Abstract
We have previously shown that the HCA/ALCAM (CD166) glycoprotein, a member of the immunoglobulin family that mediates both homophilic and heterophilic cell-cell adhesion, via the CD6 ligand, is expressed at the surface of all of the most primitive CD38−/lo, Thy-1+, rho123lo, CD34+hematopoietic cells in human fetal liver and fetal and adult bone marrow. In the present report we show that HCA is also expressed by subsets of stromal cells in the primary hematopoietic sites that sequentially develop in the human embryo and fetus, ie, the paraaortic mesoderm, liver, thymus, and bone marrow. Adult bone marrow stromal cells established in vitro, including those derived from Stro-1+ progenitors and cells from immortalized cell lines, express HCA. In contrast, no HCA expression could be detected in peripheral lymphoid tissues, fetal spleen, and lymph nodes. HCA membrane molecules purified from marrow stromal cells interact with intact marrow stromal cells, CD34+ CD38−hematopoietic precursors, and CD3+ CD6+peripheral blood lymphocytes. Finally, low but significant levels of CD6 are here for the first time detected at the surface of CD34+ rho123med/lo progenitors in the bone marrow and in mobilized blood from healthy individuals. Altogether, these results indicate that the HCA/ALCAM surface molecule is involved in homophilic or heterophilic (with CD6) adhesive interactions between early hematopoietic progenitors and associated stromal cells in primary blood-forming organs.
HEMATOPOIESIS OCCURS IN higher vertebrates in various tissues of different embryonic derivations. Whereas T-cell differentiation requires the contact of stem cells with the epithelio-mesenchymal framework of the thymus, myeloerythroid development can occur in diverse, histologically distinct environments such as those provided by yolk sac hemangioblastic clusters, embryonic and fetal hepatocytes, or bone marrow (BM) heterogeneous stroma. In pathological conditions such as myeloproliferative disorders and chronic anemias, blood cells can also develop in atypical locations such as adult liver and spleen.
Hematopoiesis is tightly controlled by interactions between blood cells and their surrounding stromal compartments1; yet, the nature of that communication remains largely obscure. Notably, the influence exerted by blood-forming tissues on hematopoietic stem and precursor cells is undefined, although some clues have recently been given on the molecules involved in the contact of the latter with BM stroma. For example, the α4β1 integrin VLA-4 is expressed by immature hematopoietic cells and by their more differentiated progeny in the BM, whereas its ligand VCAM-1 is present at the surface of cells in the adherent layer of BM cultures.2-6 In vivo expression of VCAM-1 by reticular and endothelial cells in the murine BM stroma has also been documented.7 Antibodies against both VLA-4 and VCAM-1 have been shown to inhibit or partially affect lymphomyelopoiesis in vitro and in vivo. Treatment of BM cells with an antibody to VLA-4 significantly decreases their ability to colonize the marrow of irradiated mice on intravenous injection, as does the administration of anti–VCAM-1 antibodies to the mouse hosts.8 Furthermore, knocking out the gene encoding the α4 integrin chain in embryonic stem (ES) cells profoundly disturbs their postnatal lymphoid progeny in chimeric mice by dysregulating T- and B-cell precursor development in the BM and by impeding T-cell homing to Peyer’s patches.9 The inactivation of the β1 integrin gene results in the inability of hematopoietic stem cells to colonize the embryonic liver.10Heparan sulfate, a glycosaminoglycan of the stromal cell glycocalix, also mediates binding of mouse hematopoietic progenitors via the Mac-1 and CD45 surface molecules11 and that of CD34+human progenitors through CD31 (PECAM-1), a cell surface molecule also involved in homophilic adhesion between hematopoietic cells and their endothelium-like stromal environment.12 This nonexhaustive list of receptor/ligand pairs underlines the existence of multiple, complementary adhesion mechanisms between stromal and hematopoietic cells. These mechanisms are likely to be involved at all stages of hematopoiesis. These include stem cell retention, possibly via chemotactic factors,13 in various niches inside primary blood-forming tissues—fetal liver and BM—for renewal, expansion, and differentiation,14 but also emigration into the blood flow through sinusal walls and directed homing to other hematopoietic tissues or back to the tissue of origin.
We have presented in a previous work15 the characterization and molecular cloning of HCA, the human member of a family of homophilic adhesion proteins that includes BEN/SC1/DM-GRASP in the chicken,16-18 neurolin in the fish,19KG-CAM/F84.1 in the rat,20,21 and mALCAM in the mouse.22 Like its animal homologs, HCA is expressed transiently by developing neurons in the embryonic and early fetal central and peripheral nervous systems,15,23 as well as by subsets of hematopoietic progenitor cells. The HCA protein is present at the surface of 40% to 70% of CD34+ cells in fetal liver and in fetal and adult BM (ABM), and on virtually all CD34+ cells in peripheral blood after precursor cell mobilization.15 Importantly, all of the most primitive CD34+ cells from these sources, defined by their CD38−/lo, Thy-1+, rho123lophenotype,24-27 express HCA.15 Hematopoietic precursor cell activity is confined to the subset of CD34+cells that coexpress HCA, as measured by long-term in vitro culture and severe combined immunodeficiency–hu bone reconstitution assays.15 Glycoproteins of the BEN family exhibit typical structural features of adhesion molecules, and their homophilic adhesive properties have been documented in vitro in animal studies.17,18,28 That HCA plays a similar role at the surface of human cells has been suggested by the demonstration that HCA cDNA expression confers strong self-adhesive properties to Chinese hamster ovary (CHO) cells, which can be competed by soluble recombinant HCA polypeptides.15 In addition, homotypic interactions mediated by MEMD, a molecule identical to HCA, are essential for cell-cell adhesion in several human melanoma cell lines.29
The constitutive expression of a novel homophilic adhesion protein by human hematopoietic stem cells has prompted us to search for its conjoint presence at the surface of stromal cells in blood-forming tissues. We have thus studied, by in situ hybridization of an HCA riboprobe and antibody staining of tissue sections, the distribution of the HCA message and protein in the following different anatomical sites in which hematopoiesis occurs in the human embryo and fetus: yolk sac, embryonic aorta, liver, thymus, BM, spleen, and lymph nodes. HCA expression has been also examined by flow cytometry on stromal cells from long-term cultured ABM and immortalized lines. This study shows that HCA is present on nonhematopoietic stromal cells in all primary hematopoietic tissues, but is absent from secondary ones, ie, spleen and lymph nodes.
These data suggest that HCA is involved in adhesive interactions between hematopoietic stem and progenitor cells and their supporting stromas. However, HCA is also known to be identical to ALCAM (activated leukocyte cell adhesion molecule), a glycoprotein expressed by mesenchymal stem cells,30 and involved in the binding of thymocytes to thymic epithelial cells through their CD6 surface antigen.31 We also report here that CD6 is expressed at the surface of a subset of CD34+ cells, and hypothesize that both HCA/HCA and HCA/CD6 interactions may be involved in the adherence of human primitive hematopoietic progenitors to surrounding stromal cells in primary blood-forming tissues. In support of this assumption we show that HCA molecules purified from marrow stromal cells can bind intact marrow stromal cells, CD3+CD6+peripheral blood lymphocytes (PBL), and CD34+CD38− hematopoietic cell precursors.
MATERIALS AND METHODS
Cells and tissues.
Human embryonic and fetal tissues were obtained from voluntary or therapeutic abortions performed in compliance with the French legislation. Developmental stages, indicated in weeks postconception, were estimated from menstrual history, and confirmed on anatomic criteria. Yolk sac, embryonic and fetal bone, liver and thymus, and fetal spleen and mesenteric lymph nodes were obtained and processed as described. In addition, liver pieces were biopsied at autopsy on two stillborn infants.
ABM was aspirated from the posterior iliac crest of healthy adult volunteers after informed consent was obtained according to the criteria established by the Institutional Review Board at Stanford Medical Center (Stanford, CA), or, with informed consent, from patients undergoing cardiac surgery at Minjoz Hospital (Besançon, France). Alternatively, we used peripheral blood apheresed from healthy volunteers treated with granulocyte colony-stimulating factor (G-CSF; 10 to 15 mg/kg) at either Stanford Medical Center, Fred Hutchinson Cancer Research Center (Seattle, WA), or MD Anderson Center (Houston, TX).
K562 erythroleukemia cells were obtained from the American Tissue Collection Center (Rockville, MD). The stromal cell lines HS27A and HS2332 were obtained from B. Torok-Storb (Fred Hutchinson Cancer Research Center), L87/4 and L88/533 from P. Dörmer (Forschungszentrum für Umwelt und Gesundheit, Munich, Germany), PU-3434 from D.A. Williams (Indianapolis, IN), KM10235 from J. Greenberger (Pittsburgh, PA), and L2Ori- from A. Keating (Toronto General Hospital, Canada).
Tissue processing and section immunostaining.
Freshly dissected tissues were fixed in phosphate-buffered saline (PBS), 4% paraformaldehyde, and then impregnated and included in gelatin/sucrose medium before freezing in isopentane vapors as described previously.36 Cryostat sections, 6 to 10 μm, were incubated for 1 hour at room temperature in a humid chamber with the F84.1 antibody diluted in TBST (Tris-buffered saline, 0.25% Triton X-100 [Sigma, St Louis, MO]) with 2% fetal calf serum (FCS), followed by three washing steps in TBST. Slides were then incubated for 30 minutes with appropriately diluted rabbit anti-mouse IgG (Dako, Glostrup, Denmark), rinsed again with TBST, and incubated 30 minutes with diluted mouse anti-alkaline phosphatase coupled to alkaline phosphatase (Dako). Immune reaction was revealed using the Dako Fast Red substrate system according to manufacturer’s instructions. Endogenous alkaline phosphatase activity was inhibited by adding levamisole to the substrate solution at a final concentration of 1 mmol/L. Sections were counterstained with Gill’s hematoxylin before mounting in aqueous medium.
In situ hybridization.
Digoxygenin (DIG)-labeled sense and antisense HCA riboprobes were synthesized by transcription from flanking promoters of a linearized pGEM-T vector (Promega, Madison, WI) containing a coding sequence fragment localized between positions 1381 and 2506 of the HCA cDNA. In situ hybridization was performed on frozen tissue sections as described by Henrique et al,37 with minor modifications. Briefly, sections were dried for 16 hours at room temperature and hybridized with the digoxygenin-labeled HCA probes overnight at 65°C in a humid chamber. Slides were then rinsed three times in 50% formamide 1× SSC (saline–sodium citrate buffer) at 65°C, followed by two 30-minute washes in MABT (100 mmol/L maleic acid, 150 mmol/L NaCl, 0.1% Tween-20, pH 7.5) at room temperature. Sections were blocked for 1 hour with MABT containing 2% Boehringer’s blocking reagent (Boehringer, Mannheim, Germany) and 20% FCS, and incubated overnight at room temperature with appropriately diluted alkaline phosphatase-coupled anti-digoxygenin antibody (Boehringer). After five 20-minute washes in MABT, and rinsing twice for 10 minutes in staining buffer (100 mmol/L NaCl, 50 mmol/L MgCl2, 0.1% Tween-20, 100 mmol/L Tris, pH 9.5), the staining reaction was performed by incubating slides in nitroblue tetrazolium salt/5-bromo-4-chloro-3-indolyl-phosphate solution with 5 mmol/L levamisole for 3 to 5 hours at 37°C in the dark. The reaction was stopped by several PBS washes. Subsequent staining with the anti-CD34 HPCA-1 monoclonal antibody (MoAb) (Becton Dickinson, San Jose, CA) was performed as described above.
Cultures.
Primary stromal layers for flow cytometry analysis were established by plating 2 × 106 ABM cells/mL in the presence of 50 ng/mL leukemia-inhibitory factor (LIF), 10 ng/mL interleukin-6 (IL-6), 30 mg/mL endothelial cell growth supplement (ECGS; Collaborative Research, Franklin Lakes, NJ) and 10−6 mol/L hydrocortisone (Sigma). Cultures were fed weekly by replacing 50% of the medium consisting of 50% Iscove’s modified Dulbecco’s medium (JRH Bioscience, Lenexa, KS), 50% RPMI with 10% FCS (Hyclone Laboratories, Logan, UT), 10 mmol/L HEPES, 4 × 10−5 mol/L 2-mercaptoethanol, 10−6 mol/L hydrocortisone, 100 U/mL penicillin-streptomycin, 4 mol/L glutamine (JRH Bioscience), 50 ng/mL LIF, 10 ng/mL human IL-6, and 10 mg/mL ECGS.
Primary ABM stromal cells used for secondary colony-derived cell line (CDCL) cultures (see below) were established in vitro by plating 2 × 106 cells/mL in long-term culture medium as described previously.38 The culture medium consisted of McCoy’s 5A medium supplemented with amino acids, vitamins, antibiotics, 12.5% heat-inactivated FCS (GIBCO, Paisley, UK), 12.5% heat-inactivated prescreened horse serum (GIBCO), and 10−6 mol/L hydrocortisone. Cultures were incubated at 37°C and 4% CO2 in a fully humidified atmosphere.
Monolayers of vascular smooth muscle-like stromal cells were obtained either from isolated Stro-1+ cells or from stromal colonies grown in methylcellulose, as previously described.39,40Briefly, after 7 to 10 days of culture, primary layers from ABM were trypsinized, washed twice, and incubated with M450 beads (Dynal, Oslo, Norway) coated with Stro-1 MoAb41 (three beads per cell). The cell suspension and beads were incubated under gentle agitation for 30 minutes. Cells bound to beads were then recovered using a magnetic particle concentrator (MPC-1; Dynal). Unbound cells were removed by three washes in PBS, 0.1% bovine serum albumin (BSA). Selected cells were grown in long-term culture medium containing 2 ng/mL fibroblast growth factor-2 (FGF-2). Confluent cell layers were sacrificed for flow cytometry, immunofluorescence, and interaction studies. Alternatively, primary layers were grown for 3 to 4 weeks, and then dissociated with trypsin/EDTA and grown in methylcellulose in the presence of 20 U/mL IL-1β and 200 U/mL tumor necrosis factor-α. The cell concentration was 1 to 2 × 104 cells/mL. After 2 weeks stromal colonies were obtained using fine pipettes. CDCLs were then grown in long-term culture medium with 10 ng/mL FGF-2. Confluent layers were dissociated and analyzed by flow cytometry.
Immortalized stromal cell lines were cultured in long-term culture medium as described above. K562 cells were cultured in RPMI 1640 (Biowhittaker, Walkersville, MD) and 10% FCS.
CD34+ cell purification.
On a Hypaque-Ficoll density gradient (Pharmacia, St Quentin, France) (density = 1.077), 3 to 4 × 108nucleated marrow cells were centrifuged and mononuclear cells at the interface were collected. CD34+ cells were isolated using a magnetic separation column according to the manufacturer’s instructions (mini-Macs; Myltenyi Biotec, Bergisch Gladbach, Germany). The average final purity of the CD34+ cell fraction was, in 10 distinct experiments, 98% ± 1%.
Flow cytometry.
Primary antibodies used for the analysis of primary ABM cultures were 11-59 (anti–KG-CAM), obtained from Dr E. Geisert Jr (University of Tennessee, Memphis, TN); and phycoerythrin (PE)-conjugated anti–Thy-1, CD6, and CD49d, obtained from Pharmingen (San Diego, CA). The CD34 antigen was detected by staining cells with sulforhodamine-conjugated anti-CD34 F(ab′)2 (SyStemix, Palo Alto, CA). hHCA was detected with the F84.1 MoAb (IgG1), obtained from Dr W. Stallcup (La Jolla Cancer Foundation, La Jolla, CA), and PE-conjugated goat anti-mouse IgG1 (Caltag, South San Francisco, CA). Isotype-matched negative control antibodies were used to delineate gated populations. Cells were incubated for 20 minutes on ice for each step. After the final wash, cells were resuspended in PBS supplemented with 2% FCS containing 1 mg/mL propidium iodide (PI). Labeled cells were analyzed and sorted with a Vantage dual-laser fluorescence-activated cell sorter (FACS) (Becton Dickinson Immunocytometry Systems, San Jose, CA) at the SyStemix FACS facility. Dead cells were excluded from analysis by their PI staining characteristics.
Primary antibodies used to analyze stromal cultures were F84.1, Stro-1 (IgM), purchased from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); 6-19 (IgG1), provided by Dr C. Abboud42; 1B10 (IgM; Sigma)43; anti-CD45 (IgG1; Dako); anti-CD68 (IgG1; Dako); and irrelevant IgG1 and IgM controls (Dako). For 30 minutes at 4°C in the dark, 50,000 to 100,000 cells were labeled with the primary antibody and then washed three times with PBS. Fluorescein isothyocyanate (FITC)-conjugated F(ab′)2 goat anti-mouse IgG + IgA + IgM (Cappel, Malvern, PA) was added for 30 minutes at 4°C in the dark. Cells were washed three times with PBS and analyzed on a FACScan flow cytometer equipped with the Lysis II software (Becton Dickinson). Following the model described by Andreoni et al,44 a region with intermediate to high forward scatter and autofluorescence (FL2 channel) was defined and used in antigen distribution studies.
Primary antibodies used to analyze mononuclear cells from ABM and mobilized peripheral blood progenitors (MPB) were the same as those used for primary ABM cultures except for the 11-59 antibody. The staining procedure for rhodamine 123 (rho123) was described previously15 45 as follows: a 1-mg/mL stock solution of rho123 (Molecular Probes, Eugene, OR) was prepared in ethanol, stored at −20°C in the dark, and thawed just before use. Mononuclear cells or MPB cells were resuspended at 106/mL or less in PBS containing 2% FCS, and incubated with 0.1 mg/mL of rho123 dye for 30 minutes at 37°C. The cells were washed and incubated at 37°C for 40 minutes to allow efflux of the dye, washed again, and stained with antibodies. Rho123 fluorescence was analyzed in the FITC channel after setting compensation between the FITC and PE channels.
Purified BM CD34+ cells and lymphocytes were stained with the following IgG1 MoAbs: anti-CD34 (HPCA-2) conjugated to PE (Becton Dickinson), anti-CD38 conjugated to FITC (Coulter, Miami, FL), anti-CD6 provided by Diaclone Research (Besançon, France), anti-CD45 RB (Dako), and irrelevant controls.
T-lymphocyte preparation.
An aliquot of peripheral blood was centrifuged on a Hypaque-Ficoll density gradient. Interface mononuclear cells were collected and cultured in RPMI 1640 (Biowhittaker) with L-glutamin, penicillin, streptomycin, 10% allogeneic (AB group) serum, 500 U/mL IL-2 (Chiron, Amsterdam, The Netherlands), and 10 ng/mL OKT3 (Cilag, Levallois-Perret, France). After 10 days of culture, the cellular suspension consisting of 95% CD3+ lymphocytes was collected for adhesion studies.
Studies on HCA-mediated interactions between stromal cells and CD34+ BM cells or PBL.
Marrow stromal cells obtained from Stro-1+ cell-derived layers were incubated for 30 minutes at room temperature with the F84.1 antibody or an irrelevant mouse IgG1 antibody (Dako), and then washed three times with PBS/BSA 0.4% (wt/vol). Cells were then incubated for 15 minutes at room temperature under gentle agitation with M-450 beads (Dynal) coated with goat anti-mouse IgG at a five:one bead to cell ratio. Cells bound to beads were recovered using a magnet, as described above for Stro-1+ cells, and lysed for 15 minutes at 4°C in the dark in PBS, 0.1% (vol/vol) Triton X-100, 0.5% (wt/vol) deoxycholate to solubilize membrane molecules.46After three washes with PBS/BSA 0.4%, membrane molecules complexed with beads were incubated for 30 minutes at 4°C in the dark, under gentle agitation, with intact marrow stromal cells or fresh BM CD34+ cells, CD3+ PBL, or K562 cells (three to five beads/cell). Cells with bound beads were separated magnetically as described above. Both cells coated with beads and uncoated cells were recovered and counted using a Malassez chamber. The former represented cells capable of binding HCA membrane molecules solubilized from stromal cells, ie, cells bearing either HCA or CD6 membrane antigens (homophilic and heterophilic interactions, respectively).
After numeration, each cell fraction (bound to beads or unbound) was labeled with an anti-CD45 antibody or with anti–CD34-PE and anti–CD38-FITC antibodies, before being passaged through the flow cytometer to check for the presence of remaining intact CD45-negative cells, caused by insufficient solubilization of stromal cell membranes, and to analyze the phenotype of the bound and unbound CD34+ marrow cells.
RESULTS
HCA expression in early embryonic blood-forming tissues.
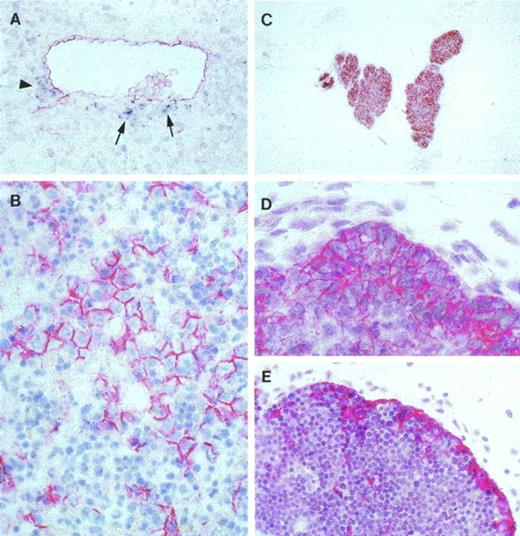
In the course of human early ontogeny, the onset of intraembryonic hematopoiesis is marked by the appearance, by the beginning of the fifth week, of CD34+ pluripotential progenitors on the ventral wall of the aorta.47 Aorta-associated CD34+ cells are arranged in clusters of several hundreds of primitive hematopoietic precursors, as inferred from phenotype and in vitro behavior.47 These cell clusters likely arise from a primitive hematogenous territory derived from the paraaortic splanchnopleura, a region of the embryo that contributes stem cells for definitive hematopoiesis in birds and mice.48,49 Therefore, aorta-associated ventral tissues in the 4- to 6-week human embryo are a site in which to study initial stem cell emergence and expansion.50 Hybridization of an HCA probe on cross sections of early human embryos in the brachial region showed the presence, immediately subjacent to the aortic endothelium, of a discrete population of HCA-expressing mesenchymal cells. In all seven cases analyzed, corresponding to 30- to 42-day embryos, the HCA message was restricted to the ventral, and, to a lesser extent, lateral mesenchyme which surround the CD34+ cell clusters, but was seldom present dorsally in regions of the aorta not associated with hematopoiesis (Fig 1A). In all instances, strong labeling of the notochord and neuronal precursors (not shown) confirmed previous observations of HCA expression by these nonhematopoietic tissues.15 23
HCA distribution in human embryonic and fetal blood-forming tissues. (A) Cross-section in the trunk region of a 5-week human embryo hybridized with a DIG-labeled antisense HCA probe (dark blue) and subsequently stained with the HPCA-1 antibody against the CD34 surface molecule (red). Note HCA-expressing mesenchymal cells (arrows) subjacent to a cluster of CD34+ hematopoietic progenitors associated with the ventral aortic endothelium, which also expresses CD34. A few HCA+ cells are also present on the lateral aspect of the aorta (arrowhead) (original magnification × 100). (B) Seventeen-week fetal liver. Hematopoietic cells are intermingled with polygonal hepatocytes displaying strong HCA surface staining (original magnification × 100). (C) Eight and one-half–week fetal thymus. The thymic epithelium appears uniformly stained (original magnification × 25). (D) Close-up view of an 8.5-week fetal thymus that shows stronger HCA density on epithelial cells in the outermost region of the rudiment (original magnification × 250). (E) Seventeen-week fetal thymus. Virtually all epithelial cells beneath the capsule are HCA+. Stained cells are also present within the inner cortex (original magnification × 100).
HCA distribution in human embryonic and fetal blood-forming tissues. (A) Cross-section in the trunk region of a 5-week human embryo hybridized with a DIG-labeled antisense HCA probe (dark blue) and subsequently stained with the HPCA-1 antibody against the CD34 surface molecule (red). Note HCA-expressing mesenchymal cells (arrows) subjacent to a cluster of CD34+ hematopoietic progenitors associated with the ventral aortic endothelium, which also expresses CD34. A few HCA+ cells are also present on the lateral aspect of the aorta (arrowhead) (original magnification × 100). (B) Seventeen-week fetal liver. Hematopoietic cells are intermingled with polygonal hepatocytes displaying strong HCA surface staining (original magnification × 100). (C) Eight and one-half–week fetal thymus. The thymic epithelium appears uniformly stained (original magnification × 25). (D) Close-up view of an 8.5-week fetal thymus that shows stronger HCA density on epithelial cells in the outermost region of the rudiment (original magnification × 250). (E) Seventeen-week fetal thymus. Virtually all epithelial cells beneath the capsule are HCA+. Stained cells are also present within the inner cortex (original magnification × 100).
At the sixth week of development, intraaortic clusters gradually disappear and the liver becomes the main hematopoietic organ. The proliferation and differentiation of blood cell progenitors occur at the contact of hepatocytes and of endothelial cells lining blood sinuses. Histochemical stainings were performed with the anti-HCA antibody on liver samples ranging from 5 weeks of gestation to 3 weeks after birth. In all 5-week liver samples analyzed, most hepatocytes were already HCA positive. HCA expression was then observed at even higher levels in all liver samples up to late fetal stages, at which fully differentiated hepatocytes were easily identifiable by their characteristic polygonal shape (Fig 1B). Strong expression of HCA was maintained in postnatal hepatocytes at 2 and 3 weeks after birth, a period when the hematopoietic activity of the liver has declined (not shown).
HCA expression by the thymic epithelium.
Pharyngeal endoderm contributes the epithelial rudiment of the thymus, the lymphoid development of which ensues on iterative colonization by migrating hematopoietic cells.48 Stem-cell seeding to the embryonic avian and mouse thymus has been timed accurately in experimental chimeras and in vitro organ cultures,51-53whereas immunodetection of hematopoietic cell antigens on tissue sections showed the onset of human embryonic thymus colonization at about 8 weeks of development.54 At 8.5 weeks, the earliest stage tested in the present study, the undifferentiated thymic epithelium is still homogenous in appearance. A strong HCA signal was then detected on most epithelial cells (Fig 1C), and the most external cell layers appeared more strongly labeled when examined at higher magnification (Fig 1D). Specialization into cortical and medullary compartments, which occurs at 13 to 15 weeks,55 was accompanied by a restriction in the expression pattern of HCA to the cortical epithelium. Thus, at the fourth month of gestation, HCA expression was mainly restricted to epithelial cells at the periphery of thymic lobules, in the external cortical region. Subcapsular epithelial cells in a 3- to 4-cell–thick layer appeared strongly stained, although some epithelial cells scattered over the cortex were also positive (Fig 1E). Hassall’s bodies in the medullary region were also HCA+ as were a few epithelial cells (not shown). This pattern remained unchanged at late fetal and early postnatal stages (not shown).
HCA expression by fetal and postnatal BM stromal cells.
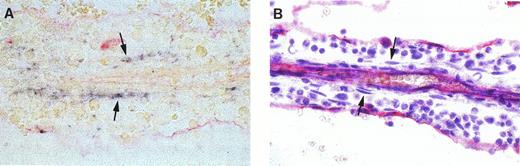
BM is the last primary blood-forming tissue that forms in human development, at the expense of the cartilaginous rudiments of long bones. After active and rapid chondrolysis in the diaphyseal area, invading osteoblasts, vascular sprouts, and mesodermal precursor cells establish from 8.5 to 10.5 weeks the cellular environment in which hematopoiesis emerges during the 11th week56 from precursor cells believed to migrate from the liver.57 Because of the simple structure of the BM at these early stages, the microenvironment of incipient hematopoiesis could be described as the “primary logette,” a mesodermal islet protruding into the sinusal lumen. That structure is a loose network of mesoderm-derived supporting cells organized around an arteriole and lined with CD34+endothelial cells.56 However, bone calcification has already started at these early stages and we were unable to define fixation/embedding conditions compatible with the preservation of both marrow structure and HCA epitope. Therefore, in situ hybridization experiments were performed on bone anlagen younger than 16 weeks of gestation, the stromal cell organization in logettes being obscured at later stages as a result of extensive blood cell proliferation. Hematopoietic cells develop within logettes in contact with three main stromal cell types, namely, endothelial cells, abluminal pericytes, and intratrabecular myoid cells that express smooth muscle-specific α-actin (α-SM actin) and extend long processes throughout the logette matrix.56 In situ hybridization on sagittal long-bone sections at 11 and 12 weeks of development showed high levels of HCA mRNA in elongated structures, parallel to the central artery, within the marrow cords (Fig 2A). Stained cells were most frequently aligned, but isolated positive cells were also observed. Because bone sections endure less well than other tissues the high temperatures required for hybridization, the resulting tissular structures were poorly preserved. To circumvent this problem, sections from the same BM samples were labeled with an anti-CD34 antibody and counterstained with hematoxylin (Fig 2B). Comparison of both series of slides suggested that the HCA-expressing elements identified by in situ hybridization corresponded to nonendothelial intratrabecular flattened cells, interspersed among developing blood progenitors. In addition to intrasinusal stromal cells, osteoblasts were also found to express the HCA message (not shown).
In situ hybridization analysis of HCA expression in fetal BM. (A) Eleven and one-half–week fetal BM logette hybridized with a DIG-labeled HCA probe. HCA+ cells (dark blue staining) can be seen running along both sides of the central arteriole. (B) Adjacent section labeled with the same CD34 antibody as in (A) and counterstained with Gill’s hematoxylin. Endothelial cells lining the central arteriole and the primary logette express CD34. The HCA+ cells in the upper section roughly correspond to rows of flattened cells located among developing blood progenitors (arrows).
In situ hybridization analysis of HCA expression in fetal BM. (A) Eleven and one-half–week fetal BM logette hybridized with a DIG-labeled HCA probe. HCA+ cells (dark blue staining) can be seen running along both sides of the central arteriole. (B) Adjacent section labeled with the same CD34 antibody as in (A) and counterstained with Gill’s hematoxylin. Endothelial cells lining the central arteriole and the primary logette express CD34. The HCA+ cells in the upper section roughly correspond to rows of flattened cells located among developing blood progenitors (arrows).
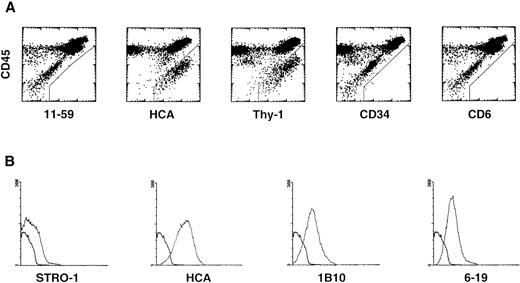
HCA expression was next examined on BM stromal layers established in vitro and endowed with the ability to support hematopoiesis.Primary cultures were first established from ABM. On enzyme dissociation and flow cytometry analysis, these cultures were observed to contain CD45+ hematopoietic cells as well as CD45− stromal cells, most of which showed a high level of autofluorescence as judged from diagonal distribution on dot plots (Fig 3A). The majority of these stromal cells—if not all of them—were stained by antibodies to HCA and to human Thy-1, whereas no expression of rat HCA (MoAb 11-59), CD34, or CD6 could be detected at their surfaces (Fig 3A). Thus, two surface molecules expressed by the most primitive hematopoietic stem cells, HCA and Thy-1, are also present on BM nonhematopoietic stromal cells established in long-term culture.
HCA expression by human BM stromal cells in primary cultures derived from (A) total ABM cells: the cells were stained with MoAbs against rat HCA (11-59), HCA, Thy-1, CD34, and CD6, and with anti-CD45 to discriminate hematopoietic cells; and (B) Stro-1+ stromal progenitors sorted from ABM. For each antibody the fluorescence histogram is presented along with that of an irrelevant antibody of the same isotype (heavy line).
HCA expression by human BM stromal cells in primary cultures derived from (A) total ABM cells: the cells were stained with MoAbs against rat HCA (11-59), HCA, Thy-1, CD34, and CD6, and with anti-CD45 to discriminate hematopoietic cells; and (B) Stro-1+ stromal progenitors sorted from ABM. For each antibody the fluorescence histogram is presented along with that of an irrelevant antibody of the same isotype (heavy line).
HCA expression was also studied on stromal cells grown in vitro either from Stro-1+ progenitors isolated by immunoaffinity from ABM or from stromal colonies selected in methylcellulose. Both methods allow the development of adherent, hematopoiesis-supporting, vascular smooth muscle-like cells expressing α-SM actin and other cytoskeletal proteins specific of myoid cells.39 40 All stromal cells grown in these conditions were HCA+. The intensity of fluorescence was strong with a mean of 25.5, versus 1 for the isotype control (Fig 3B). On in situ immunostaining of confluent layers, all cells appeared fluorescent, with more densely stained long streaks probably corresponding to membrane ruffles (not shown). Such structures are compatible with the expression of an adhesion molecule.
These data clearly indicate that vascular smooth muscle-like stromal cells developed in culture are HCA+, with a percentage of positive cells and antigen density higher than those observed after labeling with the 6-19 and 1B10 MoAbs (Fig 3B), previously shown by Li et al58 to strongly stain stromal cells. On the contrary, and as previously reported,58 expression of antigens recognized by Stro-1 in established stromal layers was restricted to a minority of cells showing weak mean fluorescence intensity (MFI = 10.3; Fig 3B). CD45+ and CD68+hematopoietic cells were not detected in these cultures, confirming the purity of the stromal cell populations present in Stro-1+cell-derived layers and CDCLs.
Finally, HCA expression was studied in immortal marrow stromal cell lines from human and nonhuman primates transformed by the early SV40 oncogenes L87/4 and L88/5,33 KM102,35L2Ori−,59 and PU-3434 or by the human papilloma virus E6 and E7 oncogenes HS23 and HS27A.32 All SV40-transformed and all E6-E7–transformed cell lines were HCA+ (Table 1), but the MFI was fourfold to fivefold more intense for L2Ori− and KM102 (22 ± 5 and 17 ± 3, respectively) than for HS27A, HS23, L87/4, L88/5, and PU-34.
Expression of HCA by Marrow Stroma From Primates
| Cells . | % HCA+ Cells (mean ± SEM) . | MFI Ratio* (mean ± SEM) . |
|---|---|---|
| Stro-1+ † | 91 ± 6 | 25 ± 9 |
| L2Ori− | 96 ± 2 | 22 ± 5 |
| HS27A | 84 ± 10 | 4 ± 0.5 |
| HS23 | 97 ± 1 | 10 ± 1 |
| L87/4 | 75 ± 5 | 3 ± 0.5 |
| L88/5 | 95 ± 1 | 5 ± 0.5 |
| KM102 | 99 ± 1 | 17 ± 3 |
| PU-34 | 75 ± 12 | 4 ± 1.5 |
| Cells . | % HCA+ Cells (mean ± SEM) . | MFI Ratio* (mean ± SEM) . |
|---|---|---|
| Stro-1+ † | 91 ± 6 | 25 ± 9 |
| L2Ori− | 96 ± 2 | 22 ± 5 |
| HS27A | 84 ± 10 | 4 ± 0.5 |
| HS23 | 97 ± 1 | 10 ± 1 |
| L87/4 | 75 ± 5 | 3 ± 0.5 |
| L88/5 | 95 ± 1 | 5 ± 0.5 |
| KM102 | 99 ± 1 | 17 ± 3 |
| PU-34 | 75 ± 12 | 4 ± 1.5 |
Each value is the mean of three distinct experiments.
For each MoAb the MFI was related to that of the corresponding irrelevant isotype control.
Cells from Stro-1+ cell-derived stroma.
Lack of HCA expression by spleen and lymph node stromal cells.
Whereas the HCA molecule was expressed by stromal cells in all primary hematopoietic organs and tissues analyzed, as summarized in Table 2, it was absent from secondary ones. No HCA+ cells were present on antibody-treated frozen sections of 4- and 5-month spleen and mesenteric lymph nodes which at that stage are already fully colonized by hematolymphoid cells (not shown).
HCA Expression in Stromal Compartments of Human Hematopoietic Tissues
| Organ/Tissue . | Expression . | Cell Type/Localization . |
|---|---|---|
| Yolk sac | + | Endoderm |
| Paraaortic embryonic mesoderm | + | Ventrolateral mesenchyme* |
| Embryonic liver | + | Hepatocytes |
| Fetal liver | + | Hepatocytes, Kupffer cells |
| Fetal thymus | + | Subcapsular epithelium, subsets of cortical and medullary epithelial cells, Hassall’s bodies |
| Fetal bone marrow | + | Vascular pericytes, osteoblasts* |
| Adult bone marrow | + | Adherent cells in primary cultures Myofibroblasts in vitro |
| Fetal spleen | − | |
| Fetal lymph nodes | − |
| Organ/Tissue . | Expression . | Cell Type/Localization . |
|---|---|---|
| Yolk sac | + | Endoderm |
| Paraaortic embryonic mesoderm | + | Ventrolateral mesenchyme* |
| Embryonic liver | + | Hepatocytes |
| Fetal liver | + | Hepatocytes, Kupffer cells |
| Fetal thymus | + | Subcapsular epithelium, subsets of cortical and medullary epithelial cells, Hassall’s bodies |
| Fetal bone marrow | + | Vascular pericytes, osteoblasts* |
| Adult bone marrow | + | Adherent cells in primary cultures Myofibroblasts in vitro |
| Fetal spleen | − | |
| Fetal lymph nodes | − |
As determined by in situ hybridization.
Surface expression of CD6 by a primitive subset of hematopoietic CD34+ cells.
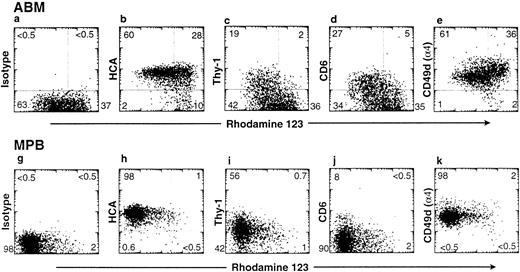
HCA is identical to ALCAM, a glycoprotein which mediates the binding of thymocytes to thymic epithelium.15,31 A known ligand for ALCAM at the surface of T cells is CD6.31 Because of the herein described wide distribution of HCA/ALCAM on stromal cells in all primary blood-forming tissues, besides the thymus, we examined by flow cytometry the expression of CD6 at the surface of hematopoietic precursor cells. Three-color immunofluorescence was performed as previously described27 on mononuclear cells from ABM or from G-CSF–mobilized blood. CD34 expression and rho123 uptake were examined together with the presence of Thy-1, HCA, CD6, and the α4 integrin chain (CD49d), a molecule known to form dimers with the β1 integrin that mediates adhesion of hematopoietic progenitors and stem cells.3,6 Confirming our previous observations,15 27 all rhomed/lowCD34+ cells express HCA in the marrow and mobilized peripheral blood (Fig 4), whereas only a subset of those are positive for Thy-1 expression. Interestingly, in both marrow and blood compartments, CD34+rhomed/low cells could be split into two subsets according to CD6 staining, one of which expressed this antigen at a low but significant level (Fig 4). Virtually all cells of both origins were stained with the CD49d-specific antibody.
Expression of adhesion-related antigens on CD34+ cells. ABM mononuclear cells and G-CSF–MPB were processed and stained with rho123 as described in Materials and Methods. Staining profiles of CD34+ gated cells with rho123 versus control isotype, HCA, Thy-1, CD6, and CD49d are shown. The percentage of cells in each quadrant of the plot is indicated.
Expression of adhesion-related antigens on CD34+ cells. ABM mononuclear cells and G-CSF–MPB were processed and stained with rho123 as described in Materials and Methods. Staining profiles of CD34+ gated cells with rho123 versus control isotype, HCA, Thy-1, CD6, and CD49d are shown. The percentage of cells in each quadrant of the plot is indicated.
Marrow stromal cell HCA membrane molecules interact with BM stromal and hematopoietic cells.
The F84.1 antibody recognizes the N-terminal region of the HCA molecule without any inhibitory effect on the formation of HCA-transfected CHO cell agregates.15 We took advantage of this property to select HCA+ and/or CD6+ cells using F84.1-HCA membrane molecule complexes. The procedure used comprised two steps: (1) collection of HCA molecules from marrow stromal cells using magnetic beads and a membrane solubilization protocol, and (2) selection of HCA+ and/or CD6+ intact cells by affinity for the HCA membrane molecules purified in step 1. HCA+ CD6− marrow stromal cells used in step 1 were either from a stromal layer generated from Stro-1+ cells, or from the L2Ori− line. In step 2, we used (1) HCA+ CD6− stromal cells, (2) HCA− CD6+ PBL, (3) HCA+and/or CD6+ BM CD34+ cells, and (4) HCA−CD6− K562 cells as a negative control.31 No contaminating stromal cells from step 1 were present among complexes because no CD45− intact cells were detected after step 2 using flow cytometry.
As shown in Table 3, bead-coated intact cells were eventually recovered only when the F84.1 antibody was used in the first step. On L2Ori− membrane extracts, we recovered 20% and 30% L2Ori− cells and PBL, respectively. On Stro-1+ cell-derived stroma extracts, 20% and 10% BM stromal and CD34+ cells, respectively, were retained. Finally, as expected, no HCA−CD6− K562 cells bound to HCA immune complexes. HCA+ and/or CD6+cells can be therefore sorted by affinity for HCA molecules, indicating that HCA:HCA or HCA:CD6 interactions occurred between membrane complexes and intact cells.
Solubilized HCA Interaction With Hematopoietic and Stromal Cells
| Step 1: Formation of HCA/Antibody Complexes3-150 . | Step 2: Selection of Intact Cells3-151 . | ||
|---|---|---|---|
| Antibody . | Cell Source . | Target Cells . | % Cells Bound to Complexes . |
| F84.1 | L2Ori− 3-152 | L2Ori− | 20; 25 (2 exp.) |
| Irrelevant | L2Ori− | L2Ori− | 0, 0 (2 exp.) |
| F84.1 | L2Ori− | PBL | 30 (1 exp.) |
| Irrelevant | L2Ori− | PBL | 0 (1 exp.) |
| F84.1 | Stro-1+ cell-derived stroma | Stro-1+ cell-derived stroma | 20; 22 (2 exp.) |
| Irrelevant | Stro-1+ cell-derived stroma | Stro-1+ cell-derived stroma | 0; 0 (2 exp.) |
| F84.1 | Stro-1+ cell-derived stroma | CD34+ | 11; 10; 11 (3 exp.) |
| Irrelevant | Stro-1+ cell-derived stroma | CD34+ | 0; 0; 0 (3 exp.) |
| F84.1 | Stro-1+cell-derived stroma | K5623-153 | 0; 0 (2 exp.) |
| Step 1: Formation of HCA/Antibody Complexes3-150 . | Step 2: Selection of Intact Cells3-151 . | ||
|---|---|---|---|
| Antibody . | Cell Source . | Target Cells . | % Cells Bound to Complexes . |
| F84.1 | L2Ori− 3-152 | L2Ori− | 20; 25 (2 exp.) |
| Irrelevant | L2Ori− | L2Ori− | 0, 0 (2 exp.) |
| F84.1 | L2Ori− | PBL | 30 (1 exp.) |
| Irrelevant | L2Ori− | PBL | 0 (1 exp.) |
| F84.1 | Stro-1+ cell-derived stroma | Stro-1+ cell-derived stroma | 20; 22 (2 exp.) |
| Irrelevant | Stro-1+ cell-derived stroma | Stro-1+ cell-derived stroma | 0; 0 (2 exp.) |
| F84.1 | Stro-1+ cell-derived stroma | CD34+ | 11; 10; 11 (3 exp.) |
| Irrelevant | Stro-1+ cell-derived stroma | CD34+ | 0; 0; 0 (3 exp.) |
| F84.1 | Stro-1+cell-derived stroma | K5623-153 | 0; 0 (2 exp.) |
Abbreviations: PBL, peripheral blood lymphocytes; exp., experiments.
Complexes formed between F84.1 antibody-coated beads and membrane molecules, as described in Materials and Methods.
Intact cells were incubated with the complexes as described in Materials and Methods.
The L2Ori− stromal cell line was used in this experiment because of its high HCA antigen expression (see Table 1).
Negative control.
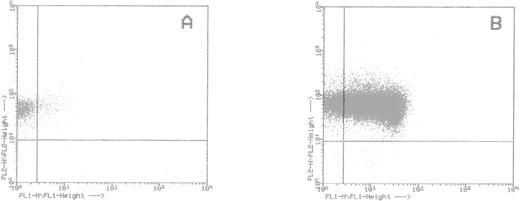
Remarkably, CD34+ BM cells selected by this method were almost exclusively CD38− (87% ± 2.3%; Fig5). This indicates that selection by HCA complexes was not random, but directed at a specific CD38− cell population. Likewise, lymphocytes sorted on the basis of their HCA affinity appeared as a homogenous population with low forward and side scatters, further showing the specificity of the HCA interactions uncovered by our cell-binding assay.
Flow cytometry analysis of CD34+ cells selected on HCA-F84.1 complexes. Bound (A) and unbound (B) cells were double-stained with anti–CD34-PE (FL2) and anti–CD38-FITC (FL1) antibodies. The upper right region was delineated as excluding any nonspecifically labeled cell after incubation with IgG1 irrelevant controls.
Flow cytometry analysis of CD34+ cells selected on HCA-F84.1 complexes. Bound (A) and unbound (B) cells were double-stained with anti–CD34-PE (FL2) and anti–CD38-FITC (FL1) antibodies. The upper right region was delineated as excluding any nonspecifically labeled cell after incubation with IgG1 irrelevant controls.
DISCUSSION
We have in the present study investigated the distribution of the HCA adhesion molecule in the stromal compartments of human embryonic and fetal blood-forming tissues. A previous report by some of us15 has shown that HCA is expressed by human CD34+ hematopoietic stem cells and myeloid progenitors. We also reported that HCA, like its avian homolog SC1/BEN,17,28 is able to mediate homophilic cell-cell adhesion.15 Based on these observations, we examined the possibility that HCA might participate in adhesive interactions between hematopoietic and stromal cells.
Hematopoietic cells develop first outside the embryo, in the wall of the yolk sac, at the expense of mesoderm-derived hemangioblastic cell clusters that also give rise to the first blood vessels. Hematopoiesis starts in the human yolk sac around 18 days of development,60,61 and we have not been able yet to assay such early tissues for HCA expression; the earliest yolk sac we have examined was 5 weeks old, a stage from which the frequency of endogenous hematopoietic clonogenic progenitors drops dramatically.62,63 HCA expression was then detected in endodermal cells (not shown). The significance of this observation is still unclear, although a key role of the yolk sac endoderm in blood island induction has been described in the chicken and mouse embryos.64-66 The finding that murine yolk sac endoderm-derived cell lines are able to sustain hematopoiesis provides additional evidence in favor of this notion,67 as does the expression of CD34 by yolk sac endoderm cells in murine embryos.68 Further examination of HCA expression in the human hematopoietic yolk sac is now pending on the unpredictable availability of 3- and 4-week samples.
Inside the 5-week embryo, HCA-expressing stromal cells were found both in the liver and in the mesenchymal region surrounding the dorsal aorta. There is now experimental evidence in animal models that the latter territory supports the emergence and expansion of stem cells for definitive hematopoiesis.69,70 At this stage of embryonic development, intraluminal clusters of primitive CD34+ blood precursors are indeed associated with the ventral walls of the human aorta47 and vitelline artery (Tavian et al, submitted for publication). Similarly, hematopoietic clusters described at an equivalent stage of development in avian and mouse embryos are always in association with the endothelial floor of the aorta, but not with its dorsal aspect.71 72 Interestingly, HCA+ cells in the human mesenchymal territory comprising the aorta were mainly restricted to the ventrolateral region lining the aortic endothelium. Thus, the distribution of HCA+paraaortic mesenchymal cells is spatially correlated with the presence of intravascular clusters of blood cell progenitors. The microanatomy of hematopoiesis in the ventral wall of the embryonic aorta is not precisely characterized yet. The discontinuous aspect of the aortic endothelium at the level of attached stem cell clusters suggests that dedifferentiated endothelial cells or underlying primitive mesenchymal cells contribute the emerging stem cells (Tavian et al, submitted for publication). Direct contact between subaortic HCA+ cells and the immediate forerunners of hematopoietic stem cells might mediate that earliest commitment to hematopoiesis.
These results, like those obtained by in situ hybridization on fetal BM sections, should be interpreted cautiously, given that we could not detect any HCA+ cells in this region on antibody staining. A simple explanation for this may be that the HCA protein is not expressed by periaortic cells underlying hematopoietic foci. Nevertheless, although the anti-HCA antibody allowed the detection by flow cytometry of positive hematopoietic cells,15 it failed to do so on the corresponding tissue sections. This might reflect the existence of distinct HCA isoforms exhibiting differential sensitivity to the tissue-processing conditions used in this study.
HCA can be added to the following list of antigens and cell adhesion molecules that have been detected on the surface of 8-week thymic epithelial cells at the time of colonization by hematopoietic progenitors: LFA-3 (CD58), VLA-1, -2, -3, -4, -6, ICAM-1 (CD54), and the TE-3 antigen.73 HCA was at this early stage uniformly expressed by undifferentiated thymic epithelial cells, but this pattern evolved with the functional specialization of thymus compartments. In the second trimester of gestation, HCA was detected on all subcapsular cells in the cortical epithelium, which is the site of entry of lymphoid progenitors, whereas scattered, often stellate, positive epithelial cells were observed within both the cortex and the medulla. This indicates that HCA-mediated adhesion to epithelial cells might concern primarily undifferentiated T-cell progenitors but also thymocytes at different maturation stages. This pattern is much the same as that reported by Patel et al74 in the adult thymus, where epithelial ALCAM mediates interactions with maturing thymocytes through the CD6 molecule. Taken together, these observations suggest that thymic HCA is involved in stem cell entry and thymocyte maturation at embryonic, fetal, and postnatal stages.
We also detected the HCA molecule in stromal cells in both fetal and ABM. In situ hybridization experiments on fetal bone sections with a DIG-labeled probe showed expression of the hcagene by a subset of flattened intrasinusal stromal cells. Although the cell morphology was poorly preserved, both the elongated aspect and the distribution within marrow sinuses of the HCA labeling was similar to that of myoid stromal cells described previously.56 These results were confirmed on in vitro cultures of ABM stroma. Primary stromal layers seemed considerably enriched in HCA+ cells, contrasting with staining profiles of fresh total BM samples, where few, if any, HCA+ CD45− cells could be detected (not shown). HCA-expressing native stromal cells may represent a very small subset of BM from aspirates, and the antigen density on that population may be too low to be readily detected by flow cytometry. BM culture in vitro may stimulate the preferential growth of a rare subset of HCA+ cells, or upregulate HCA expression. This is a likely explanation, because we could not detect HCA on endothelial cells in embryonic and fetal tissue sections, whereas high HCA levels have been reported in cultured endothelial cells.29
Purified stromal populations were also found to exhibit high surface levels of the HCA protein. We have reported before that under long-term culture conditions, most stromal cells from the BM adherent layer are vascular smooth muscle-like cells expressing α-SM actin as well as other markers of vascular smooth muscle differentiation.75This myofibroblastic population can be derived either from Stro-1+ cells40 or from stromal colonies (CDCLs).39 Most likely these cells represent the in vitro counterparts of the α-SM actin+ cells described in the hematopoietic environment of the adult and fetal BM.56,75We have also shown that these cells support the expansion and multilineage differentiation of CD34+CD38− blood cell progenitors.39,76 Thus, the results presented here establish that HCA is expressed in the BM by a subset of vascular smooth muscle-like cells endowed with the ability to support hematopoiesis. Almost all cells from primate immortalized stromal lines also showed surface HCA, indicating that HCA expression is not repressed after genomic insertion of SV40 (T and t) or human papilloma virus (E6 and E7) oncogenes. Although transformation usually dramatically affects the different components of the fibronexus,77 including integrins, it is not necessarily the case for HCA even if a low MFI can be detected for some cell lines (Table 1). Finally, HCA expression by PU-34 stromal cells indicates the presence of this molecule in Macaca mulatta as well as humans.
We examined whether high HCA expression by stromal cells might be involved in interactions with other HCA+ stromal cells or HCA+ and/or CD6+ hematopoietic primitive cells or lymphocytes. Using HCA molecules solubilized from stromal cells in a cell-binding assay, we were able to select 20% of intact BM stromal cells, 30% of PBL, and 10% of primitive CD34+ hematopoietic cells. Relatively low percentages of recovered cells may be because of technical reasons (steric hindrance by 4.5-μm beads or low amounts of antibody-bound HCA molecules with the required conformation for ligand interaction) or because of the fact that a fraction only of the target cells would be involved. Indeed, CD38− cells represented up to 87% of those selected by HCA affinity from CD34+ BM cells, whereas only 17% were found within the HCA+ subset in the starting population, as determined by double-staining with F84.1 and anti-CD38 antibodies (not shown). The preferential selection of CD38− cells, together with the homogenous scatter characteristics of the CD6+ lymphocyte population sorted on F84.1-HCA complexes indicates that our immuno-affinity procedure was effective to uncover HCA:HCA and HCA:CD6 molecular interactions.
Our previous15 and present studies show that HCA and CD6 are coexpressed on human rhomed/low CD34+cells, a population enriched in hematopoietic stem cells.26,27 Human and murine ALCAM expression is transiently upregulated in CD6+ T cells on activation.22,74 Coexpression of both members of a ligand/receptor pair in the same cell has previously been described. For instance, the ICAM-1/LFA-1 pair is present on the surface of CD34+ progenitors in cord blood and ABM,78 and thymocytes are positive for both CD2 and its counterreceptor LFA-3.79 The significance of such overlapping expressions is unknown. The so far unreported expression of CD6 by hematopoietic stem cells might be related to the phenomenon referred to as “multilineage priming.” According to this hypothesis, hematopoietic stem and progenitor cells would simultaneously activate several lineage-associated genetic programs before commitment into a particular one, as suggested for murine FDCP-mix and CD34+lin− progenitors.80 If the same applied to human cells, one would expect to find low levels of expression of lymphoid-, myeloid-, or erythroid-associated markers in blood stem cells. In this context, it is worth noticing that molecules classically considered as markers of commitment into the lymphoid (CD2, CD7, CD10, and CD19) and myeloid (CD33) lineages are expressed by uncommitted CD34+ progenitors capable of multilineage differentiation when cultured on supporting thymic stroma.81 Further investigations are required to understand whether the low levels of CD6 we have detected on human hematopoietic stem cells are relevant to their adhesion to stromal cells. It will be also important to assay the respective hematopoietic potentials of CD6+ and CD6− CD34+ cells over the long term, in vitro and in vivo.
In conclusion, this set of data emphasizes a role for HCA among other adhesion molecules expressed by the hematopoietic microenvironment. To our knowledge, HCA is the first adhesion molecule described so far in the hematopoietic system with such a widespread expression—in the mesenchyme surrounding aortic hematopoietic nests, on hepatocytes at the stage of hematopoietic development, on thymic epithelial cells, and on fetal and adult myoid marrow cells. In striking contrast, no HCA expression was detected in the framework of secondary lymphoid tissues. It should be interesting to investigate the possible induction of this molecule in pathological conditions when the adult spleen supports primary hematopoiesis, such as myeloproliferative disorders and some chronic anemias. VCAM-1 (CD102) and ICAM-1 (CD54) have also been detected on the surface of thymic and marrow-supporting cells.2,5,7,82 However, the expression of HCA is always high on marrow vascular smooth muscle-like stromal cells, at variance with that of VCAM-1 which is usually low82 unless upregulated by proinflammatory cytokines,2,3 and that of ICAM-1 which is very low if detectable.2,3,82 The intensity of HCA expression by human stroma is similar to that of SH4 and Thy-1, whose role in cell adhesion is not clearly defined, and to that of CD44, VLA-1, and VLA-5 integrins, which are mainly involved in cell to matrix adhesion and in the organization of the extracellular matrix.82 In this context, the role played by HCA in homotypic and heterotypic adhesion between stromas and hematopoietic cells and lymphocytes is significant.
ACKNOWLEDGMENT
We are indebted to C. Carrière and to Drs F. Narcy, E. Aubeny, and P. Blot for providing human embryonic and fetal tissues. We are also grateful to Drs Beverley Torok-Storb, David Williams, Joel Greenberger, Armand Keating, and Peter Dörmer for providing the cell lines and to J. M. Certoux for procuring cultured lymphocytes. We thank Dr Stallcup for the gift of the F84.1 antibody. We acknowledge the assistance of F. Viala, F. Beaujean, and H. San Clemente with the preparation of figures, and we thank M. Scaglia for typing the manuscript.
F.C. and F.D. are equal contributors to this work.
Supported in part by grants from SyStemix Inc, Association pour la Recherche sur le Cancer, Fonds d’Organisation pour la Recherche en Transfusion Sanguine and Contrat de Recherches INSERM (no 950401). F.C. was the recipient of a research fellowship from the European Commission.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bruno Péault, PhD, Institut d’Embryologie Cellulaire et Moléculaire, CNRS UPR 9064, 49bis, avenue de la Belle Gabrielle, 94736 Nogent-sur-Marne cedex, France; e-mail: peault@infobiogen.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal