Abstract
The immunodominance effect, whereby the presence of immunodominant epitopes prevents recognition of nondominant determinants presented on the same antigen-presenting cell (APC) considerably restricts the repertoire of cytotoxic T lymphocyte (CTL) responses. To elucidate the molecular basis of the immunodominance effect, we compared the interactions of a dominant (B6dom1) and a nondominant epitope (H-Y) with their restricting class I molecule (H2-Db), and their ability to trigger cognate CTLs. We found that B6dom1/Db complexes behaved as optimal T-cell receptor (TCR) ligands and triggered a more rapid in vivo expansion of cognate CTLs than H-Y/Db complexes. The superiority of the dominant epitope was explained by its high cell surface density (1,012 copies/cell for B6dom1v 10 copies/cell for H-Y) and its optimal affinity for cognate TCRs. Based on these results, we conclude that dominant class I–associated epitopes are those that have optimal ability to trigger TCR signals in CTLs. We propose that the rapid expansion of CTLs specific for dominant antigens should enable them to compete more successfully than other CTLs for occupancy of the APC surface.
WHEN CONFRONTED with numerous peptides presented in the context of self-major histocompatibility complex (MHC), T cells usually respond only to a limited number of peptide epitopes.1,2 The immunogenicity of such peptides is determined by four parameters: (1) the rate of processing (proteolysis and transport) of peptide epitopes, (2) their MHC binding affinity, (3) the presence of specific T-cell precursors, and (4) the suppression of T-cell responses to nondominant peptides by dominant epitopes (immunodominance effect).3-15 The immunodominance effect considerably restricts the repertoire of T-cell effectors. Indeed, several peptides that are adequately presented on antigen-presenting cells (APCs) are said to be nondominant because they elicit T-cell responses when they are presented alone, but are neglected when they are presented in conjunction with other peptides. Immunodominance has been found to regulate cytotoxic T lymphocyte (CTL) responses to viruses,16,17 bacteria,18 tumor antigens,19-21 and minor histocompatibility antigens (MiHAs).22-30 Based on detailed studies of in vivo and in vitro CTL responses toward MiHAs, it appears that only a very small proportion of epitopes, probably less than 10%, are dominant.22-28 A priori, immunodominance may not appear advantageous, as it signifies that by focusing exclusively on one or a few epitopes, the immune system places all its eggs in the same (or a few) basket(s). Theoretically, this should increase the risk that pathogens or tumor cells can escape from immunosurveillance.31 Therefore, it is of great importance to decipher the rules that govern immunodominance to understand why the repertoire of T-cell responses is restricted to only a few determinants when confronted with numerous immunogenic epitopes and what are the implications of this restriction.
When immunized against C57BL/6 cells, C3H.SW mice recognize a small set of class I–associated dominant MiHAs collectively called B6dom.32,33 To investigate the mechanisms of dominance, we compared immune responses towards B6dom and H-Y antigens because, in H2b mice, these MiHAs lie at opposite ends on the dominance scale. Indeed, H-Y is always nondominant when presented with one or numerous autosomal MiHAs, whereas B6dom are dominant antigens when presented with other MiHAs.32,34 Thus, when C3H.SW female mice were primed with C57BL/6 male cells, they generated anti-B6dom CTLs, but failed to respond to H-Y even though (1) C3H.SW female mice could respond to H-Y when it was presented alone (on C3H.SW male cells), and (2) C57BL/6 male cells express H-Y MiHA.32B6dom did not suppress anti–H-Y responses by acting as a T-cell receptor (TCR) antagonist for anti–H-Y CTLs because B6dom and H-Y are not cross-reactive at the TCR level. Importantly, dominance was not seen when mice (C3H.SW female) were primed with the dominant and nondominant antigens on separate APCs (ie, a mixture of C3H.SW male cells + C57BL/6 female cells).32 These results indicated that dominance results from competition for the APC surface between anti-B6dom and anti–H-Y CTLs.2
Taking advantage of the recent elucidation of the sequence of the H-Y and B6dom1 epitopes,33,35 both presented by H2-Db, we evaluated the molecular basis of the immunodominance effect. The results presented herein show that compared with H2-Db/H-Y complexes, H2-Db/B6dom1 complexes are more abundant at the surface of APCs, likely interact with optimal affinity with their specific TCR, and trigger a more rapid expansion of cognate CTLs. Coupled with previous work,32 these findings provide strong evidence that because of their rapid expansion, CTLs directed towards immunodominant antigens occupy the surface of APCs and thereby prevent the interaction of antinondominant CTLs with these same APCs. In this way, CTL responses are focused on those antigens which are the most effective at triggering T-cell activation.
MATERIALS AND METHODS
Mice.
Mice from the Jackson Laboratory (Bar Harbor, ME) were used between 6 and 16 weeks of age. They were maintained on acidified drinking water and under normal housing conditions according to the standards of the Canadian Committee for Animal Protection.
Cell lines.
The T2Db cell line (kindly provided by Dr P. Cresswell, Yale University, New Haven, CT) was created by transfection of T2 cells with the H2-Db gene.36 T2 is a human hybrid lymphoblastoid cell line with a large deletion in the MHC class II region, including genes for the TAP1/2 and LMP2/7 products. RMA-S (H2b) is a TAP2 mutant cell line expressing very low surface amounts of MHC class I molecules. SW10/B, an established AAPDNRETF-specific CD8+ cell line was generated by priming intraperitoneally (IP) C3H.SW mice with C57BL/6 spleen cells followed by four cycles of in vitro restimulation of effector cells with C3H.SW cells coated with synthetic AAPDNRETF peptide (Christianson GJ, Pion S, Roopenian DC, Perreault C, unpublished observation, October 1997).
Synthetic peptides.
Peptides were synthesized by Chiron Technologies (Victoria, Australia). AAPDNRETF (B6dom1) is an H2-Db–restricted dominant MiHA present on the surface of C57BL/6 spleen cells.33 WMHHNMDLI (H-Y) is a male specific H2-Db–restricted MiHA derived from the UTY protein.35 Purity, as determined by reversed phase–high-performance liquid chromatography (HPLC), was above 97% for both synthetic peptides.
Extraction and HPLC fractionation of natural MiHA peptides.
Natural MiHA peptides were eluted from C57BL/6 male cells by acid extraction in the presence of protease inhibitors (25 mmol/L iodoacetamide, 1 mmol/L aprotinine, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF]).32,37 Thus, 2 × 108cells were suspended in 5 mL of citrate-phosphate buffer (0.131 mol/L citric acid/0.066 mol/L Na2HPO4, pH 3.3) for 1 minute at room temperature and centrifuged. Under these experimental conditions (pH < 3.4), the efficiency of peptide extraction is ≈95%.37 After a prepurification on a C18 Sep-Pak column (Waters, Milford, MA), eluates were fractionationated on an HPLC system using a Superpac Pep-S C18 column (5 μm, 4 × 250 mm, Pharmacia, Uppsala, Sweden).32 Solvents used were 99.9% water/0.1% trifluoro-acetic acid (solvent A) and 99.9% acetonitrile/0.1% trifluoro-acetic acid (solvent B). The gradient consisted of the following linear step intervals: 0% B (0 to 5 minutes), up to 20% B at 10 minutes, up to 55% B at 55 minutes, plateau at 55% B from 55 to 60 minutes, and up to 100% B at 70 minutes. Flow rate was 1 mL/min, and 1-mL fractions were collected and lyophilized.
CTL assays.
Standard 51Cr-release assays were performed as previously described.32,38,39 Analysis of the expression of B6dom1 by various strains of H2b mice and quantification of natural AAPDNRETF epitopes expressed by C57BL/6 and 129 cells were performed with SW10/B effectors (see Fig 3). In all other experiments, effectors were polyclonal CTLs obtained after a single in vivo priming followed by a single in vitro mixed leukocyte culture (MLC).32 Target cells were either concanavalin A (Con A) blasts or T2Db cells that were either untreated or preincubated with an exogenous source of peptides in the form of HPLC fractions or synthetic peptides. In peptide sensitization assays, target cells were incubated at 26°C overnight, then sensitized with synthetic peptides or HPLC fractions for 90 minutes before being used in CTL assays. Effectors were incubated with targets for 3 hours in standard CTL assays and for 4 hours in peptide sensitization assays. All tests were performed in triplicate. Spontaneous release values varied from 5% to 20% of total release.
Interactions between H2-Db and MiHA peptides.
The binding affinity of WMHHNMDLI and AAPDNRETF for the Dbmolecule was evaluated by fluorescence-activated cell sorting (FACS) analysis with anti–H2-Db antibody (Ab) (cat no. 06114D; Cedarlane, Hornby, Canada) using T2Db and RMA-S cells, as previously described.32 The anti–H2-Db Ab used recognizes an epitope whose expression is not modified by the nature of the specific peptide bound to Db molecules.40Briefly, T2Db and RMA-S cells were sensitized with different concentrations of synthetic peptides to stabilize surface H2-Db expression. Afterwards, cells were stained with anti–H2-Db Ab, washed, and analyzed by FACS. To evaluate the half-life of H2-Db/peptide complexes, T2Dbcells were incubated for 12 hours in the presence of B6dom1(10−7 mol/L) or H-Y (10−5 mol/L) synthetic peptides at 26°C. Brefeldin A (10 μg/mL) (Sigma, Oakville, Canada) was added to the culture for the last 2.5 hours to prevent the appearance of newly synthesized class I molecules. Cells were washed five times, resuspended in Brefeldin A supplemented medium, and transferred to a 37°C incubator. Then, H2-Dbexpression was evaluated by flow cytometry at different time points. Mean fluorescence intensity (MFI) was evaluated with the MFI software program (E. Martz, University of Massachusetts, Amherst). Corrected MFI = MFIexp − MFImin, where MFIexp is the fluorescence intensity of cells incubated with one of the synthetic peptides and MFImin is the fluorescence intensity of cells that had been pulsed with medium alone before labeling with the anti–H2-Db Ab.
Limiting dilution analysis (LDA) of CTL precursor frequencies.
Limiting numbers of responder spleen cells from C3H.SW female mice primed either with C57BL/6 female (B6dom1+) or C3H.SW male (H-Y+) splenocytes (20 × 106 cells injected IP) were restimulated in vitro on days 5, 10, 15, or 20 postimmunization with 3 × 105 irradiated stimulator cells in the presence of 2.5 or 20 U/mL of interleukin-2 (IL-2). After 9 days, cultures were evaluated in a standard 4-hour 51Cr release assay. Wells were scored positive if 51Cr release exceeded the spontaneous release by more than 3 SD. The frequency of CTL precursors was determined by the χ2 minimization procedure.
RESULTS
Relative affinities of AAPDNRETF (B6dom1) and WMHHNMDLI (H-Y) for Db.
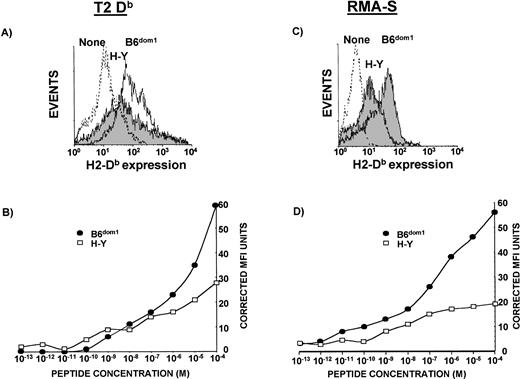
To evaluate the binding affinity for Db molecules, we compared the ability of synthetic B6dom1 and H-Y peptides to upregulate Db expression on T2Db and RMA-S cells. As they are deficient in TAP1/2 and LMP2/7 proteins (T2Db) or TAP2 (RMA-S), these cells express only low levels of unstable Db molecules on the cell surface.36However, when exogenous Db-binding peptides are added, they stabilize “empty” Db molecules, thereby causing an increased cell surface expression of this class I molecule that can be measured by flow cytometry. Studies involving a large variety of peptides have demonstrated that MHC stabilization assays can be taken as a measure of the relative peptide affinity.4,5,7,13Thus, both types of cells were incubated with titrated amounts of H-Y and B6dom1 peptides and stained with an anti-DbAb. In three experiments with T2Db cells, the concentration of H-Y required for half-maximal Db upregulation was one to two logs greater than that of B6dom1. In the representative example depicted in Fig 1B, the peptide concentrations required for similar Db expression were 2 × 10−6 mol/L for B6dom1 and 10−4 mol/L for H-Y. Similar results were observed using RMA-S cells (Fig 1D). In agreement with these findings, B6dom1 was previously shown to have a very high binding affinity for Db, even superior to that of the immunodominant Db-associated epitope of influenza nucleoprotein (ASNENMDAM).32,41 42
Relative affinities of B6dom1 (AAPDNRETF) and H-Y (WMHHNMDLI) for Db. T2Db (A and B) and RMA-S cells (C and D), precultured at 26°C, were incubated in serum-free medium with various concentrations of synthetic B6dom1 or H-Y peptide, stained for Db surface expression, and analyzed by flow cytometry. (A) H2-Dbsurface expression of T2Db cells incubated alone (dashed line), with 10−4 mol/L H-Y (solid black line, grey background), or with 10−4 mol/L B6dom1 (solid black line). (B) Corrected MFI of T2Db cells stained with anti-Db Ab after incubation with graded concentrations of peptides. (C) H2-Db surface expression of RMA-S cells incubated alone (dashed line), with 10-4 mol/L H-Y (solid black line, grey background), or with 10−4 mol/L B6dom1 (solid black line). (D) Corrected MFI of RMA-S cells stained with anti-Db Ab after incubation with graded concentrations of peptides. One representative experiment of two is shown.
Relative affinities of B6dom1 (AAPDNRETF) and H-Y (WMHHNMDLI) for Db. T2Db (A and B) and RMA-S cells (C and D), precultured at 26°C, were incubated in serum-free medium with various concentrations of synthetic B6dom1 or H-Y peptide, stained for Db surface expression, and analyzed by flow cytometry. (A) H2-Dbsurface expression of T2Db cells incubated alone (dashed line), with 10−4 mol/L H-Y (solid black line, grey background), or with 10−4 mol/L B6dom1 (solid black line). (B) Corrected MFI of T2Db cells stained with anti-Db Ab after incubation with graded concentrations of peptides. (C) H2-Db surface expression of RMA-S cells incubated alone (dashed line), with 10-4 mol/L H-Y (solid black line, grey background), or with 10−4 mol/L B6dom1 (solid black line). (D) Corrected MFI of RMA-S cells stained with anti-Db Ab after incubation with graded concentrations of peptides. One representative experiment of two is shown.
Stability of Db/peptide complexes.
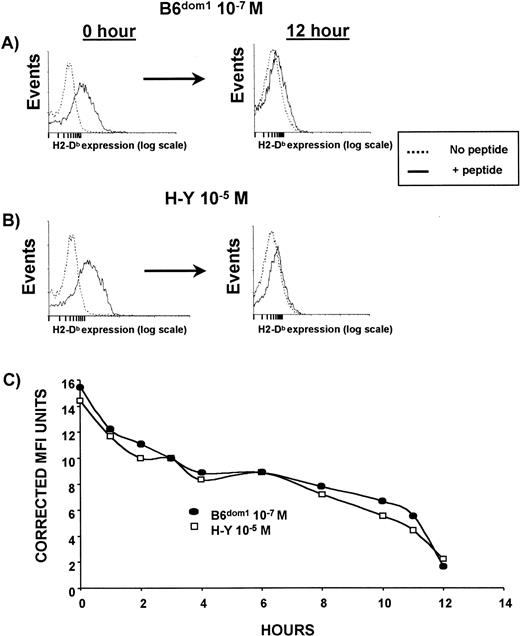
T2Db cells, precultured overnight at 26°C, were incubated with B6dom1 or H-Y peptide. Peptides were used at different molar concentrations selected to obtain an MFI of ≈15. Under these conditions, the level of Db expression at time 0 was similar for both peptides. Then, T2Db cells were washed five times, incubated at 37°C in serum-free medium supplemented with Brefeldin A, and the density of residual Db molecules was monitored over a 12-hour period. The half-life of B6dom1/Db and H-Y/Db complexes was remarkably similar (8 hours, Fig 2C) and longer than that of most viral peptides.32,41,42 As these estimates of Db/peptide stability reflect relative dissociation rates,5 we conclude that the greater affinity of B6dom1 for Db (Fig 1) is due to a faster association rate rather than to a slower dissociation rate. It has previously been found that for a number of peptides, a close correlation existed between the MHC-binding affinity and the dissociation rate32,41. Therefore, it is interesting to note that in the case of B6dom1 and H-Y, the major difference in Db-binding affinity can be ascribed to different association rates. However, this is not unprecedented, as similar findings have been reported with Kb-binding ovalbumin (OVA) peptides.5
Stability of Db/peptide complexes. T2Db cells, precultured at 26°C in the presence of Brefeldin A, were incubated with peptides in complete medium. Peptides were used at different molar concentrations selected to obtain a similar MFI value. In these conditions, the level of H2-Dbexpression at time 0 was equivalent for both B6dom1 and H-Y. T2Db cells were then washed five times, incubated at 37°C in serum-free medium supplemented with Brefeldin A, and the density of residual Db molecules was measured by staining with anti-Db Ab at various time points. (A) B6dom1/Db expression at 0 and 12 hours. (B) H-Y/Db expression at 0 and 12 hours. (C) Downregulation of peptide/Db complexes over a 12-hour time frame. One representative experiment of two is shown.
Stability of Db/peptide complexes. T2Db cells, precultured at 26°C in the presence of Brefeldin A, were incubated with peptides in complete medium. Peptides were used at different molar concentrations selected to obtain a similar MFI value. In these conditions, the level of H2-Dbexpression at time 0 was equivalent for both B6dom1 and H-Y. T2Db cells were then washed five times, incubated at 37°C in serum-free medium supplemented with Brefeldin A, and the density of residual Db molecules was measured by staining with anti-Db Ab at various time points. (A) B6dom1/Db expression at 0 and 12 hours. (B) H-Y/Db expression at 0 and 12 hours. (C) Downregulation of peptide/Db complexes over a 12-hour time frame. One representative experiment of two is shown.
Cell surface density of naturally processed B6dom1 and H-Y epitopes.
Peptides associated with cell surface MHC molecules were obtained by acid elution from C57BL/6 male splenocytes, fractionated by HPLC, and their biologic activity was compared with that of synthetic peptides (AAPDNRETF and WMHHNMDLI) in CTL sensitization assays using T2Db targets with anti–H-Y- and anti–B6dom1-specific effectors.32,37Polyclonal anti–H-Y CTLs, obtained after a single in vivo priming and in vitro restimulation of C3H.SW female cells with C3H.SW male cells, were used for quantification of natural H-Y epitopes because such effectors recognize a single peptide-Dbcomplex.43 B6dom1 activity was detected with an AAPDNRETF-specific C3H.SW-derived CTL line (SW10/B). The retention times of the natural B6dom1 and H-Y peptides were identical to those of the synthetic peptides: 18 minutes for AAPDNRETF and 27 minutes for WMHHNMDLI (data not shown). As a control, peptides extracted from C3H.SW female cells did not sensitize target cells to lysis by anti-B6dom1 or anti–H-Y effectors (data not shown).
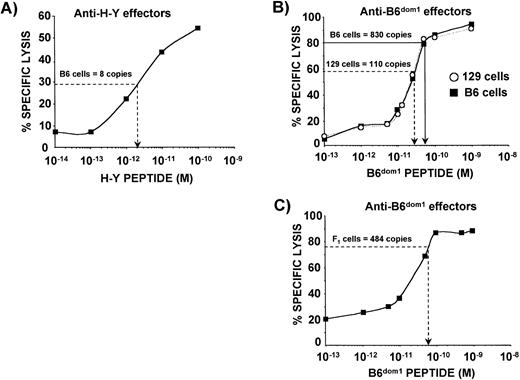
Theoretically, an assay based on the comparison of the CTL-sensitizing activity of a synthetic peptide with the activity in cell extracts is prone to underestimate copy number because other peptides present in the extract can act as competitive inhibitors of MHC binding.44 Therefore, we modified the assay to eliminate this bias which, in principle, could be more important for H-Y than for B6dom1 because the former peptide has a lower MHC binding affinity (Fig 1). Thus, we compared the activity of peptide extracts from a given number (N) antigenpos cells with the activity of various concentrations of synthetic peptide mixed with peptide extracts from N antigenneg cells. Cell extracts and synthetic peptides were subjected to the same HPLC fractionation procedure and the fraction of interest (no. 18 for B6dom1and no. 27 for H-Y) was used in CTL assays. For H-Y, the CTL-sensitizing activity of natural peptides extracted from 1.6 × 107 C57BL/6 male cells was compared with a dose response curve of graded concentrations of synthetic H-Y peptide mixed with a fixed amount of nonantigenic peptide extract from 1.6 × 107 C57BL/6 female cells. In the representative experiment shown in Fig 3A, the activity of fraction 27 extracted from 1.6 × 107 C57BL/6 male cells was equivalent to that of 2 × 10−12 mol/L of synthetic H-Y peptide. By multiplying the molar amount of synthetic peptide in a given well by Avogadro’s number and dividing by the number of H-Ypos cells required to have the same percent killing, we calculated that the number of H-Y epitopes per cell ranged from 3 to 12 in 12 experiments. The same strategy was used for B6dom1. The activity of natural peptides extracted from 4 × 106 C57BL/6 male cells was compared with the titration curve of synthetic B6dom1 peptide mixed with a nonantigenic peptide extract from 4 × 106 C3H.SW male cells (Fig 3B). Here we found that the number of B6dom1epitopes/C57BL/6 cell was 824 to 1,200 (12 experiments). Interestingly, the epitope density of B6dom1 was decreased by ≈50% on (C57BL/6 × C3H/HeJ)F1 hybrids relative to C57BL/6 cells (Fig 3C). Epitope density of B6dom1 as expressed on C57BL/6 cells place this peptide among the most abundant natural class I–restricted epitopes44-48 and show that it is much more abundant than H-Y. The low H-Y epitope density is similar to that of the H13 MiHA whose structure has recently been discovered.45
Abundance of naturally expressed B6dom1 and H-Y epitopes. Synthetic B6dom1 and H-Y peptides, as well as cell extracts, were fractionated by RP-HPLC. Both synthetic and natural B6dom1 peptides were recovered in HPLC fraction no. 18, while synthetic and natural H-Y peptides were recovered in fraction no. 27. (A) The activity of natural H-Y peptide extracted from 1.6 × 107 C57BL/6 male cells is plotted on a dose response curve showing the activity of graded concentrations of synthetic H-Y peptide mixed with competitor peptides extracted from 1.6 × 107C57BL/6 female cells. Also evaluated were the activity of 4 × 106, 8 × 106, and 1.2 × 107cell extracts from C57BL/6 male cells giving 3, 7, and 12 copies per cell, respectively (data not shown). One representative experiment of three is shown. (B) The activity of natural B6dom1 peptide extracted from 4 × 106 C57BL/6 male cells or 1.6 × 107 129 male cells is plotted on dose response curves depicting the activity of graded concentrations of synthetic B6dom1 peptide mixed with competitor peptides extracted from 4 × 106 (▪) or 1.6 × 107 (○) C3H.SW male cells, respectively. Also evaluated were the activities of extracts from 8 × 106, 1.2 × 107, or 1.6 × 107 C57BL/6 male cells and 4 × 106, 8 × 106, or 1.2 × 107 129 male cells. The number of epitope copies per cell was established at 824, 903, and 1,200, respectively for C57BL/6, and at 112, 126, and 107 copies for 129 cells (data not shown). One representative experiment of three is shown. (C) The activity of natural B6dom1 peptide extracted from 8 × 106 (C57BL/6J × C3H.HeJ)F1cells is plotted on a dose response curve depicting the activity of graded concentrations of synthetic B6dom1 peptide mixed with competitor peptides extracted from 8 × 106 C3H.SW male cells, respectively. One representative experiment of two is shown. Targets were T2Db cells. E:T ratio was 5:1 for anti-B6dom1 effectors (SW10/B cell line) and 50:1 for anti–H-Y effectors.
Abundance of naturally expressed B6dom1 and H-Y epitopes. Synthetic B6dom1 and H-Y peptides, as well as cell extracts, were fractionated by RP-HPLC. Both synthetic and natural B6dom1 peptides were recovered in HPLC fraction no. 18, while synthetic and natural H-Y peptides were recovered in fraction no. 27. (A) The activity of natural H-Y peptide extracted from 1.6 × 107 C57BL/6 male cells is plotted on a dose response curve showing the activity of graded concentrations of synthetic H-Y peptide mixed with competitor peptides extracted from 1.6 × 107C57BL/6 female cells. Also evaluated were the activity of 4 × 106, 8 × 106, and 1.2 × 107cell extracts from C57BL/6 male cells giving 3, 7, and 12 copies per cell, respectively (data not shown). One representative experiment of three is shown. (B) The activity of natural B6dom1 peptide extracted from 4 × 106 C57BL/6 male cells or 1.6 × 107 129 male cells is plotted on dose response curves depicting the activity of graded concentrations of synthetic B6dom1 peptide mixed with competitor peptides extracted from 4 × 106 (▪) or 1.6 × 107 (○) C3H.SW male cells, respectively. Also evaluated were the activities of extracts from 8 × 106, 1.2 × 107, or 1.6 × 107 C57BL/6 male cells and 4 × 106, 8 × 106, or 1.2 × 107 129 male cells. The number of epitope copies per cell was established at 824, 903, and 1,200, respectively for C57BL/6, and at 112, 126, and 107 copies for 129 cells (data not shown). One representative experiment of three is shown. (C) The activity of natural B6dom1 peptide extracted from 8 × 106 (C57BL/6J × C3H.HeJ)F1cells is plotted on a dose response curve depicting the activity of graded concentrations of synthetic B6dom1 peptide mixed with competitor peptides extracted from 8 × 106 C3H.SW male cells, respectively. One representative experiment of two is shown. Targets were T2Db cells. E:T ratio was 5:1 for anti-B6dom1 effectors (SW10/B cell line) and 50:1 for anti–H-Y effectors.
Relative avidity of CTL target cell recognition of B6dom1and H-Y epitopes.
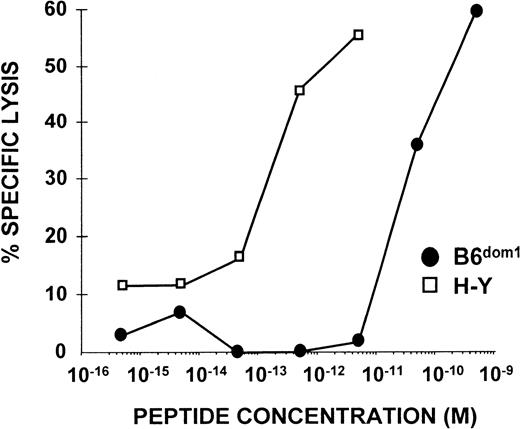
The avidity of CTL/APC interaction is the product of the cell surface density of the peptide/MHC complex on the APC and the intrinsic affinity between these complexes and their specific TCR. To compare the relative avidity of target cell recognition by specific CTLs, polyclonal anti-B6dom and anti–H-Y CTLs were tested in cytotoxicity assays with female C3H.SW targets sensitized with graded concentrations of synthetic B6dom1 and H-Y peptides. The concentration of H-Y peptide necessary to sensitize targets for 50% lysis was very low (10−12 mol/L; Fig 4) when compared with other class I–associated peptides,5 and was 100-fold lower than that required for B6dom1 (2 × 10−10mol/L; Fig 4). These results were highly reproducible in four of four experiments. This indicates that Db/H-Y complexes interact with high-affinity TCRs, whereas TCRs that recognize Db/B6dom1 show a lower intrinsic affinity for their ligand. These results, together with the demonstration that the cell surface density of B6dom1 is 100-fold higher than that of H-Y (Fig 3), support the concept that interactions between TCRs and peptide/MHC complexes obey the law of mass action, even though these reactants are confined to their respective cell membranes. Thus, for a designated level of lysis, the product of the required number of complexes per target cell (epitope density) and TCR affinity for peptide/MHC complexes is constant, low-density ligands usually interact with high-affinityTCRs and vice versa.49
Activity of B6dom1 and H-Y peptides in CTL sensitization assays. 51Cr-labeled T2Db targets that had been preincubated with various concentrations of H-Y or B6dom1 synthetic peptides were incubated for 4 hours with polyclonal anti–H-Y or anti-B6dom1 CTLs (E:T ratio 50:1). One representative experiment of four is shown.
Activity of B6dom1 and H-Y peptides in CTL sensitization assays. 51Cr-labeled T2Db targets that had been preincubated with various concentrations of H-Y or B6dom1 synthetic peptides were incubated for 4 hours with polyclonal anti–H-Y or anti-B6dom1 CTLs (E:T ratio 50:1). One representative experiment of four is shown.
In vivo expansion of anti-B6dom1 and anti–H-Y CTLs.
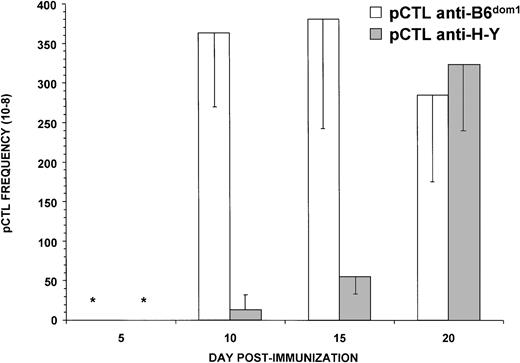
Epitope density and TCR affinity regulate the rate of expansion of cognate CTLs.50-52 To evaluate the kinetics of the primary response, we monitored the frequencies of B6dom1- and H-Y-specific CTLs at various time points after immunization using LDA. This approach has been widely used to evaluate the kinetics of CTL responses.53,54 Thus, C3H.SW female mice were primed with C3H.SW male cells (H-Y+) or C57BL/6 female cells (B6dom1+), their spleen cells were harvested on day 5, 10, 15, or 20, and cultured under limiting dilution conditions in IL-2–supplemented medium with the same cell type as for priming. After 9 days, CTL activity was tested on T2Db targets coated with optimal concentrations of synthetic B6dom1 or H-Y peptide. Assays performed after culture in the presence of low concentrations of IL-2 (2.5 U/mL) showed that the expansion of anti-B6dom1CTLs was much more rapid than that of anti–H-Y CTLs (Fig 5). Thus, on day 5, the mean frequency of anti-B6dom1 CTL precursors was 20-fold greater than that of anti–H-Y precursors. The difference was still significant on day 15 (7.5-fold) and disappeared on day 20. CTL precursor frequencies were similar, however, when the culture medium was supplemented with high concentrations of IL-2 (20 U/mL); in the latter case, anti–H-Y and anti-B6dom1 precursor frequencies reached 2 × 10-5 on day 20 (data not shown). Thus, unless large amounts of IL-2 were added, the proliferation rate of anti-B6dom1CTLs was higher than that of anti–H-Y CTLs. The fact that under these assay conditions H-Y–specific CTLs were more dependent on the supply of exogenous IL-2 than B6dom1-specific CTLs is consistent with previous evidence that in vivo expansion of anti–H-Y CTLs requires CD4 help, whereas expansion of anti-B6dom1 CTLs does not.33 55
Anti-B6dom1 CTLs expand more rapidly than anti–H-Y CTLs. Limiting numbers of responder spleen cells from C3H.SW female mice primed either with C57BL/6 female (B6dom1+) or C3H.SW male (H-Y+) splenocytes (20 × 106cells injected IP) were restimulated in vitro on days 5, 10, 15, or 20 postimmunization with 3 × 105 irradiated stimulator cells in culture medium supplemented with 2.5 U/mL of IL-2. After 9 days, cultures were evaluated in a 4-hour cytotoxicity release assay. Targets were T2Db cells coated with optimal concentrations of H-Y or B6dom1 peptide. Mean ± SD of three mice per group. *, Not detectable.
Anti-B6dom1 CTLs expand more rapidly than anti–H-Y CTLs. Limiting numbers of responder spleen cells from C3H.SW female mice primed either with C57BL/6 female (B6dom1+) or C3H.SW male (H-Y+) splenocytes (20 × 106cells injected IP) were restimulated in vitro on days 5, 10, 15, or 20 postimmunization with 3 × 105 irradiated stimulator cells in culture medium supplemented with 2.5 U/mL of IL-2. After 9 days, cultures were evaluated in a 4-hour cytotoxicity release assay. Targets were T2Db cells coated with optimal concentrations of H-Y or B6dom1 peptide. Mean ± SD of three mice per group. *, Not detectable.
Is B6dom1 expressed by cells with a different genetic background always a dominant epitope?
The results presented above strongly suggest that the high cell surface density of B6dom1 has a determining influence on the fact that this antigen is a dominant MiHA recognized by C3H.SW mice when primed against C57BL/6 cells. Thus, it is logical to assume that if the expression of the B6dom1 epitope was decreased below a certain threshold, B6dom1 would lose its dominant status. The next series of experiments were designed to test this possibility and to evaluate what could be the expression threshold required for B6dom1 to be a dominant MiHA.
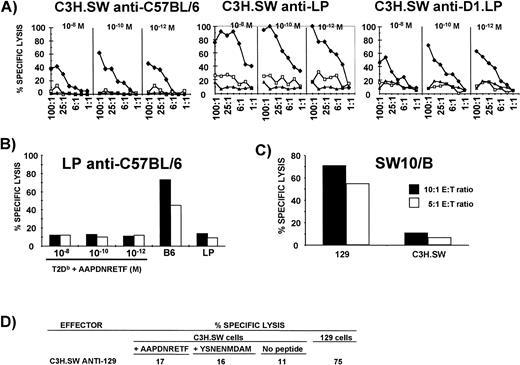
First, to assess the strain distribution of B6dom1, CML assays were performed with SW10/B effectors and Con A blast targets from six strains of H2b mice. Results were negative with C3H.SW, A.BY, and BALB.B, but strongly positive with C57BL/6, 129, D1.LP, and LP targets (data not shown). To determine if B6dom1 was a dominant MiHA, C3H.SW mice were primed IP and their splenocytes were restimulated in MLC with cells from the six strains of H2b mice mentioned above. Effectors from these cultures were tested on T2Db cells coated with synthetic B6dom1 peptide. T2Db cells coated with the YSNENMDAM peptide were used as a negative control. The latter nonapeptide is a variant of the dominant influenza nucleoprotein epitope presented by H2Db and its ability to stabilize Db molecules on the surface of T2Db cells is similar to that of B6dom1.32 As expected, effectors primed with B6dom1-negative cells (C3H.SW, A.BY, and BALB.B) did not kill target cells coated with B6dom1(data not shown). In contrast, C3H.SW-derived CTLs primed with C57BL/6, D1.LP and LP cells (all H2b) killed T2Dbtargets coated with B6dom1 peptide (Fig 6A); thus, for these strains, B6dom1 is not only present, but is also dominant. The fact that LP-derived anti-C57BL/6 CTLs did not kill targets coated with B6dom1 suggests that only B6dom1-negative mice can generate CTLs that kill these targets and that CTLs specific for other antigens present on B6dom1-expressing cells do not cross-react with B6dom1 (Fig 6B). The most interesting results were observed with C3H.SW-derived CTLs primed with 129 cells (the latter are B6dom1-positive, Fig 6C), as these effectors did not kill targets coated with B6dom1 (Fig 6D). Thus, in the latter case, B6dom1 is expressed at the cell surface, but is not immunodominant. The nature of the dominant MiHAs recognized by C3H.SW CTLs on 129 APCs is currently unknown.
B6dom1 is a dominant epitope recognized by C3H.SW-derived CTLs on C57BL/6, LP, and D1.LP, but not on 129 cells. (A) B6dom1 is dominant on C57BL/6, LP, and D1.LP cells. C3H.SW mice were primed IP and their splenocytes were restimulated in MLC with cells from C57BL/6, LP, or D1.LP mice. Effectors were tested at various E:T ratios on C3H.SW Con A blasts that had been incubated with graded concentrations of B6dom1 peptide (⧫), control YSNENMDAM peptide (□), or culture medium alone (◂). (B) LP-derived anti-C57BL/6 CTLs do not kill T2Db targets coated with B6dom1 peptide. E:T ratio was 50:1 (closed bar) or 25:1 (open bar). (C) B6dom1-specific CTLs (SW10/B cell line) kill 129 Con A blasts. (D) C3H.SW-derived anti-129 CTLs do not kill C3H.SW Con A blast targets coated with optimal concentration of B6dom1 peptide (AAPDNRETF; 10−10 mol/L).
B6dom1 is a dominant epitope recognized by C3H.SW-derived CTLs on C57BL/6, LP, and D1.LP, but not on 129 cells. (A) B6dom1 is dominant on C57BL/6, LP, and D1.LP cells. C3H.SW mice were primed IP and their splenocytes were restimulated in MLC with cells from C57BL/6, LP, or D1.LP mice. Effectors were tested at various E:T ratios on C3H.SW Con A blasts that had been incubated with graded concentrations of B6dom1 peptide (⧫), control YSNENMDAM peptide (□), or culture medium alone (◂). (B) LP-derived anti-C57BL/6 CTLs do not kill T2Db targets coated with B6dom1 peptide. E:T ratio was 50:1 (closed bar) or 25:1 (open bar). (C) B6dom1-specific CTLs (SW10/B cell line) kill 129 Con A blasts. (D) C3H.SW-derived anti-129 CTLs do not kill C3H.SW Con A blast targets coated with optimal concentration of B6dom1 peptide (AAPDNRETF; 10−10 mol/L).
Why do C3H.SW CTLs recognize B6dom1 as a dominant MiHA when it is presented on C57BL/6, D1.LP, or LP, but not when presented on 129 cells? One possibility would be that the epitope density of B6dom1 is identical on C57BL/6 and 129 cells, but that the immunodominant MiHA recognized by C3H.SW-derived anti-129 CTLs (hereafter referred to as 129dom) dominates B6dom1 expressed at ≈103 copies per cell. This possibility cannot be directly evaluated because 129dom has not been defined. An alternative explanation would be that the expression of B6dom1 at the cell surface is lower in 129 mice than in C57BL/6 mice. To evaluate this possibility, we assessed the abundance of B6dom1 at the surface of 129 cells. B6dom1 was recovered in the same fraction (ie, no. 18) after HPLC fractionation of eluates from C57BL/6 and 129 splenocytes (data not shown), but the number of epitopes per cell was only 107 to 126 in 129 splenocytes (12 experiments), ie, sevenfold less than for C57BL/6 cells (Fig 3B). This suggests that an 88% decrease in the cell surface expression of B6dom1 is sufficient for this antigen to lose its dominant status. Theoretically, quantitative assessment of B6dom1 expression could be biased if there were a variation in the structure of the B6dom1 peptide expressed in 129 versus C57BL/6 cells. However, this possibility is very unlikely because both peptides had exactly the same elution profile when extracts from B6 and 129 cells were fractionated with three different previously described high-resolution HPLC gradient systems (data not shown).32
DISCUSSION
B6dom1/Db complexes are optimal CTL ligands.
The results presented herein (summarized in Table 1) disclose major differences between the nondominant and the dominant antigens recognized by C3H.SW-derived CTLs on C57BL/6 cells: H-Y/Db complexes show a low cell surface density, but are recognized by TCRs with very high relative affinity, whereas B6dom1/Db complexes are more abundant and interact with lower affinity TCRs.
Characteristics of B6dom1 and H-Y
| . | B6dom1 . | H-Y . |
|---|---|---|
| Relative affinity for Db (Fig 1) | High | Moderate |
| Half-life of peptide/MHC complexes (Fig 2) | Long | Long |
| Epitope density on C57BL/6 spleen cells (Fig 3) | High | Low |
| Relative TCR affinity (deduced from Figs 1 and 4) | Moderate | High |
Efficient T-cell triggering requires sustained signaling, is correlated with the number of TCR engaged, and is dependent on optimal kinetics of TCR interaction with peptide/MHC complexes.50,52,56-58 A high epitope density, such as found for B6dom1 when compared with H-Y, is an advantage because the number of triggered TCRs is a function of the logarithm of the number of complexes offered.52,56 Indeed, antigen dose has a determinant influence on the level of proliferation of cognate T cells, and high epitope density can even overcome the requirement for costimulation (“signal two”).50,51,59 The fact that B6dom1 is not dominant on 129 cells, where its expression is only ≈117 copies/cell (v ≈1,012 for C57BL/6 cells) strongly suggests that epitope density plays a pivotal role in dominance. The relation between TCR affinity and T-cell triggering is, however, more complex because optimal affinity does not correspond to maximal affinity, but rather to “intermediate” affinity. Indeed, acceleration of TCR-ligand complex dissociation allows serial TCR triggering and increases the efficiency of antigen recognition by CTL up to a critical threshold, beyond which a too rapid dissociation results in aberrant TCR signaling and TCR antagonism.60Whereas epitopes with an optimal off-rate can trigger several TCRs serially, high-affinity ligands, such as anti-CD3 antibodies, are inefficient because their incapacity to dissociate does not allow serial triggering.58 According to this model, the intermediate affinity of B6dom1/Db complexes for their cognate TCRs should be more advantageous for T-cell activation than the high-affinity of Db/H-Y complexes.
The concept that B6dom1 triggers stronger signal 1 in CTL than H-Y could well explain the observation that B6dom1-specific CTLs expanded more rapidly and were less dependent on exogenous IL-2 than H-Y–specific CTLs (Fig 5). Indeed, potent helper-independent T-cell responses can only be triggered by ligands that, because of their abundance and optimal TCR affinity, induce strong TCR signals.51,61 This concept would also provide a plausible explanation for the fact that, whereas CTL responses toward B6dom1 and H-Y appear to be of similar magnitude when evaluated in standard in vitro CTL assays (percent lysis at various E:T ratios), the in vivo consequences of responses elicited by these MiHAs are quite different. Indeed, in vivo responses toward H-Y/Db are biologically attenuated; they cannot elicit graft-versus-host disease and are not sufficient to cause skin graft rejection (in H2b mice, male skin graft rejection is elicited mainly by class II, as opposed to class I-associated H-Y epitopes).30,62 In contrast, anti-B6dom1 CTLs are sufficient to provoke graft-versus-host disease.33However, it must be remembered that the dominance effect results from competition between dominant and nondominant epitopes and is, therefore, defined in relative terms. Thus, the fact that other MiHAs are neglected when presented with B6dom1 does not imply that, when presented alone, they cannot elicit biologically significant CTL responses. Nevertheless, other reports confirm the differential ability of dominant versus nondominant epitopes to generate protective responses against other types of antigens, ie, viruses or cancer cells.63-65 Collectively, these observations provide compelling evidence that dominant antigens elicit more vigorous CTL responses than nondominant epitopes. Furthermore, the fact that H-Y/Db is an adequate CTL target in vitro, but that in vivo, it elicits only a slow expansion of cognate CTLs (Fig 5), which is of little biological significance30,62 illustrates that the ability to kill target cells in vitro is not predictive of in vivo efficacy.66,67 This can be explained by the existence of more stringent requirements for the afferent arm of CTL responses (proliferation) than for the effector phase of the killing process.57
How can expression of B6dom1 inhibit anti–H-Y CTL responses?
Previous analyses of CTL responses toward B6dom1 and H-Y showed that the most critical characteristic of the immunodominance effect is that “suppression” of T-cell responses to nondominant epitopes by dominant epitopes is observed only when both types of determinants are presented on the same APC.32,34 This observation indicates that differential generation of T-cell help cannot be responsible for B6dom1 immunodominance over H-Y/Db. Indeed, help from other T cells, particularly from the CD4+ subset, is available to all CD8+ CTLs that recognize epitopes presented on the same APC, irrespective of the specificity of helper cells and CTLs.55 68-70 Thus, after immunization with C57BL/6 male cells, anti-B6dom1 and anti–H-Y CTLs receive the same help because both MiHAs are presented on the same APC. Therefore, the fact that dominance takes place only when the dominant and the nondominant epitopes are presented on the same APC suggests that the immunodominance effect results from a competition for the APC surface.
The observation that unless excessive amounts of exogenous IL-2 are added, B6dom1-specific CTLs expand more rapidly than anti–H-Y CTLs could well explain the immunodominance effect. Indeed, a direct consequence is that early in the course of the immune response, anti-B6dom1 CTLs become activated more efficiently and are likely to swiftly outnumber anti–H-Y CTLs. This should not influence CTL responses when both MiHAs are presented on separate APCs. However, when both MiHAs are presented on the same APC, B6dom1-specific CTLs could prevent triggering of H-Y–specific CTLs in two ways. First, the activation status and the numerical supremacy of anti-B6dom1 CTLs should enable them to compete more successfully for occupancy of the APC surface and for cytokines generated at the T-cell/APC interface. Competition for the APC surface and locally produced cytokines (mainly IL-2) may be a recurring theme in the regulation of immune responses because these are precisely the mechanisms whereby anergic T cells inhibit responses to linked epitopes, ie, epitopes presented on the same APC as the tolerizing determinant.71-73 Second, B6dom1-specific CTLs may simply kill the APCs before anti–H-Y CTLs have a chance to find them. Killing of APCs by cognate CTLs has been documented both in vitro and in vivo and is likely instrumental in limiting the duration of immune responses.74-77
One limitation to the interpretation of our model is that we could not ascertain whether the more rapid expansion of anti-B6dom1versus anti–H-Y CTLs in vivo depended solely on the differential strength of signal 1 delivered to CTLs by the dominant versus nondominant epitope. Although differential generation of help cannot explain B6dom1 dominance over H-Y when both MiHAs are presented on the same APC (see above), expansion of MiHA-specific CTLs could only be evaluated under experimental conditions where the two MiHAs were presented on separate APCs (the presence of B6dom1 inhibits the generation of H-Y–specific CTLs when both epitopes are on the same APC). We cannot exclude the possibility that, under these conditions, CTLs specific for B6dom1 and H-Y could receive unequal amounts of help from T cells that recognize other epitopes, such as class II–associated MiHAs. We believe that the best strategy to address this issue would be to measure in vivo expansion of anti-B6dom1 and anti–H-Y CTLs after priming with APCs presenting only B6dom1/Db or H-Y/Db, preferably on a class II–deficient background. This will be possible only when B6dom1 congenic mice are available.
What is the raison d’être of the immunodominance effect?
Is it a good strategy for the immune system to restrict the repertoire of CTL responses to a few dominant epitopes and hence to suppress responses to other nondominant epitopes expressed by APCs? Analysis of CTL responses to B6dom1 and H-Y shows that the immunodominance effect is not a stochastic process. Indeed, based on the characteristics of B6dom1, we conclude that dominant epitopes are those, which have optimal ability to trigger TCR signals in CTLs. Thus, whether the critical final event is competition for the APC surface + cytokines or APC killing, the dominance effect can serve two important purposes. First, considering that naive T cells require approximately 20 hours of sustained signaling to be committed to proliferation78 and that APCs disappear less than 48 hours after interaction with cognate T cells in vivo,79 the dominance effect may help to minimize the time required to mount a protective immune response by devoting the APC surface to CTLs specific for the best TCR ligands during the brief period of priming. This could be extremely important considering how critical time limitations are for CTL to mediate early control of a microbial infection in vivo.80,81 Secondly, taking into account that TCRs are degenerate receptors and that T lymphocytes responding to nonself-antigens can cross-react with self-peptides,4,82 83restricting the repertoire of T-cell responses may decrease the risk of autoimmunity. Thus, the immunodominance phenomenon may, in fact, represent an astute “low risk high efficiency” strategy for the immune system: restricting the diversity of the immune response limits the potential for autoimmune recognition, while focusing on the best epitopes confers good chances to rapidly eliminate pathogens.
Supported by the National Cancer Institute of Canada (C.P.) and the National Institute of Allergy and Infectious Diseases (D.C.R.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Claude Perreault, MD, Research Center, Maisonneuve-Rosemont Hospital, 5415 de l’Assomption Blvd, Montreal, Quebec, Canada H1T 2M4; e-mail: c.perreault@videotron.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal