1,0 |
| Kidney | 0,0,0 | 0,0,0 |
| Muscle | 4,4,0 | 0.5,0,0.5 |
| Bone marrow | 0,0,0 | 1,0,0 |
| Bone shaft | 2,0.3,1 | 4,0.5,4 |
| Uterus | 0 | 0 |
| Brain | 0,0,0 | 0,0,0 |
| Organ . | GM-CSF ng/mL . | |
|---|---|---|
| βc+/+ . | βc−/− . | |
| Salivary gland | 0,0 | 0.3,0 |
| Thymus | 16,16,8 | 16,32,16 |
| Lungs | 32,1024,256 | 32,512,256 |
| Heart | 16,32,2 | 4,8,1 |
| Liver | 0,0,0 | 0,0,0 |
| Spleen | 1,4,0.5 | 2,1,0 |
| Kidney | 0,0,0 | 0,0,0 |
| Muscle | 4,4,0 | 0.5,0,0.5 |
| Bone marrow | 0,0,0 | 1,0,0 |
| Bone shaft | 2,0.3,1 | 4,0.5,4 |
| Uterus | 0 | 0 |
| Brain | 0,0,0 | 0,0,0 |
Individual values represent data from the bioassay of medium conditioned for 4 days by the entire organ from separate female mice aged 2 to 3 months.
Both sets of data suggested that βc−/− and βc+/+ tissues had an equivalent capacity to produce GM-CSF under the conditions tested.
DISCUSSION
The present results document the fallacy of attempting to use serum CSF concentrations to reach conclusions regarding the involvement or noninvolvement of a CSF in responses to infections. The intravenous injection of endotoxin was used as a surrogate model of a microbial infection and was able to elicit a dramatic rise in serum G-CSF concentrations in normal mice, but in contrast, only a minor response in serum GM-CSF concentrations. These data paralleled earlier observations on the lack of elevation of GM-CSF levels in infections,10-13 observations that have led some to the conclusion that GM-CSF is likely to be of only minor importance in systemic responses to infections.
The simple maneuver of using βc−/− mice, whose cells lack high-affinity receptors for GM-CSF and have little capacity to internalize GM-CSF, resulted in a very different outcome. Following the injection of endotoxin, serum GM-CSF levels rose approximately 30-fold above preinjection levels and achieved a mean concentration of 9 ng/mL, a concentration in excess of that required to stimulate maximal numbers of granulocyte-macrophage colonies to develop in vitro. It is of interest that these GM-CSF responses always occurred slightly more rapidly than did G-CSF rises, with near-maximal responses already being evident 1 hour after injection. The very rapid rise in serum GM-CSF levels suggests that release of preformed GM-CSF to the circulation may have been the basis for the initial rapid rise. An increase in GM-CSF mRNA has been noted in macrophages after exposure to endotoxin or other inducing agents in vitro, commencing within 1 to 2 hours.24 If this response pattern is typical for other cell types, an increased synthesis of GM-CSF in vivo in response to endotoxin, presumably therefore supervenes to maintain the observed elevations in serum GM-CSF levels, because the half-life of GM-CSF in serum is only 21minutes.15
The present experiments indicated that βc−/−and βc+/+ tissues have a similar capacity to produce GM-CSF and, while not investigating in detail the metabolic fate of GM-CSF, eliminated abnormally rapid loss of GM-CSF in the urine of endotoxin-injected βc+/+ mice as a possible basis for their failure to develop high serum GM-CSF levels.
The most reasonable conclusion is therefore that mice do in fact respond promptly to challenge by endotoxin by elevating GM-CSF levels but that this GM-CSF is usually efficiently removed by high-affinity receptors, resulting in the apparent occurrence of only a very weak response to challenge.
Earlier studies noted the production of GM-CSF locally in the peritoneal cavity in response to the combined injection of casein and nonviable or saphrophytic organisms as a model of local infection and inflammation.18 The present studies showed that the magnitude of this response is also underestimated in mice with high-affinity GM-CSF receptors, not because of lower production rates of GM-CSF but again presumably because of receptor-mediated endocytosis.
Because it is reasonable to suppose that hematopoietic cells are a major receptor-bearing population in the body, the data further suggest that these cells are likely to be very effectively stimulated by the GM-CSF released or produced in response to endotoxin or local infections. It therefore appears to be a mistake to regard GM-CSF as an unresponsive regulator in the context of infections and indeed the converse may be true, that it is an efficient and effective agent during such responses. This conclusion is in line with the observations that GM-CSF–deficient mice are prone to lung infections supervening on their state of alveolar proteinosis7,8 and die prematurely with a variety of infections.9
The present data reinforce previous observations19 21 that dramatic serum G-CSF responses occur following the injection of endotoxin, with levels reaching more than 1 μg/mL, a more than 1,000-fold rise above baseline levels. In view of the much lower concentrations of G-CSF that are adequate to elicit obvious hematopoietic responses in vivo and in vitro, such extreme elevations are remarkable and, if this type of response has a design purpose, it will be of interest to determine what type of cellular responses actually require such extreme concentrations.
The present data show that both GM-CSF and G-CSF levels respond dramatically to the injection of endotoxin or local infections, but with a differing pattern, local GM-CSF responses being higher and systemic responses lower than G-CSF responses. The data from βc−/− mice suggest that much of the CSF released or produced promptly binds to receptor-bearing cells. This will lead to activation of the receptor-bearing cells and is thus a rapid and effective signaling response able to activate appropriate hematopoietic cells in response to microorganisms.
The phenomenon of receptor-mediated removal from the circulation of GM-CSF is likely to apply equally to other comparable regulators and should caution against overinterpretation of the significance of observed regulator levels in the serum both in normal and abnormal situations.
Supported by the Carden Fellowship Fund of the Anti-Cancer Council of Victoria; the National Health and Medical Research Council, Canberra; the AMRAD Corporation, Melbourne, Australia; and Grant No. CA-22556 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Donald Metcalf, MD, The Walter and Eliza Hall Institute of Medical Research, PO Royal Melbourne Hospital, Victoria 3050, Australia.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal