Abstract
Neutrophil-derived 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) is a potent activator of neutrophils and eosinophils. In the present study we examined the biosynthesis and metabolism of this substance by platelets. Although platelets contain an abundant amount of 5-hydroxyeicosanoid dehydrogenase, the enzyme responsible for the formation of 5-oxo-ETE, they synthesize only very small amounts of this substance from exogenous 5-hydroxyeicosatetraenoic acid (5-HETE) unless endogenous NADPH is converted to NADP+ by addition of phenazine methosulfate. Similarly, relatively small amounts of 5-oxo-ETE were formed by A23187-stimulated mixtures of platelets and neutrophils, which instead formed substantial amounts of two 12-hydroxy metabolites of this substance, 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE, which were identified by comparison with authentic chemically synthesized compounds. These metabolites were also formed from 5-oxo-ETE by platelets stimulated with thrombin or A23187. In contrast, unstimulated platelets converted 5-oxo-ETE principally to 5-HETE. Neither 5-oxo-12-HETE nor 8-trans-5-oxo-12-HETE had appreciable effects on neutrophil calcium levels or platelet aggregation at concentrations as high as 10 μmol/L, but both blocked 5-oxo-ETE–induced calcium mobilization in neutrophils with IC50 values of 0.5 and 2.5 μmol/L, respectively. We conclude that platelets can biologically inactivate 5-oxo-ETE. Unstimulated platelets convert 5-oxo-ETE to 5-HETE, with a 99% loss of biological potency, whereas stimulated platelets convert this substance to 12-hydroxy metabolites, which possess antagonist properties.
ARACHIDONIC ACID IS metabolized in neutrophils primarily by the 5-lipoxygenase (5-LO) pathway, resulting in the formation of 5-hydroxy-6,8,11,14-eicosatetraenoic acid (5-HETE) and leukotriene B4 (LTB4).1,25-HETE is further metabolized to the biologically active compound 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE)3 by these cells, whereas LTB4 is converted to ω-oxidation products with reduced biological activity.4-6 In contrast to neutrophils, platelets do not possess 5-LO, but instead convert arachidonic acid to products of the 12-lipoxygenase (12-LO) and cyclooxygenase pathways. The major 12-LO product formed by these cells is 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-HETE), whereas the major cyclooxygenase products are thromboxane B2 and 12-hydroxy-5,8,10-heptadecatrienoic acid (12-HHTrE).7 8
Although platelets cannot synthesize 5-LO products from arachidonic acid, they can metabolize a number of neutrophil-derived 5-LO products. For example, platelets convert 5-HETE to 5S,12S-dihydroxy-6E,8Z,10E,14Z-6,8,10,14-eicosatetraenoic acid (5S,12S-diHETE) by the action of 12-LO.9 Platelets can also transform neutrophil-derived leukotriene A4(LTA4) to leukotriene C4(LTC4) because, unlike neutrophils, they contain LTC4 synthase.10-12 Furthermore, neutrophils can convert platelet-derived 12-HETE to its ω-oxidation product 12,20-diHETE13 as well as to 5S,12S-diHETE.14Thus, the profile of products formed by mixtures of platelets and neutrophils is considerably more complex than the sum of products formed by each of the two types of cells alone because of the formation of additional products by transcellular metabolism.
The objective of the present study was to determine whether platelets can synthesize and metabolize 5-oxoeicosanoids. We have shown that various leukocytes, including neutrophils,3eosinophils,15 monocytes, and lymphocytes16 can convert 5-HETE to 5-oxo-ETE by the action of 5-hydroxyeicosanoid dehydrogenase. 5-oxo-ETE is a potent chemoattractant for eosinophils15,17,18 and neutrophils19 and induces a variety of other responses in these cells including calcium mobilization,19,20 adherence,21 integrin expression,21 actin polymerization,21,22 and degranulation.20 There is considerable evidence that its actions are mediated by a specific G protein-linked receptor.22-24 We report here that platelets contain 5-hydroxyeicosanoid dehydrogenase. However, in resting platelets this enzyme seems to act in the reverse direction, converting 5-oxo-ETE to the less biologically active 5-HETE. Stimulated platelets, on the other hand, convert 5-oxo-ETE to its 12-hydroxy metabolite, 5-oxo-12S-hydroxy-6E,8Z,10E,14Z-eicosatetraenoic acid (5-oxo-12-HETE), which has little or no effect on neutrophils, and can antagonize 5-oxo-ETE–induced calcium mobilization. Thus, platelets could make an important contribution to the biological inactivation of 5-oxo-ETE.
MATERIALS AND METHODS
Eicosanoids.
5-HETE was synthesized by incubation of arachidonic acid (NuChek Prep Inc, Elysian, MN) with an ammonium sulfate fraction from potatoes as described in the literature.25 5S,12S-diHETE was prepared by incubating arachidonic acid with porcine leukocytes in the presence of A23187 (Calbiochem, La Jolla, CA).6 LTB4 was prepared similarly from porcine leukocytes except that incubations were performed in the presence of 5 μmol/L of 5,8,11,14-eicosatetraenoic acid.26 All products were extracted on octadecylsilyl (ODS)-silica Sep-Paks (Waters Associates, Milford, MA) and purified by high pressure liquid chromatography (HPLC).
5-oxo-ETE and its 8-trans isomer27 as well as 6-trans-LTB4 were prepared by total chemical synthesis as described previously. 5-oxo-12S-HETE and 8-trans-5-oxo-12S-HETE were also prepared by total chemical synthesis.28 Prostaglandin B2 (PGB2) was purchased from Sigma Chemical Co (St Louis, MO), whereas 12-epi-6-trans-LTB4 was obtained from Cayman Chemicals (Ann Arbor, MI).
Preparation of human neutrophils.
Human neutrophils were prepared by treatment of whole blood with Dextran T-500, followed by centrifugation over Ficoll-Paque and removal of remaining red blood cells by hypotonic lysis. The cells were resuspended in calcium and magnesium-free Dulbecco’s phosphate-buffered saline (PBS) containing 137 mmol/L NaCl, 2.7 mmol/L KCl, 1.5 mmol/L KH2PO4, and 8.1 mmol/L Na2HPO4 at a pH of 7.4.
Preparation of human platelets.
Blood was treated with Dextran T-500 (final concentration, 1%) for 45 minutes, and the resulting supernatant centrifuged for 10 minutes at 200g at room temperature. EDTA (final concentration, 1.54 mmol/L) was added to the supernatant, which was centrifuged at 700g for 20 minutes at room temperature. The top white portion of the pellet was resuspended in PBS (without Ca++ and Mg++) at a concentration of 2 × 108 platelets/mL unless otherwise indicated.
Preparation of subcellular fractions from platelets.
Platelets (65 × 106 cells/mL) were sonicated in PBS containing phenylmethylsulfonyl fluoride (2 mmol/L), dithiothreitol (1 mmol/L), and EDTA (1 mmol/L). The supernatant obtained by centrifugation at 1,500g at 4°C for 10 minutes was centrifuged at 10,000g at 4°C for 10 minutes. The supernatant was centrifuged at 200,000g at 4°C for 60 minutes. The 10,000g and 200,000g pellets were resuspended in PBS containing CaCl2 (1.8 mmol/L) and MgCl2 (1 mmol/L) at concentrations equivalent to 50 × 106 and 20 × 106 cells/mL, respectively.
Analysis of eicosanoids by precolumn extraction/reversed-phase (RP)-HPLC.
Suspensions (1 mL) of platelets, platelet-neutrophil mixtures, or subcellular fractions derived from platelets were incubated in PBS containing calcium (1.8 mmol/L) and magnesium (1 mmol/L) in the presence of various eicosanoids or agonists. The incubations were terminated by addition of methanol (0.6 mL) and cooling to 0°C. Before analysis, the concentration of methanol in the samples was adjusted to 15% by addition of water and PGB2 (200 ng) was added as an internal standard. Eicosanoids were quantitated by precolumn extraction/RP-HPLC29 using a Waters gradient controller, WISP automatic injector, WAVS automated switching valve, Model 991 diode array detector, and a Model 600 solvent delivery system (Waters Associates, Milford, MA). The mobile phase was a linear gradient between a mixture of 70% solvent A (water/acetonitrile/acetic acid [80:20:0.02]) and 30% solvent B (water/acetonitrile/methanol/acetic acid [7.5:38.5:54:0.02]) and a mixture of 15% solvent A and 85% solvent B over 50 minutes unless otherwise indicated. The flow rate was 1 mL/minute. The stationary phase was a column of Novapak C18 (3.9 × 150 mm; Waters). Products were quantitated by comparing the areas of their peaks of ultraviolet (UV) absorbance at their λmaxwith that of the internal standard, PGB2. The extinction coefficients used were: 5-HETE (23,000), 5-oxo-ETE (20,500), LTB4 (39,500), 5S,12S-diHETE (40,000), 5-oxo-12-hydroxyeicosanoids (40,000), and PGB2(28,680). The identities of the products measured were confirmed by examination of their complete UV spectra.
Mass spectrometry.
Mass spectrometry was performed using a Quattro II instrument (Micromass, Manchester, UK) with an electrospray interface, which was situated in the Biomedical Mass Spectrometry Unit of McGill University (Montreal, Quebec, Canada). Before analysis, 5-oxo-ETE metabolites were dissolved in acetonitrile/methanol/water (2:1:1) containing 1.25 mmol/L ammonium acetate, and were introduced into the ion source via direct inlet.
Measurement of cytosolic calcium levels.
Neutrophils (107 cells/mL) were preincubated for 5 minutes at 37°C in PBS and then incubated with the acetoxymethyl ester of Calcium Green-1 (1 μmol/L; Molecular Probes, Eugene, OR) for a further 30 minutes. The cells loaded with Calcium Green-1 were then washed twice in PBS and resuspended in the same medium to obtain a final cell concentration of 3.22 × 106 cells/mL. Fluorescence was measured at 37°C using a Photon Technology International (Monmouth Junction, NJ) Deltascan 4000 spectrofluorometer with a temperature-controlled cuvette holder equipped with a magnetic stirrer. The excitation and emission wavelengths were set at 506 nm and 531 nm, respectively. Before the addition of agonists, CaCl2 and MgCl2 in 56 μL of PBS were added to an aliquot of the cell suspension (934 μL) to give final concentrations of 1 mmol/L of each, a final concentration of cells of 3 × 106/mL, and a final volume of 1 mL. Responses to agonists were measured after stabilization of the baseline fluorescence. Fmax was determined by adding digitonin (final concentration, 0.1%), whereas Fmin was calculated after determination of autofluorescence as described in the literature.30 31
Measurement of platelet aggregation.
Platelet aggregation was measured as described in the literature.32 Washed platelets were prepared by centrifugation of whole blood, collected in citrate, at 180gfor 15 minutes at room temperature. The supernatant was diluted with an equal amount of citrate buffer (93 mmol/L sodium citrate, 7 mmol/L citric acid, and 140 mmol/L dextrose) and centrifuged at 1,000gfor 10 minutes and the pellet resuspended in Ca++/Mg++-free Hanks’ Balanced Salt Solution. Aggregation of platelets (0.5 mL; 300 × 106/mL) was measured in a Payton aggregometer (Payton Associates, Scarborough, Ontario, Canada), after addition of calcium and magnesium to give final concentrations of 1 mmol/L of each. After stabilization of the baseline, 5-oxo-eicosanoids or vehicle (2.5 μL dimethyl sulfoxide [DMSO]) were added 2 minutes before addition of the minimal maximally effective concentration of arachidonic acid (2 to 4 μmol/L), which was determined for each experiment.
RESULTS
Metabolism of 5-HETE by subcellular fractions from platelets.
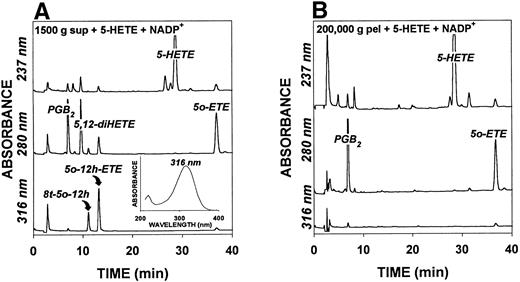
We previously showed that 5-hydroxyeicosanoid dehydrogenase in neutrophils was localized in the microsomal fraction of these cells.3 To determine whether this enzyme is also present in platelets, 5-HETE was incubated with subcellular fractions prepared from sonicates of these cells. Platelet sonicates were centrifuged successively at 1,500g, 10,000g, and 200,000g. The chromatographic profiles of products formed after incubation of 5-HETE with the 1,500g supernatant fraction and the 200,000g pellet in the presence of NADP+(1 mmol/L) are shown in Fig1. The 1,500g supernatant fraction converted 5-HETE to both 5-oxo-ETE and 5S,12S-diHETE, indicating that it contained both 5-hydroxyeicosanoid dehydrogenase and 12-LO. In addition, two products with retention times of 24.8 and 28.1 minutes, respectively, were detected at 316 nm. These products had identical UV spectra with λmax values at 316 nm (Fig 1A, inset). Because it seemed likely that these two products were 12-hydroxy metabolites of 5-oxo-ETE, both 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE were prepared by total chemical synthesis. The product with the longer retention time (13.2 minutes) cochromatographed with 5-oxo-12-HETE, whereas that with the shorter retention time (11.1 minutes) cochromatographed with 8-trans-5-oxo-12-HETE. The platelet-derived products had identical UV spectra to the corresponding chemically synthesized compounds. To provide further support for the identities of the above two products, their retention times were compared with those of the authentic chemically synthesized compounds using both normal phase and RP-HPLC (Table 1). In this case, the two 5-oxo-12-hydroxy isomers were prepared by incubation of 5-oxo-ETE with platelets in the presence of A23187 (see below). The chemically and biologically derived products had identical retention times under each of the three conditions used (Table 1), and, when mixed together, gave single peaks (data not shown). The identities of biologically prepared 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE were further confirmed by electrospray mass spectrometry, which showed M-1 ions at m/z 333 for both compounds (data not shown).
HPLC profiles of products formed by platelet subcellular fractions. Platelet sonicates were centrifuged at 1,500g for 10 minutes and the supernatant centrifuged successively at 10,000gfor 10 minutes and 200,000g for 60 minutes as described in Materials and Methods. The 1,500g supernatant (A) and the 200,000g pellet (B) fractions (equivalent to 2 × 107 platelets/mL) were incubated for 40 minutes at 37°C with 5-HETE (2 μmol/L) in the presence of NADP+ (1 mmol/L). The products were analyzed by precolumn extraction/RP-HPLC on a Novapak C18 column (3.9 × 300 mm) using a gradient between water/acetonitrile/acetic acid (50:50:0.02) and water/acetonitrile/acetic acid (40:60:0.02) over 30 minutes, followed by isocratic elution with 60% acetonitrile for a further 10 minutes. PGB2 (90 ng) was added as an internal standard. The inset to (A) shows the UV spectrum of biologically synthesized 5-oxo-12-HETE, which was identical to that of the chemically synthesized compound.
HPLC profiles of products formed by platelet subcellular fractions. Platelet sonicates were centrifuged at 1,500g for 10 minutes and the supernatant centrifuged successively at 10,000gfor 10 minutes and 200,000g for 60 minutes as described in Materials and Methods. The 1,500g supernatant (A) and the 200,000g pellet (B) fractions (equivalent to 2 × 107 platelets/mL) were incubated for 40 minutes at 37°C with 5-HETE (2 μmol/L) in the presence of NADP+ (1 mmol/L). The products were analyzed by precolumn extraction/RP-HPLC on a Novapak C18 column (3.9 × 300 mm) using a gradient between water/acetonitrile/acetic acid (50:50:0.02) and water/acetonitrile/acetic acid (40:60:0.02) over 30 minutes, followed by isocratic elution with 60% acetonitrile for a further 10 minutes. PGB2 (90 ng) was added as an internal standard. The inset to (A) shows the UV spectrum of biologically synthesized 5-oxo-12-HETE, which was identical to that of the chemically synthesized compound.
Retention Times of 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE Under Different HPLC Conditions
| Stationary Phase* . | Mobile Phase† . | Retention Time (min) . | |
|---|---|---|---|
| 8t-5o-12-HETE . | 5o-12-HETE . | ||
| Silica | 3% iPrOH | 18.5 | 8.2 |
| ODS-silica | 40% MeCN | 33.6 | 41.2 |
| ODS-silica | 65% MeOH | 24.9 | 32.5 |
| Stationary Phase* . | Mobile Phase† . | Retention Time (min) . | |
|---|---|---|---|
| 8t-5o-12-HETE . | 5o-12-HETE . | ||
| Silica | 3% iPrOH | 18.5 | 8.2 |
| ODS-silica | 40% MeCN | 33.6 | 41.2 |
| ODS-silica | 65% MeOH | 24.9 | 32.5 |
5-oxo-12-HETE and 8-trans-5-oxo-12-HETE were prepared either chemically28 or biologically, by incubating 5-oxo-ETE with platelets in the presence of A23187. The chemically and biologically prepared compounds had identical retention times and cochromatographed with one another.
The stationary phases were a 5-μm particle size Econosphere column (250 × 4.6 mm; Alltech Associates, Deerfield, IL), a 5-μm particle size Spherisorb ODS-2 (250 × 3.2 mm; Phenomenex, Torrance, CA).
The mobile phases consisted of hexane/isopropanol/acetic acid (97:3:0.1) at 2 mL/min, water/acetonitrile/acetic acid (60:40:0.02) at 0.75 mL/min, and water/methanol/acetic acid (35:65:0.02) at 0.75 mL/min.
The 5-hydroxyeicosanoid dehydrogenase activity of subcellular fractions from platelets was estimated by adding together the amounts of all the 5-oxo-eicosanoids formed by these fractions (ie, 5-oxo-ETE + 5-oxo-12-HETE + 8-trans-5-oxo-12-HETE). Although 5-oxo-ETE was the most abundant product in all cases, significant amounts of 12-hydroxy products were formed by the 1,500g supernatant (Fig 1A) and 10,000g pellet fractions. The fraction containing the highest 5-hydroxyeicosanoid dehydrogenase activity was the 200,000gpellet fraction (Fig 1B), which, in the presence of NADP+, showed about three times the specific activity of the 1,500gsupernatant (Table 2). In contrast, the 10,000g pellet fraction showed about the same activity as the 1,500g pellet, whereas the 200,000g supernatant fraction had little 5-hydroxyeicosanoid dehydrogenase activity.
The effects of cofactors on 5-hydroxyeicosanoid dehydrogenase activity were also investigated. In the absence of any cofactors, the 200,000g pellet fraction showed little activity (Table 2). Although addition of NAD+ resulted in markedly increased enzyme activity, it was only about one-fifth as effective as NADP+ in serving as a cofactor for the dehydrogenase.
Profile of eicosanoids released by mixtures of platelets and neutrophils.
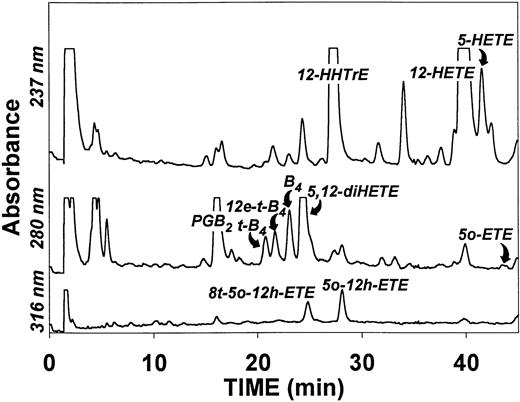
Although platelets contain 5-hydroxyeicosanoid dehydrogenase, they would not be expected to synthesize 5-oxo-ETE or other 5-oxo-eicosanoids from endogenous substrate because they do not contain 5-LO. However, addition of platelets to neutrophils could increase the formation of 5-oxo-eicosanoids by transcellular metabolism. To test this hypothesis, a mixture of platelets (2 × 108/mL) and neutrophils (5 × 106/mL) were incubated with the calcium ionophore A23187 (5 μmol/L) and phorbol 12-myristate 13-acetate (PMA; 30 nmol/L) for 30 minutes at 37°C. Analysis of the resulting products by RP-HPLC showed the presence of a large number of substances derived from the two types of cells (Fig 2). The major compounds detected by UV absorption were the platelet-derived products 12-HETE and 12-HHTrE, along with 5S,12S-diHETE, a product of transcellular metabolism. The neutrophil-derived products 5-HETE and LTB4 and its 6-trans isomers were also detected, along with similar amounts of 5-oxo-12-HETE and its 8-trans isomer.
HPLC profile of products formed after incubation of a mixture of platelets and neutrophils with A23187 and PMA. Platelets (2 × 108/mL) and neutrophils (5 × 106/mL) were incubated for 30 minutes at 37°C with A23187 (5 μmol/L) and PMA (30 nmol/L). After addition of the internal standard (200 ng PGB2), the products were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods. Abbreviations: B4, LTB4; t-B4, 6-trans-LTB4; 12e-t-B4, 12-epi-6-trans-LTB4; 5o-12h-ETE, 5-oxo-12-HETE; 8t-5o-12h-ETE, 8-trans-5-oxo-12(RS)-HETE.
HPLC profile of products formed after incubation of a mixture of platelets and neutrophils with A23187 and PMA. Platelets (2 × 108/mL) and neutrophils (5 × 106/mL) were incubated for 30 minutes at 37°C with A23187 (5 μmol/L) and PMA (30 nmol/L). After addition of the internal standard (200 ng PGB2), the products were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods. Abbreviations: B4, LTB4; t-B4, 6-trans-LTB4; 12e-t-B4, 12-epi-6-trans-LTB4; 5o-12h-ETE, 5-oxo-12-HETE; 8t-5o-12h-ETE, 8-trans-5-oxo-12(RS)-HETE.
Effects of different ratios of platelets and neutrophils on eicosanoid production.
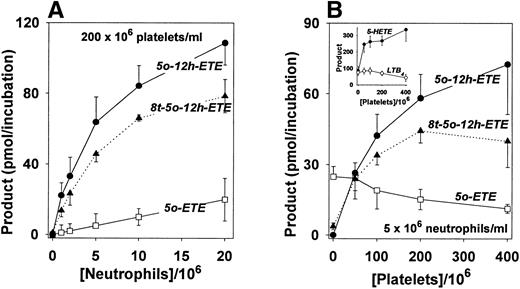
The effects of different ratios of platelets to neutrophils on the synthesis of 5-oxo-eicosanoids from endogenous substrate were investigated. Stimulation of a suspension of platelets alone (200 × 106 cells/mL) with A21387 (5 μmol/L) and PMA (30 nmol/L) failed to result in the release of detectable amounts of 5-oxo-eicosanoids (Fig 3A). However, addition of increasing numbers of neutrophils resulted in progressive increases in the amounts of these compounds. The amounts of 5-oxo-12-hydroxy metabolites far exceeded that of 5-oxo-ETE at all concentrations of neutrophils tested (Fig3A).
Effects of different concentrations of platelets and neutrophils on the formation of 5-oxo-eicosanoids. Mixtures of platelets and neutrophils were incubated for 30 minutes at 37°C with A23187 (5 μmol/L) and PMA (30 nmol/L). The products were quantitated by RP-HPLC with PGB2 as an internal standard as shown in Fig 1. (A) Formation of 5-oxo-12-HETE (•), 8-trans-5-oxo-12-HETE (▴), and 5-oxo-ETE (□) by platelets (2 × 108/mL) in the presence or absence of various concentrations of neutrophils. (B) Formation of 5-oxo-12-HETE (•), 8-trans-5-oxo-12-HETE (▴), and 5-oxo-ETE (□) by neutrophils (5 × 106/mL) in the presence or absence of various concentrations of platelets. The inset shows the formation of 5-HETE (•) and LTB4 (○) by neutrophils (5 × 106/mL) in the presence or absence of various concentrations of platelets. The results are means ± standard error (SE) of experiments on mixtures of platelets and neutrophils from four different subjects.
Effects of different concentrations of platelets and neutrophils on the formation of 5-oxo-eicosanoids. Mixtures of platelets and neutrophils were incubated for 30 minutes at 37°C with A23187 (5 μmol/L) and PMA (30 nmol/L). The products were quantitated by RP-HPLC with PGB2 as an internal standard as shown in Fig 1. (A) Formation of 5-oxo-12-HETE (•), 8-trans-5-oxo-12-HETE (▴), and 5-oxo-ETE (□) by platelets (2 × 108/mL) in the presence or absence of various concentrations of neutrophils. (B) Formation of 5-oxo-12-HETE (•), 8-trans-5-oxo-12-HETE (▴), and 5-oxo-ETE (□) by neutrophils (5 × 106/mL) in the presence or absence of various concentrations of platelets. The inset shows the formation of 5-HETE (•) and LTB4 (○) by neutrophils (5 × 106/mL) in the presence or absence of various concentrations of platelets. The results are means ± standard error (SE) of experiments on mixtures of platelets and neutrophils from four different subjects.
Metabolism of 5-HETE by Different Subcellular Fractions in the Presence and Absence of NAD+ and NADP+
| Fraction (n = 7) . | Cofactor . | 5h Dehydrogenase (pmol/mg protein/min) . |
|---|---|---|
| 1,500g supernatant | NADP+ | 68.7 ± 6.8 |
| 10,000g pellet | NADP+ | 82.7 ± 7.8 |
| 200,000g pellet | None | 2.4 ± 1.1 |
| 200,000g pellet | NAD+ | 41.6 ± 6.3 |
| 200,000g pellet | NADP+ | 192.9 ± 29.3 |
| 200,000g supernatant | NADP+ | 2.4 ± 0.3 |
| Fraction (n = 7) . | Cofactor . | 5h Dehydrogenase (pmol/mg protein/min) . |
|---|---|---|
| 1,500g supernatant | NADP+ | 68.7 ± 6.8 |
| 10,000g pellet | NADP+ | 82.7 ± 7.8 |
| 200,000g pellet | None | 2.4 ± 1.1 |
| 200,000g pellet | NAD+ | 41.6 ± 6.3 |
| 200,000g pellet | NADP+ | 192.9 ± 29.3 |
| 200,000g supernatant | NADP+ | 2.4 ± 0.3 |
Platelets were sonicated and subcellular fractions prepared as described in Materials and Methods. Fractions (1 mL) equivalent to either 20 × 106 (1,500g supernatant and 200,000g pellet) or 50 × 106 (10,000gpellet and 200,000g supernatant) platelets/mL were incubated for 40 minutes at 37°C with 5-HETE (2 μmol/L) in the presence or absence of either NAD+ (1 mmol/L) or NADP+ (1 mmol/L). Eicosanoids were measured by RP-HPLC as shown in Fig 1. 5-hydroxyeicosanoid dehydrogenase activity was calculated from the total amounts of 5-hydroxyeicosanoids (ie, 5-oxo-ETE + 5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) formed. The values are means ± SE (n = 7).
Abbreviation: 5h dehydrogenase, 5-hydroxyeicosanoid dehydrogenase.
The effects of adding increasing numbers of platelets to a constant number of neutrophils (5 × 106 cells/mL) were somewhat more complicated. In the absence of platelets, the principal 5-oxo-eicosanoid formed was 5-oxo-ETE (Fig 3B), along with very small amounts of 8-trans-5-oxo-12-HETE. The latter substance was presumably formed by oxidation of 6-trans isomers of LTB4 by 5-hydroxyeicosanoid dehydrogenase in neutrophils to give 5-oxo-12S-hydroxy and 5-oxo-12R-hydroxy metabolites, which would not be separated by RP-HPLC. The 5S,12S-diHETE metabolite 5-oxo-12-HETE (retention time, 28.1 minutes) was not detected in the absence of platelets. Addition of platelets caused dramatic increases in the formation of both 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE, but reduced the amount of 5-oxo-ETE detected. The total amount of 5-oxo-eicosanoids (5-oxo-ETE + 5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) synthesized by a mixture of neutrophils with the highest concentration of platelets tested (400 × 106/mL) was 124 ± 31 pmol/mL, compared with 29 ± 5 pmol/mL in the presence of neutrophils alone (P < .05). Addition of platelets to neutrophils also resulted in increased formation of 5-HETE (P < .05) (Fig 3B, inset). In contrast, LTB4 production seemed to be somewhat lower in the presence of platelets, but this difference was not significant.
As shown above, changes in the ratios of platelets to neutrophils affected the distribution of products. However, the absolute numbers of cells could also be important, as has been shown to be the case for the synthesis of LTB433 and 20-hydroxy-LTB46 by neutrophils. To test this hypothesis, mixtures of increasing numbers of platelets and neutrophils at a constant ratio of 40:1 were incubated with A23187 and PMA as described above. The amounts of the two 5-oxo-12-HETE isomers increased nearly linearly with cell concentration, whereas the amount of 5-oxo-ETE increased only modestly. Thus at a relatively low concentration of cells (50 × 106 platelets; 1.25 × 106 neutrophils), the total amount of 5-oxo-12-HETE was 5.4 ± 0.5 times greater than the amount of 5-oxo-ETE. This ratio increased markedly as the total numbers of cells were increased so that at a high concentration of cells (400 × 106platelets; 10 × 106 neutrophils) the total amount of 5-oxo-12-HETEs was 26.6 ± 3.0 times greater than that of 5-oxo-ETE. This presumably reflects an increased rate of metabolism of 5-oxo-ETE at higher concentrations of platelets.
Metabolism of 5-HETE and related compounds by intact platelets.
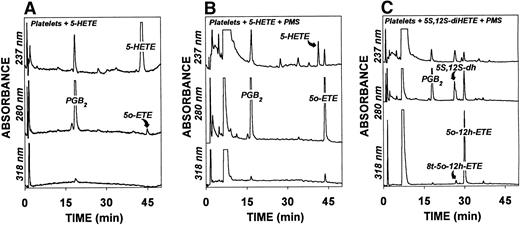
The increased amounts of 5-oxo-eicosanoids formed by addition of platelets to neutrophils suggest that intact platelets can convert 5-HETE or 5S,12S-diHETE to their 5-oxo metabolites. To test this hypothesis more directly, 5-HETE (2 μmol/L) was incubated with platelets (200 × 106 cells/mL) for 30 minutes at 37°C. As shown in Fig 4A, platelets converted 5-HETE to only small amounts of 5-oxo-ETE. It is possible that the modest metabolism of 5-HETE could have been related to incorporation into membranes, as occurs in neutrophils,34but this is probably not a major consideration, because most of the 5-HETE was recovered unmetabolized after incubation with unstimulated platelets (Fig 4A). It is also possible that 5-HETE may not readily be taken up by platelets, but subsequent experiments showed that the metabolism of this substance was markedly enhanced by activation by thrombin or A23187 (see below). Previous studies with neutrophils had shown that conversion of 5-HETE to 5-oxo-ETE was tightly regulated by intracellular NADP+ levels.35 Therefore, platelets were incubated with 5-HETE in the presence of phenazine methosulfate (PMS; 100 μmol/L), which nonenzymatically converts NADPH to NADP+36 without oxidizing 5-HETE.35 Under these conditions, the formation of 5-oxo-ETE was dramatically increased (Fig 4B). In addition to 5-HETE, platelets also oxidized 5S,12S-diHETE in the presence of PMS to 5-oxo-12-HETE, along with a small amount of the corresponding 8-trans isomer (Fig 4C). Both 6-trans-LTB4 and 12-epi-6-trans-LTB4 were also converted by platelets to their 5-oxo metabolites (ie, 8-trans-5-oxo-12R-HETE and 8-trans-5-oxo-12S-HETE) which both had retention times similar to that of chemically synthesized 8-trans-5-oxo-12S-HETE (data not shown). Only small amounts of 5-oxo-12-hydroxy metabolites were formed from the above compounds in the absence of PMS (data not shown). Unlike all of the above 5S-hydroxy-6-trans eicosanoids, LTB4 was not converted to significant amounts of products absorbing at 316 nm in the presence of PMS (data not shown).
HPLC profiles of 5-oxo-eicosanoids formed by platelets in the presence and absence of phenazine methosulfate. Platelets (2 × 108/mL) were preincubated for 5 minutes at 37°C in the absence (A) or presence (B and C) of PMS (100 μmol/L) and then incubated for a further 30 minutes with 5-HETE (2 μmol/L; A and B) or 5S,12S-diHETE (2 μmol/L; C). The products were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods.
HPLC profiles of 5-oxo-eicosanoids formed by platelets in the presence and absence of phenazine methosulfate. Platelets (2 × 108/mL) were preincubated for 5 minutes at 37°C in the absence (A) or presence (B and C) of PMS (100 μmol/L) and then incubated for a further 30 minutes with 5-HETE (2 μmol/L; A and B) or 5S,12S-diHETE (2 μmol/L; C). The products were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods.
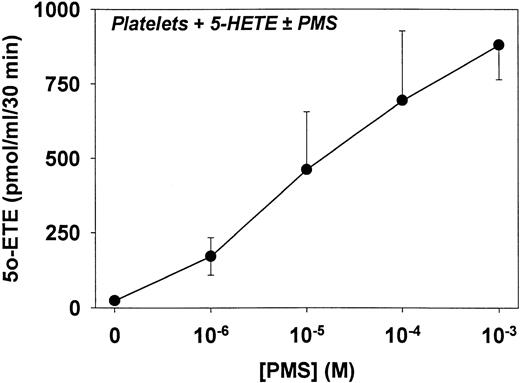
The effects of different concentrations of PMS on the formation of 5-oxo-ETE by platelets are shown in Fig 5. As in Fig 4A, only small amounts of 5-oxo-ETE were formed in the absence of PMS. Formation of 5-oxo-ETE seemed to be increased at concentrations of PMS as low as 1 μmol/L, although this effect was not statistically significant. However, all concentrations of PMS higher than 1 μmol/L significantly and dramatically increased the production of 5-oxo-ETE from 5-HETE by platelets. These results clearly show that intact platelets have a high capacity to synthesize 5-oxo-ETE, even though it may not normally be realized.
Concentration-response for the effect of PMS on the formation of 5-oxo-ETE. Platelets (2 × 108/mL) were preincubated for 5 minutes at 37°C in the absence or presence of various concentrations of PMS and then incubated for a further 30 minutes with 5-HETE (2 μmol/L). The products were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods. The only product formed in appreciable amounts under these conditions was 5-oxo-ETE. The results are means ± SE of determinations on platelets from three different individuals.
Concentration-response for the effect of PMS on the formation of 5-oxo-ETE. Platelets (2 × 108/mL) were preincubated for 5 minutes at 37°C in the absence or presence of various concentrations of PMS and then incubated for a further 30 minutes with 5-HETE (2 μmol/L). The products were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods. The only product formed in appreciable amounts under these conditions was 5-oxo-ETE. The results are means ± SE of determinations on platelets from three different individuals.
Metabolism of 5-HETE by stimulated platelets.
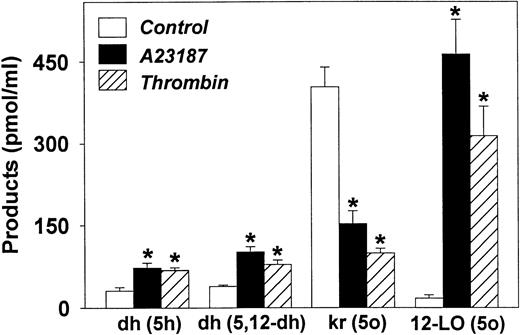
The modest amounts of 5-oxo-eicosanoids formed after incubation of 5-HETE with platelets in the absence of PMS compared with the amounts synthesized by stimulated mixtures of platelets and neutrophils suggested that the synthesis of these compounds could be enhanced in stimulated platelets. To test this hypothesis, platelets were incubated with 5-HETE, 5S,12S-diHETE, or 5-oxo-ETE for 10 minutes in the presence or absence of A23187 (5 μmol/L) or thrombin (1 U/mL). Unstimulated platelets converted 5-HETE to small amounts of 5-oxo-ETE but little or no 5-oxo-12-hydroxy-ETEs (Fig 4A), whereas platelets stimulated with either A23187 or thrombin formed mainly the latter compounds (data not shown). Both A23187 and thrombin stimulated the dehydrogenase-catalyzed oxidation of 5-HETE to 5-oxo-eicosanoids (ie, 5-oxo-ETE + 5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) by over twofold (Fig 6). Similarly, these two agonists increased the conversion of 5S,12S-diHETE to its 5-oxo metabolites by twofold to threefold. These results show that the formation of 5-hydroxyeicosanoid dehydrogenase products by platelets is enhanced as a result of platelet stimulation.
Effects of platelet stimulation on the metabolism of 5-HETE, 5S,12S-diHETE, and 5-oxo-ETE. Platelets (2 × 108) were incubated with either 5-HETE (2 μmol/L; 5h), 5S,12S-diHETE (2 μmol/L; 5,12-dh), or 5-oxo-ETE (2 μmol/L; 5o) for 10 minutes at 37°C in the absence (□) or presence of A23187 (5 μmol/L; ▪) or thrombin (1 U/mL; ▨). The products were quantitated by precolumn extraction/RP-HPLC as described in Materials and Methods. The four groups of bars (from left to right) show: (1) the amounts of 5-hydroxyeicosanoid dehydrogenase (dh) products (5-oxo-ETE + 5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) formed from 5-HETE, (2) the amounts of dehydrogenase products (5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) formed from 5S,12S-diHETE, (3) the amounts of 5-ketoreductase (kr) products (5-HETE + 5S,12S-diHETE) formed from 5-oxo-ETE, and (4) the amounts of 12-LO products (5-oxo-12-HETE + 8-trans-5-oxo-12-HETE + 5S,12S-diHETE) formed from 5-oxo-ETE. The values are means ± SE of determinations on platelets from four different donors.
Effects of platelet stimulation on the metabolism of 5-HETE, 5S,12S-diHETE, and 5-oxo-ETE. Platelets (2 × 108) were incubated with either 5-HETE (2 μmol/L; 5h), 5S,12S-diHETE (2 μmol/L; 5,12-dh), or 5-oxo-ETE (2 μmol/L; 5o) for 10 minutes at 37°C in the absence (□) or presence of A23187 (5 μmol/L; ▪) or thrombin (1 U/mL; ▨). The products were quantitated by precolumn extraction/RP-HPLC as described in Materials and Methods. The four groups of bars (from left to right) show: (1) the amounts of 5-hydroxyeicosanoid dehydrogenase (dh) products (5-oxo-ETE + 5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) formed from 5-HETE, (2) the amounts of dehydrogenase products (5-oxo-12-HETE + 8-trans-5-oxo-12-HETE) formed from 5S,12S-diHETE, (3) the amounts of 5-ketoreductase (kr) products (5-HETE + 5S,12S-diHETE) formed from 5-oxo-ETE, and (4) the amounts of 12-LO products (5-oxo-12-HETE + 8-trans-5-oxo-12-HETE + 5S,12S-diHETE) formed from 5-oxo-ETE. The values are means ± SE of determinations on platelets from four different donors.
Metabolism of 5-oxo-ETE by platelets.
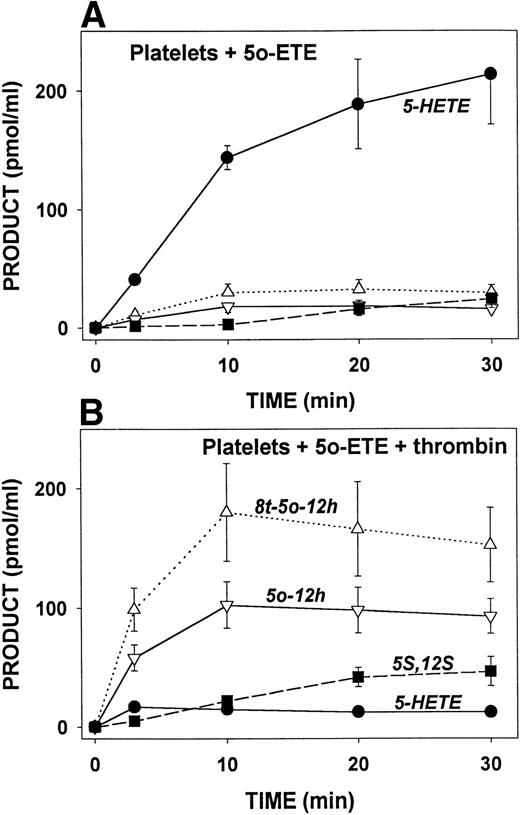
5-oxo-ETE was incubated with platelets in the presence or absence of thrombin (1 U/mL) for various times and the products analyzed by RP-HPLC. As shown in Fig 7A, the major 5-oxo-ETE metabolite formed by unstimulated platelets was 5-HETE, which reached near maximal levels by 10 minutes, and then increased slowly with time thereafter. Only small amounts of 5-oxo-12-HETE isomers were formed by unstimulated platelets. 5S,12S-diHETE was nearly undetectable for the first 10 minutes, and then increased slowly with time. In contrast, in the presence of thrombin, only small amounts of 5-HETE were detected (Fig 7B), and instead, the major 5-oxo-ETE metabolites were the cis and trans isomers of 5-oxo-12-HETE, which reached maximal levels by 10 minutes. In this case, the trans isomers seemed to predominate. The ratios of the cis and trans isomers varied somewhat from one experiment to another, probably because of nonenzymatic conversion of the cis to the trans, which occurs very easily. Little or no 5S,12S-diHETE was formed after 3 minutes, but its concentration gradually increased over 30 minutes.
Time course for the formation of 5-oxo-ETE metabolites by resting and activated platelets. Platelets (2 × 108/mL) were incubated with 5-oxo-ETE (2 μmol/L) for various times at 37°C in the absence (A) or presence (B) of thrombin (1 U/mL). 5-HETE (•), 5-oxo-12-HETE (▿; 5o-12h), and 8-trans-5-oxo-12-HETE (▵; 8t-5o-12h), and 5S,12S-diHETE (▪; 5S,12S) were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods. All values are means ± SE of determinations on platelets from four different donors.
Time course for the formation of 5-oxo-ETE metabolites by resting and activated platelets. Platelets (2 × 108/mL) were incubated with 5-oxo-ETE (2 μmol/L) for various times at 37°C in the absence (A) or presence (B) of thrombin (1 U/mL). 5-HETE (•), 5-oxo-12-HETE (▿; 5o-12h), and 8-trans-5-oxo-12-HETE (▵; 8t-5o-12h), and 5S,12S-diHETE (▪; 5S,12S) were analyzed by precolumn extraction/RP-HPLC as described in Materials and Methods. All values are means ± SE of determinations on platelets from four different donors.
The effects of incubation of platelets with A23187 or thrombin for 10 minutes on the metabolism of 5-oxo-ETE by the 5-ketoreductase and 12-LO pathways are shown in Fig 6. In agreement with Fig 7, both agents inhibit the formation of 5-ketoreductase metabolites of 5-oxo-ETE, and strongly enhance its conversion to its 12-hydroxy metabolites by 12-LO. Thus stimulated platelets metabolize 5-oxo-ETE principally via the 12-LO pathway.
Effects of 12-hydroxy metabolites of 5-oxo-ETE on calcium mobilization in neutrophils.
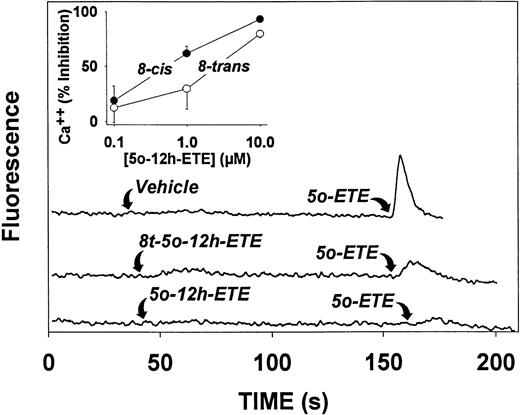
Because 5-oxo-ETE is a potent stimulus of calcium mobilization in neutrophils, we examined the effects of its 12-hydroxy metabolites on this response. Because the 5-oxo-12-hydroxy-ETE isomers absorbed strongly above 300 nm, they interfered with the fluorescence emission of indo-1, the calcium indicator used in our previous studies.19 We therefore used calcium green-1, which has much higher excitation and emission wavelengths. The results we obtained for 5-oxo-ETE with this indicator were identical with those obtained with indo-1. In contrast to 5-oxo-ETE, which stimulates calcium mobilization in neutrophils with an EC50of about 4 nmol/L,23 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE have little effect on cytosolic calcium levels, even at concentrations as high as 10 μmol/L (Fig 8). However, both of these 12-hydroxy metabolites block the effects of 5-oxo-ETE on calcium mobilization in neutrophils. 5-oxo-12-HETE (EC50 0.5 μmol/L) is somewhat more potent than 8-trans-5-oxo-12-HETE (EC50 2.5 μmol/L) in this respect (Fig 8, inset).
Effects of 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE on cytosolic calcium levels in human neutrophils. Neutrophils (3 × 106 cells/mL) loaded with calcium green-1 were treated with either vehicle, 5-oxo-12-HETE (10 μmol/L; 8-cis) or 8-trans-5-oxo-12-HETE (10 μmol/L; 8-trans). Approximately 2 minutes later, 5-oxo-ETE (10 nmol/L) was added. Fluorescence was measured as described in Materials and Methods. The inset shows the inhibitory effects of different concentrations of 5-oxo-12-HETE (•) and 8-trans-5-oxo-12-HETE (○) on calcium mobilization induced by addition of 5-oxo-ETE (10 nmol/L). The values are means ± SE of determinations on platelets from three different donors.
Effects of 5-oxo-12-HETE and 8-trans-5-oxo-12-HETE on cytosolic calcium levels in human neutrophils. Neutrophils (3 × 106 cells/mL) loaded with calcium green-1 were treated with either vehicle, 5-oxo-12-HETE (10 μmol/L; 8-cis) or 8-trans-5-oxo-12-HETE (10 μmol/L; 8-trans). Approximately 2 minutes later, 5-oxo-ETE (10 nmol/L) was added. Fluorescence was measured as described in Materials and Methods. The inset shows the inhibitory effects of different concentrations of 5-oxo-12-HETE (•) and 8-trans-5-oxo-12-HETE (○) on calcium mobilization induced by addition of 5-oxo-ETE (10 nmol/L). The values are means ± SE of determinations on platelets from three different donors.
Effects of 5-oxo-eicosanoids on platelet aggregation.
The effects of 5-oxo-ETE, 5-oxo-12-HETE, and 8-trans-5-oxo-12-HETE on platelet aggregation were investigated. None of these 5-oxo-eicosanoids induced aggregation of washed platelets at concentrations up to 20 μmol/L (Fig 9A). However, 5-oxo-ETE (20 μmol/L) completely prevented aggregation induced by a maximally effective concentration (ca. 3 μmol/L) of arachidonic acid (Fig 9A). 5-oxo-12-HETE (20 μmol/L) also inhibited arachidonate-induced aggregation, but only by about 50%. Its 8-trans isomer was ineffective. The effects of 5-oxo-ETE and 5-oxo-12-HETE were concentration dependent, with much smaller effects observed at concentrations of 10 μmol/L, and little or no effect at 3 μmol/L (Fig 9B).
Effects of 5-oxo-eicosanoids on platelet aggregation. (A) The effects of vehicle (veh; 2.5 μL DMSO), 5-oxo-ETE (5o), 5-oxo-12-HETE (cis), and 8-trans-5-oxo-12-HETE (tr) on platelet aggregation induced by a maximally effective concentration of arachidonic acid (AA; 4 μmol/L), added 2 minutes later. The ticks on the bar below the tracings are 1 minute apart. (B) Concentration-response curves for the effects of 5-oxo-ETE (•; 5o-ETE), 5-oxo-12-HETE (○; 5o-12h [cis]), and 8-trans-5-oxo-12-HETE (▪; 5o-12h [tr]) on platelet aggregation induced by the lowest maximally effective concentration of arachidonic acid (2 to 4 μmol/L). All values are means ± SE of determinations on platelets from three different donors.
Effects of 5-oxo-eicosanoids on platelet aggregation. (A) The effects of vehicle (veh; 2.5 μL DMSO), 5-oxo-ETE (5o), 5-oxo-12-HETE (cis), and 8-trans-5-oxo-12-HETE (tr) on platelet aggregation induced by a maximally effective concentration of arachidonic acid (AA; 4 μmol/L), added 2 minutes later. The ticks on the bar below the tracings are 1 minute apart. (B) Concentration-response curves for the effects of 5-oxo-ETE (•; 5o-ETE), 5-oxo-12-HETE (○; 5o-12h [cis]), and 8-trans-5-oxo-12-HETE (▪; 5o-12h [tr]) on platelet aggregation induced by the lowest maximally effective concentration of arachidonic acid (2 to 4 μmol/L). All values are means ± SE of determinations on platelets from three different donors.
DISCUSSION
Platelets possess 5-hydroxyeicosanoid dehydrogenase activity and can thus convert 5-HETE and related eicosanoids to their 5-oxo metabolites. The properties of this enzyme are very similar to those of the corresponding enzyme that we previously identified in neutrophils.3 The enzyme is localized in the microsomal fraction of both neutrophils and platelets and requires NADP+ as a cofactor. It can oxidize 5-HETE and other 5-hydroxyeicosanoids in which the 5-hydroxyl group is followed by a 6-trans double bond. However, it cannot oxidize LTB4, which has a 6-cis double bond.
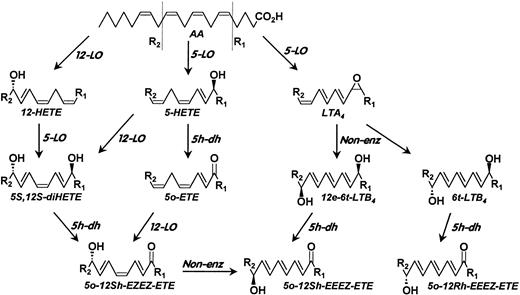
The presence of 5-hydroxyeicosanoid dehydrogenase in platelets raised the possibility that these cells could convert neutrophil-derived 5-HETE to 5-oxo-ETE by transcellular metabolism. However, mixtures of platelets and neutrophils synthesized relatively small amounts of 5-oxo-ETE, but instead formed substantial amounts of its 12-hydroxy metabolites. There are several pathways for the formation of the two 5-oxo-12-hydroxy metabolites, as shown in Fig 10. The 8-cis isomer, which is normally the more abundant of the two, can be formed either by oxidation of 5S,12S-diHETE by 5-hydroxyeicosanoid dehydrogenase or by the action of 12-LO on 5-oxo-ETE. The 8-trans isomer is probably a mixture of 12R-hydroxy and 12S-hydroxy epimers, formed by the oxidation of 6-trans-LTB4 or 12-epi-6-trans-LTB4 by 5-hydroxyeicosanoid dehydrogenase. Because the two 5-oxo-12-hydroxy-8-trans isomers possess only one chiral center, they would not be separated by RP-HPLC. In agreement with this, stimulated neutrophils alone synthesized very small amounts of 8-trans-5-oxo-12(RS)-HETE but not the corresponding 8-cis isomer (Fig3B). It is likely that some of the 8-trans-5-oxo-12-HETE formed in the presence of platelets was formed nonenzymatically from the 8-cis isomer, because the latter substance is rather unstable and can decompose to the trans form under certain conditions. We have also detected the formation of 8-trans-5-oxo-ETE in incubations with neutrophils,23 and it is also possible that isomerization, either enzymatic or nonenzymatic, of the 8-cis double bond of 5-oxo-ETE precedes the formation of the 12-hydroxy metabolite.
Scheme for the formation of 5-oxo-12S-HETE and 8-trans-5-oxo-12(RS)-HETE by mixtures of platelets and neutrophils. Arachidonic acid (AA) is converted to 12-HETE by 12-LO and to 5-HETE and LTA4 by 5-LO. 12-HETE and 5-HETE can be converted to 5S,12S-diHETE by neutrophil 5-LO or platelet 12-LO, respectively, whereas 5-HETE can also be converted to 5-oxo-ETE by 5-hydroxyeicosanoid dehydrogenase (5h-dh) in neutrophils or platelets. 5-oxo-12S-HETE can be formed either by the action of 5h-dh on 5S,12S-diHETE or the actions of 12-LO on 5-oxo-ETE. The 8-trans isomers of 5-oxo-12-HETE can be formed by the actions of 5h-dh on 6-trans-LTB4, which is converted to 8-trans-5-oxo-12R-HETE, and 12-epi-6-trans-LTB4, which is converted to 8-trans-5-oxo-12R-HETE.
Scheme for the formation of 5-oxo-12S-HETE and 8-trans-5-oxo-12(RS)-HETE by mixtures of platelets and neutrophils. Arachidonic acid (AA) is converted to 12-HETE by 12-LO and to 5-HETE and LTA4 by 5-LO. 12-HETE and 5-HETE can be converted to 5S,12S-diHETE by neutrophil 5-LO or platelet 12-LO, respectively, whereas 5-HETE can also be converted to 5-oxo-ETE by 5-hydroxyeicosanoid dehydrogenase (5h-dh) in neutrophils or platelets. 5-oxo-12S-HETE can be formed either by the action of 5h-dh on 5S,12S-diHETE or the actions of 12-LO on 5-oxo-ETE. The 8-trans isomers of 5-oxo-12-HETE can be formed by the actions of 5h-dh on 6-trans-LTB4, which is converted to 8-trans-5-oxo-12R-HETE, and 12-epi-6-trans-LTB4, which is converted to 8-trans-5-oxo-12R-HETE.
It is clear that the total amounts of 5-oxo-eicosanoids formed by stimulated neutrophils increased considerably as increasing numbers of platelets were added. This is likely to be caused at least in part by the presence of 5-hydroxyeicosanoid dehydrogenase in platelets. However, addition of platelets to neutrophils also increased the amounts of 5-HETE that were formed (Fig 3B, inset), and this could have resulted in increased 5-oxo-eicosanoid formation by both neutrophils and platelets. The stimulatory effect of platelets on neutrophil 5-HETE formation could have been because of the utilization of platelet-derived arachidonic acid by neutrophil 5-LO. It has been shown that when platelets and neutrophils are coincubated and stimulated, platelets release arachidonic acid, which is converted to 5-HETE and other lipoxygenase products by neutrophils.14,37 Stimulated platelets could also increase the formation of 5-LO products through the release of 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid, which, along with other hydroperoxy fatty acids, activates this enzyme.38 In contrast to the production of 5-HETE, the production of LTB4 was not enhanced, and was perhaps even diminished, by the presence of platelets. The difference in the effects of platelets on 5-HETE and LTB4 formation could possibly be caused by the existence of different pools of 5-LO in neutrophils, because it was previously shown that platelet-derived arachidonic acid was used preferentially for the synthesis of 5-HETE rather than LTB4.39 Platelets could also divert LTA4, released by neutrophils,33 to cysteinyl-LTs, because they possess LTC synthase activity,10-12 as well as to lipoxins.37 40
Platelets can interact with neutrophils in multiple ways to form eicosanoids by transcellular metabolism. They possess two enzymes that are specific for 5-LO products, namely LTC synthase, and, as shown in the present study, 5-hydroxyeicosanoid dehydrogenase, which interconverts 5-HETE and 5-oxo-ETE. In addition, platelets can metabolize a variety of 5-LO products by the 12-LO pathway. 5-HETE is converted to 5S,12S-diHETE by this enzyme,41 whereas LTA4 is converted to lipoxins.42 As shown here, 5-oxo-ETE is also a substrate for platelet 12-LO.
To determine whether the major 5-oxo-ETE metabolites formed by activated platelets are biologically active, their effects on calcium mobilization in neutrophils were tested. As shown in Fig 8, neither 5-oxo-12-HETE nor 8-trans-5-oxo-12-HETE at concentrations as high as 10 μmol/L has a significant effect on cytosolic calcium levels in neutrophils. In contrast, concentrations of 5-oxo-ETE as low as 1 nmol/L mobilize calcium in these cells.19 Thus, addition of a 12-hydroxyl group and replacement of the 11-cis double bond of 5-oxo-ETE with a 10-trans double bond result in a loss of potency of over 10,000-fold. This adds support to the concept that neutrophils possess a highly specific recognition mechanism for 5-oxo-ETE. We19,23 and others43 have shown that relatively minor changes in the structure of 5-oxo-ETE result in substantial losses in biological activity. This, along with evidence implicating the involvement of a G-protein in 5-oxo-ETE-induced responses,22-24 suggests that the actions of this substance are mediated by its interaction with a specific G-protein coupled 7-transmembrane domain receptor.
Although both 5-oxo-12-HETE and its 8-trans isomer lack significant agonist activity, both substances seem to have some antagonist activity, because they were able to substantially reduce 5-oxo-ETE–induced calcium mobilization. In the case of the 8-cis derivative, the calcium response to 5-oxo-ETE was virtually abolished at a concentration of 10 μmol/L and reduced by over 50% at a concentration of 1 μmol/L. These results raise the possibility that it may be feasible to develop specific antagonists to 5-oxo-ETE that could be used to investigate its physiological role.
In contrast to its potent effects on neutrophils and eosinophils, 5-oxo-ETE had only a very modest effect on human platelets. None of the 5-oxo-eicosanoids synthesized by platelets induced platelet aggregation at concentrations up to 20 μmol/L. However, relatively high concentrations of 5-oxo-ETE and 5-oxo-12-HETE inhibited arachidonate-induced aggregation. It is interesting that there was some structural specificity for this inhibitory response, because 8-trans-5-oxo-12-HETE was ineffective. However, the high concentrations of 5-oxo-eicosanoids required for this response would not support a physiological role for these compounds in regulating platelet function, and it is likely that they are more relevant to granulocytes.
It would seem unlikely that the role of 5-hydroxyeicosanoid dehydrogenase in platelets is to synthesize 5-oxo-ETE. Although platelets possess a high capacity to synthesize this compound, as shown by the experiments with PMS shown in Figs 4 and 5, this capacity may not be realized under normal circumstances. Unstimulated platelets synthesize relatively small amounts of 5-oxo-ETE, presumably because insufficient levels of NADP+ are available in the resting state. Thus, even if platelets were exposed to 5-HETE released by inflammatory cells, little 5-oxo-ETE would be formed unless the platelets were stimulated. Although increased amounts of 5-oxo-eicosanoids are formed by stimulated platelets, this also results in the activation of 12-LO, which converts 5-oxo-ETE to its biologically inactive 12-hydroxy metabolites, which, if present in high concentrations, could antagonize the effects of 5-oxo-ETE. Thus it would seem unlikely that platelets could be a significant source of biologically active 5-oxo-ETE under physiological conditions.
The small amounts of 5-oxo-ETE formed by platelets could be caused by the reversibility of 5-hydroxyeicosanoid dehydrogenase, which would explain the high 5-ketoreductase activity observed in these cells (Fig6). Thus, when unstimulated platelets are presented with either 5-oxo-ETE or 5-HETE, the equilibrium would always be on the side of 5-HETE. We have previously shown that neutrophil microsomes stereospecifically convert 5-oxo-ETE to 5S-HETE in the presence of NADPH.3 It is therefore possible that platelet 5-hydroxyeicosanoid dehydrogenase serves to inactivate 5-oxo-ETE by converting it to 5-HETE, which has only about 1% of the biological potency of the former substance.19 Thus, resting platelets could inactivate 5-oxo-ETE by converting it to 5-HETE, whereas stimulated platelets could inactivate it by converting it to 5-oxo-12-HETE.
In conclusion, we have shown that platelets possess 5-hydroxyeicosanoid dehydrogenase, which displays properties similar to the neutrophil enzyme. In spite of this, intact resting platelets form relatively little 5-oxo-ETE and instead may use this enzyme as a 5-ketoreductase to inactivate 5-oxo-ETE released by inflammatory cells. Activated platelets convert 5-oxo-ETE to biologically inactive 12-hydroxy metabolites, which at high concentrations seem to act as 5-oxo-ETE antagonists. Thus, platelets may be a site for the biological inactivation of 5-oxo-ETE in blood.
ACKNOWLEDGMENT
We are grateful to Dr Daniel Boismenu, Biomedical Mass Spectrometry Unit, McGill University, for assistance with the mass spectrometry.
Supported by the Medical Research Council of Canada (W.S.P.; grant no. MT-6254), the Quebec Heart and Stroke Foundation (W.S.P.), the J.T. Costello Memorial Research Fund, and the National Institutes of Health (J.R.; grant no. DK44730). J.R. also wishes to acknowledge the National Science Foundation for an AMX-360 NMR instrument (grant no. CHE-90-13145).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to William S. Powell, PhD, Meakins-Christie Laboratories, 3626 St Urbain St, Montreal, Quebec, Canada H2X 2P2; e-mail: Bill@Meakins.LAN.McGill.ca.

![Fig. 9. Effects of 5-oxo-eicosanoids on platelet aggregation. (A) The effects of vehicle (veh; 2.5 μL DMSO), 5-oxo-ETE (5o), 5-oxo-12-HETE (cis), and 8-trans-5-oxo-12-HETE (tr) on platelet aggregation induced by a maximally effective concentration of arachidonic acid (AA; 4 μmol/L), added 2 minutes later. The ticks on the bar below the tracings are 1 minute apart. (B) Concentration-response curves for the effects of 5-oxo-ETE (•; 5o-ETE), 5-oxo-12-HETE (○; 5o-12h [cis]), and 8-trans-5-oxo-12-HETE (▪; 5o-12h [tr]) on platelet aggregation induced by the lowest maximally effective concentration of arachidonic acid (2 to 4 μmol/L). All values are means ± SE of determinations on platelets from three different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1086/5/m_blod40309009x.jpeg?Expires=1763465752&Signature=EHw05-fvKpDL5mR8Pn5pSGWcFyoCK1nP7PEnK3B5YiKX5XOse3LihEA1BjlsNqNWObkvhbKY8-cEJaWuBOIw5QitfCHTNwaRgaM24Y4O~Sborj-9OHbZ~DUPBGPcYwPAMEkDOt-7y4tLbSja9fvMETITzxHSY58cqKBmPi0lFR-TreXfSvFJ-UFzjlI0XaGmQ~tYLsQyzEi3zUIFMv5YYleNkR8AS6OBFI-HBSE60TPCTuadMcGSRBDFoHFGH-hG~4fmNBurz4z3m9P0IUa5phM-g-~sbMc2lZjLP02vTkEBvSDGB~8AM7wImmoVwfYmDKEm54QgwUoW6FVgd7S4CQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal