Abstract
The proto-oncogene c-fos was transiently upregulated in primitive hematopoietic stem (Lin−Sca-1+) cells stimulated with stem cell factor, interleukin-3 (IL-3), and IL-6. To investigate a role of the c-fos in hematopoietic stem cells, we used bone marrow (BM) cells from transgenic mice carrying the c-fos gene under the control of the interferon-/β–inducible Mx-promoter (Mx–c-fos), and fetal liver cells from c-fos–deficient mice. Prolonged expression of the c-fos in Lin−Sca-1+ BM cells inhibited factor-dependent colony formation and hematopoiesis on a stromal cell layer by keeping them at G0/G1 phase of the cell cycle. These Lin−Sca-1+ BM cells on a stromal layer entered into the cell cycle whenever exogenous c-fos was downregulated. However, ectopic c-fos did not perturb colony formation by Lin−Sca-1+ BM cells after they entered the cell cycle. Furthermore, endogenous c-fos is not essential to cell cycle progression of hematopoietic stem cells because the factor-dependent and the stroma-dependent hematopoiesis by Lin−Sca-1+ fetal liver cells from c-fos–deficient mice was not impaired. These results suggest that the c-fos induced in primitive hematopoietic stem cells negatively controls cell cycle progression and maintains them in a dormant state.
IT IS WIDELY CONSIDERED that pluripotent hematopoietic stem cells (PHSC) are highly quiescent in normal steady state bone marrow (BM), and that dormancy plays an important role in their preservation.1 However, mechanisms involved in retaining PHSC at a dormant state are not well understood. Several cytokines are involved in initiation of cell cycle progression of the dormant PHSC.1 Those cytokines activate signal transduction pathways via their own receptors in the PHSC to induce expression of immediate early genes. Those early gene products function as transcription factors that regulate expression of target genes. Many transcription factors control proliferation and differentiation of PHSC.2,3 Although lineage-restricted transcription factors such as tal-1/SCL and rtbn2/LMO2 may be candidates as key regulator for differentiation of PHSC,4-9 widely or even ubiquitously expressed transcription factors may have a special role in maintaining PHSC in a dormant state.2 3
The c-fos proto-oncogene, one of the immediate early genes, is transiently expressed on stimulation by external stimuli leading to cell cycle progression.10 Its product (c-Fos) forms a complex with the product of another proto-oncogene c-jun (AP-1) that regulates expression of AP-1–binding genes at their transcriptional level.10-12 Thus, c-Fos may play a key role in the transduction of signals induced by external stimuli.12-14 c-Fos is known to be critical for the G0/G1 transition and cell cycle progression in fibroblasts.13,14The overexpression of c-fos in transgenic mice leads to a deregulated bone growth and results in sarcomas,15,16 and the overexpression in several cell lines leads to acceleration of cell cycle progression.17,18 On the contrary, overexpression of c-Fos negatively regulates cell cycle progression in some cell types.19 Thus, functions of c-Fos in cell cycle progression have remained open to question.
c-Fos is thought to be important in various differentiation processes as well as in development.2,10-12 Although the developmental capacity of PHSC lacking the c-fos gene appears to be fairly normal,20-23 functions of c-Fos in controlling proliferation and differentiation of PHSC are unknown. Inducible type transgenic mice are powerful tools to investigate the gene function at certain stages of development.24 Using the transgenic mice carrying the c-fos gene under the control of the interferon (IFN)-α/β–inducible Mx-promoter (Mx–c-fos mice),25 we have shown that c-Fos interferes cell cycle progression of mature B cells at the G1/S transition of the cell cycle.26Furthermore, c-Fos induces apoptosis in pro–B cells27 and germinal-center B cells28 from Mx–c-fos mice. Thus, functions of c-Fos in regulating proliferation and differentiation of PHSC can be investigated using PHSC from Mx–c-fos mice.
Recent progress in the stem-cell biology revealed that PHSC and progenitors can be identified in BM cells, based on their surface marker profile.29 They lack lineage-specific antigens (Lin−) and express c-kit, H-2K, low level of Thy-1, and a high affinity to WGA.30-32 PHSC can be further isolated from committed progenitors by cell-surface staining with monoclonal antibody against Sca-1 or CD34, or by nuclear staining with supravital staining dye (rhodamine-123, Hoechst-33342).29 33-36 This method has made feasible studies on the nature of PHSC at the clonal level. We investigated the role of c-Fos in cell cycle progression of PHSC using primitive hematopoietic stem (Lin−Sca-1+) cells isolated from Mx–c-fos mice and also c-fos–deficient mice. We show here that the c-fos gene is transiently expressed in Lin−Sca-1+ BM cells stimulated with stem cell factor (SCF), interleukin-3 (IL-3), and IL-6. The prolonged expression of c-fos inhibits Lin−Sca-1+ BM cells from entering the cell cycle. The role of c-Fos in maintenance of PHSC in a dormant state is discussed.
MATERIALS AND METHODS
Mice.
C57BL/6CrSlc mice were purchased from Japan SLC Co, Ltd (Hamamatsu, Japan). Transgenic mice carrying the mouse c-fos gene under the control of the Mx gene promoter (Mx–c-fos)25 and c-fos–deficient mice,20 provided by Dr E.F. Wagner (IMP, Vienna, Austria), were maintained by heterozygous mating in our animal facility.
Reverse-transcribed polymerase chain reaction (RT-PCR) analysis.
Total RNA was extracted from 1 × 104 of Lin−Sca-1+ BM cells using an ISOGEN total RNA isolating kit (Waco, Tokyo, Japan). RNAs were reverse-transcribed using Superscript (Life Technologies, Grand Island, NY) and oligo(dT) (Pharmacia, Piscataway, NJ), in a final volume of 20 μL, and 1 μL of cDNA was used for PCR. After an initial 7-minute incubation at 95°C, 22 cycles of PCR were performed using the following conditions: c-fos cDNA, denaturation at 95°C for 1.5 minutes, annealing at 55°C for 1.5 minutes, and polymerization at 72°C for 1.5 minutes; G3PDH cDNA, denaturation at 95°C for 1 minute, annealing at 60°C for 1 minute, and polymerization at 72°C for 1 minute. PCR primers for the cDNA amplification were as follows: the c-fos primers,375′-TTCTCGGGTTTCAACGCC-3′ and 5′-GGCGTTGAAACCCGAGAA-3′; and the G3PDH primers,38 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ and 5′-CATGTAGGCCATGAGGTCCACCAC-3′.
PCR products were separated on a 1.5% agarose gel, transferred onto a nylon membrane (Boehringer Mannheim, Mannheim, Germany) and fixed by cross-linking with ultraviolet irradiation and by baking at 80°C for 3 hours. The filter was hybridized overnight with the digoxigenin (DIG)-labeled probe at 42°C. Following hybridization, the filter was washed twice with 0.1× standard saline citrate/0.1% sodium dodecyl sulfate at 68°C for 15 minutes. The probe on the filter was detected by sheep anti-DIG antibody conjugated with alkaline phosphatase. The antibody detection reaction was performed using the enhanced chemiluminescent detection system (Boehringer Mannheim GmbH, Mannheim, Germany). The probes were the full-length murine c-fos cDNA and murine G3PDH cDNA that were subcloned into pGEM vectors and labeled by DIG using PCR with T7 and SP6 primers.
Hematopoietic growth factors.
Purified recombinant human IL-6, purified recombinant murine IL-3, and conditioned medium (CM) of Chinese hamster ovary (COS) cells that had been transfected with an expression plasmid containing the murine SCF cDNA, were provided by Dr Tetsuo Sudo (Toray Industries, Kamakura, Japan). Concentration of SCF CM was assessed as 3 μg/mL in the proliferation assay by BaF cells transfected with mouse c-kit receptor. Recombinant murine SCF was purchased from Pepro Tech (Rocky Hill, NJ). Unless specified otherwise, SCF CM was used as a source of SCF. Recombinant human erythropoietin (Epo) was provided by Kirin Brewery (Tokyo, Japan). Concentration of the cytokines used here was as follows: IL-3, 200 U/mL; IL-6, 20 ng/mL; SCF CM, 5%; SCF, 100 ng/mL; Epo, 2 U/mL.
Antibodies.
Biotinylated monoclonal antibodies against B220 (RA3-6B2), Mac-1 (M1/70), Gr-1 (RB6-8C5), CD4 (GK1.5), CD8 (53-6.72), and TER119 were purchased from PharMingen (San Diego, CA) and used to detect lineage markers. Fluorescein isothiocyanate (FITC)-conjugated anti–Sca-1 (Ly6A/E) antibody was purchased from PharMingen. Biotinylated antibodies were visualized using Streptavidin-phycoerythrin (PE; PharMingen).
Isolation of Lin−Sca-1+ cells from BM cells or fetal liver (FL) cells.
Total BM cells from Mx–c-fos mice and their littermates were stained with a cocktail of biotinylated monoclonal antibodies against lineage markers for 20 minutes at 4°C. FL cells isolated from c-fos–deficient embryos and their littermates on day 14.5 postcoitus were stained with a cocktail of biotinylated monoclonal antibodies against lineage markers except for anti–Mac-1 antibody, as described.39 After washing the cells 3 times with staining medium (phosphate-buffered saline with 3% fetal calf serum [FCS] and 0.1% sodium azide), the cells were treated with streptavidin-conjugated immunomagnetic beads (BioMag; Perceptive Diagnostics, Cambridge, MA) for 30 minutes to remove lineage marker highly positive cells. The remaining cells were collected and stained with FITC–anti–Sca-1 antibody and Streptavidin-PE at 4°C for 20 minutes. After washing, the cells were resuspended in staining medium supplemented with propidium iodide (PI; 1 μg/mL). Stained cells were analyzed by FACS Vantage (Becton Dickinson, San Jose, CA), and the Lin−Sca-1+ cells were sorted and used as a primitive hematopoietic stem-cell fraction.40
In vitro colony assay.
Methylcellulose culture was performed using the modified method32 described by Iscove.41 Briefly, 1 mL of culture medium contained an adequate number of total BM cells or sorted Lin−Sca-1+ cells, 1.2% methylcellulose (Shin-etsu Chemical Co, Tokyo, Japan), alpha-medium (Flow Laboratories, North Ryde, Australia), 30% FCS (Flow Laboratories), 1% deionized bovine serum albumin (Sigma Chemical, St Louis, MO), 0.1 mmol/L β-2-mercaptoethanol (Eastman Organic Chemical, Rochester, NY), and appropriate concentrations of growth factors in the presence or absence of IFN-α/β (Sigma Chemical). The cultures were prepared in 35-mm nontissue culture dishes (Beckton Dickinson Labware, Lincoln Park, NJ) and incubated at 37°C in a humidified atmosphere of 5% CO2. The number of colonies was counted using an inverted microscope. When IFN-α/β was added in the culture on day 4, 100 μL of alpha-medium with 200 U of IFN-α/β was added to the methylcellulose culture dish.
Short-term liquid culture.
Total BM cells or sorted Lin−Sca-1+ cells were cultured in a 24-well plate (Beckton Dickinson Labware) with 1 mL alpha-medium containing 20% FCS and appropriate concentrations of growth factors in the presence (200 U/mL) or absence of IFN-α/β under a humidified 5% CO2 atmosphere at 37°C. After 7 days of culture, cells were obtained and nucleated cells were counted using Trypan blue (Life Technologies).
Spleen colony assay.
The spleen colony assay of Till and McCulloch42 was used. Freshly isolated or cultured Lin−Sca-1+cells were injected into lethally irradiated mice (9.0 Gy total body irradiation). The spleens were removed on day 8 or day 12 after transplantation, fixed in Bouin’s solution, and macroscopically visible colonies were counted and scored as colony forming unit in spleen (CFU-S).
Coculture of Lin−Sca-1+ cells with stromal cells.
PA-6 stromal cells support myelopoiesis.43 PA-6 cells (3 × 105/well) were seeded in a 6-well plate (PRIMARIA, Beckton Dickinson Labware) 1 day before coculture. Five hundred sorted Lin−Sca-1+ cells were cultured on the PA-6 stromal layer with 3 mL of alpha-medium containing 10% FCS in the presence (200 U/mL) or absence of IFN-α/β.
Cell cycle analysis.
Cell cycle analysis was performed as described by Nicoletti.44 Briefly, sorted Lin−Sca-1+ cells were cultured for 24 hours with SCF, IL-3, and IL-6 in the presence or absence of IFN-α/β. Those cells were incubated in hypotonic lysing buffer (0.1% sodium citrate, 0.01% Triton X, 0.1 mg/mL RNase, and 0.1 mg/mL PI). DNA content in each nuclei was analyzed on FACScalibur (Becton Dickinson, Mountain View, CA) using Cell Quest software (Becton Dickinson) for Macintosh (Apple Computet Inc, Cupertino, CA).
5-bromo-2′-deoxyuridine (BrdU) incorporation assay.
Sorted Lin−Sca-1+ cells were cultured with SCF, IL-3, and IL-6 in the presence or absence of IFN-α/β for 36 hours and pulsed with 10 μmol/L BrdU (Sigma) for 3 hours. Those cells were spread on a slide glass by Cytospin (Shandon Southern Instruments Inc, Sewickley, PA) and fixed with cold acetone for 10 minutes. The cells were then incubated with 2N HCl for 1 hour, followed by reaction with mouse monoclonal antibody to BrdU (Boehringer Mannheim, Indianapolis, IN). These cells were further incubated with F(ab′)2 fragment of anti-mouse Ig labeled with horseradish peroxidase (Nycomed Amersham plc, Buckinghamshire, UK). The DAB kit (Nichirei, Tokyo, Japan) was used to visualize peroxidase, and counter staining was done using hematoxylin.45
RESULTS
Expression of the c-fos gene in primitive hematopoietic stem cells stimulated with SCF, IL-3, and IL-6.
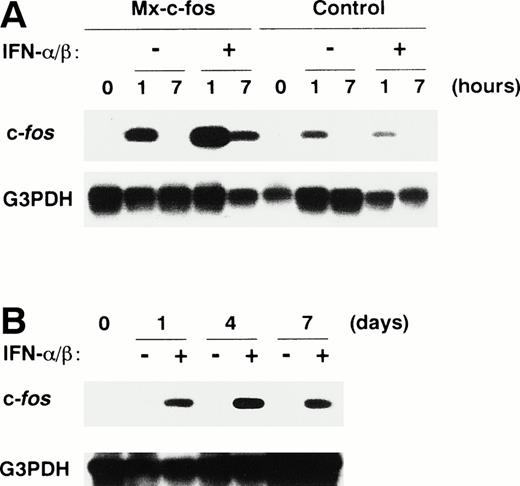
To examine expression of the c-fos gene in primitive hematopoietic stem cells after stimulation, Lin−Sca-1+ BM cells from Mx–c-fos mice and their control littermates were stimulated with SCF, IL-3, and IL-6 in the presence or absence of IFN-α/β. Expression of c-fos was analyzed by RT-PCR with Southern blotting (Fig 1A). c-fos RNA was detected in the Lin−Sca-1+ cells from both Mx–c-fos and control mice at 1 hour but not at 7 hours after stimulation in the absence of IFN-α/β, thereby indicating transient expression induced in these hematopoietic stem cells. When the cells were stimulated with SCF, IL-3, and IL-6 in the presence of IFN-α/β (200 U/mL), a large amount of c-fos RNA was evident in the Mx–c-fos but not in control stem cells, even 7 hours after stimulation. When IFN-α/β was added to those cultures on day 0 and day 4, c-fos mRNA was continuously detected until day 7 of culture (Fig 1B). Because IFN-α/β receptor is present in almost every cell type, including hematopoietic stem cells,46 we concluded that all of the cells derived from Mx–c-fos Lin−Sca-1+cells in the culture express the exogenous c-fos in the presence of IFN-α/β.
Prolonged expression of c-fos mRNA in primitive hematopoietic stem cells from Mx–c-fos mice. Lin−Sca-1+ BM cells were cultured with SCF, IL-3, and IL-6 in the presence (200 U/mL) or absence of IFN-/β for 7 hours (A), or by the addition of IFN-/β (200 U/mL) on day 0 and day 4 (B). Levels of c-fos mRNA were measured by RT-PCR analysis followed by Southern blotting, as described in Materials and Methods. G3PDH mRNA served as an internal control for the amount of RNA.
Prolonged expression of c-fos mRNA in primitive hematopoietic stem cells from Mx–c-fos mice. Lin−Sca-1+ BM cells were cultured with SCF, IL-3, and IL-6 in the presence (200 U/mL) or absence of IFN-/β for 7 hours (A), or by the addition of IFN-/β (200 U/mL) on day 0 and day 4 (B). Levels of c-fos mRNA were measured by RT-PCR analysis followed by Southern blotting, as described in Materials and Methods. G3PDH mRNA served as an internal control for the amount of RNA.
Effect of the prolonged expression of c-fos on colony formation by primitive hematopoietic stem cells stimulated with SCF, IL-3, and IL-6.
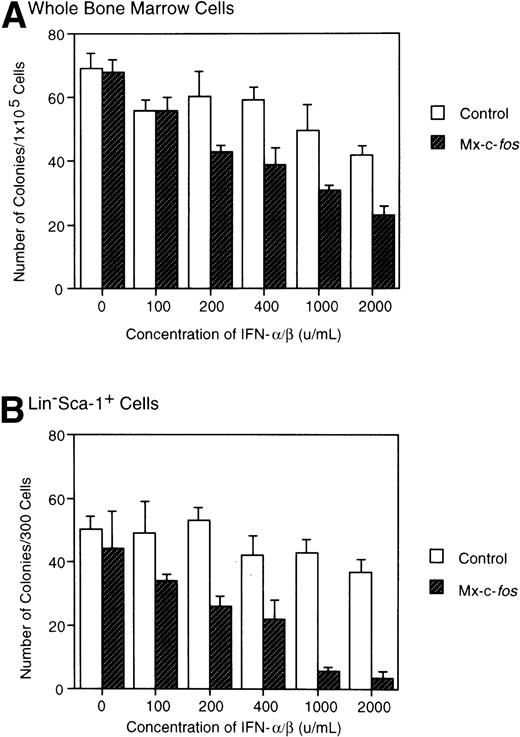
Total BM cells or Lin−Sca-1+ BM cells from Mx–c-fos mice and their control littermates were cultured with SCF, IL-3, and IL-6 in the presence of various concentrations of IFN-α/β, and the number of colonies was counted in those cultures on day 8. As shown in Fig 2A, colony formation by BM cells from Mx–c-fos mice was suppressed in the presence of IFN-α/β at more than 200 U/mL. However, the suppression was not so significant in control cultures even at 2,000 U/mL of IFN-α/β, although size of each colony was reduced at more than 1,000 U/mL of IFN-α/β (data not shown). The suppression was more evident in the Lin−Sca-1+ cell cultures from Mx–c-fos mice in the presence of IFN-α/β (Fig 2B), which means that c-fos suppressed on onset of colony formation from primitive hematopoietic stem cells but not from progenitors.
Inhibitory effect of c-fos on colony formation by primitive hematopoietic stem cells. Total BM cells or Lin−Sca-1+ BM cells from Mx–c-fos mice or control littermates were cultured with SCF, IL-3, and IL-6 with various concentrations of IFN-/β. On day 8 of culture, the number of colonies was scored. Results represent mean and SD of four dishes. The data presented are representative of two independent experiments.
Inhibitory effect of c-fos on colony formation by primitive hematopoietic stem cells. Total BM cells or Lin−Sca-1+ BM cells from Mx–c-fos mice or control littermates were cultured with SCF, IL-3, and IL-6 with various concentrations of IFN-/β. On day 8 of culture, the number of colonies was scored. Results represent mean and SD of four dishes. The data presented are representative of two independent experiments.
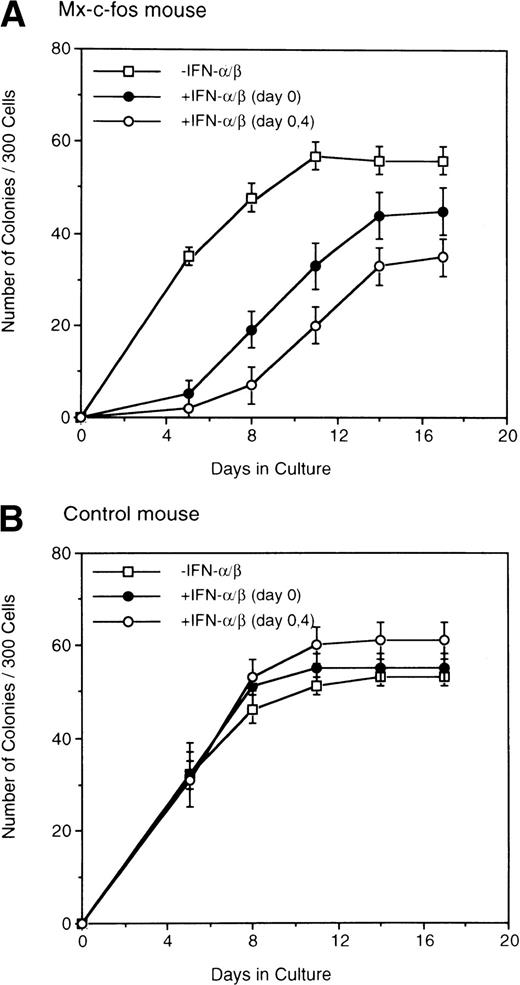
Kinetics of colony formation by Lin−Sca-1+ cells was further examined by adding IFN-α/β to the cultures on day 0 and day 4 (Fig 3). The number of colonies increased and reached a plateau in both Mx–c-fos and control cultures on day 8 of culture in the absence of IFN-α/β. When IFN-α/β (200 U/mL) was added to the culture on day 0, colony formation was delayed and the plateau level was slightly reduced in the Mx–c-fos but not in the control cultures. Furthermore, the addition of IFN-α/β to those cultures on day 0 and day 4 slowed down the colony formation and lowered the plateau level in the Mx–c-fos cultures but not in the control cultures. These observations suggest that prolonged expression of c-fos suppresses the onset of colony formation by primitive hematopoietic stem cells stimulated with SCF, IL-3, and IL-6.
Kinetics of colony formation by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells (3 × 102) from Mx–c-fos mice or control littermates were cultured with SCF, IL-3, and IL-6 (□). IFN-/β (200 U/mL) was added on day 0 (•), or on day 0 and day 4 (○). The number of colonies was scored every 4 days. Results represent mean and SD of four dishes. The data presented are representative of two independent experiments.
Kinetics of colony formation by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells (3 × 102) from Mx–c-fos mice or control littermates were cultured with SCF, IL-3, and IL-6 (□). IFN-/β (200 U/mL) was added on day 0 (•), or on day 0 and day 4 (○). The number of colonies was scored every 4 days. Results represent mean and SD of four dishes. The data presented are representative of two independent experiments.
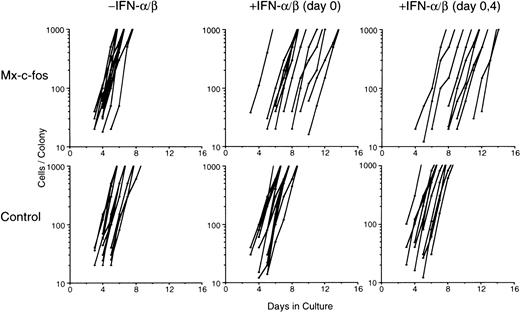
To further analyze the inhibitory effect of c-fos on growth of the colony, we serially plotted growth rate of each colony through colony mapping study, as described by Ikebuchi.47 One hundred and fifty of Lin−Sca-1+ cells per dish were cultured with SCF, IL-3, IL-6, and Epo, and emergence of a new colony later showed the CFU-Mix and its subsequent rate of proliferation were analyzed (Fig 4). Although the number of CFU-Mix was about 13 to 16 per dish in both Mx–c-fos and control cultures in the presence (200 U/mL) or absence of IFN-α/β, the onset of each colony was delayed in Mx–c-fos cultures but not in control cultures when IFN-α/β was added on day 0 of culture. When IFN-α/β was added to those cultures on day 0 and day 4, the onset of each colony was further delayed in the Mx–c-fos cultures. However, the proliferation rate of each colony developed in the Mx–c-fos cultures did not differ significantly from that in the control cultures in the presence of IFN-α/β. Becuase 200 U/mL of IFN-α/β can induce expression of the exogenous c-fos gene in BM cells from Mx–c-fos mice for more than 2 days,26 28 these results suggest that after entering the cell cycle c-fos does not inhibit growth of colonies derived from primitive hematopoietic stem cells.
Mapping study of colony formation by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells (1 × 102) from Mx–c-fos mice or control littermates were cultured with SCF, IL-3, IL-6, and Epo. IFN-/β (200 U/mL) was added on day 0, or on day 0 and day 4. Graphic presentation indicates cell number changes in individual colonies that later became a mixed colony (CFU-Mix). The data represent colonies identified in two plates. The data presented are representative of two independent experiments.
Mapping study of colony formation by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells (1 × 102) from Mx–c-fos mice or control littermates were cultured with SCF, IL-3, IL-6, and Epo. IFN-/β (200 U/mL) was added on day 0, or on day 0 and day 4. Graphic presentation indicates cell number changes in individual colonies that later became a mixed colony (CFU-Mix). The data represent colonies identified in two plates. The data presented are representative of two independent experiments.
Effect of c-fos on cell growth of primitive hematopoietic stem cells stimulated with various combinations of cytokines in the liquid culture.
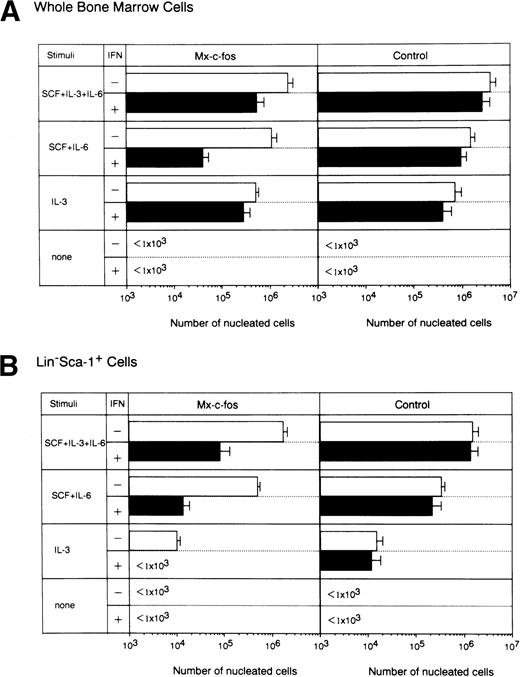
The combination of SCF, IL-3, and IL-6 provides one of the strongest signals for differentiation of stem cells and progenitors.1,36 Stimulation of SCF and IL-6 is required for expansion of immature cells48 and that of IL-3 alone supports differentiation of stem cells and progenitors in a cycling state.1 47 To examine which cytokine signals were inhibited by c-fos, we did short-term liquid cultures of stem cells from Mx–c-fos mice, using various combinations of cytokines by adding IFN-α/β (200 U/mL) on day 0 and day 4 of culture, and the number of nucleated cells was counted on day 7 of culture (Fig 5). When total BM cells were cultured with combinations of cytokines, cell growth in the Mx–c-fos cultures with SCF+IL-6 stimulation was suppressed in the presence of IFN-α/β. However, cell growth in Mx–c-fos cultures with SCF+IL-3+IL-6 or IL-3 alone was not suppressed, suggesting that the prolonged expression of c-fos inhibits cell growth of primitive hematopoietic stem cells. Indeed, cell growth in cultures of Lin−Sca-1+ BM cells from Mx–c-fosmice but not from control littermates with all of the combinations of cytokines used was inhibited in the presence of IFN-α/β.
Inhibitory effect of c-fos on cell proliferation by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells from Mx–c-fos or control littermates were cultured with SCF+IL-3+IL-6, SCF+IL-6, or IL-3. IFN-/β (200 U/mL) was added to the culture on day 0 and day 4. On day 7 of culture, the number of viable cells was counted by Trypan blue dye exclusion. Results represent mean and SD of four dishes. The data presented are representative of three independent experiments. Purified recombinant SCF was used in this experiment.
Inhibitory effect of c-fos on cell proliferation by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells from Mx–c-fos or control littermates were cultured with SCF+IL-3+IL-6, SCF+IL-6, or IL-3. IFN-/β (200 U/mL) was added to the culture on day 0 and day 4. On day 7 of culture, the number of viable cells was counted by Trypan blue dye exclusion. Results represent mean and SD of four dishes. The data presented are representative of three independent experiments. Purified recombinant SCF was used in this experiment.
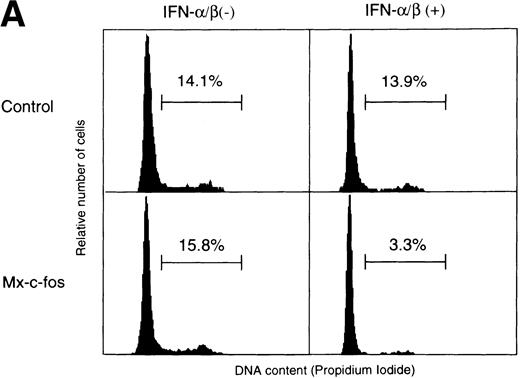
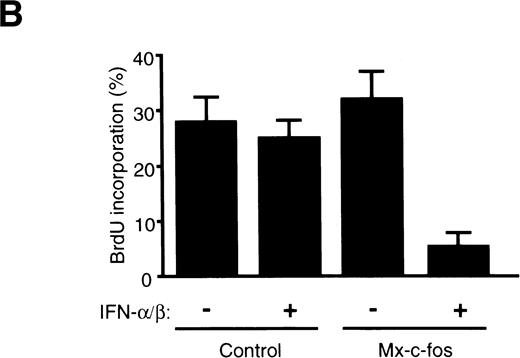
To confirm the suppressive effect of c-fos on cell growth of primitive hematopoietic stem cells, cell cycle analysis of Lin−Sca-1+ BM cells stimulated with SCF, IL-3, and IL-6 in the liquid culture was done using fluorescence-activated cell sorter (FACS). As shown in Fig 6A, the percentage of cells in the S/G2/M phase was approximately 15% in Mx–c-fos and control cultures in the absence of IFN-α/β and in control cultures in the presence of IFN-α/β (200 U/mL) 24 hours after stimulation. In contrast, only 3.3% of the Lin−Sca-1+ cells from Mx–c-fos mice were in the S/G2/M phase in the presence of IFN-α/β. Similar results were obtained in the case of BrdU incorporation assay (Fig 6B). Approximately 30% of cells were BrdU positive in cultures from Mx–c-fos and from control mice in the absence of IFN-α/β and in control cultures in the presence of IFN-α/β 36 hours after stimulation. However, only 5% of cells were positive for BrdU in Mx–c-fos cultures in the presence of IFN-α/β.
Cell cycle analysis of primitive hematopoietic stem cells after stimulation. Lin−Sca-1+ BM cells from Mx–c-fos or control littermates were cultured with SCF, IL-3, and IL-6 in the presence (200 U/mL) or absence of IFN-/β. (A) After 24 hours, the cells were lysed and the nuclei were stained with propidium iodide. DNA content in the nuclei was determined by FACS. Percentage of PI-labeled nuclei in S/G2/M phase of the cell cycle is indicated. (B) After 36 hours, cells were pulsed with BrdU for 3 hours. Incorporated BrdU was detected by anti-BrdU monoclonal antibody and positive cells were counted. Results represent mean and SD of four dishes. The data presented are representative of three independent experiments.
Cell cycle analysis of primitive hematopoietic stem cells after stimulation. Lin−Sca-1+ BM cells from Mx–c-fos or control littermates were cultured with SCF, IL-3, and IL-6 in the presence (200 U/mL) or absence of IFN-/β. (A) After 24 hours, the cells were lysed and the nuclei were stained with propidium iodide. DNA content in the nuclei was determined by FACS. Percentage of PI-labeled nuclei in S/G2/M phase of the cell cycle is indicated. (B) After 36 hours, cells were pulsed with BrdU for 3 hours. Incorporated BrdU was detected by anti-BrdU monoclonal antibody and positive cells were counted. Results represent mean and SD of four dishes. The data presented are representative of three independent experiments.
To examine the suppressive effect of c-fos on differentiation of hematopoietic stem cells, we performed the spleen colony assay.42 Five hundred Lin−Sca-1+ BM cells were cultured with SCF+IL-3+IL-6 in the presence (200 U/mL) or absence of IFN-α/β. On day 4 of culture, cells were collected and injected into lethally irradiated mice. As shown in Table 1, generation of day-8 CFU-S but not day-12 CFU-S was suppressed in mice injected with these cultured cells from Mx–c-fos mice in the presence of IFN-α/β. The number of day-8 CFU-S and that of day-12 CFU-S reflect the number of hematopoietic progenitor cells and that of more primitive hematopoietic stem cells,42 49 respectively. Therefore, these results indicate that prolonged expression of c-fos suppresses differentiation of hematopoietic stem cells.
Spleen Colony Formation by Lin−Sca-1+ BM Cells Following Prolonged Expression of c-fos
| Mice . | IFN-α/β . | Day-8 CFU-S . | Day-12 CFU-S . | Day-8/Day 12 CFU-S Ratio . |
|---|---|---|---|---|
| Control | − | 5.3 ± 1.5 | 11.0 ± 3.7 | 0.48 |
| + | 7.5 ± 2.9 | 10.8 ± 2.9 | 0.69 | |
| Mx–c-fos | − | 6.4 ± 2.5 | 10.3 ± 4.2 | 0.62 |
| + | 1.0 ± 1.4 | 13.7 ± 5.1 | 0.07 |
| Mice . | IFN-α/β . | Day-8 CFU-S . | Day-12 CFU-S . | Day-8/Day 12 CFU-S Ratio . |
|---|---|---|---|---|
| Control | − | 5.3 ± 1.5 | 11.0 ± 3.7 | 0.48 |
| + | 7.5 ± 2.9 | 10.8 ± 2.9 | 0.69 | |
| Mx–c-fos | − | 6.4 ± 2.5 | 10.3 ± 4.2 | 0.62 |
| + | 1.0 ± 1.4 | 13.7 ± 5.1 | 0.07 |
Five hundred Lin−Sca-1+ BM cells from Mx–c-fos or control littermates were cultured with SCF + IL-3 + IL-6 in the presence (200 U/mL) or absence of IFN-α/β. On day 4 of culture, those cells were collected and injected into lethally irradiated mice. Spleens from the mice were removed on day 8 or day 12 after the injection, fixed in Bouin’s solution, and the number of macroscopically visible spleen colonies was determined. Results represent mean and SD of 5 mice. The data presented are representative of two independent experiments. Purified recombinant SCF was used in this experiment.
Effect of c-fos on hematopoiesis by primitive hematopoietic stem cells cultured on a stromal cell layer.
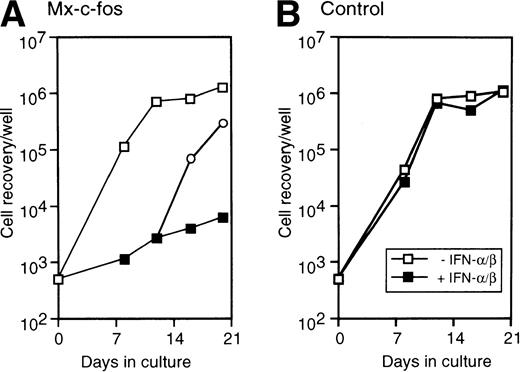
To investigate the suppressive effect of c-fos on hematopoiesis by primitive hematopoietic stem cells on a stromal cell layer, Lin−Sca-1+ BM cells were cultured on a layer of PA-6 stromal cells. Medium in the cultures was changed twice a week, and the number of nonadherent cells was counted by Trypan blue exclusion. As shown in Fig 7, the number of cells in both Mx–c-fos and control cultures exponentially increased and reached a plateau after day 12 of culture. When IFN-α/β (200 U/mL) was added to medium from the start of culture, the number of cells in Mx–c-fos cultures did not clearly increase. When addition of IFN-α/β in the medium was ceased after day 12 of culture, the number of cells increased and caught up to the control level in the Mx–c-fos cultures within 1 week. These data suggest that prolonged expression of c-fos to primitive hematopoietic stem cells also inhibits stroma-dependent hematopoiesis.
Inhibitory effect of c-fos on the stroma-dependent hematopoiesis by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells (3 × 102 cells/well in 6-well tissue culture plate) were cultured on a PA-6 stromal-cell layer in the presence (closed square; 200 U/mL) or absence (□) of IFN-/β. On day 12 of culture, the cells from Mx–c-fos culture with IFN-/β were obtained and washed with medium, and continued to culture on the PA-6 stromal layer with (▪) or without (○) IFN-/β. Nonadherent cells obtained from at least four dishes by gentle pipetting at every medium change were rinsed once with medium, pooled, and counted. The data presented are representative of three independent experiments.
Inhibitory effect of c-fos on the stroma-dependent hematopoiesis by primitive hematopoietic stem cells. Lin−Sca-1+ BM cells (3 × 102 cells/well in 6-well tissue culture plate) were cultured on a PA-6 stromal-cell layer in the presence (closed square; 200 U/mL) or absence (□) of IFN-/β. On day 12 of culture, the cells from Mx–c-fos culture with IFN-/β were obtained and washed with medium, and continued to culture on the PA-6 stromal layer with (▪) or without (○) IFN-/β. Nonadherent cells obtained from at least four dishes by gentle pipetting at every medium change were rinsed once with medium, pooled, and counted. The data presented are representative of three independent experiments.
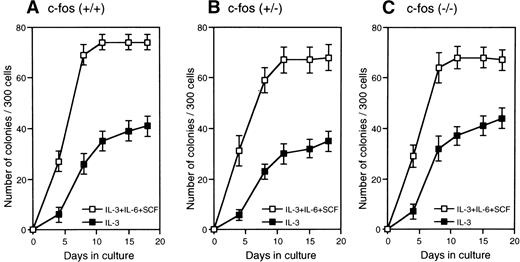
Factor-dependent and stroma-dependent hematopoiesis by primitive hematopoietic stem cells from c-fos–deficient mice.
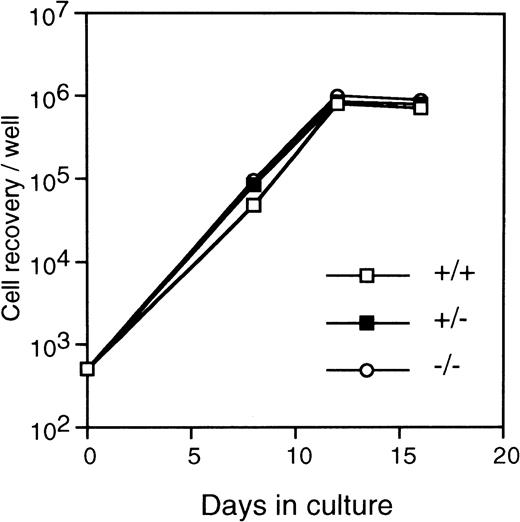
FL cells from c-fos–deficient mice was used as the source of primitive hematopoietic stem cells because of the osteopetrosis in c-fos–deficient mice.20 21 Kinetics of colony formation by Lin−Sca-1+ cells was examined in the cultures with SCF+IL-3+IL-6 or IL-3 alone. As shown in Fig 8, kinetics of colony formation did not significantly differ between c-fos–deficient and control littermates. In addition, stromal-dependent hematopoiesis by Lin−Sca-1+ cells from c-fos–deficient mice was normal because kinetics of cell growth on a PA-6 stromal layer by the Lin−Sca-1+ cells from c-fos–deficient mice showed similar features to those seen in case of control littermates (Fig9). These results suggest that endogenous c-fos is not required for cell cycle progression of primitive hematopoietic stem cells.
Kinetics of colony formation by primitive hematopoietic stem cells from c-fos–deficient mice. Lin−Sca-1+ cells (3 × 102) from FL of c-fos–deficient mice or control littermates were cultured with SCF, IL-3, and IL-6 (□), or IL-3 alone (▪). The number of colonies was scored and plotted. Results represent mean and SD of four dishes. The data presented are representative of three independent experiments.
Kinetics of colony formation by primitive hematopoietic stem cells from c-fos–deficient mice. Lin−Sca-1+ cells (3 × 102) from FL of c-fos–deficient mice or control littermates were cultured with SCF, IL-3, and IL-6 (□), or IL-3 alone (▪). The number of colonies was scored and plotted. Results represent mean and SD of four dishes. The data presented are representative of three independent experiments.
Kinetics of the stroma-dependent hematopoiesis by primitive hematopoietic stem cells from c-fos–deficient mice. Lin−Sca-1+ cells (3 × 102cells/well in 6-well tissue culture plate) from FL of c-fos–deficient mice (○) or control littermates (c-fos+/+, □; c-fos+/−, ▪) were cultured on a PA-6 stromal cell layer. The number of nonadherent cells was counted (see Fig 7 legend). The data presented are representative of three independent experiments.
Kinetics of the stroma-dependent hematopoiesis by primitive hematopoietic stem cells from c-fos–deficient mice. Lin−Sca-1+ cells (3 × 102cells/well in 6-well tissue culture plate) from FL of c-fos–deficient mice (○) or control littermates (c-fos+/+, □; c-fos+/−, ▪) were cultured on a PA-6 stromal cell layer. The number of nonadherent cells was counted (see Fig 7 legend). The data presented are representative of three independent experiments.
DISCUSSION
In the steady state, the majority of hematopoietic stem cells reside in BM at a dormant state,1,49,50 and only a few stem cells supply all of the hematopoietic cells at a given time.1,51Because hematopoietic stem cells in BM are always exposed to various forms of stimuli, mechanisms to maintain the stem cells in a dormant state no doubt exist. We found that c-fos negatively controls cell cycle progression of primitive hematopoietic stem cells. Initiation of cell cycling and subsequent proliferation of stem cells appear to require collaboration of early acting cytokines. Ogawa proposed that cytokines regulating proliferation of primitive hematopoietic progenitors may be separated into three arbitrary groups.1 The combination among these three groups such as SCF, IL-3, and IL-6 is one of the most effective stimuli supporting the early process of hematopoiesis.1 36 These stimulations transiently induce expression of c-fos in primitive hematopoietic stem cells (Fig 1). Because prolonged expression of c-fos inhibits G0/G1 transition of dormant hematopoietic stem cells in both cytokine-dependent (Figs 2 to 5) and stroma-dependent (Fig 8) hematopoiesis, downregulation of the c-fos may initiate G0/G1 transition. Indeed, cell proliferation began in the stem cell culture from Mx–c-fos mice whenever addition of IFN-α/β to the culture was stopped (Fig 8). Therefore, c-Fos may be a gate keeper for cell cycle entry of dormant hematopoietic stem cells.
c-Fos is an important positive regulator of cell growth and notably of the G0/G1 transition.2,10-12,14 Expression of c-fosantisense RNA or injection of anti-Fos antibodies inhibit serum-stimulated cells to enter into S phase of the cell cycle and to reduce the growth rate of asynchronously growing cells.52In addition, overexpression of c-fos in fibroblasts and myeloid cell lines accelerates growth rate.17,18 On the other hand, Balsalobre and Jolicoeur19 showed that G0-S progression of rat-1 fibroblasts was delayed by overexpression of c-fos. We reported that prolonged expression of c-fos also perturbs cell cycle progression of mature B cells by surface Ig cross-linking.26 The c-fos gene is transiently induced in B cells, and prolonged expression inhibits B cells from passing into S phase of the cell cycle. This perturbation of cell cycle progression is due to poor degradation of the cyclin kinase inhibitor p27kip1 in the G1 phase. However, prolonged expression of c-fos accelerates cell cycle progression of mature B cells stimulated with LPS.53 Taken together, c-Fos may act as a negative and as a positive regulator of cell growth that is dependent on cell types, stimulation signals, differentiation stages, and cell cycle states.
The inhibitory effect of c-fos on cell cycle progression of dormant hematopoietic stem cells was also shown in a mapping study (Fig4). However, growth rate was not significantly affected by the expression after colony formation began. This is supported by the finding that colony formation by total BM cells was affected by c-fos much less than that by purified Lin−Sca-1+ BM cells because the majority of colony-forming cells in total BM are committed progenitors. These results suggest that endogenous c-fos negatively regulates cell cycle progression of dormant hematopoietic stem cells.
Embryonal stem cells and 3T3-type fibroblasts lacking the c-fosgene divide at a normal rate.54,55 Furthermore, c-fos–deficient mice are viable but do have osteopetrosis, as a primary pathology.20,21 Although they have extramedullary hematopoiesis in the spleen, B lymphopenia, and thymic atrophy,20,21 we have found that hematopoietic stem cells can appear normal,22 except for failure differentiation into functional osteoclasts.56 In the present study, we found that both factor-dependent and stroma-dependent hematopoiesis by primitive hematopoietic stem cells from c-fos–deficient mice are normal (Fig 8 and 9). Colony formation by stem cells stimulated with IL-3+IL-6+SCF or IL-3 alone was almost the same as that seen in control littermates (Fig 8). These results indicate that c-fos is not essential for cell proliferation in several cell types and that redundancy with other members of the fosgene family may exist.11 12 These results also support the notion that c-Fos is a gate keeper for cell cycle entry of primitive hematopoietic stem cells.
c-Fos is also implicated in apoptosis.11,57 Treatment of cells with antisense oligonucleotide against c-fos increased survival of growth factor–derived lymphoid cells,37suggesting that expression of c-fos may represent an early event in activating programmed cell death. High levels of c-fosexpression were also observed in mouse tissues in which apoptosis is part of normal development.57 We also reported that overexpression of c-fos induced apoptosis of CD43+pro–B cells.27 On the other hand, c-fos plays a protective function from apoptosis of fibroblasts in response to stress such as ultraviolet irradiation.58 Mapping study (Fig 4) and spleen colony assay (Table 1) showed that significant number of primitive hematopoietic stem cells could survive despite the prolonged expression of c-fos. Furthermore, hematopoietic stem cells with long-term repopulating ability remain after the liquid culture for 7 days with SCF, IL-3, and IL-6 in the presence of IFN-α/β (data not shown). These results indicate that c-fos is not related to apoptosis in the dormant-state hematopoietic stem cells. Alternatively, as SCF, IL-3, and IL-6 are survival factors for primitive hematopoietic stem cells,59 60 these factors may contribute to survival of hematopoietic stem cells with overexpression of c-fos.
In summary, the c-fos was transiently induced in primitive hematopoietic stem cells stimulated with SCF, IL-3, and IL-6. The prolonged expression of c-fos inhibited cell-cycle entry of primitive hematopoietic stem cells stimulated with SCF, IL-3, and IL-6 as well as in case of cultures on a stromal cell layer. Hematopoietic stem cells with the c-fos expression in culture survived in a dormant state and entered the cell cycle after c-fos was downregulated. We propose that c-Fos plays the role of gate keeper in cell cycle progression of dormant hematopoietic stem cells.
ACKNOWLEDGMENT
We thank Drs T. Suda and M. Hatano for helpful discussions, Dr E.F. Wagner for c-fos–deficint mice, Dr T. Sudo for reagents, E. Furusawa and N. Fujita for secretarial services, and M. Ohara for comments on the manuscript.
Supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan and a Research Grant from the Inohana Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Takeshi Tokuhisa, MD, Department of Developmental Genetics, Chiba University Graduate School of Medicine, Chiba 260-8670, Japan; e-mail: tokuhisa@med.m.chiba-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal