Abstract
Although it is well established that long-term heparin therapy causes osteoporosis, it is unknown whether heparin-induced bone loss is reversible when heparin treatment is stopped. To address this question, we randomized rats to once daily subcutaneous injections of either unfractionated heparin (1.0 U/g or 0.5 U/g) or saline for 28 days and then followed the rats for an additional 28 days off treatment. Based on histomorphometric analysis of the distal third of the right femur proximal to the epiphyseal growth plate, 1.0 U/g heparin caused a 30% loss in cancellous bone volume over the first 28 days. This was accompanied by a 137% increase in osteoclast surface and a 60% decrease in both osteoblast and osteoid surface. One month after cessation of heparin treatment, no recovery in these parameters was observed. Similarly, serum levels of alkaline phosphatase, a biochemical marker of bone formation, which continued to decrease over the course of heparin treatment, showed no signs of recovery in the subsequent 28 days off treatment. To explore the mechanism responsible for the prolonged effect of heparin on bone, we repeated the experiment giving 125I-labeled heparin in place of unlabeled heparin.125I-labeled heparin was found to accumulate in bone during the course of its administration, and be retained in bone for at least 56 days after stopping heparin treatment. These findings suggest that heparin-induced osteoporosis is not rapidly reversible because heparin is sequestered in bone for an extended period.
OSTEOPOROSIS IS A well-recognized complication of long-term heparin therapy.1-10 Although only 2% to 3% of patients receiving protracted heparin therapy develop symptomatic fractures, up to one third have subclinical reductions in bone density.5,8 9 It is unknown whether the effects of heparin on bone are reversible when treatment is discontinued. This is an important question because if irreversible, heparin could lower peak bone mass, thereby contributing to osteoporosis later in life.
In previous studies, we used a rat model to examine the in vivo effects of heparin on bone.11,12 Histomorphometric analysis of the distal third of femurs from heparin-treated animals demonstrated a significant loss of cancellous bone accompanied by increased numbers of osteoclasts, and decreased numbers of osteoblasts, lining the trabecular bone surface. Biochemical markers of bone turnover supported these findings suggesting that heparin causes bone loss not only by increasing osteoclastic bone resorption, but also by decreasing osteoblastic bone formation.11 12
In the present study, we used the same rat model of heparin-induced osteoporosis to determine whether the effects of heparin on cancellous bone are reversible when heparin treatment is stopped. Thus, we treated rats on a daily basis with pharmacologically relevant doses of heparin or saline for 28 days. On day 28, half of the rats were killed and their femurs subjected to histomorphometric analysis. The remaining rats were kept off treatment for an additional 28 days before subjecting their femurs to histomorphometric analysis. Herein we report that the effects of heparin on cancellous bone loss are not reversible within the 28-day period after treatment is stopped. To explore the mechanism responsible for the prolonged effect of heparin on bone, the experiment was repeated using 125I-labeled heparin in place of unlabeled heparin. 125I-labeled heparin accumulated in bone during the course of its administration and was retained in bone for at least 56 days after stopping treatment. This suggests that the lack of recovery in bone volume was due to sequestration of heparin within the bone microenvironment.
MATERIALS AND METHODS
Materials.
Specific pathogen-free female Sprague-Dawley rats, 4 to 5 months old, and weighing 300 to 325 g were purchased from Charles River Laboratories (St Constant, Quebec). Serum alkaline phosphatase (ALP) was measured using an assay from Sigma Chemical Co (St Louis, MO). Unfractionated heparin was generously provided by Rhone-Poulenc Rorer (Montreal, Quebec) and was iodinated as described previously.13
Experimental design.
To examine the effects of heparin on bone morphology, a total of 56 animals was studied. Rats were randomized into one of three treatment groups, each consisting of 16 rats. Two groups were given daily subcutaneous injections of heparin at doses of either 0.5 U/g/day or 1.0 U/g/day for a total of 28 days. The third group served as an age-matched control, and rats were given an equivalent volume of saline instead of heparin. On day 28, half of the rats in each treatment group were killed with 5% isofluorane and after exsanguination, their right femurs were removed for histologic evaluation. Remaining rats were kept for an additional 28 days with no further treatment. Eight rats were also killed at day 0 (body weight of 325 ± 5 gm; mean ± standard deviation [SD]) and served as baseline controls. To permit measurements of bone apposition rates and the calculation of dynamic parameters, all animals received two intraperitoneal injections of fluorescent markers. The first, demeclocycline (15 mg/kg), was given 10 days before the animals were killed, while the second, calcein (8 mg/kg), was given 3 days before the animals were killed.
In a second study that also included 56 rats, we examined the retention of I125-labeled heparin in bone as a function of time. Briefly, 56 rats (28 rats/group) were given daily subcutaneous injections of I125-labeled heparin (0.1 U/g; 4.8 × 106 cpm/μg) mixed with unlabeled heparin (0.9 U/g) or an equal amount of I125-labeled bovine serum albumin (BSA; 5.0 × 106 cpm/μg) as a control, for a period of 28 days. At various intervals over the first 28 days and for the next 56 days, four rats from each group were killed and the amount of I125-heparin or I125-BSA retained in the right femur determined by counting the entire bone in a γ-counter (Clinigamma, model 1272; Fisher Scientific, Nepean, Ontario, Canada).
Bone histomorphometry.
Bone histomorphometry was performed as described previously.11,12 Briefly, the undecalcified distal third of the right femur of each rat was embedded in glycolmethacrylate (JB-4 embedding medium; Analychem, Toronto, Ontario, Canada). Histologic sections (6 to 8 μm) were obtained using a Riechert Jung microtome (model K4), mounted, and then stained with either 1% toluidine blue or hematoxylin and eosin (H&E) before being subjected to morphometric analysis. In each case, a region 800 μm below the epiphyseal growth plate that included the entire metaphysis was subjected to light microscopy using a Merz grid.14 Sections examined in this fashion encompassed a total tissue area of approximately 10 to 15 mm2. The following parameters were determined: (1) cancellous bone volume, (2) osteoblast surface, (3) osteoid surface, and (4) osteoclast surface. For each section, cancellous bone volume was calculated from a total of > 1,600 point measurements (45 fields; 400× magnification), which were selected at random using the Merz grid. The percent osteoblast, osteoid or osteoclast surface was calculated under oil immersion (1,000×) by recording the presence or absence of each where the hemispherical grid of the Merz radicule crossed cancellous bone. Osteoblasts were identified morphologically as distinct cuboidal-shaped cells lining the cancellous bone surface, whereas osteoclasts were identified as large multinucleated cells that stained with tartrate-resistant acid phosphatase (Sigma Chemical Co; Procedure No. 386) and were located close to the cancellous bone surface. The histomorphometric parameters of trabecular width, number, and separation were measured directly using an epifluorescent microscope (Leica Laborlux; Willowdale, Ontario, Canada) coupled to an IBM computer (Hewlett Packard, Toronto, Ontario, Canada). Images were captured using a CCD video camera module electronically linked to a computer imaging software system (Northern Exposure; Empix Inc, Mississauga, Ontario, Canada). Measurements of erosion depth were also determined from captured images by random measurement of the depth of resorption lacunae that were associated with tartrate resistant acid phosphatase-positive cells. All histological analyses were done by a single investigator who was blinded to treatment allocation.
To quantify bone mineralization, cancellous bone surface intersecting with the grid of the Merz radicule was scored as either labeled or unlabeled depending on whether or not the cancellous bone surface was fluorescently labeled. Fluorescent bone surface was further characterized as having either single or double label according to the number of distinct lines observed on the labeled surface. Double-labeled perimeter was then used to calculate the dynamic variables of mineral apposition rate and bone formation rate (surface-based) according to the standard nomenclature described by Jee et al15 and Parfitt et al.16
Statistical analysis.
Analysis of variance was used to compare the results in the experimental groups with those in the controls. The significance of differences was determined using an unpaired Student’s t-test with a Bonferroni correction for multiple comparisons. All data are expressed as a mean ± standard error of mean (SEM).
RESULTS
Effect of heparin on body weight and alkaline phosphatase levels.
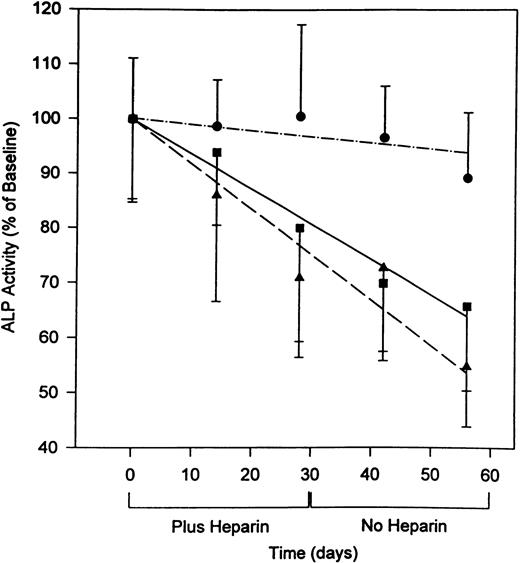
During the course of the study, both heparin-treated and control rats gained similar amounts of weight (data not shown). A time-dependent decrease in serum ALP levels was observed in rats treated with either 0.5 U/g/day or 1.0 U/g/day heparin (Fig 1). Serum ALP levels decreased during heparin treatment and remained decreased 28 days after stopping treatment. Thus, at day 56, there was a 44.3% ± 5.1% (P < .001) and a 33.2% ± 7.0% (P < .001) reduction in ALP in rats given 1.0 U/g/day and 0.5 U/g/day heparin, respectively.
Serum ALP levels both during and after the discontinuation of heparin treatment. Rats were injected for 28 days with vehicle alone (•) or unfractionated heparin at concentrations of either 0.5 U/g/day (▪) or 1.0 U/g/day (▴) and then allowed to live for an additional 28 days with no further treatment. During the course of this experiment, tail vein blood samples were collected weekly and assayed for ALP activity as an index of bone formation.
Serum ALP levels both during and after the discontinuation of heparin treatment. Rats were injected for 28 days with vehicle alone (•) or unfractionated heparin at concentrations of either 0.5 U/g/day (▪) or 1.0 U/g/day (▴) and then allowed to live for an additional 28 days with no further treatment. During the course of this experiment, tail vein blood samples were collected weekly and assayed for ALP activity as an index of bone formation.
Reversibility of heparin-induced cancellous bone loss.
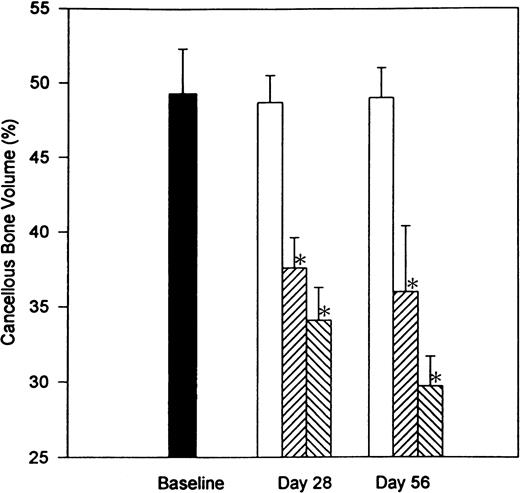
To examine the reversibility of heparin-induced bone loss, we compared the extent of bone loss in the undecalcified right femur of rats that had been treated with heparin for 28 days with that in rats that had a 28-day recovery period after a 28-day course of heparin treatment. A region 800 μm below the epiphyseal growth plate that included the entire metaphysis was analyzed for cancellous bone. As shown in Fig 2, no significant difference in cancellous bone volume (BV/TV) was found between baseline and age-matched control rats. In contrast, heparin, at doses of either 0.5 or 1.0 U/g/day, produced a significant reduction (P < .001) in cancellous bone by day 28. This remained unchanged 28 days after stopping heparin treatment. Thus, after 28 days treatment with 1.0 U/g heparin, there was a 30.0% ± 4.5% reduction in cancellous bone volume when compared with age-matched controls. Twenty-eight days after stopping heparin treatment, cancellous bone volume was still decreased by 39.0% ± 4.1% as compared with age-matched controls.
The reversibility of heparin-induced cancellous bone loss. Rats were injected with vehicle alone (open bars) or unfractionated heparin at a concentration of 0.5 U/g/day (cross-hatched; rising to the right) or 1.0 U/g/day (cross-hatched; rising to the left). On day 28, half of the rats from each group were killed while the other half were allowed to live for an additional 28 days with no further treatment before determining the total epiphyseal area occupied by cancellous bone. Data are expressed as mean ± SEM. *P < .005 when compared with either baseline or control values.
The reversibility of heparin-induced cancellous bone loss. Rats were injected with vehicle alone (open bars) or unfractionated heparin at a concentration of 0.5 U/g/day (cross-hatched; rising to the right) or 1.0 U/g/day (cross-hatched; rising to the left). On day 28, half of the rats from each group were killed while the other half were allowed to live for an additional 28 days with no further treatment before determining the total epiphyseal area occupied by cancellous bone. Data are expressed as mean ± SEM. *P < .005 when compared with either baseline or control values.
Similar results were obtained when the parameters of trabecular width, number, and separation were evaluated (Table 1). Thus, heparin significantly decreased both trabecular number and width when compared with either age-matched controls or baseline values. Age-matched controls had a mean of 9.8 trabeculae/mm2 with a mean width of 104.4 μm. Heparin treatment (1.0 U/g/day) for 28 days reduced the number of trabeculae to 7.5 ± 0.3 trabeculae/mm2 (P < .01), with the remaining trabeculae having a mean width of 73.7 ± 3.3 μm (P < .001). This resulted in the distance between adjacent trabeculae increasing from 261.6 ± 9.8 μm to 296.8 ± 12.4 μm, an increase of 13.5% ± 4.7% (P < .001). Similar findings were obtained 28 days after stopping heparin treatment (Table 1).
The Reversibility of Heparin’s Effect on Trabecular Thickness, Number, and Separation
| . | Trabecular Width (μm) . | Trabecular No. (#/mm2) . | Trabecular Separation (μm) . |
|---|---|---|---|
| Baseline | 107.7 ± 2.7 | 9.6 ± 0.2 | 256.4 ± 6.2 |
| Day 28 | |||
| Control | 104.4 ± 1.7 | 9.8 ± 0.4 | 261.6 ± 9.8 |
| Heparin (0.5 U/g) | 85.4 ± 2.7* | 7.1 ± 0.4* | 282.2 ± 14.5* |
| Heparin (1.0 U/g) | 73.7 ± 3.3* | 7.5 ± 0.3* | 296.8 ± 12.4* |
| Day 56 | |||
| Control | 104.4 ± 1.7 | 9.8 ± 0.4 | 261.6 ± 9.8 |
| Heparin (0.5 U/g) | 84.5 ± 7.5*,† | 7.3 ± 0.5*,† | 279.5 ± 31.0*,† |
| Heparin (1.0 U/g) | 73.7 ± 1.7*,† | 6.8 ± 0.7*,† | 313.6 ± 24.2*,† |
| . | Trabecular Width (μm) . | Trabecular No. (#/mm2) . | Trabecular Separation (μm) . |
|---|---|---|---|
| Baseline | 107.7 ± 2.7 | 9.6 ± 0.2 | 256.4 ± 6.2 |
| Day 28 | |||
| Control | 104.4 ± 1.7 | 9.8 ± 0.4 | 261.6 ± 9.8 |
| Heparin (0.5 U/g) | 85.4 ± 2.7* | 7.1 ± 0.4* | 282.2 ± 14.5* |
| Heparin (1.0 U/g) | 73.7 ± 3.3* | 7.5 ± 0.3* | 296.8 ± 12.4* |
| Day 56 | |||
| Control | 104.4 ± 1.7 | 9.8 ± 0.4 | 261.6 ± 9.8 |
| Heparin (0.5 U/g) | 84.5 ± 7.5*,† | 7.3 ± 0.5*,† | 279.5 ± 31.0*,† |
| Heparin (1.0 U/g) | 73.7 ± 1.7*,† | 6.8 ± 0.7*,† | 313.6 ± 24.2*,† |
Rats were injected daily with vehicle alone or unfractionated heparin as described in Materials and Methods. On days 28 and 56, rats were exsanguinated and their right femurs removed for histologic examination. The region extending from the epiphyseal growth plate and including the entire metaphysis was analyzed for (1) trabecular width, (2) trabecular number, and (3) trabecular separation. Data are expressed as mean ± SEM.
P < .005 when compared with either baseline or control values.
P > .05 when compared with values obtained from heparin-treated animals at day 28.
Reversibility of surface-based data.
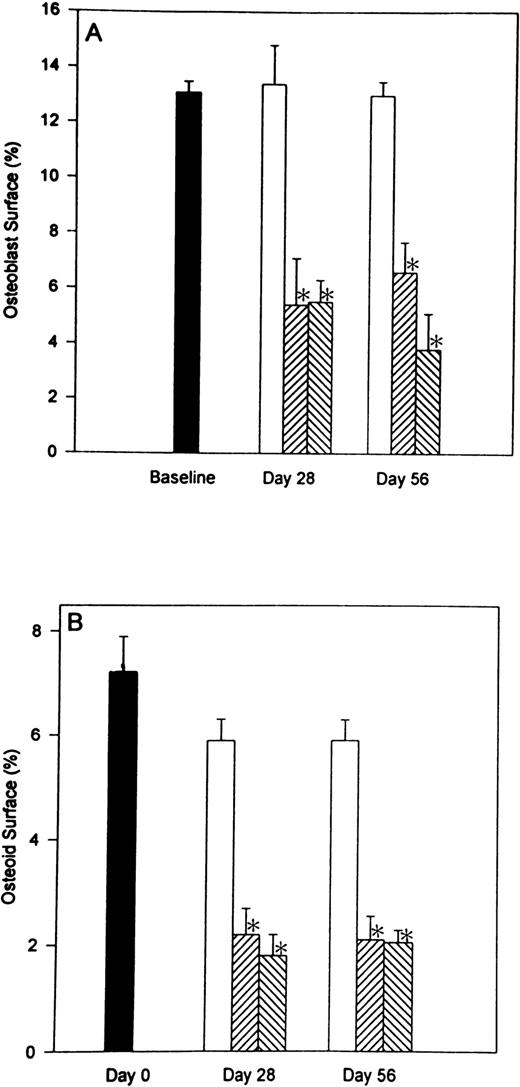
In previous studies,11 12 we demonstrated that heparin has profound effects on the numbers of both osteoblasts and osteoclasts. To determine whether these effects were reversible, we compared surface-based data from rats allowed to recover for 28 days after heparin therapy was stopped with the results obtained immediately after 28 days of heparin treatment. The parameters that were evaluated included: (1) percent osteoblast surface (Ob.S/BS), (2) percent osteoid surface (OS/BS), and (3) percent osteoclast surface (Oc.S/BS). As shown in Fig 3, heparin treatment caused a decrease in both osteoblast and osteoid surface. At a heparin dose of 1.0 U/g/day, there was a 59.0% ± 6.0% (P < .001) decrease in osteoblast surface and a 68.3% ± 12.2% (P < .001) decrease in the percentage of cancellous bone covered by osteoid. Similar decreases in osteoblast and osteoid surfaces were found 28 days after stopping heparin treatment (Fig 3).
The reversibility of heparin’s effects on the percentage of cancellous bone surface length occupied by either osteoblasts or osteoid. Rats were injected daily with vehicle alone (open bars) or unfractionated heparin at a concentration of either 0.5 U/g/day (cross hatched; rising to the right) or 1.0 U/g/day (cross hatched; rising to the left). On day 28, half of the rats from each group were killed while the other half were allowed to live for an additional 28 days with no further treatment before determining the percentage of cancellous bone surface lined with either osteoblasts (A) or osteoid (B). Data are expressed as mean ± SEM. *P < .005 when compared with either baseline or control values for both (A and B).
The reversibility of heparin’s effects on the percentage of cancellous bone surface length occupied by either osteoblasts or osteoid. Rats were injected daily with vehicle alone (open bars) or unfractionated heparin at a concentration of either 0.5 U/g/day (cross hatched; rising to the right) or 1.0 U/g/day (cross hatched; rising to the left). On day 28, half of the rats from each group were killed while the other half were allowed to live for an additional 28 days with no further treatment before determining the percentage of cancellous bone surface lined with either osteoblasts (A) or osteoid (B). Data are expressed as mean ± SEM. *P < .005 when compared with either baseline or control values for both (A and B).
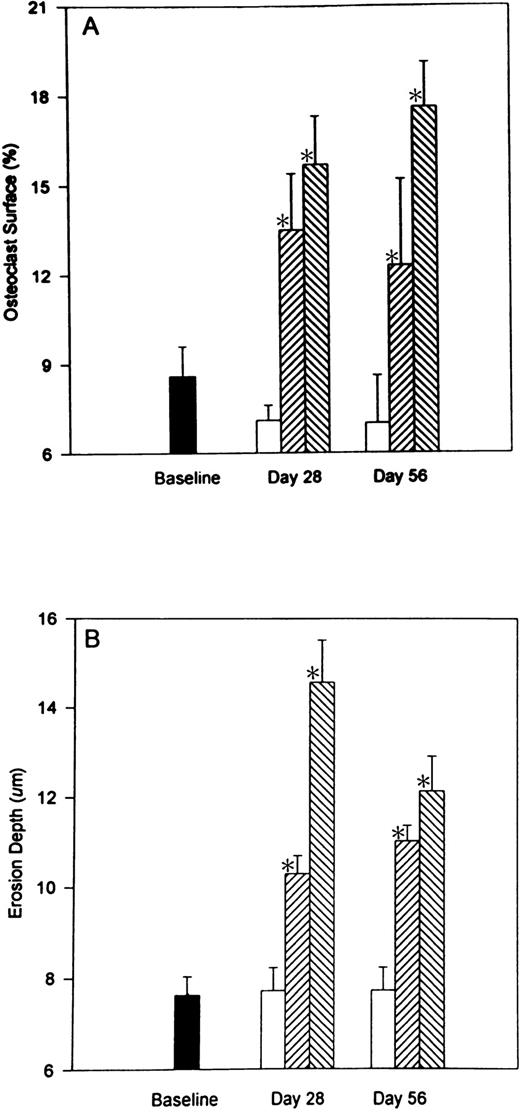
Heparin treatment causes an increase in osteoclast surface (Oc.S/BS). As illustrated in Fig 4A, heparin (1.0 U/g/day) caused a 137.3% ± 14% (P < .001) increase in the percentage of cancellous bone covered by osteoclasts. A similar increase in osteoclast surface was found 28 days after stopping heparin. Erosion depth also increased by 47.9% ± 5.4% (P< .001) in rats given 1.0 U/g heparin (Fig 4B) and remained elevated 28 days after stopping heparin treatment.
The reversibility of heparin’s effects on osteoclast surface and erosion depth. Rats were injected with vehicle alone (open bars) or unfractionated heparin at a concentration of 0.5 U/g/day (cross-hatched; rising to the right) or 1.0 U/g/day (cross-hatched; rising to the left). On day 28, half of the rats from each group were killed while the other half were allowed to live for an additional 28 days with no further treatment before determining osteoclast surface (A) and/or erosion depth (B). Data are expressed as mean ± SEM. * P < .005 when compared with either baseline or control values for both (A and B).
The reversibility of heparin’s effects on osteoclast surface and erosion depth. Rats were injected with vehicle alone (open bars) or unfractionated heparin at a concentration of 0.5 U/g/day (cross-hatched; rising to the right) or 1.0 U/g/day (cross-hatched; rising to the left). On day 28, half of the rats from each group were killed while the other half were allowed to live for an additional 28 days with no further treatment before determining osteoclast surface (A) and/or erosion depth (B). Data are expressed as mean ± SEM. * P < .005 when compared with either baseline or control values for both (A and B).
Reversibility of the heparin effect on dynamic measurements of bone mineralization.
Bone mineralization was measured by fluorescent labeling over a 7-day period. As shown in Table 2, heparin had no effect on mineral apposition rates. In contrast, heparin treatment significantly decreased the percentage of cancellous bone covered by double-labeled surface (MS/BS) (29.3% ± 6.1%; P < .01) and lowered the bone formation rate (BFR/BS) from 25.9 ± 2.7 to 19.2 ± 2.2 (P < .001). There was no significant change in these values 28 days after stopping heparin treatment (Table 2).
The Reversibility of Heparin’s Effect on Dynamic Measurements of Bone Mineralization
| . | Labeled Surface (%) . | Mineral Apposition Rate (μm/day) . | Bone Formation Rate BoneSurface Based (μm3/μm2/d) . |
|---|---|---|---|
| Baseline | — | — | — |
| Day 28 | |||
| Control | 18.1 ± 2.0 | 1.44 ± 0.01 | 25.9 ± 2.7 |
| Heparin (0.5 U/g) | 14.9 ± 1.4 | 1.52 ± 0.02 | 24.5 ± 2.7 |
| Heparin (1.0 U/g) | 12.8 ± 1.1* | 1.52 ± 0.04 | 19.2 ± 2.2* |
| Day 56 | |||
| Control | 18.1 ± 2.0 | 1.44 ± 0.01 | 25.9 ± 2.7 |
| Heparin (0.5 U/g) | 16.9 ± 1.0 | 1.41 ± 0.06 | 24.3 ± 2.5 |
| Heparin (1.0 U/g) | 12.7 ± 1.4*,† | 1.49 ± 0.05 | 18.4 ± 2.3*,† |
| . | Labeled Surface (%) . | Mineral Apposition Rate (μm/day) . | Bone Formation Rate BoneSurface Based (μm3/μm2/d) . |
|---|---|---|---|
| Baseline | — | — | — |
| Day 28 | |||
| Control | 18.1 ± 2.0 | 1.44 ± 0.01 | 25.9 ± 2.7 |
| Heparin (0.5 U/g) | 14.9 ± 1.4 | 1.52 ± 0.02 | 24.5 ± 2.7 |
| Heparin (1.0 U/g) | 12.8 ± 1.1* | 1.52 ± 0.04 | 19.2 ± 2.2* |
| Day 56 | |||
| Control | 18.1 ± 2.0 | 1.44 ± 0.01 | 25.9 ± 2.7 |
| Heparin (0.5 U/g) | 16.9 ± 1.0 | 1.41 ± 0.06 | 24.3 ± 2.5 |
| Heparin (1.0 U/g) | 12.7 ± 1.4*,† | 1.49 ± 0.05 | 18.4 ± 2.3*,† |
Rats were injected daily with vehicle alone or unfractionated heparin as described in Materials and Methods. On days 28 and 56, rats were exsanguinated and their right femurs removed for histologic examination. To permit measurements of bone apposition rates and the calculation of dynamic parameters, all animals received two interperitoneal injections of fluorescent markers. The first, demeclocycline (15 mg/kg), was given 10 days before sacrifice, while the second, calcein (8 mg/kg), was given 3 days before sacrifice. Data are expressed as mean ± SEM.
P < .01 when compared with control values.
P > .05 when compared with values obtained from heparin-treated animals at day 28.
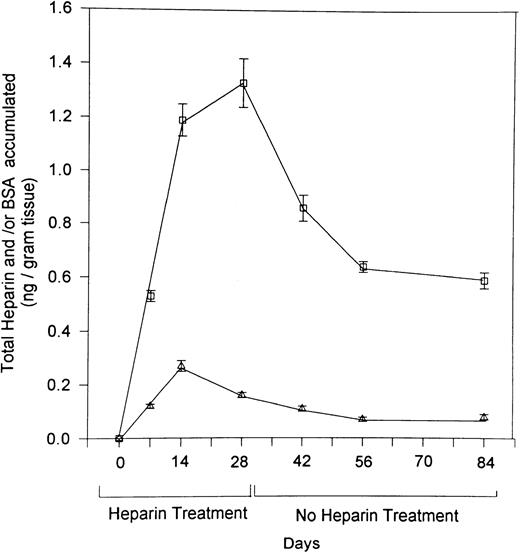
Retention of I125-labeled heparin in bone.
To explore the mechanism responsible for the prolonged effect of heparin on bone, we gave rats daily injections of I125-labeled heparin or I125-labeled BSA for 28 days. Radioactivity in the femurs was determined at various intervals during and after the course of heparin treatment. As illustrated in Fig 5, accumulation of radioactive heparin in the femur during the 28-day course of treatment was significantly greater than that of radiolabeled BSA. After cessation of heparin treatment, radiolabeled heparin levels plateaued at a significantly higher level than radiolabeled BSA, which approached baseline.
The retention in bone of 125I-labeled heparin over time. Rats were injected with 125I-labeled heparin (□) or 125I-labeled BSA (▵) over a 28-day period. At various intervals over the 28 days of heparin treatment and for an additional 56 days thereafter, four rats from each group were killed, their femurs isolated. The amount of heparin that had accumulated in each femur was then determined by use of a γ-counter. Counts were corrected over time for the decay of 125iodine and converted to ng of heparin or BSA/g tissue. Data are expressed as mean ± SEM.
The retention in bone of 125I-labeled heparin over time. Rats were injected with 125I-labeled heparin (□) or 125I-labeled BSA (▵) over a 28-day period. At various intervals over the 28 days of heparin treatment and for an additional 56 days thereafter, four rats from each group were killed, their femurs isolated. The amount of heparin that had accumulated in each femur was then determined by use of a γ-counter. Counts were corrected over time for the decay of 125iodine and converted to ng of heparin or BSA/g tissue. Data are expressed as mean ± SEM.
DISCUSSION
In this study, we used a well-established rat model of heparin-induced osteoporosis11 12 to determine whether the decrease in cancellous bone produced by heparin is reversible once heparin therapy is discontinued. Here we report no evidence of recovery in the 4 weeks after stopping heparin treatment, and we hypothesize that this reflects the fact that heparin accumulates in bone and is retained long after heparin treatment is discontinued.
Heparin could reduce cancellous bone volume by either decreasing bone growth or by influencing the rate of bone remodeling (ie, increasing rates of bone resorption and/or decreasing rates of bone formation). In this study, we used mature 4- to 5-month old rats to minimize potential effects of heparin on growth. Previously, we used 2-month old rats, which made it difficult to determine whether the heparin-induced reduction in cancellous bone was caused by an effect on bone growth or by effects on bone remodeling. In this study, rats given 1.0 U/g/day heparin for 28 days demonstrated a 30.0% ± 4.5% reduction in cancellous bone; a value comparable to the 31.9% ± 3.2% reduction that we previously reported.12 This suggests that heparin causes cancellous bone loss by influencing bone remodeling rather than growth.
There is evidence in humans that heparin-induced reduction in bone density is not rapidly reversible. For example, 5 of 14 women given long-term heparin during pregnancy had a 10% or greater decrease in femoral bone density, and none showed a significant increase 6 months later.9 Similar results were reported in a second study.8 A more recent case-control study compared spinal and radial bone mineral densities in 61 premenopausal women treated with long-term heparin therapy with those in age-matched controls. Although there were no significant differences in mean radial and spinal bone densities, a significantly greater proportion of women who had received heparin 2 years previously had bone densities below a predefined minimal level.17 Sequestration of heparin in bone provides a plausible explanation for these results.
Spinal fractures have been reported in patients treated with long-term (≥ 2 months) subcutaneous heparin at doses as low as 10,000 IU/day.7,10 Doses as high as 50,000 IU/day have been used when treating pregnant women with venous thromboembolism.7-9 In the current study, we used dosages of 0.5 and 1.0 U/g/day, which are equivalent in a 70 kg patient to doses of 35,000 and 70,000 IU/day. Thus, while our dose of 0.5 U/g/day is within the range used for prophylaxis and treatment, our dose of 1.0 U/g/day is higher than that which is normally recommended therapeutically.
The site of heparin sequestration in bone is unknown. Heparin has been reported to bind to endothelial cells and macrophages, as well as to a variety of plasma proteins.18-21 Such binding explains heparin’s poor bioavailability at low doses and the variable anticoagulant response that it produces when used therapeutically.20-22 Moreover, the phenomenon of heparin resistance may also be directly attributable to nonspecific binding of heparin by plasma proteins.23 Our findings suggest that heparin binds to bone marrow cells or to the cancellous bone surface. Thus, we compared the accumulation of 125I-labeled heparin in rat femurs with that of 125I-labeled BSA and found that only heparin accumulated to significant levels in bone where it remained at relatively high levels once treatment was discontinued. Were this radioactivity a result of catabolized 125iodine, we would expect that radioactivity levels in the125I-labeled BSA group would also be elevated. As this was not the case, we conclude that radioactivity in the femurs of heparin-treated animals is likely a result of heparin sequestering in the bone microenvironment. The sequestration of heparin within the bone microenvironment may explain the lack of recovery of bone loss over the 28-day study period after heparin therapy was stopped.
The choice of a 28-day recovery period after heparin treatment should have been sufficient to observe an improvement in cancellous bone volume after stopping heparin treatment. Both corticosteroid-induced bone loss24-26 and bone loss due to limb immobilization27,28 are, at least to some extent, reversible within a similar time frame. For example, Tuukkanen et al27 reported that rat femur mineral mass recovered 40% within the first 3 weeks of remobilization after 3 weeks of cast immobilization. In contrast, we found no evidence of recovery within the first 28 days after stopping heparin treatment. Moreover, heparin reduces cancellous bone volume not only by reducing the mean width of individual trabeculae, but also by reducing the number of trabeculae. This suggests that even if there is delayed recovery in cancellous bone volume, the distorted bone architecture would compromise bone strength. If our findings in rats can be translated to humans, long-term heparin therapy may contribute to osteoporosis later in life. This may be particularly important in women, given the significant bone loss that occurs in the postmenopausal period.
Supported by the Heart and Stroke Foundation of Ontario. J.I.W. is a Career Investigator of the Heart and Stroke Foundation of Ontario. Partial salary support for J.M.M. was obtained from the Canadian Memorial Chiropractic College.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephen G. Shaughnessy, PhD, Hamilton Civic Hospitals Research Centre, 711 Concession St, Hamilton, Ontario, Canada L8V 1C3.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal