Abstract
We investigated the mechanisms responsible for severe factor VII (FVII) deficiency in homozygous Italian patients with either Gly97Cys or Gln100Arg mutations in the second epidermal growth factor domain of FVII. Transient expression of complementary DNA coding for the mutations in COS-1 cells showed impaired secretion of the mutant molecules. Using stably transfected Chinese hamster ovary (CHO) cells, we performed pulse-chase labeling studies, immunohistochemistry, and experiments with inhibitors of protein degradation, showing that FVII-Cys97 did not accumulate intracellularly but was degraded in a pre-Golgi, nonlysosomal compartment by a cysteine protease. In stably transfected CHO cells expressing FVII-Arg100, the level of intracellular FVII was not increased by several inhibitors of protein degradation, but FVII-Arg100 was retained in the endoplasmic reticulum for a longer period of time than wild-type FVII. FVII-Arg100 had a lower apparent molecular weight than did wild-type FVII under nondenaturing conditions, which is attributable to misfolding due to abnormal disulfide bond formation.
HUMAN FACTOR VII (FVII) is a vitamin K–dependent glycoprotein that normally circulates in plasma at a concentration of 0.5 μg/mL.1 FVII in association with tissue factor initiates blood coagulation by activating factor IX and factor X.2,3 The mature FVII molecule is a single chain of 406 amino acids and is comprised of several discrete domains including the Gla domain, two epidermal growth factor (EGF)-like domains and a large catalytic domain.4 It undergoes several post-translational modifications before its secretion by the liver including γ-carboxylation of 10 glutamic acid residues in the Gla domain, N-glycosylation of residues Asn145 and Asn322,5 and O-glycosylation of residues Ser52 and Ser60 in the first EGF domain.6
Hereditary FVII deficiency is a rare autosomal recessive bleeding disorder.7,8 Patients with FVII deficiency have been classified with respect to the plasma level of FVII antigen (VII:Ag) or crossreacting material (CRM) as either CRM− (low or absent antigen), CRMR (reduced antigen), or CRM+ (normal antigen). More than 30 different naturally occurring mutations in the FVII gene have been reported.9Most are point mutations that alter FVII function, but others interfere with FVII biosynthesis. In this paper, we investigated the mechanisms responsible for FVII deficiency in two homozygous Italian patients who were CRM− and CRMR as a result of mutations in the molecule’s second EGF domain.
MATERIALS AND METHODS
Collection and processing of blood samples.
Blood was collected by atraumatic venipuncture into plastic tubes containing 1/10th volume 0.129 mol/L buffered trisodium citrate. Plasma was obtained by centrifugation at 2,500g for 15 minutes at 4°C, transferred into plastic tubes, and stored along with the cellular elements at −80°C until use.
FVII assays.
FVII coagulant activity (VII:C) and VII:Ag were measured by one-stage clotting assay using recombinant human tissue factor (RecombiPlastin, Ortho Diagnostic Systems, Raritan, NJ) and an enzyme-linked immunoabsorbent assay (ELISA) (American Bioproducts Co, Parsippany, NJ), respectively. A normal pool was constructed by mixing equal volumes of plasma from 30 healthy control subjects.
DNA isolation and in vitro amplification using polymerase chain reaction (PCR).
DNA was purified by standard techniques from leukocyte nuclei obtained from whole blood.10 Oligonucleotides and PCR conditions used to amplify the entire coding sequence of the FVII gene have been described in detail.11 PCR amplifications12were performed using a DNA Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT). PCR products were generated in 20 μL reaction mixtures that contained 200 ng of genomic DNA, 0.4U of Taq DNA polymerase (Perkin Elmer Cetus), oligonucleotide primers at a concentration of 0.5 μmol/L each, dNTPs at a concentration of 100 μmol/L each,1 mmol/L MgCl2, 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, and 0.01 mg/mL of autoclaved gelatin.
Cloning and sequencing of PCR fragments.
Amplified PCR fragments were purified and ligated into PT7Blue T-vectors (Novagen, Madison, WI). Cloned inserts were sequenced by the dideoxy chain termination method on an Applied Biosystems 373A DNA Sequencer (Foster City, CA). All sequence alterations were confirmed in at least two independent clones. Sequence analyses were performed using the GCG Sequence Analysis Software Package (Genetics Computer Group, Inc, Madison, WI) from the Molecular Biology Computer Research Resource (Boston University, Boston, MA).
Restriction enzyme analysis.
Restriction enzyme digestion of PCR fragments was used to detect mutations that introduced or abolished a restriction site. Ten μL of PCR–amplified product were digested with 0.5 U of enzyme in a final volume of 20 μL for 16 hours. Restriction fragments were subjected to electrophoresis in nondenaturing 8% (wt/vol) polyacrylamide gels. After completion of the electrophoretic procedure, gels were stained in 0.5 μg/mL ethidium bromide for 5 minutes and photographed under ultraviolet (UV) transillumination.
Antibodies.
Construction of expression vectors and site-directed mutagenesis.
A 2.4 Kb complete human FVII complementary DNA (cDNA)4 withEcoRI/BamHI linkers at each end was provided by Dr Earl W. Davie (Seattle, WA). This cDNA was cloned into the EcoRI site of the PT7-BlueT vector to obtain the plasmid PT7EcoRIFVIIwtEcoRI and subsequently modified as previously reported to obtain PT7Sal IFVIIwtEcoRI.14The FVII cDNA was then isolated and cloned into pED-mtxrprovided by Dr Randal J. Kaufman15 to obtain the plasmid pEDFVIIwt, a dicistronic messenger RNA mammalian expression vector carrying the dihydrofolate reductase (DHFR) gene at the 3′ open reading frame. To investigate the influence of the Gly97Cys and Gln100Arg substitutions on FVII levels, pEDFVIICys97 and pEDFVIIArg100 were obtained by site-directed mutagenesis of PT7Sal IFVIIwtEcoRI using a commercially available kit (Transformer Site-Directed Mutagenesis Kit, Clontech, Palo Alto, CA). Oligonucleotides (5′-GAACGGCTGCTGTGAGCAGTACTGCAGTGATCACACG-3′) and (5′-GAACGGCGGCTGTGAGCGGTACTGCAGTGATCACACG-3′) spanning nucleotides 7817 to 7853 of the human FVII cDNA were used to introduce a G to T at position 7824 and an A to G at position 7834 (bold letters) coding for Gly97Cys or Gln100Arg, respectively. These primers also introduced aBclI restriction site (underlined), arising from a silent GAC to GAT mutation at nucleotide 7847, to facilitate screening for clones carrying the mutations. The mutant FVII cDNA were then inserted into the pED expression vector to obtain pEDFVIICys97 and pEDFVIIArg100.
Several restriction enzyme digests were performed to confirm that we had produced vectors containing the mutations. A SalI-EcoRI digest released a 2.4 kb fragment containing the complete FVII cDNA and 1.2 kb of the gene’s 3′ untranslated region, thereby confirming that the entire cDNA had been introduced into the vector. A HindIII digest of the vector generated three fragments of 955, 2735, and 4000 bp resulting from three sites in pED and none in the FVII cDNA. EcoRI digestion linearized the 7.7 kb construct (5.3 kb pED + insert 2.4 kb cDNA) by cleavage at a single site in pED. To confirm the presence of the mutations, we amplified from the construct a 893 bp fragment with the oligonucleotides 5′-CCCGGTCGACTCAACAGGCAGGGGCAGCACT-3′ (position −94 to −74) introducing a Sal I site (bold letters) and 5′-CAGGCGGAGCAGCG-3′ (position 10648-10661 in exon 8). This PCR product coded for the first 248 amino acids of FVII excluding exon 1b. Because the mutagenic primers introduced a BclI site in addition to the mutation, a Bcl I digest of the product gave two fragments of 695 and 198 bp for the FVIIwt construct, whereas the FVIICys97 and FVIIArg100 constructs yielded fragments of 458, 237, and 198 bp confirming that the mutations had been introduced. Moreover, because the G to T transversion at position 7824 resulting in Gly97Cys introduces a BbvI site, Bbv I digestion of the 893 bp fragment from pEDFVIICys97 led to the generation of a 713 bp product that was cleaved into pieces of 445 and 268 bp in the presence of the mutation. As the A to G transversion at position 7834 resulting in Gln100Arg introduces a BsrBI site, BsrBI digestion of the 893 bp product from pEDFVIIArg 100generates fragments of 447 and 446 bp fragments in the presence of the mutation.
Cell culture and transfection assays.
For transient transfection experiments, Monkey COS-1 cells (ATCC CRL1650; American Type Culture Collection, Rockville, MD) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 2 mmol/L L-glutamine, 10 mmol/L HEPES pH 7.2, 100 units/mL of penicillin G, 100 μg/mL streptomycin, and 5 μg/mL of vitamin K1 (AquaMEPHYTON, Merck & Co Inc, West Point, PA) in a 5% CO2atmosphere at 37°C. Twenty hours before transfection, COS-1 cells were plated on 60 mm culture dishes at a density of 1 × 106 cells/dish. Five micrograms of the pEDFVII constructs was transfected into cells by Lipofectamine (GIBCO-BRL, Gaithersburg, MD) according to the manufacturers’ instructions. After 16 hours, medium was changed, and 36 hours later, supernatants and cell lysates were harvested and assayed for VII:C and VII:Ag. Results of transient assays are expressed as the percentage of FVIIwt and represent the mean ± SE.
To obtain stable cell lines expressing recombinant FVIIwt, FVIICys97, and FVIIArg100, we used DHFR-deficient Chinese ovary (CHO) cells (CHO-DUKX-B11)16provided by Dr Barbara C. Furie (Boston, MA). These cells were grown in alpha-modified essential medium (AMEM) supplemented with 10% FBS, 2 mmol/L L-glutamine, 10 mmol/L HEPES pH 7.2, 100 units/mL of penicillin G, 100 μg/mL streptomycin, 5 μg/mL of vitamin K1, 10 μg/mL adenosine, 10 μg/mL deoxy-adenosine, and 10 μg/mL thymidine. CHO-DUKX-B11 cells were plated on 100-mm culture dishes at a density of 3 × 106 cells/dish. Transfections were performed as described above with 20 μg of plasmid and 45 μL of Lipofectamine. Two days after transfection, cells were divided at a 1 to 8 ratio and selected for DHFR expression using medium deficient in ribonucleosides and deoxyribonucleosides. Twelve days after transfection, 24 colonies were picked at random and isolated in 12-well (24 mm) plates. At day 20, when the cells achieved confluence, each well was split into two 35 mm dishes. Two days later, cell lysates of the wells were harvested and assayed for VII:Ag. A single clone stably transfected with each construct and expressing high levels of FVII was selected for further experiments. The rates of intracellular FVII synthesis for the selected clones expressing FVIIwt, FVIICys97, and FVIIArg100 were 33.3 ± 2.1, 52.5 ± 5.8, and 47.7 ± 5.5 ng/mL/h (mean ± SEM, n = 9), respectively.
Metabolic labeling studies.
Nearly confluent 60 mm dishes of CHO cells stably expressing recombinant FVII were used for pulse-chase experiments. Fresh media with FBS was added 4 hours before cells were deprived of methionine for 45 minutes and labeled for 15 minutes with 0.4 mL of methionine-free AMEM (GIBCO-BRL, Gaithersburg, MD) containing 110 μCi Expre35S Protein Labeling Mix (∼73% L-[35S]methionine and ∼22% L-[35S]cysteine; DuPont NEN Research Products, Billerica, MA) in a 5% CO2 atmosphere at 37°C. A chase was then performed in 1 mL medium containing an excess of unlabeled L-methionine (GIBCO-BRL, Gaithersburg, MD) for various time periods. At each time point, medium was harvested and phenylmethyl sulfonyl fluoride (PMSF) added to a final concentration of 1 mmol/L. Cell extracts were prepared in 350 μL ice-cold NP-40 lysis buffer (50 mmol/L NaCl, 50 mmol/L Tris pH 8.0, 1% (wt/vol) NP-40) supplemented with 1 mmol/L PMSF. The cell lysates were precleared overnight at 4°C with 50 μL of 20% (vol/vol) fixed Staphylococcus aureus Cowan I (SAC) coupled with a rabbit antimouse IgG (Sigma, St Louis, MO) in NP-40 lysis buffer. Immunoprecipitation of FVII was accomplished by incubating precleared cell lysates and conditioned media with 4 μg of MoAb MC1476 for 2 hours at 4°C. The resulting immune complexes were adsorbed with 30 μL of 20% (vol/vol) Protein A Sepharose FF (Sigma) coupled 5:1 (vol/vol) with rabbit antimouse IgG antiserum in NP-40 lysis buffer. Pellets were washed four times in NP-40 lysis buffer and resuspended either in buffer for further enzymatic digestion (see below) or in polyacrylamide gel electrophoresis (PAGE) sample buffer with or without reducing agents, and denatured by heating to 95°C for 5 minutes. The immunoprecipitated proteins were resolved by sodium dodecyl sulfate (SDS)-PAGE in 8% (wt/vol) gels, and analyzed by fluorography on X-OMAT-AR film (Eastman-Kodak Co, Rochester, NY) after treatment with En3Hance (DuPont NEN Research Products, Billerica, MA). To quantitate the relative amount of FVII immunoprecipitated at each time, the radioactivity incorporated into bands containing FVII was analyzed with the Umax PowerlookII Imaging Analyzer (Umax Data System Inc, Taiwan).
Immunohistochemistry.
CHO cell lines expressing FVII were grown overnight at 150,000 cells/glass coverslip (Baxter Scientific products, McGawpark, IL). The coverslips were washed once in phosphate-buffered saline (PBS) and fixed for 1 hour in 3% (vol/vol) paraformaldehyde (Fisher Scientific, Pittsburgh, PA) in PBS. The cells were sequentially washed, permeabilized for 3 minutes in 0.1% (vol/vol) Triton X-100 (Sigma), and washed three more times. They were then incubated in 0.15% (wt/vol) glycine containing 0.1% (wt/vol) bovine serum albumin (BSA) for 15 minutes followed by anti-FVII Mab (4 μg in 0.5 mL of PBS containing 1% BSA) for 30 minutes. The cells were again washed three times and then incubated with fluorescein isothiocyanate (FITC)-labeled goat-antimouse IgG (1/1000 in PBS containing 1% BSA) (Cappel, Durham, NC) for 30 minutes. After further washing, the coverslips were mounted on glass slides with Airvol (Air Products, Allentown, PA). Immunofluorescence microscopy was performed on a Zeiss axioplan fluorescence microscope at 630× magnification (Zeiss, Thornwood, NY).
Effect of protein degradation inhibitors on FVIIwt, FVIICys97, and FVIIArg100 levels.
To study the effect of various protein degradation inhibitors on FVII biosynthesis, confluent stably transfected CHO cells grown in 60 mm dishes were incubated with media containing either lactacystin (10 μmol/L) (Calbiochem, La Jolla, CA), ammonium chloride (50 mmol/L), leupeptin (100 μmol/L), N-acetyl-Leu-Leu-Norleucinal (50 μg/mL), or brefeldin A (10 μg/mL) (Sigma) dissolved according to the manufacturers’ recommendations and used at previously published concentrations.17-21 Four hours later, cell lysates were harvested and assayed for VII:Ag.
Analysis of N-linked glycosylation and sialation.
Immunoprecipitated proteins were incubated with 100 mU/mL Endo-β-N-acetylglucosaminidase H (Endo H) or 9.4 U/mL Peptide-N4-(N-acetyl-β-glucosaminyl) asparagine amidase (N-glycanase) for 18 hours at 37° C according to the manufacturer’s instructions. For sialation analysis, immunoprecipitated proteins were incubated for 1 hour at 37°C with 4 U/mL neuraminidase in 1 mmol/L PMSF, 20 mmol/L Tris-maleate pH 6.0, 10 mmol/L calcium acetate, and 1.75% (vol/vol) NP-40. All enzymes were from Genzyme (Cambridge, MA). The reactions were terminated by adding 5× PAGE sample buffer, and were analyzed by SDS-PAGE.
Informed consent.
Informed consent to perform research studies was obtained from the patients. The study was approved by the Human Studies Committee of the Brockton-West Roxbury VA Affairs Medical Center.
RESULTS
Patients and genomic studies.
We investigated the molecular defects in two Italian patients with FVII deficiency. Patient 1 is a 28-year-old male with a mild hemorrhagic diathesis manifested by epistaxis and excessive bleeding after dental extractions.22 Patient 2 is a 53-year-old woman with severe bleeding evidenced by recurrent hemarthrosis with chronic arthropathy and an episode of cerebral hemorrhage.22 The levels of VII:Ag and VII:C in the two patients were 2% and less than 1% of normal and 12% and less than 1% of normal, respectively (Table 1).
Coagulation Data and Genetic Alterations in Two Italian Patients With FVII Deficiency
| Patient . | VII:Ag (%) . | VII:C (%) . | Gly97Cys . | Gln100Arg . | Arg353Gln . | −323 Insert . |
|---|---|---|---|---|---|---|
| 1 | 2 | <1 | +/+ | −/− | +/+ | +/+ |
| 2 | 12 | <1 | −/− | +/+ | −/− | −/− |
| Patient . | VII:Ag (%) . | VII:C (%) . | Gly97Cys . | Gln100Arg . | Arg353Gln . | −323 Insert . |
|---|---|---|---|---|---|---|
| 1 | 2 | <1 | +/+ | −/− | +/+ | +/+ |
| 2 | 12 | <1 | −/− | +/+ | −/− | −/− |
Presence or absence of the sequence alteration in each allele is denoted by + or − signs, respectively.
For each patient, we subcloned and sequenced the entire coding region and the exon/intron boundaries of the FVII gene as well as its 5′-flanking region. In patient 1, a G to T transition at position 7824 (GGC to TGC) and a C to T transition at position 7880 (CAC to CAT) in exon 5 were identified resulting in Gly97Cys and a neutral dimorphism in the codon for His115, respectively. Two previously described FVII polymorphisms known to influence FVII levels, a C to A substitution at position 10976 (CGG to CAG) in exon 8 resulting in Arg353 Gln and the insertion of a decanucleotide at position −323 in the 5′-flanking region of the FVII gene, were also present. In patient 2, an A to G transversion at position 7834 (CAG to CGG) in exon 5 resulting in Gln100Arg was identified. No alleles with wild-type sequence were identified in either patient. To definitively establish that the patients were homozygous, we tested for the presence of the various sequence alterations using restriction enzyme analysis. The G to T transversion at position 7824, resulting in Gly97Cys, introduces a single Bbv I site. Digestion of a 313 bp PCR product spanning exon 5 with Bbv I generates fragments of 170 and 143 bp in the presence of the mutation. The A to G transversion at position 7834, resulting in Gln100Arg, introduces a BsrBI site and digestion with this enzyme generates fragments of 147 and 166 bp in the presence of the mutation.Msp I restriction analysis was used to identify the Arg353Gln polymorphism,23 whereas the decanucleotide insert at position −323 was assessed by visualization of a 10 bp difference in fragment size afterEcoRI digestion of a PCR fragment spanning this region.24 Based on the results of these restriction analyses (data not shown), patient 1 was homozygous for Gly97Cys and the two polymorphisms whereas patient 2 was homozygous for only Gln100Arg (Table 1).
Transient transfection assays in COS-1 cells.
To investigate the influence of the Gly97Cys and Gln100Arg substitutions on FVII biosynthesis, transient transfections were performed in COS-1 cells using the dicistronic pED vectors containing either wild-type or mutant FVII cDNAs. Assays of VII:Ag in cell lysates showed that FVIICys97 and FVIIArg100 were reduced to 38% and 54% of FVIIwt, respectively, whereas the levels in the conditioned media were decreased to 6.6% and 16.7% of FVIIwt, respectively (n=8). The small amount of FVIICys97 released into the media was functionally active as assessed by VII:C assay (6.1% ± 0.3% of wt) because the level was similar to VII:Ag (6.6% ± 0.5%). In contrast, the Gln100Arg mutation impaired the molecule’s function because the VII:C level in the media (2.8% ± 0.2%) was considerably lower than VII:Ag (16.7% ± 2.1%) (Table 2).
Transient Expression Assays of pEDFVIIwt, pEDFVIICys97, and pEDFVIIArg100 in COS-1 Cells
| . | FVIIwt . | FVIICys97 . | FVIIArg100 . |
|---|---|---|---|
| Cell lysate, VII:Ag | 100 ± 2.8 | 38.1 ± 3.9 | 54.6 ± 3 |
| Conditioned media, VII:Ag | 100 ± 3 | 6.6 ± 0.5 | 16.7 ± 2.1 |
| Conditioned media, VII:C | 100 ± 2.9 | 6.1 ± 0.3 | 2.8 ± 0.2 |
| . | FVIIwt . | FVIICys97 . | FVIIArg100 . |
|---|---|---|---|
| Cell lysate, VII:Ag | 100 ± 2.8 | 38.1 ± 3.9 | 54.6 ± 3 |
| Conditioned media, VII:Ag | 100 ± 3 | 6.6 ± 0.5 | 16.7 ± 2.1 |
| Conditioned media, VII:C | 100 ± 2.9 | 6.1 ± 0.3 | 2.8 ± 0.2 |
FVII levels were measured in cell lysates and conditioned media 36 hours after 8 independent transfections of cells from a single clone transfected with each construct. Results are expressed as the percentage of FVII (mean ± SE) produced by the wild-type construct.
Expression studies in stably transfected cell lines.
Based on the results of the transient transfection experiments, it appeared that the Gly97Cys and Gln100Arg mutations led to impaired FVII biosynthesis. To study these defects, pEDFVIIwt, pEDFVIICys97, and pEDFVIIArg100were transfected into DHFR-deficient CHO cells to obtain stably transfected cell lines. It can be observed that the amounts of FVII in cell lysates of the stable cell lines transfected with pEDFVIICys97 and pEDFVIIArg100 were actually greater than FVIIwt. This is attributable to the selection of high-level FVII producers. After a 15 minute pulse with [35S] methionine, the recombinant FVIIwt in cell lysates was maximal at 30 to 60 minutes and decreased as the protein was secreted (Fig 1, top). FVIICys97 was synthesized at a rate similar to FVIIwt with maximal accumulation at 60 minutes, but remained in the cell for a longer period of time. Approximately, 40% of the maximal amount of intracellular FVIICys97 was still present at 240 minutes as compared with 12% for FVIIwt (Fig 1, top). FVIIArg100 was retained intracellularly even longer with maximal accumulation at 60 to 120 minutes, and 55% of the protein was retained in the cell at 240 minutes. In the conditioned media, FVIIwt was barely detectable at 30 minutes and then proceeded to accumulate (Fig 1, bottom). The levels of FVIICys97 and FVIIArg100 were much lower than that of FVIIwt at all time points. At 240 minutes, the levels of FVIICys97 and FVIIArg100 in the media were 30% and 16% of FVIIwt, respectively (Fig 1, bottom).
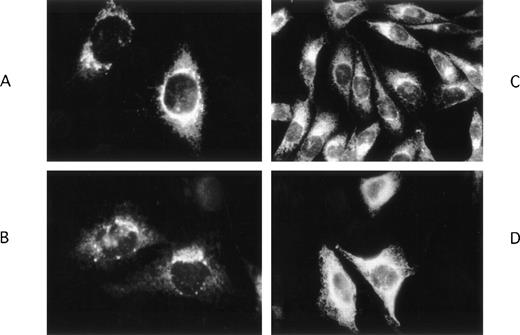
Using immunohistochemical techniques to detect intracellular VII:Ag, we found different patterns of localization for FVIIwt, FVIICys97, and FVIIArg100. Staining of FVIIwt (Fig 2A and B) and FVIICys97 (Fig 2C) was mostly perinuclear suggesting that these molecules were localized primarily in the Golgi apparatus. In contrast, FVIIArg100 (Fig 2D) staining was predominantly diffuse without perinuclear enhancement, suggesting that it was retained for a longer duration of time in the endoplasmic reticulum (ER) than the other recombinant proteins.
Whereas the acquisition of Endo H resistance is frequently employed to monitor protein transit to the Golgi complex from the ER, the radioactive bands that were observed for FVIICys97 and FVIIArg100 in pulse-chase experiments were generally of low intensity that precluded interpretation of these experiments (data not shown).
Effects of protein degradation inhibitors on FVII biosynthesis.
We next analyzed the effects of various inhibitors of protein degradation on intracellular FVII levels in stably transfected cells expressing FVIIwt, FVIICys97, and FVIIArg100. Inhibitors of lysosomal degradation, including NH4 Cl, which inactivates lysosomal enzymes by pH modification, and leupeptin, which inhibits cathepsins B, D, H, and L, did not increase intracellular FVII levels (Table 3) suggesting that FVIICys97 and FVIIArg100 were not degraded in the lysosome. Lactacystin, a potent specific inhibitor of proteasome degradation, also did not increase FVII levels (Table 3). However, ALLN, a neutral inhibitor of the cysteine proteases, calpain, and cathepsin D, induced a significant increase in FVIICys97levels (147% with ALLN versus 100% without ALLN, P = .0004) without altering the levels of FVIIwt or FVIIArg100. This data therefore suggested that FVIICys97 is degraded intracellularly.
Effect of Various Inhibitors of Protein Degradation and Brefeldin A on Intracellular Levels of FVIIwt, FVIICys97, and FVIIArg100
| . | FVIIwt . | FVIICys97 . | FVIIArg100 . |
|---|---|---|---|
| Media alone (n = 6) | 100 ± 1.1 | 100 ± 3.1 | 100 ± 4.2 |
| NH4Cl, 50 mmol/L (n = 3) | 107 ± 4.2 | 83.4 ± 3.6 | 113 ± 2.7 |
| Leupeptin, 100 μmol/L (n = 3) | 98 ± 1.2 | 100.5 ± 3.9 | 84 ± 3.6 |
| Lactacystin, 10 μmol/L (n = 3) | 88 ± 1.6 | 91.4 ± 2.8 | 98.1 ± 5.5 |
| ALNN, 50 μg/mL (n = 9) | 96.4 ± 5.7 | 147 ± 8.93-150 | 113 ± 4.1 |
| Brefeldin A, 10 μg/mL (n = 6) | 190 ± 10.43-151 | 125 ± 7.53-152 | 151 ± 10.83-153 |
| . | FVIIwt . | FVIICys97 . | FVIIArg100 . |
|---|---|---|---|
| Media alone (n = 6) | 100 ± 1.1 | 100 ± 3.1 | 100 ± 4.2 |
| NH4Cl, 50 mmol/L (n = 3) | 107 ± 4.2 | 83.4 ± 3.6 | 113 ± 2.7 |
| Leupeptin, 100 μmol/L (n = 3) | 98 ± 1.2 | 100.5 ± 3.9 | 84 ± 3.6 |
| Lactacystin, 10 μmol/L (n = 3) | 88 ± 1.6 | 91.4 ± 2.8 | 98.1 ± 5.5 |
| ALNN, 50 μg/mL (n = 9) | 96.4 ± 5.7 | 147 ± 8.93-150 | 113 ± 4.1 |
| Brefeldin A, 10 μg/mL (n = 6) | 190 ± 10.43-151 | 125 ± 7.53-152 | 151 ± 10.83-153 |
90% confluent stably transfected CHO cells expressing recombinant FVIIwt, FVIICys97, or FVIIArg100 were incubated 4 hours in fresh media containing 10% FBS in the presence or absence of the different agents. The figures in parentheses are the number of independent transfections performed on cells from a single clone expressing each construct. The levels of VII:Ag were then measured in cell lysates and the results are expressed as the percentage of FVII (mean ± SE) produced in the absence of the agent.
P = .0004.
P = .0001.
P = .02.
P = .002.
To localize the site of degradation, we investigated the effect of brefeldin A on intracellular levels of FVII in the three cell lines. Brefeldin A blocks protein transport from the ER to the Golgi complex and causes translocation of Golgi components back to the ER. After incubation with brefeldin A, FVIIwt and FVIIArg100increased significantly within the cells to 190% and 151% of control levels, respectively, consistent with normal translocation of these two proteins from the ER to the Golgi in the absence of the drug. In contrast, the intracellular level of FVIICys97 increased only modestly in the presence of brefeldin A to 125% of control levels, thereby suggesting that the degradation of this molecule occurred primarily in a pre-Golgi compartment. Analysis of immunoprecipitates obtained after a 45 minute pulse with 110 μCi [35S] methionine and 2 hours of chase in the presence and absence of 10 μg/mL of brefeldin A confirmed that FVIIwt and FVIIArg100 levels in cell lysates increased to 188% and 180% of control levels respectively as measured by imaging analysis of radioactive bands, whereas FVIICys97 levels were unaffected (105% versus 100% in control cells) (data not shown).
Analysis of altered mobility of FVIIArg100.
In Fig1, FVIIwt and FVIICys97 from conditioned media are visualized by nondenaturing PAGE as a single band with an apparent mol wt of 48 kD. This is slightly greater than that for the intracellular FVII species and results from post-transcriptional modifications of the protein. However, FVIIArg100 from the conditioned media appeared as a single band with a faster electrophoretic mobility than FVIIwt on nonreducing 8% SDS-PAGE. A smaller difference in electrophoretic mobility between FVIIwt and FVIIArg100 was also observed in the cell lysates. We hypothesized that this difference in electrophoretic mobility resulted from either a change in glycosylation or alteration of the molecule’s secondary structure.
Pulse-chase labeling experiments in CHO cells transfected with pEDFVIIwt, pEDFVIICys97, and pEDFVIIArg100. After a 15-minute pulse with [35S] methionine, the cells were chased for 30, 60,120, 180, and 240 minutes. Equivalent amounts of cell lysate (top) or conditioned media (bottom) for FVIIwt (wt), FVIICys97(97) and FVIIArg100 (100) were immunoprecipitated using a Mab against FVII and analyzed by 8% SDS-PAGE under nonreducing conditions. The location of molecular weight markers in kD is denoted at the left-hand side of the figure. In the conditioned media (bottom), the 77 kD band was nonspecific as it was observed with equal intensity in untransfected CHO cells (data not shown).
Pulse-chase labeling experiments in CHO cells transfected with pEDFVIIwt, pEDFVIICys97, and pEDFVIIArg100. After a 15-minute pulse with [35S] methionine, the cells were chased for 30, 60,120, 180, and 240 minutes. Equivalent amounts of cell lysate (top) or conditioned media (bottom) for FVIIwt (wt), FVIICys97(97) and FVIIArg100 (100) were immunoprecipitated using a Mab against FVII and analyzed by 8% SDS-PAGE under nonreducing conditions. The location of molecular weight markers in kD is denoted at the left-hand side of the figure. In the conditioned media (bottom), the 77 kD band was nonspecific as it was observed with equal intensity in untransfected CHO cells (data not shown).
Immunohistochemical localization of wild-type and mutant FVII molecules in stably transfected CHO cells. FVIIwt (A, B) and FVIICys 97 (C) were mostly localized in the perinuclear area, whereas FVIIArg100 (D) was present diffusely throughout the cytoplasm without perinuclear enhancement. Untransfected CHO cells did not react with either the anti-FVII Mab or the fluorescent second antibody indicating that the observed labeling was specific for FVII (data not shown).
Immunohistochemical localization of wild-type and mutant FVII molecules in stably transfected CHO cells. FVIIwt (A, B) and FVIICys 97 (C) were mostly localized in the perinuclear area, whereas FVIIArg100 (D) was present diffusely throughout the cytoplasm without perinuclear enhancement. Untransfected CHO cells did not react with either the anti-FVII Mab or the fluorescent second antibody indicating that the observed labeling was specific for FVII (data not shown).
Analysis of the altered electrophoretic mobility of FVIIArg100. After a 15-minute pulse with [35S] methionine, stably transfected cells expressing FVIIwt and FVIIArg100 were chased for 60 minutes and 120 minutes, respectively, to analyze FVII in cell lysates. In the conditioned media, FVIIwt and FVIIArg100 were both investigated after 180 minutes of chase. FVIIwt (wt) and FVIIArg100 (100) were immunoprecipitated using a MoAb against FVII. (A) Equivalent amounts of cell lysate were analyzed by 8% SDS-PAGE before (-) or after treatment with Endo H and N-Glycanase (N-Gly). (B) Conditioned media were analyzed before (-) or after treatment with neuraminidase (Neura) by 10% SDS-PAGE. (C) Conditioned media were evaluated under nonreducing conditions (control) or after reduction by 100 mmol/L Dithiothreitol (DTT). The 77 kD band was nonspecific because it was also observed in conditioned media from untransfected CHO cells (data not shown). Molecular weight markers in kD are indicated on the left.
Analysis of the altered electrophoretic mobility of FVIIArg100. After a 15-minute pulse with [35S] methionine, stably transfected cells expressing FVIIwt and FVIIArg100 were chased for 60 minutes and 120 minutes, respectively, to analyze FVII in cell lysates. In the conditioned media, FVIIwt and FVIIArg100 were both investigated after 180 minutes of chase. FVIIwt (wt) and FVIIArg100 (100) were immunoprecipitated using a MoAb against FVII. (A) Equivalent amounts of cell lysate were analyzed by 8% SDS-PAGE before (-) or after treatment with Endo H and N-Glycanase (N-Gly). (B) Conditioned media were analyzed before (-) or after treatment with neuraminidase (Neura) by 10% SDS-PAGE. (C) Conditioned media were evaluated under nonreducing conditions (control) or after reduction by 100 mmol/L Dithiothreitol (DTT). The 77 kD band was nonspecific because it was also observed in conditioned media from untransfected CHO cells (data not shown). Molecular weight markers in kD are indicated on the left.
To investigate N-linked glycosylation, radiolabeled FVII was immunoprecipitated and incubated with Endo-H or N-Glycanase which respectively cleaves either high-mannose and certain hybrid-type carbohydrates at the GlcNAcβ1-4GlcNAc linkage to leave a single GlcNAc residue attached to asparagine25 or all N-linked carbohydrates by hydrolyzing the asparginyl-oligosaccharide bond.26 As the secretion of FVIIwt and FVIIArg100 occur at different rates, we evaluated lysates from the two stably transfected cell lines at different chase times. After a 15-minute pulse with [35S] methionine and 120 minutes of chase time, FVIIwt migrated more rapidly after digestion with either Endo H or N-Glycanase, reflecting the removal of N-linked high mannose oligosaccharides from the nascent 46 kD protein (Fig 3A). FVIIArg100 behaved similarly to FVIIwt at 180 minutes, suggesting that N-linked glycosylation of this molecule was occurring normally. To detect the presence of sialic acid on the glycans of FVIIwt and FVIIArg100, we performed digestion with neuraminidase. Recombinant FVIIwt and FVIIArg100 both migrated more rapidly after digestion with neuraminidase, but the difference in electrophoretic mobility of FVIIArg100 as compared with FVIIwt remained (Fig 3B). This suggested that modification of the glycans with sialic acid was similar for the two molecules. However, after reduction of the disulfide linkages with 100 mmol/L dithiothreitol, FVIIwt and FVIIArg100 migrated with a similar electrophoretic mobility (Fig 3C), thereby suggesting that disulfide bond formation of the FVII molecule was affected by the Gln100 Arg substitution. Similar results were obtained after reduction with 5% (vol/vol) β-Mercaptoethanol (data not shown).
DISCUSSION
We investigated the mechanisms responsible for FVII deficiency in two unrelated Italian patients with a bleeding diathesis. Sequencing of the coding sequences and intron/exon boundaries of the FVII gene showed that they were homozygous for different mutations within the coding region for exon 5. Patient 1 had a G to T mutation at position 7824 and patient 2 had an A to G mutation at position 7834 resulting in Gly97Cys and Gln100Arg, respectively. These two mutations were previously reported in association with other mutations in doubly heterozygous patients.27-29 Patient 1, who was homozygous for Gly97Cys, was also homozygous for two polymorphisms known to reduce FVII levels, Arg353Gln in exon 8 and the decanucleotide insert in the 5′ flanking region of the FVII gene.23,30 31
To study the mechanism by which the two mutations reduce FVII levels, we performed transient expression studies in COS-1 cells with cDNA encoding FVIIwt, FVIICys97, and FVIIArg100 and showed that the secretion of the two mutant proteins was impaired. The levels of VII:Ag and VII:C in the conditioned media were reduced concordantly for FVIICys97, whereas a higher level of VII:Ag relative to VII:C was observed for FVIIArg100. The results were similar to those in the patients’ plasmas. We also observed that the intracellular levels of VII:Ag were reduced in transient transfection assays performed with the mutant cDNA.
To study the intracellular processing of the mutant proteins, we performed pulse-chase experiments and immunohistochemical staining of stably transfected CHO cells expressing FVIICys97 and FVIIArg100 in comparison to FVIIwt. Our results showed that the proteins did not accumulate intracellularly in spite of major secretion defects. A potential mechanism to account for reduced amounts of FVII protein in the cells is degradation. Intracellular degradation of abnormal proteins can result from lysosomal proteolysis or from pre-Golgi or ER degradation, which has been termed the “quality control function” of the ER.32,33 Protein degradation within the ER is a complex, poorly understood process and occurs either within the ER lumen or on the cytoplasmic side. Some of the proteolytic events are adenosine triphosphate (ATP)-dependent and several of the enzymes involved in this process are sensitive to serine protease inhibitors. Some proteins with an abnormal conformation are degraded by a soluble ATP-dependent pathway in which conjugation occurs between the abnormal protein and multiple molecules of ubiquitin followed by hydrolysis by a 26S proteolytic enzyme complex. The proteolytic core of this structure is the 20S proteasome, which has been localized to the cytoplasmic surface of the ER by immunoelectron microscopy.34
To investigate potential mechanisms of intracellular degradation, we examined the biosynthesis of FVIICys97 using inhibitors of lysosomal pH (NH4Cl), lysosomal proteolytic enzymes (leupeptin), and the 20S-proteasome (lactacystin). We also evaluated the effect of ALLN, a common inhibitor of neutral Ca2+-dependent cysteine proteases, which inhibits the ubiquitin-proteasome pathway.35 The intracellular level of FVIICys97 did not change in the presence of inhibitors of lysosomal function (ie, NH4Cl and leupeptin) or lactacystin, whereas a significant increase was observed with ALLN. These data indicate that FVIICys97 degradation does not occur in lysosomes and involves a cysteine protease, which is, however, independent of the proteasome pathway.
Brefeldin A inhibits protein secretion in a pre-Golgi compartment such that proteins destined for secretion remain in the ER.36Whereas the level of FVIIwt rose by 90% of baseline in the presence of brefeldin A, the intracellular level of FVIICys97 increased by less than 30%. The Gly97Cys mutation is in the second EGF domain of FVII and results in substitution of a small nonpolar side chain by a polar side chain. Based on the crystal structure of activated FVII bound to the extracellular domain of tissue factor,37 Gly97 helps position the Cys98-Cys112 loop to facilitate intramolecular disulfide bonding. We postulate that the resulting alteration in protein conformation causes FVIICys97 to undergo degradation in a pre-Golgi compartment.
For FVIIArg100, pulse-chase experiments showed that the mutant protein was retained for a longer time interval in stably transfected CHO cells than FVIIwt and the level was not increased by the various inhibitors of protein degradation. In the presence of brefeldin A, the levels of FVIIArg100 increased by 51% as compared to FVIIwt that increased by 90%. This data coupled with the immunohistochemical demonstration that FVIIArg100 is present diffusely throughout the cytoplasm is consistent with retention of a substantial portion of the mutant protein in the ER.
The more rapid electrophoretic mobility of FVIIArg100 as compared with FVIIwt suggested that FVIIArg100 might be incompletely glycosylated or have an abnormal tertiary structure. To investigate alterations in N-linked glycosylation and sialation, radiolabelled FVIIwt and FVIIArg100 from conditioned media were treated with Endo H, N-Glycanase, and neuraminidase. The difference in electrophoretic mobility remained between FVIIwt and FVIIArg100 after digestion with the three enzymes, implying that N-linked glycosylation and sialation of FVIIArg100were unaffected. We did not evaluate whether O-linked sugars on residues Ser52 and Ser60 of FVII6,38 were altered because FVIIAla52, a mutant molecule lacking glycosylation at this residue, has been reported to have the same electrophoretic mobility as FVIIwt.38 Because the second EGF domain contains three intramolecular disulfide bonds (Cys91-Cys102, Cys98-Cys112, and Cys114-Cys127), we compared the electrophoretic mobility of FVIIwt and FVIIArg100 under reducing as well as nonreducing conditions. After reduction, the difference in electrophoretic mobility between the two molecules was no longer present, leading us to speculate that FVIIArg100 does not undergo normal disulfide bonding during its biosynthesis. To confirm this, chemical determination of the disulfide bonding pattern of FVIIArg100 would need to be performed and the results compared with those for FVIIwt. The mutation replaces a neutral Gln residue with a larger positively-charged Arg, which may sterically interfere with Tyr118 and electrostatically interfere with the positively-charged side chain of His115.37We therefore postulate that the Gln100Arg mutation in FVII results in a protein with an abnormal conformation that remains in the ER for an extended period during its biosynthesis and reduces its secretion.
Whereas defective biosynthesis is a major defect conferred by the Gln100Arg mutation, a small amount of dysfunctional protein is secreted as evidenced by the low specific activity of the FVII (ie, ratio of VII:C to VII:Ag) that is present in the patient’s plasma as well as conditioned medium of transfected COS-1 cells. Our data is in agreement with studies of FVIIArg100 reported by Kemball-Cook et al,39 who showed that the activated form of the mutant recombinant protein had markedly diminished affinity for tissue factor and, in complex with soluble tissue factor, had less than 5% of the ability of FVIIwt to activate factor X. Orning et al40 have also provided data that the peptide sequence, Glu99-Gln100-Tyr101, in the second EGF domain of FVII, inhibits the ability of FVIIwt to mediate tissue factor–dependent factor X activation. Based on the crystal structure of the FVIIa–tissue factor complex, Gln100 is located at the interface of the second EGF and the protease domain, but is not in contact with tissue factor.
Whereas Gly97Cys and Gln100Arg have previously been reported in double heterozygotes with FVII deficiency, this is the first report of homozygous patients with these mutations. The severe clinical phenotype of patient 2 with Gln100Arg is consistent with studies showing a major secretion defect as well as markedly impaired function of the small amount of FVII that is released from cells. It is unclear why patient 1 with Gly97Cys does not have a similarly severe bleeding diathesis. Because it is not possible to obtain sufficient FVIICys97 protein to perform detailed biochemical studies, we cannot evaluate whether a very small amount of circulating FVII protein posseses sufficient biologic function to attenuate the bleeding diathesis.
ACKNOWLEDGMENT
The authors thank Dr Michael Yaffee (Beth Israel Deaconess Medical Center, Boston, MA) for his assistance in the analysis of the coordinates of FVIIa from the FVIIa–soluble tissue factor crystal structure.
Supported by the Medical Research Service of the Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth A. Bauer, MD, Department of Veterans Affairs Medical Center, 1400 VFW Pkwy, West Roxbury, MA 02132; e-mail: bauer_md.kenneth_a+@brockton.va.gov.

![Fig. 1. Pulse-chase labeling experiments in CHO cells transfected with pEDFVIIwt, pEDFVIICys97, and pEDFVIIArg100. After a 15-minute pulse with [35S] methionine, the cells were chased for 30, 60,120, 180, and 240 minutes. Equivalent amounts of cell lysate (top) or conditioned media (bottom) for FVIIwt (wt), FVIICys97(97) and FVIIArg100 (100) were immunoprecipitated using a Mab against FVII and analyzed by 8% SDS-PAGE under nonreducing conditions. The location of molecular weight markers in kD is denoted at the left-hand side of the figure. In the conditioned media (bottom), the 77 kD band was nonspecific as it was observed with equal intensity in untransfected CHO cells (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1237/5/m_blod404020011w.jpeg?Expires=1767910495&Signature=ukG2x0zswQav~gXeySxcbn~hWX~LTsCurPHCGUaDHLysuu~pbvcoOZxtoddKLwmVxpfkgQStWaaHx2gspZYGgxLbrInE2fGiw6HFQlO0x~a~vmu6y-nan8R2zdilzVRqXxjcg3fphWMYpudsNu3ukPegO8Qjfu7AyM1ZQe-Ntkz7dNMT2T4IM7eQC4v55U8eboTAhD3b7uZxFJpow8sceqLtACB9DHR1AwW66arC-UG135ZYDRnno2WSAvs-Hpl6prSCJI0KvRdFkdVnS0iUHiNVjiA-qJZ7lUeJN2YzUKUg5C-xuIYWdji2qNAOgBqU-M8~OMx2ZfJYn0Og~L93xQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Pulse-chase labeling experiments in CHO cells transfected with pEDFVIIwt, pEDFVIICys97, and pEDFVIIArg100. After a 15-minute pulse with [35S] methionine, the cells were chased for 30, 60,120, 180, and 240 minutes. Equivalent amounts of cell lysate (top) or conditioned media (bottom) for FVIIwt (wt), FVIICys97(97) and FVIIArg100 (100) were immunoprecipitated using a Mab against FVII and analyzed by 8% SDS-PAGE under nonreducing conditions. The location of molecular weight markers in kD is denoted at the left-hand side of the figure. In the conditioned media (bottom), the 77 kD band was nonspecific as it was observed with equal intensity in untransfected CHO cells (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1237/5/m_blod404020012w.jpeg?Expires=1767910495&Signature=o7K5N1zoPJgdQ9iiex9~pyTWP9IY7oPW5X4dN6WA4k1OL-ToKeOkg6WvspJWlyi5ifIBtuArYoY3qKKFsUPDi4kTdMgU1LxvfBjqbJKZs~e8mYnn-b9pY-d0ke46D--7G0iEqU~~PdH5GcyFd5RzaCx2oxdk7sWmG6ZZVH7r-R2JfTdqYxYqzbFtggB~XV7fjB02AEAd5jUAlX5h8hmvlHoKiyTYVnIcOHrUi5hgjvftydRxpoDwxRo9kENYRxS4G82M6CCU2ER3pxpEBitC80EhXfBR97S6CORsPhZMSF3DlFaNK8n9fvFPhApl0xFzcuXCKKlK7wydJRV9R05h4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Analysis of the altered electrophoretic mobility of FVIIArg100. After a 15-minute pulse with [35S] methionine, stably transfected cells expressing FVIIwt and FVIIArg100 were chased for 60 minutes and 120 minutes, respectively, to analyze FVII in cell lysates. In the conditioned media, FVIIwt and FVIIArg100 were both investigated after 180 minutes of chase. FVIIwt (wt) and FVIIArg100 (100) were immunoprecipitated using a MoAb against FVII. (A) Equivalent amounts of cell lysate were analyzed by 8% SDS-PAGE before (-) or after treatment with Endo H and N-Glycanase (N-Gly). (B) Conditioned media were analyzed before (-) or after treatment with neuraminidase (Neura) by 10% SDS-PAGE. (C) Conditioned media were evaluated under nonreducing conditions (control) or after reduction by 100 mmol/L Dithiothreitol (DTT). The 77 kD band was nonspecific because it was also observed in conditioned media from untransfected CHO cells (data not shown). Molecular weight markers in kD are indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1237/5/m_blod40402003w.jpeg?Expires=1767910495&Signature=hU9R41lAKymL7IwUX4-fa2sanzpdPua-4hU2gMzMsPVpnahQSE26SWU05fg-q6RUOV9nCby9CqnlEgANjf87dksF1m4oQ1ZWKKXLKFi1RQhYTE-EFEM9-fQDeFCSwf0SZjt~i4H7hcM00Ke0JUE~i4Yb-vOxpOElYXWuVo1QzGm3yjAtP9dXZtH5c2jG4hcVM-6TaltcUa-bjsK~nulTDLy9Lx~tfx64baZk-ut0th8m0cZhuBX~xXHSXCHL3OynLq26Wq5ecH6ZCf3E9BYFRF~RIk8Dogh1CGM5QR9Os174~Cin6aR-D4lIw6sML7i8XxF8j~w5xhtj4gK~ra85xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal