Abstract
Multiple myeloma (MM) cells express idiotypic proteins and other tumor-associated antigens which make them ideal targets for novel immunotherapeutic approaches. However, recent reports show the presence of Kaposi’s sarcoma herpesvirus (KSHV) gene sequences in bone marrow dendritic cells (BMDCs) in MM, raising concerns regarding their antigen-presenting cell (APC) function. In the present study, we sought to identify the ideal source of DCs from MM patients for use in vaccination approaches. We compared the relative frequency, phenotype, and function of BMDCs or peripheral blood dendritic cells (PBDCs) from MM patients versus normal donors. DCs were derived by culture of mononuclear cells in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4. The yield as well as the pattern and intensity of Ag (HLA-DR, CD40, CD54, CD80, and CD86) expression were equivalent on DCs from BM or PB of MM patients versus normal donors. Comparison of PBDCs versus BMDCs showed higher surface expression of HLA-DR (P = .01), CD86 (P = .0003), and CD14 (P = .04) on PBDCs. APC function, assessed using an allogeneic mixed lymphocyte reaction (MLR), demonstrated equivalent T-cell proliferation triggered by MM versus normal DCs. Moreover, no differences in APC function were noted in BMDCs compared with PBDCs. Polymerase chain reaction (PCR) analysis of genomic DNA from both MM patient and normal donor DCs for the 233-bp KSHV gene sequence (KS330233) was negative, but nested PCR to yield a final product of 186 bp internal to KS330233 was positive in 16 of 18 (88.8%) MM BMDCs, 3 of 8 (37.5%) normal BMDCs, 1 of 5 (20%) MM PBDCs, and 2 of 6 (33.3%) normal donor PBDCs. Sequencing of 4 MM patient PCR products showed 96% to 98% homology to the published KSHV gene sequence, with patient specific mutations ruling out PCR artifacts or contamination. In addition, KHSV-specific viral cyclin D (open reading frame [ORF] 72) was amplified in 2 of 5 MM BMDCs, with sequencing of the ORF 72 amplicon revealing 91% and 92% homology to the KSHV viral cyclin D sequence. These sequences again demonstrated patient specific mutations, ruling out contamination. Therefore, our studies show that PB appears to be the preferred source of DCs for use in vaccination strategies due to the ready accessibility and phenotypic profile of PBDCs, as well as the comparable APC function and lower detection rate of KSHV gene sequences compared with BMDCs. Whether active KSHV infection is present and important in the pathophysiology of MM remains unclear; however, our study shows that MMDCs remain functional despite the detection of KSHV gene sequences.
MULTIPLE MYELOMA (MM) is a fatal cancer, and will be responsible for 11,300 (2%) of cancer deaths in 1998.1 Despite the use of aggressive therapy up-front, including high-dose therapy and transplantation, the median overall survival does not exceed 3 to 4 years.2 Recent advances in our understanding of disease biology and pathogenesis3 have not yet translated to the bedside. However, MM would appear to be an ideal tumor for novel immune therapeutic strategies because in vitro, animal, and early clinical trials have shown that it may be possible to induce both allogeneic and autologous immune responses to MM cells.4-8 Evidence for allogeneic anti-MM immunity is twofold: immunization of the allogeneic marrow donor against the patient’s idiotypic protein results in the transfer of donor anti-idiotype–specific immunity at the time of subsequent allografting7; and donor lymphocyte infusions can mediate a graft-versus-myeloma effect that effectively treats relapsed MM post allografting.4,9 Early attempts to generate autologous immunity to MM have included immunization with either idiotypic protein or dendritic cells (DCs) pulsed with idiotype.6,8 The use of DCs as carriers of tumor-associated antigens in such immune-based vaccination strategies is based on their unique capacity to take up, process, and present Ags, triggering a T-cell response in otherwise immune-compromised tumor-bearing hosts. In patients with MM, a recent report shows that functionally pure DCs for use in such immune therapies can be readily generated from apheresis cells using a combination of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor-α (TNF-α).10
A potential limitation of immune-based strategies in MM stems from recent reports that cells of DC lineage may be infected with Kaposi’s sarcoma herpesvirus (KSHV). In particular, Rettig et al11demonstrated the presence of KSHV DNA, using polymerase chain reaction (PCR) with primers that detect KSHV gene sequence (KS330233), in long-term bone marrow stromal cells (BMSCs) from patients with MM. In this study, KSHV PCR+ cells were identified to be of DC lineage by virtue of expression of CD83, fascin, and CD68 coupled with lack of CD31 and CD34 antigens. The detection of KSHV in MM has been shrouded in controversy and several groups have reported conflicting results. Three subsequent reports have detected KSHV DNA, using in situ hybridization and PCR, within BM core biopsy specimens from patients with MM.12-14 In contrast, several groups failed to detect KSHV DNA in either freshly isolated BM mononuclear cells (BMMCs), BMSCs, or BM biopsy specimens from patients with MM,15-18 and several other studies have not detected antibodies to KSHV in sera from MM patients despite humoral responses to other herpesviruses.15,16,19-23 In an attempt to confirm the results of Rettig et al,11 our prior studies included PCR amplification of KS330233 in DNA from MM BMSCs.24 Our results showed 92% of MM long-term BMSC samples to be positive for open reading frame (ORF) 26 KSHV gene sequence in a nested PCR reaction. In this study using a nested PCR approach, we have amplified T 1.1, another KSHV-specific gene sequence, and viral cyclin D. However, despite PCR detection of KSHV gene sequences, antibodies for both latent and lytic phase infection were negative.
In the report by Rettig et al11 and our prior studies,24 KSHV gene sequence positive cells in MM have the cell-surface phenotype of DCs. However, this is also controversial because two recent reports have suggested that clinical-grade functional DCs generated from peripheral blood (PB) from patients with MM are not infected with KSHV, based on lack of KS330233PCR amplification product.25,26 Given the potential for innovative immune-based vaccination strategies in MM and the controversy as to whether KSHV is present in MM DCs or affects their function, we in the present study compared the relative frequency, phenotype, and function of BMDCs or PBDCs obtained from MM patients versus normal donors. An important endpoint was to determine the ideal source of DCs, ie, BM versus PB, for vaccination strategies in MM. DCs were derived from BM of 18 MM patients and 8 normal donors and from PB of 5 MM patients and 6 normal donors by culture of mononuclear cells in the presence of GM-CSF and IL-4. The yield of DCs was not statistically different from MM BM than from normal donor BM (P = .19), with 107 DCs expressing HLA class II, CD40, CD80, and CD86 antigens readily obtainable from either source. There were no differences in either the pattern or intensity of Ag expression on PBDCs from MM patients versus normal donors, but HLA-DR (P = .01), CD86 (P = .0003), and CD14 (P = .04) were more highly expressed on PBDCs than on BMDCs. Importantly, DCs from either BM or PB of MM patients showed APC function equivalent to normal donor DCs in allogeneic mixed lymphocyte reaction (MLR) at DC:T-cell ratios of 1:20 to 1:180. Although PCR analysis of genomic DNA from both MM and normal donor DCs using primers specific for KS330233 was negative, nested PCR using primers specific for a 186-bp product internal to KS330233was positive in 16 of 18 (88.8%) MM BMDCs, 3 of 8 (37.5%) normal BMDCs, 1 of 5 (20%) MM PBDCs, and 2 of 6 (33.3%) normal donor PBDCs. Sequencing of the 186-bp product from 4 MM patients and 1 normal donor showed 96% to 98% homology to the published KSHV sequence,27 and patient-specific mutations were noted in all samples, ruling out the possibility of artifact or contamination. The amplification of viral cyclin D sequences28 in 2 MM BMDCs, with sequencing demonstrating 91 and 92% homology to the viral cyclin D gene sequence, further supports the presence of KSHV gene. Therefore, our study shows that DCs can be readily obtained either from the BM or PB of MM patients that are phenotypically and functionally equivalent to DCs from normal donors. Most importantly, these DCs remain functional, despite the presence of KSHV gene sequences, providing the rationale for novel immune-based therapeutic strategies in MM.
MATERIALS AND METHODS
Patients.
BM aspirate samples were freshly obtained from 8 normal donors and 18 MM patients after written informed consent according to institutional guidelines. There were 13 men and 5 women with a median age of 57 (range, 38 to 77) years (Table 1). The majority of patients presented with advanced disease at diagnosis: Durie-Salmon stage IIA (3 patients), IIIA (11 patients), and IIIB (3 patients). A single patient presented with plasmacytoma originating in bone. Therapy included combination chemotherapy as well as high-dose alkylating agents with or without total body irradiation followed by autologous or allogeneic stem cell grafting; 2 patients were untreated. The median time from diagnosis to BM sampling was 13 (range, 0 to 50) months. The percentage of monoclonal plasma cells in BM biopsy specimens varied as follows: <5% (10 patients); 30% to 40% (3 patients); 50% to 60% (1 patient); 70% (2 patients); and 90% (2 patients). PB samples were obtained from 5 of these 18 MM patients, as well as from 6 normal donors.
Patient Characteristics
| Patient No. . | Age . | Stage Dx . | BM Status (%plasma cells) . | Time From Diagnosis (mo) . | Prior Therapy . |
|---|---|---|---|---|---|
| 1 | 62 | IIIA | 30-40 | 3 | D/VAD |
| 2 | 45 | IIIA | <5 | 28 | VAD/allogeneic BMT |
| 3 | 50 | IIA | <5 | 7 | D |
| 4 | 43 | IIA | <5 | 18 | D/C/EDAP/CD34+ PBSCT |
| 5 | 66 | IIIA | <5 | 5 | D/VAD |
| 6 | 49 | IIIA | 30 | 15 | D/CD34+ PBSCT |
| 7 | 52 | IIIA | <5 | 11 | MP/D |
| 8 | 38 | IIIA | <5 | 13 | VAD/CD34+ PBSCT |
| 9 | 60 | IIIB | 70 | 0 | None |
| 10 | 56 | IIIA | <5 | 39 | VAD/allogeneic BMT |
| 11 | 63 | IIIA | 30 | 10 | C |
| 12 | 57 | IIA | <5 | 10 | VAD/CD34+ PBSCT |
| 13 | 50 | IIIB | >90 | 15 | MP/VAD/CD34+ PBSCT |
| 14 | 69 | * | <5 | 17 | XRT/VAD/CD34+ PBSCT |
| 15 | 58 | IIIB | 50-60 | 50 | MP/VAD/C |
| 16 | 62 | IIIA | 70-80 | 30 | VAD/CD34+ PBSCT |
| 17 | 60 | IIIA | <5 | 24 | VAD/CD34+ PBSCT |
| 18 | 77 | IIIA | 80-90 | 0 | None |
| Patient No. . | Age . | Stage Dx . | BM Status (%plasma cells) . | Time From Diagnosis (mo) . | Prior Therapy . |
|---|---|---|---|---|---|
| 1 | 62 | IIIA | 30-40 | 3 | D/VAD |
| 2 | 45 | IIIA | <5 | 28 | VAD/allogeneic BMT |
| 3 | 50 | IIA | <5 | 7 | D |
| 4 | 43 | IIA | <5 | 18 | D/C/EDAP/CD34+ PBSCT |
| 5 | 66 | IIIA | <5 | 5 | D/VAD |
| 6 | 49 | IIIA | 30 | 15 | D/CD34+ PBSCT |
| 7 | 52 | IIIA | <5 | 11 | MP/D |
| 8 | 38 | IIIA | <5 | 13 | VAD/CD34+ PBSCT |
| 9 | 60 | IIIB | 70 | 0 | None |
| 10 | 56 | IIIA | <5 | 39 | VAD/allogeneic BMT |
| 11 | 63 | IIIA | 30 | 10 | C |
| 12 | 57 | IIA | <5 | 10 | VAD/CD34+ PBSCT |
| 13 | 50 | IIIB | >90 | 15 | MP/VAD/CD34+ PBSCT |
| 14 | 69 | * | <5 | 17 | XRT/VAD/CD34+ PBSCT |
| 15 | 58 | IIIB | 50-60 | 50 | MP/VAD/C |
| 16 | 62 | IIIA | 70-80 | 30 | VAD/CD34+ PBSCT |
| 17 | 60 | IIIA | <5 | 24 | VAD/CD34+ PBSCT |
| 18 | 77 | IIIA | 80-90 | 0 | None |
DCs were generated from a range of MM patients during different stages of disease with variable treatment histories. Treatment included D (Dexamethasone), VAD (Vincristine, Adriamycin, Dexamethasone), EDAP (Etoposide, Dexamethasone, Ara-C, and Cisplatinum), C (Cyclophosphamide), MP (Melphalan, Prednisolone), BMT (bone marrow transplant), PBSCT (peripheral blood stem cell transplant), and XRT (Radiotherapy).
Patient 14 had a plasmacytoma of the bone.
Culture of DCs.
BMMCs were isolated by centrifugation on Ficoll-Paque (Pharmacia Biotech, Piscataway, NJ). BMMCs were suspended in RPMI 1640 medium containing 10% heat-inactivated human AB serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (10% RPMI); plated on 6 well, flat-bottomed tissue culture plates (Becton Dickinson, Franklin Lakes, NJ); and incubated for 1 to 2 hours at 37°C in a humidified 5% CO2 atmosphere. Nonadherent cells were harvested, and fresh 10% RPMI medium containing GM-CSF (1,000 U/mL) and IL-4 (800 U/mL) (Genzyme, Boston, MA) was added to the adherent layer. After 12 to 14 days, loosely adherent cells were harvested and used for further studies. DCs were also cultured either from PB progenitor cells (PBPCs), collected by apheresis after mobilization with cyclophosphamide and G-CSF in 3 MM patients, or from PBMCs in 2 MM patients. Cells were washed three times, resuspended in 10% RPMI medium, and incubated at 37°C in a 5% CO2 atmosphere in 750-mL Falcon tissue-culture flasks (Becton Dickinson). After overnight incubation, adherent cells were collected and resuspended in fresh 10% RPMI medium with GM-CSF and IL-4 as described above, and plated on 6-well flat-bottomed tissue culture plates. Loosely adherent cells were harvested after 8 to 10 days for further studies.
Characterization of DC phenotype.
Indirect immunofluorescence flow cytometry using the Coulter Epics XL (Coulter Corp, Miami, FL) was done to assay for expression of DC lineage Ags (CD14, CD40, CD80, CD86, and HLA-DR [Pharmingen, San Diego, CA] and CD83 [Immunotech, Marseille, France]) as well as MM-associated Ags (CD38). Cells were washed in phosphate-buffered saline (PBS) and incubated in PBS with 20% human AB serum at room temperature for 20 minutes to eliminate nonspecific Fc receptor binding. After washing with PBS, cells were incubated with primary murine monoclonal antibodies (MoAbs) reactive with the above Ags for 30 minutes on ice. After several washes, the cells were developed with goat anti-murine antibody conjugated with fluorescein isothiocyanate (FITC). Cells were then washed and fixed with 2% paraformaldehyde and evaluated by flow cytometry.
APC function of DCs in allogeneic MLR.
We next examined the function of DCs from MM and normal BM and PB as APCs in allogeneic MLRs. Graded numbers of irradiated (3 Gy) DCs were added to 2 × 105 allogeneic T cells in 96-well U-bottomed culture plates for 5 days. T cells were obtained from PBMCs using T-cell enrichment columns (R & D Systems, Minneapolis, MN) via high-affinity negative selection. T-cell proliferation was determined by measuring uptake of tritiated thymidine (3[H]TdR) by cells pulsed with 1 μCi of 3[H]TdR (GBq/mmol; Du Pont-New England Nuclear, Wilmington, DE) for the last 12 hours of 5-day cultures. Results were expressed as mean counts per minute (cpm) ±SD in triplicates.
Detection of KSHV DNA sequences by PCR.
DNA was extracted from BMDCs derived from 8 normal donors and 18 MM patients, as well as from PBDCs derived from 6 normal donors and 5 MM patients, using a Promega kit (Madison, WI) according to the manufacturer’s instructions. PCR was first performed using primers that recognize and amplify a region within the KSHV gene sequence to yield a 233-bp fragment (KS330233), as reported by Chang et al.27 An aliquot from the first PCR amplification product was used as a template in a subsequent nested PCR using primers (forward primer, 5′-CTC GAA TCC AAC GGA TTT GA-3′; reverse primer, 5′-ATA TGT GCG CCC CAT AAA TG-3′) that recognize sequences internal to this 233-bp fragment to yield a final PCR product of 186 bp. The thermal cycling conditions, similar for both initial and nested PCR reactions, were as follows: 95°C for 3 minutes (1 cycle); 94°C for 1 minute, 60°C for 1 minute, 72°C for 1 minute (45 cycles); and 72°C extension for 5 minutes (1 cycle). Each PCR reaction uses 800 ng of genomic DNA and 30 pmol of each primer. The PCR reactions were performed in Ready-To-Go PCR beads (Pharmacia Biotech AB, Uppsala, Sweden) with a final volume of 25 μL reaction mixture, and amplifications were performed in a Perkin-Elmer 480 Thermocycler (Applied Biosystems, Foster City, CA). Fifteen microliters of the PCR product was analyzed on 1.5% agarose gels containing ethidium bromide and photographed under UV light. Genomic DNA from body cavity–based lymphoma-1 (BCBL-1) cell line infected with KSHV29 served as a positive control for the detection of KSHV gene sequences. PCR with β-actin primers was performed on all samples to assure quality of genomic DNA.
To further assay for the presence of KSHV gene sequences in DCs, we performed additional PCR on genomic DNA from 5 MM BMDCs using primers which amplify KSHV specific viral cyclin D.28 A nested PCR approach was used to yield a 115-bp product. The thermal cycling conditions were as previously described.
PCR product purification and sequencing.
PCR products for the 186-bp amplicon from 4 MM BMDCs and 1 normal BMDC were purified using the QIAquick Gel Extraction Kit (Qiagen, Chatsworth, CA). Sequencing was done using the Sanger method,30 with dye-labeled dideoxy nucleotides as terminators. Samples were analyzed on an Applied Biosystems model 373A automated DNA sequencer.31 Viral cyclin D 116-bp amplicon was similarly purified and sequenced from 2 MM BMDCs.
Statistical methods.
A Student’s t-test was used to evaluate for statistically significant differences between MM BMDCs and normal BMDCs for mean fluorescence intensity of cell-surface Ag expression and MLR activity. A similar analysis was performed for MM PBDCs and normal PBDCs. Finally, a comparison was made between BMDCs versus PBDCs. Results were expressed as means ± SD; P < .05 was considered significant.
RESULTS
Dendritic cell yield from normal versus MM BMMCs.
BMMCs from 8 normal donors and 18 MM patients were cultured with IL-4 and GM-CSF to generate DCs. A mean of 7.1 × 107(range, 4.2 to 11.2 × 107) BMMCs from normal donors and a mean of 3.9 × 107 (range, 1.9 to 9.7 × 107) BMMCs from MM patients were plated. The final DC yield was calculated as the number of DCs (HLA class II+ CD40+CD80+CD86+cells)/number of adherent cells. The DC yield from normal donors was 49.5% (range, 21.6% to 74.4%), which was not statistically different (P = .19) from that in MM patients (26.8% [range, 8.8% to 55.4%]).
Phenotypic characterization of DCs by flow cytometry.
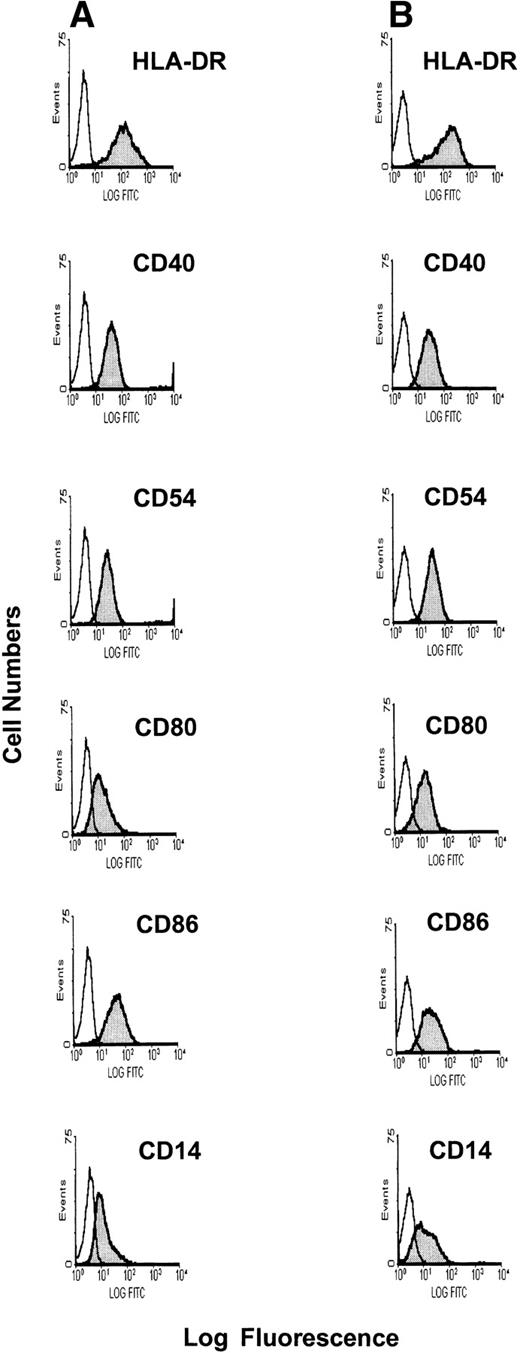
The cell-surface phenotype of DCs was assessed by indirect immunofluorescence flow cytometry. After culture of BMMCs in the presence of GM-CSF and IL-4 for 12 to 14 days, the loosely adherent cell population had a typical veiled DC morphology. As can be seen in Fig 1, the majority of DCs expressed HLA class II and the costimulatory molecules CD40, CD80, and CD86. Few, if any, DCs stained for CD14, a mature monocyte marker expressed on some immature DC. Staining with anti-CD38 MoAb to identify tumor cell contamination was negative, as was anti-CD83, a mature DC marker (data not shown). The expression of both class II MHC molecules and costimulatory molecules was comparable in BMDCs derived from MM patients (Fig 1A) versus normal donors (Fig 1B). There were no statistical differences either in the pattern or mean fluorescence intensity of cell-surface Ag expression (Table2) on these BMDC populations.
Phenotypic profile of BMDCs generated from MM patients versus normal donors. BMDCs were generated by culture of BMMCs from MM patients and normal donors in the presence of GM-CSF and IL-4 for 12 to 14 days. Phenotype was determined using indirect immunofluorescence flow cytometry with MoAbs specific for CD14, CD40, CD80, CD86, and HLA-DR developed with goat anti-mouse FITC antibody for MM BMDCs (A) and normal donor BMDCs (B).
Phenotypic profile of BMDCs generated from MM patients versus normal donors. BMDCs were generated by culture of BMMCs from MM patients and normal donors in the presence of GM-CSF and IL-4 for 12 to 14 days. Phenotype was determined using indirect immunofluorescence flow cytometry with MoAbs specific for CD14, CD40, CD80, CD86, and HLA-DR developed with goat anti-mouse FITC antibody for MM BMDCs (A) and normal donor BMDCs (B).
Phenotypic Profile of DCs From MM Patients Versus Normal Donors
| Cell-Surface Antigen . | Normal Donor DC (% of Ag expression) . | MMDC (% of Ag expression) . | P Value . |
|---|---|---|---|
| BM | |||
| HLA-DR | 88.6 ± 5.1 | 87.4 ± 5.4 | .67 |
| CD40 | 64 ± 19.8 | 79.9 ± 11.8 | .44 |
| CD54 | 77.5 ± 12.2 | 72.7 ± 10.5 | .59 |
| CD80 | 69.8 ± 10.8 | 75.0 ± 10.1 | .43 |
| CD86 | 54.8 ± 30.9 | 65.8 ± 16.3 | .48 |
| CD14 | 8.8 ± 11.5 | 8.3 ± 6.5 | .99 |
| CD38 | 3.3 ± 1.5 | 2.6 ± 0.9 | .46 |
| PB | |||
| HLA-DR | 94.7 ± 2.1 | 91.5 ± 5.1 | .24 |
| CD40 | 76.9 ± 26.4 | 80.7 ± 15.3 | .87 |
| CD54 | 84.9 ± 15.1 | 58.2 ± 26.0 | .14 |
| CD80 | 60.2 ± 32.1 | 47.3 ± 23.0 | .56 |
| CD86 | 91.9 ± 3.9 | 88.1 ± 7.4 | .41 |
| CD14 | 47.1 ± 13.7 | 35.3 ± 19.9 | .44 |
| CD38 | 3.5 ± 0.7 | 2.3 ± 0.6 | .18 |
| Cell-Surface Antigen . | Normal Donor DC (% of Ag expression) . | MMDC (% of Ag expression) . | P Value . |
|---|---|---|---|
| BM | |||
| HLA-DR | 88.6 ± 5.1 | 87.4 ± 5.4 | .67 |
| CD40 | 64 ± 19.8 | 79.9 ± 11.8 | .44 |
| CD54 | 77.5 ± 12.2 | 72.7 ± 10.5 | .59 |
| CD80 | 69.8 ± 10.8 | 75.0 ± 10.1 | .43 |
| CD86 | 54.8 ± 30.9 | 65.8 ± 16.3 | .48 |
| CD14 | 8.8 ± 11.5 | 8.3 ± 6.5 | .99 |
| CD38 | 3.3 ± 1.5 | 2.6 ± 0.9 | .46 |
| PB | |||
| HLA-DR | 94.7 ± 2.1 | 91.5 ± 5.1 | .24 |
| CD40 | 76.9 ± 26.4 | 80.7 ± 15.3 | .87 |
| CD54 | 84.9 ± 15.1 | 58.2 ± 26.0 | .14 |
| CD80 | 60.2 ± 32.1 | 47.3 ± 23.0 | .56 |
| CD86 | 91.9 ± 3.9 | 88.1 ± 7.4 | .41 |
| CD14 | 47.1 ± 13.7 | 35.3 ± 19.9 | .44 |
| CD38 | 3.5 ± 0.7 | 2.3 ± 0.6 | .18 |
DCs from MM patients and normal donors, from either BM or PB, were tested by immunofluorescence for cell-surface expression of HLA-DR, CD40, CD54, CD80, CD86, CD14, and CD38. Statistical differences were tested using the Student’s t-test.
We next determined the cell-surface phenotype of PBDCs from 6 normal donors and 5 MM patients. After culturing PBMCs in the presence of GM-CSF and IL-4 for 8 to 10 days, PBDCs were harvested and stained for expression of HLA-DR, CD40, CD54, CD80, CD86, CD14, CD83, and CD38 Ags. All of these Ags except CD38 and CD83 were expressed on PBDCs from both MM patients (Fig 2A) and normal donors (Fig 2B). As noted for BMDCs, there were no significant differences in the profile of Ag expression on PBDCs from normal donors versus PBDCs from MM patients (Table 2).
Phenotypic profile of PBDCs generated from MM patients versus normal donors. PBDCs were generated by culture of PBDCs from MM patients and normal donors for 8 to 10 days in the presence of GM-CSF and IL-4. Phenotype was determined by indirect immunofluorescence flow cytometry with MoAbs specific for CD14, CD40, CD54, CD80, CD86, and HLA-DR developed with goat anti-mouse FITC antibody for MM PBDCs (A) and normal donor PBDCs (B).
Phenotypic profile of PBDCs generated from MM patients versus normal donors. PBDCs were generated by culture of PBDCs from MM patients and normal donors for 8 to 10 days in the presence of GM-CSF and IL-4. Phenotype was determined by indirect immunofluorescence flow cytometry with MoAbs specific for CD14, CD40, CD54, CD80, CD86, and HLA-DR developed with goat anti-mouse FITC antibody for MM PBDCs (A) and normal donor PBDCs (B).
We next compared BMDCs with PBDCs as sources for generation of DCs in numbers sufficient for clinical use. Specifically, because there were no differences in the phenotype of BMDCs or PBDCs from normal donors versus MM patients, we have compared BMDCs with PBDCs. As can be seen in Table 3, HLA-DR (P = .01), CD86 (P = .0003), and CD14 (P = .04) were more highly expressed on PBDCs than on BMDCs.
Phenotypic Profile of DCs From BM Versus PB
| Cell-Surface Antigen . | BM (% of Ag expression) . | PB (% of Ag expression) . | P Value . |
|---|---|---|---|
| HLA-DR | 87.8 ± 5.2 | 92.1 ± 4.2 | .01 |
| CD40 | 69.0 ± 21.1 | 79.2 ± 17.2 | .40 |
| CD54 | 74.3 ± 10.6 | 69.7 ± 24.8 | .65 |
| CD80 | 73.6 ± 10.2 | 54.7 ± 27.2 | .11 |
| CD86 | 62.8 ± 21.0 | 88.1 ± 10.3 | .0003 |
| CD14 | 8.8 ± 7.8 | 31.8 ± 24.4 | .04 |
| CD38 | 2.8 ± 1.1 | 2.8 ± 0.86 | .9 |
| Cell-Surface Antigen . | BM (% of Ag expression) . | PB (% of Ag expression) . | P Value . |
|---|---|---|---|
| HLA-DR | 87.8 ± 5.2 | 92.1 ± 4.2 | .01 |
| CD40 | 69.0 ± 21.1 | 79.2 ± 17.2 | .40 |
| CD54 | 74.3 ± 10.6 | 69.7 ± 24.8 | .65 |
| CD80 | 73.6 ± 10.2 | 54.7 ± 27.2 | .11 |
| CD86 | 62.8 ± 21.0 | 88.1 ± 10.3 | .0003 |
| CD14 | 8.8 ± 7.8 | 31.8 ± 24.4 | .04 |
| CD38 | 2.8 ± 1.1 | 2.8 ± 0.86 | .9 |
Dendritic cells from BM and PB were compared with respect to cell-surface expression of HLA-DR, CD40, CD54, CD80, CD86, CD14, and CD38 by flow cytometry, using the Student’s t-test.
APC function of DCs in allogeneic MLR.
To determine whether these phenotypic differences correlated with variations in APC functional repertoire, we next assessed the relative function of BMDCs and PBDCs, derived from both normal donors and MM patients, as APCs in MLR. T cells were cultured with graded numbers of irradiated DCs for 5 days, and T-cell proliferation was measured by3[H]TdR uptake. Results from 3 normal BMDCs and 3 MM BMDCs are shown in Fig3A.3[H]TdR uptake was not statistically different for normal BMDCs versus MM BMDCs at DC:T-cell ratios of 1:20 (P = .85), 1:60 (P = .86), 1:120 (P = .69), and 1:180 (P = .25).
APC function of DCs in allogeneic MLR. Graded numbers of irradiated DCs (3 Gy) were cultured with allogeneic T cells, and proliferation measured by 3[H]TdR incorporation during the last 12 hours of 5 day cultures. BMDCs from 3 normal donors (BM1, BM2, BM3) and 3 MM patients (MM1, MM2, MM3) are shown in (A), and PBDCs from 2 normal donors (PB1, PB2) and 3 MM patients (MM1, MM2, MM3) are shown in (B).
APC function of DCs in allogeneic MLR. Graded numbers of irradiated DCs (3 Gy) were cultured with allogeneic T cells, and proliferation measured by 3[H]TdR incorporation during the last 12 hours of 5 day cultures. BMDCs from 3 normal donors (BM1, BM2, BM3) and 3 MM patients (MM1, MM2, MM3) are shown in (A), and PBDCs from 2 normal donors (PB1, PB2) and 3 MM patients (MM1, MM2, MM3) are shown in (B).
PBDCs from normal donors and PBDCs from MM patients were similarly tested for their APC function in MLR (Fig 3B). As noted for BMDCs, there were no statistical differences in 3[H]TdR uptake at DC:T-cell ratios of 1:20 (P = .8), 1:60 (P = .72), 1:120 (P = .81), and 1:180 (P = .15) for normal donor PBDCs versus MM patient PBDCs. We next compared APC function of BMDCs with PBDCs, including both MM and normal DCs. APC function of BMDCs and PBDCs were equivalent at DC:T-cell ratios of 1:20 (P = .38), 1:60 (P = .33), 1:120 (P = .49), and 1:180 (P = .70).
PCR analysis for KSHV gene sequences.
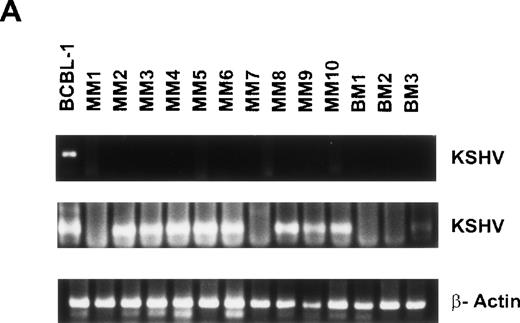
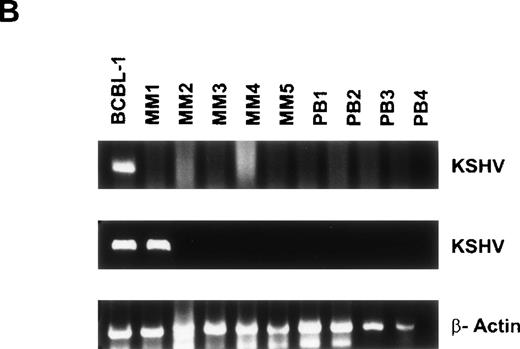
Given recent reports suggesting detection of KSHV gene sequences in MM BMDCs,11-14 we next isolated DNA from BMDCs or PBDCs to assay for the presence of KSHV gene sequence by PCR. Initial PCR was performed using primers specific for the 233-bp KSHV gene sequence (KS330233),27 followed by a nested PCR with a second set of primers to yield a 186-bp amplification product internal to the 233-bp fragment, as in our prior studies.24 Genomic DNA from the KSHV-infected BCBL-1 cell line served as a positive control.29 Representative results from 10 MM BMDCs and 3 normal BMDCs are shown in Fig 4A. All samples are negative for the 233-bp product, except BCBL-1 (lane 1), which represents the positive control. Nested PCR yields a 186-bp amplification product detectable in KSHV infected BCBL-1 cell line (lane 1) and in DCs generated from 8 of 10 MM BMs (lanes 3 through 6, 7, 9, 10, and 11), which is absent in normal donor BMDCs (lanes 12 through 14). Amplification with β actin primers confirmed adequacy and integrity of DNA in each lane. Overall, KSHV-specific amplicons were detected by nested PCR in BMDCs from 16 of 18 (88.8%) MM patients and 3 of 8 (37.5%) normal donors.
Detection of KSHV gene sequences in BMDCs and PBDCs from MM patients and normal donors. DNA isolated from BMDCs from 15 MM patients and 6 normal donors was assayed for KSHV gene sequences by PCR using primers that amplify KS330233, followed by a nested PCR using primers internal to this sequence to yield a final PCR product of 186 bp. Results from 10 MM patient BMDCs (lanes 2 through 11) and 3 normal donor BMDCs (lanes 12 through 14) are shown in (A). (B) Results of PCR on PBDCs DNA from 5 MM patients (lanes 2 through 6) and 4 normal donors (lanes 7 through 10). In each case DNA from BCBL-1 cell line (lane 1) served as the positive control. β-Actin was amplified in all samples to ensure adequacy and integrity of DNA.
Detection of KSHV gene sequences in BMDCs and PBDCs from MM patients and normal donors. DNA isolated from BMDCs from 15 MM patients and 6 normal donors was assayed for KSHV gene sequences by PCR using primers that amplify KS330233, followed by a nested PCR using primers internal to this sequence to yield a final PCR product of 186 bp. Results from 10 MM patient BMDCs (lanes 2 through 11) and 3 normal donor BMDCs (lanes 12 through 14) are shown in (A). (B) Results of PCR on PBDCs DNA from 5 MM patients (lanes 2 through 6) and 4 normal donors (lanes 7 through 10). In each case DNA from BCBL-1 cell line (lane 1) served as the positive control. β-Actin was amplified in all samples to ensure adequacy and integrity of DNA.
DNA isolated from 5 MM PBDCs and 6 normal donor PBDCs was similarly tested for the presence of the KSHV gene sequences. Results from 4 normal donor PBDCs and 5 MM patient PBDCs are shown in Fig 4B. Lane 1 represents the BCBL-1 cell line, which served as a positive control. Lanes 2 through 6 represent DNA from MM patient PBDCs and lanes 7 through 10 represent DNA from normal donor PBDCs. All samples are negative for KS330233 except the BCBL-1 cell line (lane 1). Lanes 1 (BCBL-1) and 2 (MM PBDC) are positive for the 186-bp product of nested PCR, but the remainder of PBDCs from both MM patients and normal donors are PCR−. Amplification with β actin primers confirmed integrity of DNA. Overall KSHV gene sequences were detected in PBDCs from 1 of 5 (20%) MM patients and 2 of 6 (33%) normal donors.
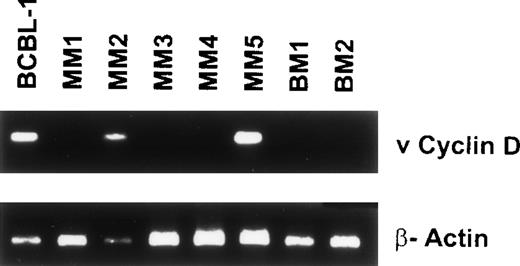
To probe for other KSHV gene sequences in BMDCs, we next performed nested PCR on genomic DNA from 5 MM BMDCs and 2 normal donor BMDCs using primers that amplify gene sequences specific for viral cyclin D (Fig 5).28 The BCBL-1 cell line (lane 1) and 2 MM patient samples (lanes 3 and 6) were positive for viral cyclin D. Amplification with β actin primers confirmed integrity of DNA.
Detection of ORF 72 (viral cyclin D) gene sequences in BMDCs from MM patients. DNA isolated from 5 MM BM DCs were assayed for ORF 72 gene sequences. BCBL-1 served as the positive control (lane 1) and lanes 2 through 6 represent MM patient samples. Lanes 7 and 8 are normal BMDC controls.
Detection of ORF 72 (viral cyclin D) gene sequences in BMDCs from MM patients. DNA isolated from 5 MM BM DCs were assayed for ORF 72 gene sequences. BCBL-1 served as the positive control (lane 1) and lanes 2 through 6 represent MM patient samples. Lanes 7 and 8 are normal BMDC controls.
Sequencing of the 186-bp product confirmed 96% to 98% homology to the published KSHV gene sequence,27 with patient-specific point mutations ruling out artifact and possible contamination. Sequencing of the viral cyclin D product confirmed 91% and 92% homology to the published sequence28 with distinct patient specific point mutations.
DISCUSSION
MM cells express specific idiotypic proteins and other associated Ags, which may serve as potential targets for immunotherapy. However, patients with MM are known to be immunocompromised, with defects in both humoral and cellular immunity.32 Moreover, early attempts at immunization with pneumococcal and other vaccines in patients with MM were unsuccessful. However, it has recently been shown that MM cells themselves can serve as effective APCs,33 and that vaccination with idiotypic protein7,8 or using DCs pulsed with idiotype6 can induce specific responses. The recent report of KSHV infection in cells of DC lineage within long-term BMSCs and BM biopsy specimens from patients with MM,11,13although controversial, suggested potential sequelae of viral infection on MM APC function. Previous studies have shown the feasibility of generating large numbers of functional DCs from apheresis cells from MM patients10,25 and have compared PBDCs from MM patients with those from normal donors.34 However, in these studies DCs were either KSHV PCR− or not examined. Given the great potential of novel immune therapeutic approaches in MM, we in the present study compared phenotype, functional repertoire, and KSHV gene expression in DCs from either BM or PB of MM patients with those derived from normal donors. We have shown that DCs from either BM or PB of patients with MM are equivalent in phenotypic profile and function with those from normal donors, despite the presence of KSHV gene sequences. These studies provide the framework for incorporation of DCs in novel immune treatment strategies for MM.
In our previous studies, we identified KSHV DNA sequences in long-term BMSC cultures from 92% (24 of 26) MM patients, in cells expressing CD68, CD83, and fascin.24 Furthermore, these PCR results were confirmed with southern blotting and DNA sequence analysis. Given these and Rettig et al’s11 prior report, we in this study examined DCs from BM and PB cultured with GM-CSF and IL-4, in terms of phenotype, function, and KSHV gene expression. Patients at various stages of therapy, including those who were heavily treated and had persistent infiltration of BM with MM cells, were examined. Our results demonstrate that DCs can be readily generated from either BM or PB of MM patients in the presence of IL-4 and GM-CSF. These DCs are monocyte derived with an immature DC phenotype, evidenced by lack of cell-surface expression of CD83. Interestingly, we found that generation of BM-derived DCs, identified by both antigenic profile and functional repertoire, required 12 to 14 days of culture compared with only 7 to 10 days for PBDCs. Importantly, DCs from either source were equivalent in terms of phenotypic profile and APC function; moreover, they were equivalent to DCs derived from either BM or PB from normal donors. Therefore, our studies show that the presence of KSHV gene sequences does not adversely impact on function of DCs grown from either BM or PB using GM-CSF and IL-4.
Whether KSHV gene sequences are present in MM remains controversial. We previously found KSHV gene sequences to be present in the majority (92%) of long-term MM BMSCs.24 In this study DNA sequencing confirmed specificity of the PCR results. In addition, amplification and sequencing of other KSHV gene sequences including T1.1 and viral cyclin D further confirmed the presence of KSHV DNA. In the present study, we have amplified two distinct sequences in the KSHV genome, ORF 26 and viral cyclin D, using a nested PCR approach. Stringent PCR conditions were used to prevent contamination. Sequencing of the PCR products in 5 MM patients and 1 normal donor for orf 26 and 2 MM patients for viral cyclin D confirmed specificity of the PCR. In addition, patient-specific mutations were noted, ruling out PCR contamination. Therefore, these results confirm the detection of KSHV gene sequences by PCR and are consistent with prior reports.11,13,14 The contrasting results reported by several groups11-13,15-17,26 may be attributable to differences in PCR methodology and the sensitivity of the techniques used. For example, the recent report25 that functional clinical-grade DCs from patients with MM are not infected with KSHV used PCR for KS330233, which also failed to detect KSHV gene sequences in our study. Only with nested PCR did we detect KSHV sequences in MMDCs. It is possible that the virus is present in a very low copy number and at the threshold of PCR detection.
In contrast to the report by Rettig et al,11 we have in the present study identified the KSHV gene sequence in 3 of 8 normal BMDCs and 2 of 6 normal PBDCs, suggesting that use of nested PCR with enhanced sensitivity detects KSHV sequences in a significant fraction of normal individuals. KSHV gene sequences have also been previously identified in PBMCs of children and healthy donors.35Moreover, although controversial, this virus has also been identified in a number of tissue specimens, including lymph nodes, brain, prostate, and semen, in clinical settings other than human immunodeficiency virus infection (HIV) and immunosuppression, suggesting that it may be ubiquitous as is EBV.36-38 These findings, coupled with the lack of serologic response to KSHV in patients with MM in our previous report24 and multiple other studies,15-17,19-22 further highlight the need to look for evidence other than PCR detection to answer the important question of the role of KSHV in the pathophysiology of MM. The finding that viral IL-6, like human IL-6,39-42 induces DNA synthesis of human MM cell lines in vitro,43 suggests a potential role for KSHV in tumor cell proliferation and survival, which is the object of ongoing studies. A recent report44 showing KSHV antibodies in MM patients with the use of improved latent nuclear antigen immunoblot assay and ORF 65 immunoblot assay further supports an association between MM and KSHV infection. However, this improved assay needs to be used to confirm these results in a larger series of MM patients. Until evidence is provided demonstrating either the presence of viral transcripts and biologically active viral gene products in MM or other functional sequelae of KSHV infection, PCR detection of KSHV gene sequences must be interpreted with caution.
The lack of clinically evident anti-MM immunity in MM patients, coupled with our finding that DCs in MM are effective APCs in vitro, suggests that defects in Ag presentation may be uniquely present in vivo in these patients. For example, it is possible that DCs in MM patients may not take up, process, and present Ag due to an inhibitory effect of cytokines, ie, IL-10 or vascular endothelial growth factor secreted by tumor cells. The ability to generate large numbers DCs using different cytokine cocktails ex vivo, as described in this study, will facilitate their use in various immunization protocols.45-48 To exploit their APC function, DCs are being pulsed with whole-tumor Ag, naked DNA, or whole-tumor RNA49-51; fused with tumor cells52; or genetically modified before vaccination.53,54 Already, these modified DCs have induced Ag-specific, major histocompatibility complex–restricted cytotoxic T lymphocyte responses in animals, with associated antitumor activity in both prophylaxis and treatment models. Therefore, the stage is set to test analogous approaches in clinical trials. Indeed, vaccinations using DCs pulsed with idiotype have already triggered tumor-specific response in patients with non-Hodgkin’s lymphoma and chronic myeloid leukemia, and similar studies are presently ongoing in MM patients.55 56
Supported by National Institutes of Health Grants No. CA50947 and CA98378, and the Kraft Family Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth C. Anderson, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02215; e-mail:kenneth_anderson@dfci.harvard.edu.


![Fig. 3. APC function of DCs in allogeneic MLR. Graded numbers of irradiated DCs (3 Gy) were cultured with allogeneic T cells, and proliferation measured by 3[H]TdR incorporation during the last 12 hours of 5 day cultures. BMDCs from 3 normal donors (BM1, BM2, BM3) and 3 MM patients (MM1, MM2, MM3) are shown in (A), and PBDCs from 2 normal donors (PB1, PB2) and 3 MM patients (MM1, MM2, MM3) are shown in (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1487/4/m_blod40535003x.jpeg?Expires=1769397796&Signature=ocelzK0iY0BuUwy5O0H1s5eLKl-lP3S30XyxqFFvvGPcl1HmrxOqPP2elrcC4GXoITOkb~ANQ35ft4K5Zhw4Wpn061YP5BUkCz-Sf9laV9SI-lKBtCiaxH~oPwmaXV3ciQoWi1do6WAO1cWAMnmZupd3lxeGtktOBxzT7aFRri6iIwkHjxyPGRwueUJ1zpyw8Gk0McWVjXiYRYzRXdul21idp7fLpd8EuzltMKk6CMBxLsOFSUHnZo4uoGKIjwZanqzecnSWv3o9cjFSUyLcjX7lFP05fZO1VzA18XLUUj74G3Srff0~XhjSYa4P631rDLlZjpK-Vzy3WcGTsqzBMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal