To the Editor:
In a recent issue of Blood, Chalmers et al1communicated their findings of intracellular cytokine profiles of cord and adult blood. Analyzing cytokine synthesis at a single-cell level, they showed that cord blood (CB) lymphocytes produced less interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) than adult peripheral blood (AB) lymphocytes. Further subset analysis indicated that in CB lymphocytes, the majority of cytokine-producing cells were CD4+ CD45RA+in contrast to AB lymphocyte, which were both CD4+CD45RO+ and CD8+CD45RO+. Thus, they concluded that the reduced incidence of graft-versus-host disease (GVHD) in CB stem cell transplantation (CBSCT) could be partly due to the observed altered cytokine profile in CB lymphocytes.
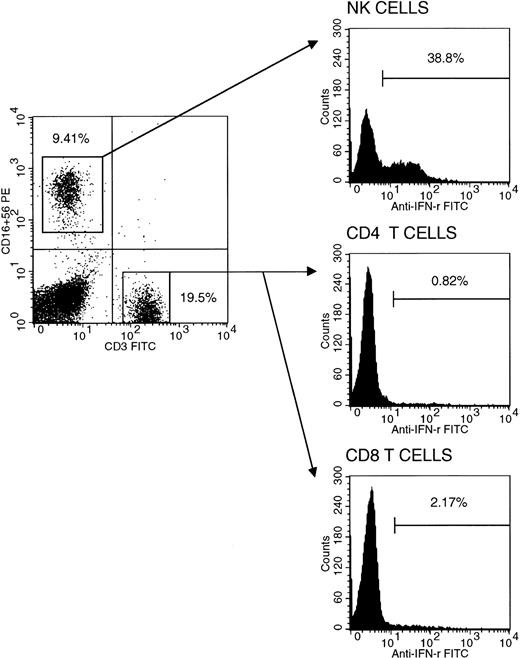
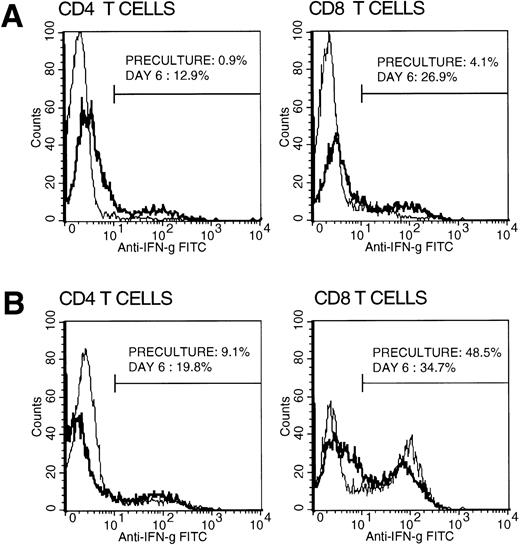
Interestingly, we also recently conducted a similar study assessing intracellular cytokine profiles of purified CB CD4+ and CD8+ T-cell subsets in comparison with those of healthy older children and adults. We have found and reported IFN-γ production capability to be altered and significantly lower (<5% of total T cells) in CB compared with that of older children and adults.2 However, in contrast, our findings revealed normal and comparably high IL-2 production capability, at a single-cell level, relative to older children and adults with even significantly higher mean cell percentage values of the cytokine-producing cell populations in CB. In a concurrent assessment of CB mononuclear cell population able to produce IFN-γ, we found that a large proportion of natural killer (NK) cells had the ability to produce IFN-γ with virtually no CD4+ cells (<1%) being able to do so (Fig1). Thus, the few IFN-γ–producing CB mononuclear cells were almost exclusively not T but largely NK cells. We observed that CB T lymphocytes, as indicated by their almost exclusive expression of CD45RA antigen and sole production of IL-2, were comprised almost exclusively of T helper (Thp) and T cytotoxic (Tcp) precursor cells, explaining in part the observed altered low IFN-γ production. However, when CB mononuclear cells were cultured with anti-CD3 monoclonal antibody and exogenous IL-2, the IFN-γ–producing cells markedly increased, reaching levels comparable to those of AB (Fig 2). This suggests that CB T cells may not, as such, be hyporesponsive in an in vivo alloantigen setting such as during CBSCT. Indeed, recent reports3 4 together with our in vitro studies (manuscript in preparation) suggest that the neonatal T cell, though naive, is immunologically and comparably competent. Thus, factors other than altered cytokine profiles, not yet sought and investigated, may fully explain the reported infrequent GVHD associated with CBSCT.
Intracellular IFN-γ staining in cord blood NK cells, CD4+, and CD8+ T cells. NK cells with CD3−/CD16+/CD56+ phenotype isolated by fluorescence-activated cell sorting using FACS vantage flow cytometer (purity >90%), together with CD4+ (purity >98%) and CD8+ (purity >85%) separated from the CD16/56-negative fraction by positive immunomagnetic separation were separately stimulated and stained for cytokine production capability as reported.2 Representative histograms are shown with respective percentages of IFN-γ+cells. Mean populations (±SD) of IFN-γ–producing NK cells (n = 9), CD4+ (n = 17), and CD8+ (n = 8) T cells in cord blood are 19.12% ± 9.53%, 1.15% ± 0.62%, and 3.70% ± 1.58%, respectively.
Intracellular IFN-γ staining in cord blood NK cells, CD4+, and CD8+ T cells. NK cells with CD3−/CD16+/CD56+ phenotype isolated by fluorescence-activated cell sorting using FACS vantage flow cytometer (purity >90%), together with CD4+ (purity >98%) and CD8+ (purity >85%) separated from the CD16/56-negative fraction by positive immunomagnetic separation were separately stimulated and stained for cytokine production capability as reported.2 Representative histograms are shown with respective percentages of IFN-γ+cells. Mean populations (±SD) of IFN-γ–producing NK cells (n = 9), CD4+ (n = 17), and CD8+ (n = 8) T cells in cord blood are 19.12% ± 9.53%, 1.15% ± 0.62%, and 3.70% ± 1.58%, respectively.
Intracellular IFN-γ staining in cultured T cells. CB and AB mononuclear cells were cultured over 6 days with anti-CD3 MoAb (1:5,000 diluted OKT3 immune ascities) and IL-2 (20 U/mL). Preculture and on day 6, cells were harvested separating CD4+ and CD8+ T cells by positive selection, stimulated, and stained for intracellular IFN-γ production capability as described.2 Representative histograms of preculture (plain line) and day 6 (bold line) CB (A) and AB (B) T cells are shown with percentages of positive cells.
Intracellular IFN-γ staining in cultured T cells. CB and AB mononuclear cells were cultured over 6 days with anti-CD3 MoAb (1:5,000 diluted OKT3 immune ascities) and IL-2 (20 U/mL). Preculture and on day 6, cells were harvested separating CD4+ and CD8+ T cells by positive selection, stimulated, and stained for intracellular IFN-γ production capability as described.2 Representative histograms of preculture (plain line) and day 6 (bold line) CB (A) and AB (B) T cells are shown with percentages of positive cells.
Finally and understandably, the variation in intracellular IL-2 and IFN-γ values between the report of Chalmers et al and our report, among other factors, could be due to intracellular staining protocol modifications. Indeed, Jason and Larned5 have recently illustrated how slight protocol modification in intracellular cytokine flow cytometry can lead to diverse variations of fluorescence stain results. Chalmers et al used 5 ng/mL phorbol 12-myristate 13-acetate (PMA), whereas we used 50 nmol/L (30.8 ng/mL) to induce cytokine production. It is documented that a high concentration of PMA may be necessary to induce a more profound T-cell response, such as the expression of IL-2 receptor.6 However, despite the use of a high concentration of PMA in our study, CB IFN-γ–producing cells were consistently low in both CD4+ and CD8+T-cell subsets.
Response
Studies looking at the secretion of cytokines by cord blood mononuclear cells have shown either similar, low, or high levels of secretion compared with cells from adults depending on the experimental approach used for the analysis. Chipeta et al1-1 have investigated the intracellular cytokine profiles of purified CD4+ and CD8+ cell subsets at a single cell level and, in contrast to our data, have found normal and comparable levels of interleukin-2 (IL-2) secretion between cord blood (CB) and adult blood (AB) cells.
As the authors rightly acknowledge, the most likely explanation for these discrepancies could be the variation in the intracellular staining protocols. First is the different concentration of mitogen used. In their study, a 6× higher concentration of phorbol 12-myristate 13-acetate (PMA) was used. Secondly, the analysis was performed using purified CD4+ and CD8+ subsets of cells. In our study the whole mononuclear cells (MNCs) were stimulated and then individual subsets were gated and analyzed. The activation of MNCs could include cells secreting cytokines with a regulatory effect on the CD4+ and CD8+ subsets (although we have no evidence that this is the case).
The different concentration of the mitogenic stimulus seems to be crucial since it has been shown that cord blood CD4+CD45RA+ has a lower level of activation than adult cells1-2 and, therefore, a higher concentration of PMA may be required to achieve levels of secretion comparable to those obtained in adult cells.
We have also obtained indirect evidence of the reduced IL-2 secretion by CB MNC by assessing the cellular responses to allogeneic stimulation in the mixed lymphocyte reaction (MLR). The results showed that whereas both CB and AB MNC were able to mount similar cellular responses in the primary MLR, only AB and not CB were able to proliferate in response to a secondary allogeneic stimulation as determined by the PLT assay. However, this deficient secondary response could be overcome by the addition of exogenous IL-2. Thus, it appears that at least in response to alloantigens, CB cells secrete levels of IL-2 that are insufficient to support a secondary allogeneic response in contrast to the response of AB cells.1-3 1-4
The results on the differential effect of the various concentrations of mitogens of the IL-2 and not interferon-γ (IFN-γ) secretion could be explained by the existence of different regulatory pathways involved in the secretion of both these cytokines. IL-2 and IL-2R synthesis are regulated by c-fos and c-jun proteins,1-5whereas a separate regulatory pathway for IFN-γ has been reported.1-6
With regard to the results of the predominant IFN-γ secretion by NK cells, it is difficult for us to comment because we did not analyze NK cells. However, in our study, although there was a markedly reduced secretion of IFN-γ by CB as compared with AB cells, there was a proportion of CD45RO cells (mostly CD8) secreting IFN-γ.
Finally, we agree with their comment regarding the immunological competence of CB T cells. The differences observed in vitro are probably due to the predominance of naive T cells in CB whose requirement for activation is more stringent.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal