Abstract
In human immunodeficiency virus (HIV)-1 infection, decrease of telomere length is mainly found in CD8+ T cells and not in CD4+ T cells. Telomerase, a ribonucleoprotein enzyme that can synthesize telomeric sequence onto chromosomal ends, can compensate for telomere loss. Here, we investigated if telomerase activity could explain differential telomere loss of CD4+and CD8+ T cells in HIV-1 infection. Telomerase activity was higher in CD8+ than in CD4+ T cells from HIV-infected patients, but still in the same range as in healthy controls, and upregulation after stimulation was comparable to normal. Telomerase activity in lymph node CD4+ and CD8+ T cells from HIV-infected patients was in the same range as that in CD4+ and CD8+ T cells from peripheral blood (PB) and was normal in unseparated bone marrow cells. Thus, our study did not provide evidence for compartmentalized elongation of telomeres in HIV infection. In patients treated with reverse transcriptase inhibitors, telomerase activity was inhibited, but this did not lead to accelerated loss of telomere length in vivo. Thus, differential telomere loss in CD4+ and CD8+ T cells in HIV-1 infection cannot be explained by telomerase activity.
HUMAN IMMUNODEFICIENCY virus (HIV-1) infection is characterized by a decrease in CD4+ T-cell numbers, an increase in CD8+ T-cell numbers, and progressive immune dysfunction eventually leading to acquired immunodeficiency syndrome (AIDS). It has been proposed that progression to AIDS is related to exhaustion of CD4+ T-cell renewal capacity due to persistently enhanced CD4+ T-cell turnover.1 However, longitudinal analysis of CD4+ T-cell telomere length showed that telomere loss was not accelerated in the course of HIV-1 infection, suggesting that turnover of CD4+ T cells might not be increased in HIV infection.2 Telomeres are the extreme ends of chromosomes consisting of TTAGGG repeats, which shorten progressively during cell division in vitro and with aging.3-5 Telomerase, a ribonucleoprotein enzyme that synthesizes telomeric DNA repeats onto chromosomal ends, can compensate for telomere shortening.6In germline cells and immortalized cells, telomerase activity is high and telomeres do not shorten.4,7 Initially, telomerase activity was only found in neoplastic somatic cells.8 More recently, several reports showed that hematopoietic cells have low telomerase activity, which can be upregulated by cell activation.9-12 However, after activation in vitro, increase of telomerase activity was transient and did not prevent telomere shortening in long-term culture.13,14 It has been suggested that telomerase provides partial compensation for telomere loss during cell division.12 Therefore, changes in telomere length might be caused by proliferation, but could also be related to altered telomerase activity. We and others previously addressed the question whether normal telomere length in CD4+ T cells from HIV-infected individuals could be due to increased telomerase activity.2 15 Here, we compared telomerase activity in lymphocyte subsets from HIV-infected individuals with controls and studied upregulation, inhibition by antiviral therapy with reverse transcriptase inhibitors, and telomerase activity in tissues from HIV-infected individuals.
MATERIALS AND METHODS
Patients.
For telomerase activity in CD4+ and CD8+ T-cell subsets, sequential cryopreserved samples were used from HIV-infected individuals participating in the Amsterdam cohort study on HIV infection in homosexual men. Patient clinical and laboratory characteristics are described in Table 1. To study telomerase activity during treatment, eight HIV-infected cohort participants treated with azidothymidine (AZT) monotherapy (600 mg/d) or a combination of AZT (600 mg/d) and dideoxyinosine (DDI) (400 mg/d) or dideoxycytidine (DDC) (2.25 mg/d) were selected, of which frozen blood samples were used before and after the start of treatment. Patient clinical and laboratory characteristics are described in Table 2. As controls, frozen blood samples from healthy HIV− homosexual cohort participants and fresh blood samples from laboratory workers were used. Lymphoid tissue and peripheral blood (PB) were obtained from four HIV-infected individuals with CD4+ T-cell counts >400 cells/μL who did not receive treatment at the time of lymph node biopsy. Bone marrow was obtained from four AIDS patients and two HIV−individuals by aspiration for diagnostic reasons.
Cell separation and culture.
Frozen PB mononuclear cells (PBMC) from cohort participants were used either to analyze telomerase activity directly or to purify CD4+ and CD8+ T cells by magnetic separation over columns (MiniMACS; Miltenyi Biotec Inc, Sunnyvale, CA) as described previously.2 For separation in CD45RA+ and CD45RO+ T cells, MiniMACS multisort kit was used for purification of CD4+ T cells according to the manufacturer’s instructions. In brief, after purification, cells were released from the magnetic beads and the cells were further separated in CD45RA+ and CD45RO+fractions by positive selection over columns after a 15-minute incubation with 20 μL magnetic microbeads conjugated with CD45RA, respectively, CD45RO monoclonal antibodies (MoAbs) per 107cells. Purity was checked by fluorescence-activated cell sorting (FACS) staining. To analyze upregulation of telomerase activity, PBMC were stimulated for 4 days with CD3 MoAb (CLB T3/4.E 1:1,000 final dilution of ascites) in Iscove’s modified Dulbecco’s medium (IMDM) complemented with 10% fetal calf serum, 2-mercaptoethanol, and antibiotics. After stimulation, PBMC were purified for CD4+and CD8+ T cells by magnetic separation over columns (MiniMACS; Miltenyi Biotec Inc) and used for extract preparation.
Cells from tissues.
Lymphocytes from lymph nodes and bone marrow were purified on a Ficoll Hypaque (Pharmacia Biotech, Uppsala, Sweden) gradient. Lymphocytes from lymph nodes were separated in CD4+ and CD8+ T cells by sorting on a FACSVantage (Becton Dickinson, San Jose, CA) with a combination of CD3 and CD4 MoAbs or CD3 and CD8 MoAbs (Pharmingen, Hamburg, Germany). Cells were frozen until further use.
Telomeric repeat amplification protocol (TRAP) assay.
For detection of telomerase activity, a modification of the TRAP assay8 was used. PBMC or purified lymphocytes (1 × 106 to 2 × 106) were washed once with phosphate-buffered saline (PBS) and once with ice-cold wash buffer (10 mmol/L HEPES-KOH [pH, 7.5], 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L dithiothreitol), and resuspended in 100 μL/1 × 106 cells of ice-cold lysis buffer (0.5% CHAPS, 10 mmol/L Tris/HCl [pH, 7.5], 1 mmol/L MgCl2, 1 mmol/L EGTA, 10% glycerol, 5 mmol/L β-mercaptoethanol, 0.1 mmol/L phenylmethylsulfonylamide), incubated on ice for 25 minutes, centrifuged at 12,000g for 20 minutes, and the supernatant was quick-frozen in liquid nitrogen and stored at −70°C. Cell extracts (10 to 15 μL), corresponding to 100,000 to 150,000 cells, were diluted by end-point dilution. Three subsequent extract dilutions were incubated for 30 minutes at room temperature (RT) in a 50-μL reaction mixture containing 50 μmol/L each of 2′deoxynucleoside triphosphate (dNTP), 0.1 μg of TS primer, 0.2 μL [α-32P]deoxycytidine triphosphate (dCTP) (10 μCi/μL, 3,000 Ci/mmol), 3 U Taq polymerase (Promega, Leiden, The Netherlands), 20 mmol/L Tris/HCl (pH, 8.3), 1.5 mmol/L MgCl2, 63 mmol/L KCl, 1 mmol/L EGTA, and 0.1 mg/mL bovine serum albumin (BSA). After 30 minutes, 0.1 μg of CX primer was added, and polymerase chain reaction (PCR) was performed as follows: 90 seconds 90°C, and 34 cycles 30 seconds 94°C, 30 seconds 50°C, 90 seconds 72°C. The PCR product was electrophoresed on a 12.5% nondenaturing polyacrylamide gel in 0.5× TBE. Gels were fixed in 50% ethanol (vol/vol), 50 mmol/L NaCl, and 40 mmol/L NaAc, and dried. Films (Kodak, Rochester, NY) were exposed overnight (O/N). Telomerase activity was expressed as the percentage of telomerase activity in a telomerase-positive immortalized cell line.9 The minimum number of cell equivalents of the lung carcinoma cell line GLC4 needed for specific telomerase activity, usually derived from 13 cells, was taken as 100%. The cell equivalent of the last positive signal in the diluted extracts of the sample was expressed as a percentage of this. For example, the last positive signal in a sample of 10 cell equivalents represents 130% activity, and in a sample of 10,000 cell equivalents, it represents 0.13% activity. A sample was considered positive when a fragment of the typical 6-bp ladder was still present. When samples were repeatedly measured, results could differ a factor of 10. To confirm that the PCR products were the product of telomerase activity, samples were treated with 0.5 μg RNase at 37°C for 15 minutes, which resulted in abolishment of the specific signal. A modification of the Internal Telomerase Assay Standard (M-ITAS)16 was used to exclude inhibitors of Taq polymerase in the samples, which could lead to artificially low telomerase activity in the sample.
Nonradioisotopic and semiquantitative TRAP assay.
Because the original TRAP assay as described above is limited in quantitating the telomerase activity, samples were reanalyzed in a TRAP assay using fluorescence-labeled primers and an automatic sequencer where indicated.17 18 Telomerase products were represented by fluorescent curves, and the peak height, peak area, and size (bp) were calculated automatically by the Fragment Manager computer program (ALF, Pharmacia Biotech). To obtain semiquantitative levels of telomerase activity, the M-ITAS (5 attogram) was included in the TRAP buffer. As a standard, the GLC4 cell line was used. Peaks representing telomerase activity in GLC4equivalents were summed, then relatively expressed to telomerase activity of 100 GLC4 cell equivalents (set at 100%) and normalized to the M-ITAS signal. For the samples (10,000 to 100,000 cell equivalents), the peaks representing the telomerase activity were summed, normalized to the M-ITAS, and correlated to the GLC4 cell number. For example, if a sample of 10,000 cell equivalents is comparable to 10 GLC4 cells, the activity is 0.1%. After heat inactivation (10 minutes, 85°C) telomerase activity peaks disappeared, while the M-ITAS was still detected, indicating that the peaks are representing telomerase activity. The assay is highly reproducible (variation <20%).
Determination of telomeric restriction fragment (TRF) length.
TRF length was analyzed in patients treated with nucleoside analogs. DNA was isolated from 1 × 106 PBMC by the Qiagen Blood and Body Fluid Protocol according to manufacturer’s instructions (Qiagen, Hilden, Germany). Genomic DNA (5 μg) was digested with 40 U of HinfI and Rsa I (GIBCO Life Technologies, Breda, The Netherlands). The digested DNA was electrophoresed on 0.6% agarose gels (50 mA, 24 hours). Gels were then denatured in 0.25 N HCl and neutralized in 0.4 N NaOH/0.6 mol/L NaCl, and blotted to Genescreen plus (DuPont NEN, Brussels, Belgium) in 0.5 N NaOH/1.5 mol/L NaCl. Blots were washed twice in 2× saline-saturated citrate (SSC) and cross-linked (UV Stratalinker; Stratagene, Leusden, The Netherlands). The telomeric probe (TTAGGG)5 was radiolabeled with α-32P-dCTP using terminal transferase (Boehringer Mannheim, Almere, The Netherlands). Hybridization was at 65°C in 0.5 mol/L Na2HPO4/7% sodium dodecyl sulfate (SDS) (pH, 7.2). Blots were washed in buffer with decreasing salt concentration starting with 3× SSC/0.5% SDS onto a final concentration of 0.1× SSC/0.5% SDS (15 minutes at 65°C). Blots were exposed to Phosphor-Imager screens (Fuji, Kanagawa, Japan) for 4 hours or O/N, and mean telomere length was analyzed by Phosphor-Imager software (TINA, Raytest Company, Straubenhardt, Germany), which calculates the integrated signal of the area above the background. The mean value in kilobases was calculated using the molecular weight marker Lamda/HindIII.
RESULTS
Telomerase activity in blood T lymphocytes from HIV-infected individuals.
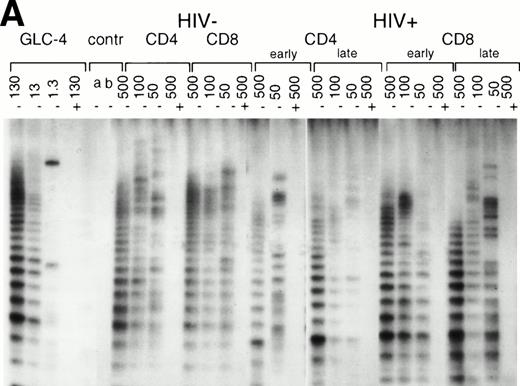
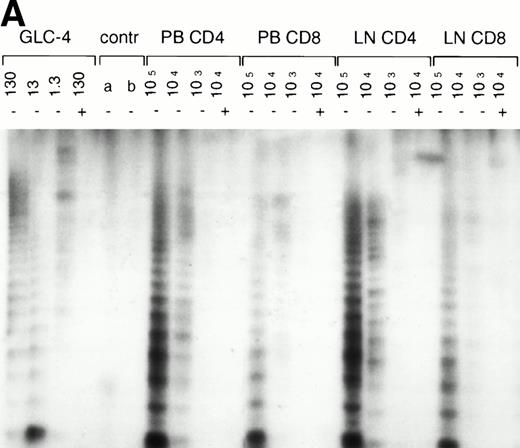
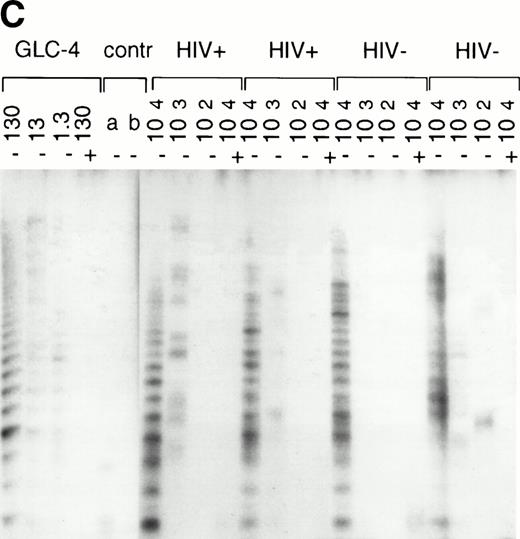
In normal human T cells, low to undetectable levels of telomerase activity are found.9-12 In healthy controls, telomerase activity in extracts from PBMC (n = 8, data not shown) and purified CD4+ and CD8+ T cells (n = 3, Table 1 and Fig 1A) was less than 1% of activity in the GLC4 cell line. To assess telomerase activity changes during HIV-1 infection, we analyzed telomerase activity in purified CD4+ and CD8+ T cells from seven HIV-infected individuals in the first year after seroconversion (SC) and 3 to 9 years after seroconversion (Table 1, Fig1A). The upper four patients remained asymptomatic during follow-up, while the lower three patients progressed to AIDS 3 to 5 years after SC. In the HIV-infected individuals both early and late in infection, telomerase activity in CD4+ T cells and CD8+ T cells was in the same range as that of healthy controls (Mann-Whitney U test, P > .4). During the course of infection, there was no consistent change of telomerase activity in CD4+ or CD8+ T cells (Wilcoxon matched-pairs signed-ranks test,P > .6). However, in HIV-infected individuals, telomerase activity in CD8+ T cells was significantly higher than telomerase activity in CD4+ T cells (Wilcoxon matched-pairs signed-ranks test, P = .016). Furthermore, telomerase activity was analyzed in purified CD45RA+ (naive) and CD45RO+ (memory) CD4+ T-cell subsets obtained from fresh blood samples of seven asymptomatic HIV-infected individuals (CD4+ T-cell counts >300 cells/μL and viral load <4 log copies/mL). These data confirmed that in HIV-1 infection telomerase activity in CD4+ T-cell subsets was in the same range as in healthy controls (Fig 1B and 1C).
Patient Clinical and Laboratory Characteristics and Telomerase Activity
| Patients . | Time (yr after SC) . | CD4 (/μL) . | RNA Load (log copies per mL) . | Telomerase Activity . | Antiviral Treatment . | Viral Phenotype . | |
|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | ||||||
| (%) . | |||||||
| H-1171 | 0.5 | 830 | 3.7 | 0.09 | 0.87 | NSI | |
| H-1171 | 8.0 | 540 | 3.0 | 0 | 0.09 | NSI | |
| H-658 | 0.1 | 950 | 6 | ND | 0.87 | NSI | |
| H-658 | 4.2 | 810 | 3 | 0.09 | 0.09 | NSI | |
| H-1024 | 0.1 | 900 | 5.5 | 0.13 | 1.3 | NSI | |
| H-1024 | 7.5 | 410 | 4.0 | 0.13 | 0.13 | NSI | |
| H-232 | 0.8 | 390 | 5.6 | 0.009 | 0.009 | NSI | |
| H-232 | 9.5 | 320 | 4.5 | 0.09 | 0.87 | AZT | NSI |
| H-412 | 0.7 | 990 | 4.8 | 0.04 | 0.2 | NSI | |
| H-412 | 4.3 | 490 | 4.8 | 0.2 | 0.2 | NSI | |
| H-39 | −1.2 | 1,160 | 0.009 | 0.09 | |||
| H-39 | 2.9 | 230 | 5.9 | 0.09 | 0.87 | SI | |
| H-8317 | 0.7 | 550 | 5.7 | 0.2 | 0.2 | NSI | |
| H-8317 | 3.6 | 50 | 5.9 | 0.04 | 0.04 | AZT | SI |
| Mean (*) early | 0.08 (0.07) | 0.51 (0.50) | |||||
| Mean (*) late | 0.09 (0.06) | 0.32 (0.37) | |||||
| Control 1 | 0.2 | 0.2 | |||||
| Control 2 | 0.13 | 0.13 | |||||
| Control 3 | 0.009 | 0.09 | |||||
| Mean (*) | 0.11 (0.09) | 0.14 (0.06) | |||||
| Patients . | Time (yr after SC) . | CD4 (/μL) . | RNA Load (log copies per mL) . | Telomerase Activity . | Antiviral Treatment . | Viral Phenotype . | |
|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | ||||||
| (%) . | |||||||
| H-1171 | 0.5 | 830 | 3.7 | 0.09 | 0.87 | NSI | |
| H-1171 | 8.0 | 540 | 3.0 | 0 | 0.09 | NSI | |
| H-658 | 0.1 | 950 | 6 | ND | 0.87 | NSI | |
| H-658 | 4.2 | 810 | 3 | 0.09 | 0.09 | NSI | |
| H-1024 | 0.1 | 900 | 5.5 | 0.13 | 1.3 | NSI | |
| H-1024 | 7.5 | 410 | 4.0 | 0.13 | 0.13 | NSI | |
| H-232 | 0.8 | 390 | 5.6 | 0.009 | 0.009 | NSI | |
| H-232 | 9.5 | 320 | 4.5 | 0.09 | 0.87 | AZT | NSI |
| H-412 | 0.7 | 990 | 4.8 | 0.04 | 0.2 | NSI | |
| H-412 | 4.3 | 490 | 4.8 | 0.2 | 0.2 | NSI | |
| H-39 | −1.2 | 1,160 | 0.009 | 0.09 | |||
| H-39 | 2.9 | 230 | 5.9 | 0.09 | 0.87 | SI | |
| H-8317 | 0.7 | 550 | 5.7 | 0.2 | 0.2 | NSI | |
| H-8317 | 3.6 | 50 | 5.9 | 0.04 | 0.04 | AZT | SI |
| Mean (*) early | 0.08 (0.07) | 0.51 (0.50) | |||||
| Mean (*) late | 0.09 (0.06) | 0.32 (0.37) | |||||
| Control 1 | 0.2 | 0.2 | |||||
| Control 2 | 0.13 | 0.13 | |||||
| Control 3 | 0.009 | 0.09 | |||||
| Mean (*) | 0.11 (0.09) | 0.14 (0.06) | |||||
Abbreviation: ND, not determined; NSI, (non)syncytium-inducing variant.
Standard deviation.
Telomerase activity in CD4+ and CD8+ T cells. Telomerase activity was measured by the TRAP assay and is expressed as a percentage of the activity detected in the lung carcinoma cell line GLC4. (A) Telomerase activity in the original TRAP assay. Telomerase activity in 130, 13, and 1.3 GLC4 cell equivalents is shown at the left. Specific telomerase activity could still be measured in 130 GLC4cell equivalents. Addition of RNAse is indicated (+). Abolishment of the signal confirmed specific telomerase activity. No cell extracts were added to the PCR controls a (lysis buffer only) and b (PCR mix). Cell extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells from one healthy control and one representative HIV-infected person early (0.1 year after seroconversion) and late (7.5 years after seroconversion) in infection were analyzed. Addition of the M-ITAS resulted in specific bands (arrow) indicating that Taq polymerase was not limiting. (B) Telomerase activity in freshly isolated purified naive (CD4RA) and memory (CD4RO) CD4+ T cells from one HIV-infected individual and one healthy control performed as described in (A), but without M-ITAS. (C) Telomerase activity in freshly isolated naive (CD4RA) and memory (CD4RO) CD4+ T cells from six healthy controls and seven HIV-infected individuals. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (D) Representative fluorescence curves showing telomerase activity and M-ITAS (at 145 bp). Lanes 1 and 2, healthy control CD4+ and CD8+ T cells (100,000 cell equivalents); lanes 3 and 4, HIV+ CD4+ T cells early and late (100,000, respectively, 75,000 cell equivalents); lanes 5 and 6, HIV+ CD8+ T cells early and late (identical [id]); lane 7, GLC4 (100 cell equivalents); and lane 8, lysis buffer.
Telomerase activity in CD4+ and CD8+ T cells. Telomerase activity was measured by the TRAP assay and is expressed as a percentage of the activity detected in the lung carcinoma cell line GLC4. (A) Telomerase activity in the original TRAP assay. Telomerase activity in 130, 13, and 1.3 GLC4 cell equivalents is shown at the left. Specific telomerase activity could still be measured in 130 GLC4cell equivalents. Addition of RNAse is indicated (+). Abolishment of the signal confirmed specific telomerase activity. No cell extracts were added to the PCR controls a (lysis buffer only) and b (PCR mix). Cell extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells from one healthy control and one representative HIV-infected person early (0.1 year after seroconversion) and late (7.5 years after seroconversion) in infection were analyzed. Addition of the M-ITAS resulted in specific bands (arrow) indicating that Taq polymerase was not limiting. (B) Telomerase activity in freshly isolated purified naive (CD4RA) and memory (CD4RO) CD4+ T cells from one HIV-infected individual and one healthy control performed as described in (A), but without M-ITAS. (C) Telomerase activity in freshly isolated naive (CD4RA) and memory (CD4RO) CD4+ T cells from six healthy controls and seven HIV-infected individuals. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (D) Representative fluorescence curves showing telomerase activity and M-ITAS (at 145 bp). Lanes 1 and 2, healthy control CD4+ and CD8+ T cells (100,000 cell equivalents); lanes 3 and 4, HIV+ CD4+ T cells early and late (100,000, respectively, 75,000 cell equivalents); lanes 5 and 6, HIV+ CD8+ T cells early and late (identical [id]); lane 7, GLC4 (100 cell equivalents); and lane 8, lysis buffer.
The original method to quantitate telomerase activity in the TRAP assay9 has been modified17 18 so that telomerase activity of the sample can be accurately correlated to the amount of GLC4 cells equaling this activity. Telomerase activity in T cells from patient 1024 and control 2 (Fig 1A) was reanalyzed by this semiquantitative method (Fig 1D). Telomerase activity in T cells from the healthy control was 0.001% (Fig 1D, lanes 1 and 2). In CD4+ T cells from the patient, activity was now 0.01%, which was 10-fold higher than in the control (Fig 1D, lanes 3 and 4). As before, the highest telomerase activity was measured in CD8+ T cells from the HIV-infected individual early in infection (Fig 1D, lane 5, activity 0.08%). In addition, reanalysis by the semiquantitative method showed a twofold difference in telomerase activity between CD45RA+ and CD45RO+CD4+ T cells from a healthy control (0.01% and 0.02%, respectively), while in the HIV-infected individual, telomerase activity was 0.01% for both subsets (data not shown). Furthermore, the amplification of the internal standard (M-ITAS, Fig 1D, right peaks) confirmed that there were no Taq inhibitors present in the cell extracts, which could lead to artificially low telomerase activity in the samples.
Thus, telomerase activity in T cells from HIV-infected individuals is low compared with activity in the GLC4 cell line, and CD4+ T cells differ less than 10-fold in activity compared with healthy controls.
Induction of telomerase activity in blood lymphocytes.
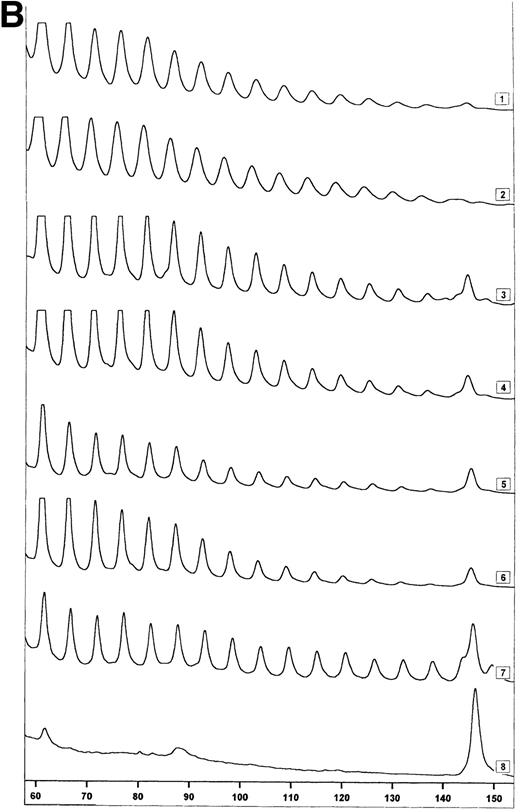
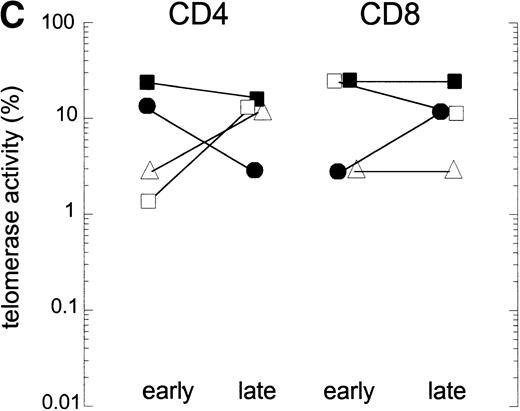
In HIV-1 infection, T-cell dysfunction is already found before CD4+ T-cell numbers decline.19 Therefore, the induction of telomerase activity after cell activation might be impaired in HIV-1 infection. However, if during activation telomerase activity is upregulated to a higher extent, proliferation-induced telomere loss might be compensated. To determine if activation of T cells from HIV-infected individuals induces telomerase activity as described for T cells from healthy controls,9 PBMC were cultured in vitro with CD3 MoAbs. After 4 days of culture, telomerase activity was measured in extracts equivalent to 500, 100, and 50 purified CD4+ and CD8+ T cells (Fig 2A) or 10,000 cell equivalents (Fig2B). Cell stimulation could upregulate telomerase activity in lymphocytes from healthy controls, resulting in telomerase activity on the order of 2% to 30% (Fig 2A and B). In the HIV-infected individual, telomerase upregulation was comparable to the healthy controls in CD8+ T cells and CD4+ T cells early in infection, while CD4+ T cells late in infection showed less activity compared with the healthy control (Fig 2A and B). Differences in upregulation of telomerase activity in lymphocytes early and late in infection were found between HIV-infected individuals (Fig2C), but activity did not exceed the level of activity in healthy controls after stimulation. Furthermore, these differences were not related to T-cell function, which was declining in three of four patients.
Upregulation of telomerase activity in CD4+and CD8+ T cells after in vitro stimulation. PBMC were cultured in vitro for 4 days with CD3 MoAbs and telomerase activity was analyzed in extracts prepared from purified CD4+ and CD8+ T cells. (A) Telomerase activity in 500, 100, and 50 cell equivalents from one healthy control (HIV-) and one representative HIV-positive individual (HIV+) early and late (3 years after seroconversion, 1 year before AIDS) in infection is shown as described in Fig 1A. (B) Representative fluorescence curves showing telomerase activity in activated CD4+ and CD8+ T cells and M-ITAS. Lanes 1 and 2, CD4+ and CD8+ T cells from a second healthy control (10,000 cell equivalents); lanes 3 and 4, CD4+and CD8+ T cells from HIV+ early in infection (id); lanes 5 and 6, CD4+ and CD8+ T cells from HIV+ late in infection (id); lanes 7 and 8, GLC4 (100 cell equivalents) and lysis buffer. (C) Telomerase activity in CD4+ and CD8+ T cells after activation in vitro in four HIV-infected individuals early and late in infection as analyzed with the original TRAP assay.
Upregulation of telomerase activity in CD4+and CD8+ T cells after in vitro stimulation. PBMC were cultured in vitro for 4 days with CD3 MoAbs and telomerase activity was analyzed in extracts prepared from purified CD4+ and CD8+ T cells. (A) Telomerase activity in 500, 100, and 50 cell equivalents from one healthy control (HIV-) and one representative HIV-positive individual (HIV+) early and late (3 years after seroconversion, 1 year before AIDS) in infection is shown as described in Fig 1A. (B) Representative fluorescence curves showing telomerase activity in activated CD4+ and CD8+ T cells and M-ITAS. Lanes 1 and 2, CD4+ and CD8+ T cells from a second healthy control (10,000 cell equivalents); lanes 3 and 4, CD4+and CD8+ T cells from HIV+ early in infection (id); lanes 5 and 6, CD4+ and CD8+ T cells from HIV+ late in infection (id); lanes 7 and 8, GLC4 (100 cell equivalents) and lysis buffer. (C) Telomerase activity in CD4+ and CD8+ T cells after activation in vitro in four HIV-infected individuals early and late in infection as analyzed with the original TRAP assay.
Telomerase activity after treatment with nucleoside analogs.
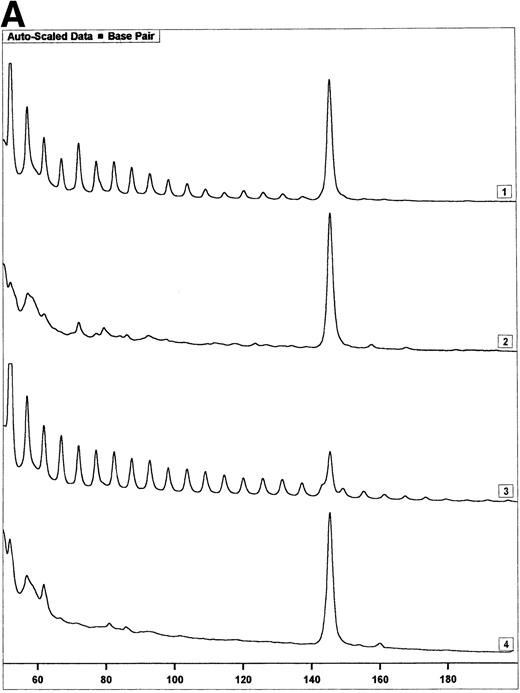
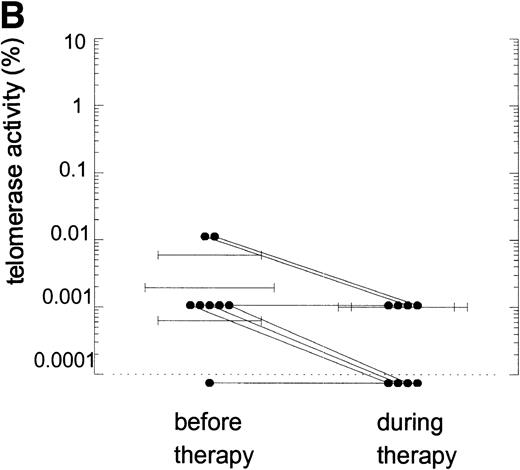
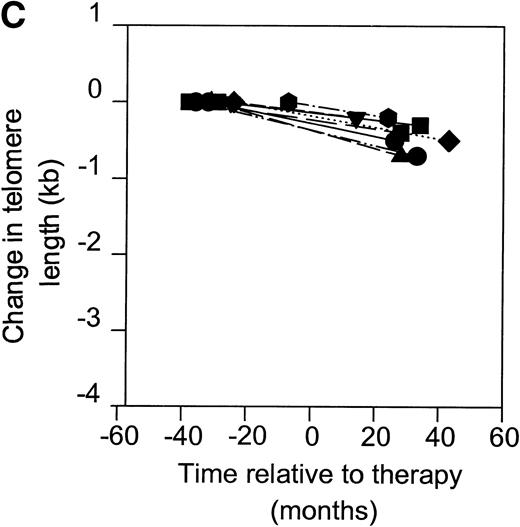
Telomerase is a reverse transcriptase, which uses its own RNA template. It has been described that reverse transcriptase inhibitors inhibit telomerase activity in ciliates and in cultured cell lines in vitro, which caused telomere shortening.20,21 In the group of HIV-infected individuals initially studied, we could not find any relation between treatment with nucleoside analogs and loss of telomere length.2 To address the question if telomerase activity is inhibited by treatment with nucleoside analogs in vivo, we analyzed telomerase activity in PBMC from eight individuals before and around 2 years after the start of treatment with AZT monotherapy or a combination of AZT and DDI or DDC (Table2). Using the semiquantitative analysis, telomerase in PBMC from these HIV-infected individuals before therapy generated a specific banding pattern, which equalled an activity of 0.001% to 0.01% (Fig 3A, lane 1 and Fig 3B). During therapy, the specific banding pattern vanished in two patients (Fig 3A, lane 2, Fig 3B). In three patients, telomerase activity disappeared (Fig 3B). This was not due to Taq inhibitors in the samples or an assay artefact, because the M-ITAS was amplified as expected. No specific differences were found in telomerase activity between patients with a different treatment regimens or different laboratory parameters, although patient groups may have been too small to allow for a good comparison. To see if diminished telomerase activity due to treatment would lead to accelerated loss of telomere length, telomere loss in PBMC was measured over the same time span. However, telomere loss over the follow-up period was within the normal range (<100 bp/yr2) in these treated HIV-infected individuals (Fig 3C).
Nucleoside Analog-Treated Patients’ Clinical and Laboratory Characteristics and Telomerase Activity
| Patients . | Therapy . | Time (mo after treatment) . | CD4 (/μL) . | Viral Load (log copies RNA per mL) . | Telomerase Activity (%) . |
|---|---|---|---|---|---|
| 1 | AZT | −32 | 480 | 3.0 | 0.001 |
| 1 | 26 | 160 | 5.0 | 0 | |
| 2 | AZT | −36 | 180 | 4.1 | 0.01 |
| 2 | 33 | 120 | 3.9 | 0.001 | |
| 3 | AZT/DDC | −31 | 670 | 3.2 | 0.001 |
| 3 | 28 | 410 | 4.1 | 0.001 | |
| 4 | AZT/DDC | −38 | 290 | 4.6 | 0.001 |
| 4 | 28 | 60 | 4.0 | 0 | |
| 5 | AZT/DDC | −25 | 650 | ND | 0.001 |
| 5 | 14 | 140 | ND | 0.001 | |
| 6 | AZT/DDI | −29 | 270 | 3.6 | 0.001 |
| 6 | 34 | 280 | 3.0 | 0 | |
| 7 | AZT/DDI | −7 | 330 | ND | 0.01 |
| 7 | 24 | 90 | ND | 0.001 | |
| 8 | AZT/DDC/DDI | −24 | 390 | 4.4 | 0 |
| 8 | 43 | 20 | 4.3 | 0 |
| Patients . | Therapy . | Time (mo after treatment) . | CD4 (/μL) . | Viral Load (log copies RNA per mL) . | Telomerase Activity (%) . |
|---|---|---|---|---|---|
| 1 | AZT | −32 | 480 | 3.0 | 0.001 |
| 1 | 26 | 160 | 5.0 | 0 | |
| 2 | AZT | −36 | 180 | 4.1 | 0.01 |
| 2 | 33 | 120 | 3.9 | 0.001 | |
| 3 | AZT/DDC | −31 | 670 | 3.2 | 0.001 |
| 3 | 28 | 410 | 4.1 | 0.001 | |
| 4 | AZT/DDC | −38 | 290 | 4.6 | 0.001 |
| 4 | 28 | 60 | 4.0 | 0 | |
| 5 | AZT/DDC | −25 | 650 | ND | 0.001 |
| 5 | 14 | 140 | ND | 0.001 | |
| 6 | AZT/DDI | −29 | 270 | 3.6 | 0.001 |
| 6 | 34 | 280 | 3.0 | 0 | |
| 7 | AZT/DDI | −7 | 330 | ND | 0.01 |
| 7 | 24 | 90 | ND | 0.001 | |
| 8 | AZT/DDC/DDI | −24 | 390 | 4.4 | 0 |
| 8 | 43 | 20 | 4.3 | 0 |
Abbreviation: ND, not detected.
Telomerase activity in PBMC from HIV-infected individuals before and after start of treatment with nucleoside analogs. (A) Representative fluorescence curves showing telomerase activity in 100,000 cell equivalents from patient 2 (Table 2). M-ITAS at 145 bp. Lane 1, before therapy; lane 2, during therapy; lanes 3 and 4, GLC4 (100 cell equivalents) and lysis buffer. (B) Telomerase activity in eight HIV-infected individuals before and after the start of treatment with nucleoside analogs. Telomerase activity was analyzed in PBMC with the semiquantitative method and expressed as percentage of activity in the GLC4 cell line. Lines connect telomerase activity before and during treatment from the same patient. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (C) Telomere length of PBMC from the eight HIV-infected patients at the same time points as in (B). Telomere length was analyzed by Southern blot. Telomere length is expressed as change of telomere length in kilobases over time.
Telomerase activity in PBMC from HIV-infected individuals before and after start of treatment with nucleoside analogs. (A) Representative fluorescence curves showing telomerase activity in 100,000 cell equivalents from patient 2 (Table 2). M-ITAS at 145 bp. Lane 1, before therapy; lane 2, during therapy; lanes 3 and 4, GLC4 (100 cell equivalents) and lysis buffer. (B) Telomerase activity in eight HIV-infected individuals before and after the start of treatment with nucleoside analogs. Telomerase activity was analyzed in PBMC with the semiquantitative method and expressed as percentage of activity in the GLC4 cell line. Lines connect telomerase activity before and during treatment from the same patient. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (C) Telomere length of PBMC from the eight HIV-infected patients at the same time points as in (B). Telomere length was analyzed by Southern blot. Telomere length is expressed as change of telomere length in kilobases over time.
Telomerase activity in lymphoid tissues.
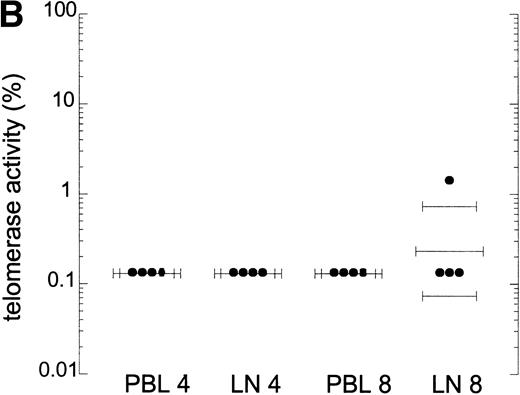
In T-cell progenitors and tonsil T cells, telomerase activity is reported to be higher than in PB T cells.12 Therefore, high turnover of T cells could lead to increased telomerase activity in other compartments than PB. Telomerase activity in CD4+ and CD8+ T cells from lymph nodes was compared with that in PB lymphocytes obtained from four HIV-infected individuals. One representative patient is shown in Fig 4A. Telomerase activity was comparable in blood and lymph node CD4+ and CD8+ T cells (Fig 4A and B). Telomerase activity in lymphoid tissue CD4+ and CD8+ T cells from these patients was similar to that in a HIV− individual (data not shown). In addition, telomerase activity in total bone marrow cells obtained from four AIDS patients and two HIV− controls was comparable, on the order of 1%, which is in agreement with previous reports9 10 (Fig 4C, two patients shown). Extracts from lymph node and bone marrow cells tested in the semiquantitative method did show amplification of the M-ITAS, indicating that there are no Taq inhibitors present in extracts from these tissue cells (data not shown), and telomerase activity measured by this method was on the order of 0.01% to 0.1%.
Telomerase activity in lymph nodes and bone marrow from HIV-infected patients. (A) Telomerase activity in extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells isolated from PB (CD4 and CD8) and lymph node tissue (LN, CD4 and CD8) shown for one representative HIV-infected individual. (B) Telomerase activity in CD4+ and CD8+ T cells isolated from PB and LN from four HIV-infected individuals, as analyzed by the original TRAP assay. (C) Telomerase activity in extracts equivalent to 104, 103, and 102 total bone marrow cells from two HIV-infected patients after AIDS diagnosis and two HIV− controls. Total bone marrow cell samples contained approximately 40% to 45% CD3+ cells, 4% CD19+ cells, and 2% CD34+ cells. In bone marrow samples from the AIDS patients, the percentage of CD4+ cells was decreased (6.5% compared with 15% in HIV− samples) and the percentage CD8+ cells was increased (30% compared with 18%).
Telomerase activity in lymph nodes and bone marrow from HIV-infected patients. (A) Telomerase activity in extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells isolated from PB (CD4 and CD8) and lymph node tissue (LN, CD4 and CD8) shown for one representative HIV-infected individual. (B) Telomerase activity in CD4+ and CD8+ T cells isolated from PB and LN from four HIV-infected individuals, as analyzed by the original TRAP assay. (C) Telomerase activity in extracts equivalent to 104, 103, and 102 total bone marrow cells from two HIV-infected patients after AIDS diagnosis and two HIV− controls. Total bone marrow cell samples contained approximately 40% to 45% CD3+ cells, 4% CD19+ cells, and 2% CD34+ cells. In bone marrow samples from the AIDS patients, the percentage of CD4+ cells was decreased (6.5% compared with 15% in HIV− samples) and the percentage CD8+ cells was increased (30% compared with 18%).
DISCUSSION
It has been found that telomere length in HIV-infected individuals is decreased in CD8+ T cells, but normal or even increased in CD4+ T cells in HIV-1 infected individuals with CD4+ T-cell numbers >200 cells/μL.2 15 Telomerase activity could play a role in compensating proliferation-induced telomere loss in HIV-1 infection, which could explain why high CD4+ T-cell turnover in CD4+ T cells is not reflected in shorter telomeres or accelerated telomere loss. We showed here that this is unlikely to be the case.
First, telomerase activity in T cells from HIV-infected individuals was low compared with activity in the GLC4 cell line and in the same range as that in healthy controls. The original TRAP assay is limited in quantification of telomerase activity because telomerase activity is quantitated by end-point dilution, while in the semiquantitative method, telomerase activity of the sample can be more accurately related to telomerase activity in a specific amount of GLC4 cells. Because of these differences in quantification, one cannot compare the percentages of activities in the different assays. However, when telomerase activity in different samples were compared, the semiquantitative method confirmed that telomerase activity was low in quiescent T cells from HIV-infected individuals and could be upregulated after stimulation (Figs 1 and 2). Now, a difference of 10-fold in telomerase activity between CD4+ T cells from the healthy control and the HIV-infected individual could be shown, and differences of less than 10-fold in telomerase activity between samples could be analyzed (Fig 1). However, the relevance of 10-fold differences in telomerase activity in quiescent cells as calculated semiquantitatively by these methods is not yet clear. A large variation already exists between telomerase activity in T cells from healthy controls. Fluctuations of 10-fold were observed in telomerase activity of CD4+ T cells during the course of infection (Table 1), but no relation to disease progression could be found in this patient group, while telomere length is stable during the course of infection in these patients.2 In addition, a 10-fold difference in telomerase activity was found between samples of different timepoints from a healthy control (data not shown). Furthermore, increases of 10-fold to 100-fold in telomerase activity during progression, as shown in the CD8+ T cells from patients 232 and 39, did not prevent these cells from losing telomere length.2 This indicates that differences in low telomerase activity levels as found in a population of quiescent T cells by the TRAP assay do not correlate with telomere length changes.
Secondly, higher levels of telomerase activity could be measured after stimulation in vitro. These levels of telomerase activity are shown to compensate telomere shortening of cells for a limited amount of population doublings.13 14 In HIV-infected patients, telomerase activity was upregulated after stimulation in vitro, but did not exceed the normal level of activation. Moreover, decreased upregulation of telomerase activity late in infection did not lead to accelerated loss of CD4+ T-cell telomere length, while normal upregulation late in infection did not prevent CD8+T cells from losing telomere length (Table 12).
Thirdly, we could find no indication for localized or compartmentalized elongation of telomeres, as telomerase activity of CD4+ and CD8+ T cells obtained from blood and lymph nodes from HIV-infected individuals was comparable, and telomerase activity in unseparated bone marrow samples from AIDS patients was not increased compared with healthy individuals. However, we can at this time formally not exclude the possibility of high telomerase activity at the single-cell level in precursor cells.
Furthermore, we showed that in patients treated with reverse transcriptase inhibitors, telomerase activity might be inhibited. Such a decrease in telomerase activity is not expected as a result of disease progression as illustrated by the changes in telomerase activity in the patients in Table 1. In addition, telomerase activity in PBMC from four untreated HIV-infected individuals did not change within 3 years of infection (unpublished observation, 1997). Alterations of the normal banding pattern in the TRAP assay is expected in the presence of nucleoside analogs, because the elongation of the TS primer by the reverse transcriptase part of telomerase will be stopped at random by competition of the nucleoside analogs. Analog-specific alterations of the normal banding pattern have been reported when telomerase activity of Tetrahymena thermophilia was assayed in the presence of nucleoside analogs.20 Culturing T-cell lines in the presence of AZT or dideoxyguanine (ddG), however, did result in inhibition of telomerase activity without a disturbed banding pattern.21 Our results showed a disturbed banding pattern in the automatic sequencer (Fig 3A) and a smear in the original TRAP assay (data not shown) when telomerase was assayed in cell extracts from two of the patients treated with nucleoside analogs, while the signal disappeared in three other treated patients. Thus, this indicates that nucleoside analogs given in therapeutic dosage are present in sufficient amounts in cell extracts to inhibit telomerase activity in the TRAP assay, which indicates that the intracellular concentration of the nucleoside analogs is sufficient to inhibit telomerase activity in vivo. Indirect effects of treatment are less likely to play a role because treatment was not potent enough to increase CD4+ T-cell counts and suppress viral load during follow-up. The decreased telomerase activity did not result in accelerated loss of telomere length after 2 years of treatment. Because telomerase antagonists have been proposed as anticancer drugs,22 it might be interesting to study the long-term effects of telomerase inhibition by nucleoside analogs on telomere length and development of AIDS-related malignancies.
In conclusion, telomerase activity and upregulation in HIV-1 infection, which are in the normal range, are not related to telomere length loss and thus cannot explain differential telomere length loss in CD4+ and CD8+ T cells.
ACKNOWLEDGMENT
We thank Dr A. van’t Wout, N. Kootstra, Dr H. Schuitemaker, and M. Westers for providing us with tissue samples; our colleagues at the Municipal Health Service, N. Albrecht and J. Maas, for providing us with fresh blood samples from cohort participants; Dr F. de Wolf and M. Bakker from the Academical Medical Hospital for data on viral load; and Dr G. Pantaleo for stimulating discussions.
Supported by Grant No. 1008 from the Dutch AIDS Foundation and by the Dutch Cancer Foundation. This study was conducted as part of the Amsterdam Cohort Studies on AIDS.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Frank Miedema, PhD, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Department of Clinical Viro-Immunology, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: miedema@clb.nl.

![Fig. 1. Telomerase activity in CD4+ and CD8+ T cells. Telomerase activity was measured by the TRAP assay and is expressed as a percentage of the activity detected in the lung carcinoma cell line GLC4. (A) Telomerase activity in the original TRAP assay. Telomerase activity in 130, 13, and 1.3 GLC4 cell equivalents is shown at the left. Specific telomerase activity could still be measured in 130 GLC4cell equivalents. Addition of RNAse is indicated (+). Abolishment of the signal confirmed specific telomerase activity. No cell extracts were added to the PCR controls a (lysis buffer only) and b (PCR mix). Cell extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells from one healthy control and one representative HIV-infected person early (0.1 year after seroconversion) and late (7.5 years after seroconversion) in infection were analyzed. Addition of the M-ITAS resulted in specific bands (arrow) indicating that Taq polymerase was not limiting. (B) Telomerase activity in freshly isolated purified naive (CD4RA) and memory (CD4RO) CD4+ T cells from one HIV-infected individual and one healthy control performed as described in (A), but without M-ITAS. (C) Telomerase activity in freshly isolated naive (CD4RA) and memory (CD4RO) CD4+ T cells from six healthy controls and seven HIV-infected individuals. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (D) Representative fluorescence curves showing telomerase activity and M-ITAS (at 145 bp). Lanes 1 and 2, healthy control CD4+ and CD8+ T cells (100,000 cell equivalents); lanes 3 and 4, HIV+ CD4+ T cells early and late (100,000, respectively, 75,000 cell equivalents); lanes 5 and 6, HIV+ CD8+ T cells early and late (identical [id]); lane 7, GLC4 (100 cell equivalents); and lane 8, lysis buffer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1011/5/m_blod40305001aw.jpeg?Expires=1769137515&Signature=UDfpKM6rdEkWHxtg1angbOHEneWO~JDBpryY658KPONYG1lOroMk4eLlnX5DGnShtwaNjBmm9Y1IrxRq8WoGoaUfmrZp7CU5gUfOaWh~TmlEG5dNBAVe2P2YHSkgX2PusNme6l-HfUo5GQMwgsK~NpzUBLl745MuZZ7ZwJiQcdSoLMnrqpKUSfKqUlFvi4QC02DI~RdxZBf~6SiyvrEAljmXxYOCnijWvdcT59l7JaG03QfurZg8voxwSyspS2KJeVtnPiKu9YuvQPtKiLiXEdjEz0HYH8BgcQVr8qVpFAg5vY3-~hwinfBe-pt1SkTEhkNK7LBp-HhzDnKVUXTM1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Telomerase activity in CD4+ and CD8+ T cells. Telomerase activity was measured by the TRAP assay and is expressed as a percentage of the activity detected in the lung carcinoma cell line GLC4. (A) Telomerase activity in the original TRAP assay. Telomerase activity in 130, 13, and 1.3 GLC4 cell equivalents is shown at the left. Specific telomerase activity could still be measured in 130 GLC4cell equivalents. Addition of RNAse is indicated (+). Abolishment of the signal confirmed specific telomerase activity. No cell extracts were added to the PCR controls a (lysis buffer only) and b (PCR mix). Cell extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells from one healthy control and one representative HIV-infected person early (0.1 year after seroconversion) and late (7.5 years after seroconversion) in infection were analyzed. Addition of the M-ITAS resulted in specific bands (arrow) indicating that Taq polymerase was not limiting. (B) Telomerase activity in freshly isolated purified naive (CD4RA) and memory (CD4RO) CD4+ T cells from one HIV-infected individual and one healthy control performed as described in (A), but without M-ITAS. (C) Telomerase activity in freshly isolated naive (CD4RA) and memory (CD4RO) CD4+ T cells from six healthy controls and seven HIV-infected individuals. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (D) Representative fluorescence curves showing telomerase activity and M-ITAS (at 145 bp). Lanes 1 and 2, healthy control CD4+ and CD8+ T cells (100,000 cell equivalents); lanes 3 and 4, HIV+ CD4+ T cells early and late (100,000, respectively, 75,000 cell equivalents); lanes 5 and 6, HIV+ CD8+ T cells early and late (identical [id]); lane 7, GLC4 (100 cell equivalents); and lane 8, lysis buffer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1011/5/m_blod40305001cx.jpeg?Expires=1769137515&Signature=SSFaP-AbaEmeivRKUWVjlIqAN178KJPUtscmdJ5i~I-TAxQzttfAiuzqTrIhMDdb96YuWUA5LQNWVDHhahXObu~nvIkGodAMjxyruT3C3OXLB2QRaNNM-e6GvUnFAsYmmbqFIv09UdIIbQY-DW0IeayDaqZb6FhEIXsMOBl1wmZoWMXK8WO8HfYpfSVoKnB0plfH2JUBMWQ1OvMvGhHlSrspuaG-J0H5H3Fiw~a3O2ogwyG2WzW1IMft-kJQj7mOfFjCH6QoXRY0kgFzJNsDC95PKGdAIQhGalILnGtBPVMLVsMB4h0-o1GKVk-UcKC7zOG6Wlm26nqFtrSBsiNZZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Telomerase activity in CD4+ and CD8+ T cells. Telomerase activity was measured by the TRAP assay and is expressed as a percentage of the activity detected in the lung carcinoma cell line GLC4. (A) Telomerase activity in the original TRAP assay. Telomerase activity in 130, 13, and 1.3 GLC4 cell equivalents is shown at the left. Specific telomerase activity could still be measured in 130 GLC4cell equivalents. Addition of RNAse is indicated (+). Abolishment of the signal confirmed specific telomerase activity. No cell extracts were added to the PCR controls a (lysis buffer only) and b (PCR mix). Cell extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells from one healthy control and one representative HIV-infected person early (0.1 year after seroconversion) and late (7.5 years after seroconversion) in infection were analyzed. Addition of the M-ITAS resulted in specific bands (arrow) indicating that Taq polymerase was not limiting. (B) Telomerase activity in freshly isolated purified naive (CD4RA) and memory (CD4RO) CD4+ T cells from one HIV-infected individual and one healthy control performed as described in (A), but without M-ITAS. (C) Telomerase activity in freshly isolated naive (CD4RA) and memory (CD4RO) CD4+ T cells from six healthy controls and seven HIV-infected individuals. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (D) Representative fluorescence curves showing telomerase activity and M-ITAS (at 145 bp). Lanes 1 and 2, healthy control CD4+ and CD8+ T cells (100,000 cell equivalents); lanes 3 and 4, HIV+ CD4+ T cells early and late (100,000, respectively, 75,000 cell equivalents); lanes 5 and 6, HIV+ CD8+ T cells early and late (identical [id]); lane 7, GLC4 (100 cell equivalents); and lane 8, lysis buffer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1011/5/m_blod40305001bw.jpeg?Expires=1769137515&Signature=hWwSrVPhy-HIT0qiK7iebMa3YzeWPgD0djwKBUrRUmlkvbcraxUq6BKee1K49Y06qJMMfklNAHUXz~tnQTLC61OayO8UMk97KKzvDfAD49LhQyTfNS~oXtac5IKqptwZ7gYK4b4tP4svZkT0zTp3RNqmAz3cPbhjJtpcizs-eBRSHQNVDnNG5yHOihcMDIAl97qu8wfp2pc91x3KFH6YMOh~T3wu98yYi89pRDRYeGkO3oCBBmwUiDT2nRvmNL9x0-nHwTzJiVS9OF5tiOR-mmqVI6pAX5NhQqkcQJI0vE4t4LV~oUpHl8qf7v-EkIE4IZkFk2UjX8sGtiK7YQPk7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Telomerase activity in CD4+ and CD8+ T cells. Telomerase activity was measured by the TRAP assay and is expressed as a percentage of the activity detected in the lung carcinoma cell line GLC4. (A) Telomerase activity in the original TRAP assay. Telomerase activity in 130, 13, and 1.3 GLC4 cell equivalents is shown at the left. Specific telomerase activity could still be measured in 130 GLC4cell equivalents. Addition of RNAse is indicated (+). Abolishment of the signal confirmed specific telomerase activity. No cell extracts were added to the PCR controls a (lysis buffer only) and b (PCR mix). Cell extracts equivalent to 105, 104, and 103 purified CD4+ and CD8+ T cells from one healthy control and one representative HIV-infected person early (0.1 year after seroconversion) and late (7.5 years after seroconversion) in infection were analyzed. Addition of the M-ITAS resulted in specific bands (arrow) indicating that Taq polymerase was not limiting. (B) Telomerase activity in freshly isolated purified naive (CD4RA) and memory (CD4RO) CD4+ T cells from one HIV-infected individual and one healthy control performed as described in (A), but without M-ITAS. (C) Telomerase activity in freshly isolated naive (CD4RA) and memory (CD4RO) CD4+ T cells from six healthy controls and seven HIV-infected individuals. Bars with error bars indicate the mean telomerase activity ± the standard deviation of the population. (D) Representative fluorescence curves showing telomerase activity and M-ITAS (at 145 bp). Lanes 1 and 2, healthy control CD4+ and CD8+ T cells (100,000 cell equivalents); lanes 3 and 4, HIV+ CD4+ T cells early and late (100,000, respectively, 75,000 cell equivalents); lanes 5 and 6, HIV+ CD8+ T cells early and late (identical [id]); lane 7, GLC4 (100 cell equivalents); and lane 8, lysis buffer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1011/5/m_blod40305001dx.jpeg?Expires=1769137515&Signature=OS8w1oUpjaq5kWTijEe4feuDlTa3kH0ECdw9Kjy0c1sCccpCmsuXGYeYRjJJLDCq1nyvKSpALZjAFaJLyVFKSDcAEJ5sR58Q01OdcPM8QcEaQj8HdcmQT2UV0gt-6NLVWjMjK7pJe74WRXUOYs6qM4X47~n6wGTLiIGBayjD5UoYn~cuz8oqlpN8DLVJOQIlPwVlYWhMF~6u6i100XdxwDiUtR7~BYDr3X8jywsl3s9MGAFOi6Uwq1KE5wRVVWhrgZZYyKnoNndQGKZzgKZjvgvsIW7so1utLD3fcgWWM~5nmGgSKhS5QTSQ0oj409WzgxDl4Ty82edw~kJdjoSROw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal