Abstract
Many viruses have evolved genes encoding proteins that regulate cell death by apoptosis. The human immunodeficiency virus type 1 (HIV-1) Nef protein alters T-cell development and signaling and is required for optimal viral replication and pathogenicity in vivo. To analyze the interference of Nef with cell survival, we used both regulated and constitutively expressed nef alleles in stably transfected T-cell lines. Nef-expressing cells were sensitized to cell death by apoptosis, which was specifically exacerbated by an anti-CD95 IgM monoclonal antibody (MoAb). Flow cytometric analysis showed that the surface expression of both CD95 and CD95 ligand (CD95L) was upregulated by endogenous Nef expression. Nef-mediated apoptosis was almost completely suppressed by the addition in culture of an anti-CD95 Fab′ IgG MoAb, which specifically blocks CD95/CD95L interactions. Lastly, mutation of a proline motif in the core region of the nef gene, which disrupts its ability to interact with cellular kinases and reduces HIV-1 replication in vitro, completely abrogated the Nef-mediated induction of apoptosis as well as its ability to upregulate surface CD95 and CD95L. These findings may provide molecular insight into the role of endogenous Nef in the T-cell depletion observed in vivo, particularly HIV-specific cytotoxic CD8+ T cells.
APOPTOTIC CELL DEATH has been proposed as one of the key mechanisms involved in T-cell depletion during the course of human immunodeficiency virus type 1 (HIV-1) disease.1-3 It has also been shown that CD95 (Fas/Apo-1) antigen stimulation induces marked apoptosis of T lymphocytes in HIV-1–infected carriers,4,5 and the lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to CD95-induced apoptosis.6
In vivo studies have clearly demonstrated that an intact nefgene is critical to attain high virus loads and the development of an acquired immune deficiency syndrome (AIDS)-like illness in rhesus monkeys infected with simian immunodeficiency virus (SIV).7-10 A similar requirement for the maintenance of a high virus load has been demonstrated for HIV-1 using the severe combined immunodeficiency (SCID-Hu mouse) model,11 and deletions of nef sequences were consistently identified in some long-term nonprogressors with HIV-1 infection.12 Of note, the Nef proteins of SIV and HIV are functionally interchangeable.13 Enhanced virus replication and infectivity are associated with nef expression upon propagation of HIV-1 in peripheral blood mononuclear cell (PBMC) cultures in vitro.14 An increased inoculum of nef-deleted virus can overcome the decreased infectivity of the viral progeny but still does not induce illness in infected animals, suggesting a role fornef in HIV-1 pathogenicity.15 Indeed, mice expressing a lymphoid-targeted nef transgene develop severe T-cell depletion with altered T-cell function.16
Besides the enhancement of virus replication, in vitro studies also indicate that the regulatory HIV-1 Nef protein plays a key role in AIDS pathogenesis. One function of Nef is to mediate downregulation of cell surface CD4, the major HIV-1 receptor.17 Nef also induces dramatic effects on T-cell activation,18-21 leading to a specific Th1 cytokine impairment, possibly via binding to cellular kinases, including serine/threonine and tyrosine kinases.21-27 Moreover, different investigators have reported difficulties in establishing cell lines constitutively expressing Nef protein. In fact, it has been shown that Nef may be either cytotoxic or cytostatic when expressed in transfected cell lines.28-30 In this context, we were able to establish stably transfected clonal T-cell lines that can be propagated in culture and allow for the controlled expression of an HIV-1–derivednef allele.21 Taken together, these observations imply that Nef has a role in perturbing T-cell activation pathways, which are presumed to influence viral replication in the host and possibly cause a dysfunction of cells in the immune system.
Virus-specific cellular immune responses were also studied. They were barely detectable, if at all, in macaques infected with pathogenic SIV, whereas they were strongly induced in animals infected with the nef-deleted virus.31,32 In this last group of animals, increased Fas expression and apoptotic cell death were noted on T-lymphocyte populations.32 Furthermore, in vitro infection of macaque PBMCs was associated with increased Fas ligand (FasL) expression in anef-dependent manner. It was thus proposed that expression of FasL may protect infected cells from cytotoxic T lymphocyte (CTL) attack, killing viral-specific CTLs in the process.
Whether nef itself can induce FasL expression and apoptosis thus remains to be established. Here, we investigated the effect of endogenous Nef on the degree of apoptosis and CD95/CD95 ligand (CD95L) expression and function in a set of stably transfected T-cell lines.21
MATERIALS AND METHODS
Cell lines.
JH6.2 and JBru.2 are CD4+ lymphoblastoid T stably transfected clonal cell lines that have been previously described.21 JBru.3 and JBru.mut.8 clonal cell lines were obtained similarly in another round of stable transfections. JBru.mut.8 was obtained by stable transfection of the Nef Bru P72A-P75A mutant (Dutartre et al, manuscript submitted for publication). Nef Bru P72A-P75A was generated by substitution of Pro residues 72 and 75 with Ala residues and yielded a construct allowing for the expression of a stable Nef protein (data not shown). Cells were cultured in RPMI 1640 (GIBCO, Grand Island, NY) plus 10% fetal calf serum ([FCS] GIBCO) at an optimal cell density of 0.3 × 106 to 1 × 106/mL. In most experiments, exponentially growing JH6.2, JBru.2, JBru.3, and JBru.mut.8 cell clones were cultured in the presence of low serum levels (RPMI + 0.1% FCS) for up to 24 hours, and cell aliquots were harvested at various time points to measure the percentage of apoptosis and the expression of CD95, CD95L, and Nef proteins by flow cytometry.
Jurkat cell clones were either untreated or treated for 4 hours with 10−5 mol/L forskolin (FK), a potent protein kinase A activator, which increases Nef expression due to the presence of CREB consensus sequences in the cytomegalovirus promoter33 driving the expression of Nef cDNA.21 In some experiments, JH6.2, JBru.2, and JBru.3 cells were cultured in the presence of 50 ng/mL anti-CD95 IgM monoclonal antibody (MoAb) (Immunotech, Marseille Cedex, France), which triggers apoptosis by interacting with surface CD95, or 100 ng/mL anti–Apo-1 (CD95) Fab′ MoAb (kindly provided by Dr Peter Kramer, Heidelberg, Germany), which specifically blocks CD95/CD95L interactions. In other experiments, cells were treated with 100 ng/mL anti-Apo-1 (CD95) Fab′ MoAb for 1 hour and supplemented with 50 ng/mL anti-CD95 IgM for an additional 23 hours.
Western blot analysis.
Samples derived from 2 × 106 cells, containing approximately 100 μg protein, were migrated in 10% acrylamide gels and blotted onto nitrocellulose filters. Blotted filters were blocked for 30 minutes in a 3% suspension of dried skimmed milk in phosphate-buffered saline (PBS) and incubated overnight at 4°C with a 1:200 dilution of anti-Nef MoAb (Transgene, Strasbourg, France) or 1:1,500 dilution of anti-tubulin MoAb (Sigma, St Louis, MO). The filters were washed and further incubated for 1 hour at room temperature with a 1:1,500 dilution of peroxidase-conjugated anti-mouse IgG (Sigma) in 1% bovine serum albumin. Specific reactions were revealed with the ECL Western blotting detection reagent (Amersham Corp, Arlington Heights, IL).
Flow cytometric analysis of surface CD95 and CD95L and intracellular Nef.
The detection of CD95 and CD95L surface expression was performed on aliquots of 3 × 105 cells using unconjugated anti-CD95 IgM MoAb (dilution 1:100; Immunotech) or anti-CD95L rabbit polyclonal antibody (dilution 1:40; Santa Cruz Biotechnology, Santa Cruz, CA), respectively, at 4°C for 30 minutes. After two washings with PBS, the cells were stained with a polyclonal goat anti-mouse IgG covalently linked to fluorescein isothiocyanate (GAM-FITC, dilution 1:100; Becton Dickinson, San Jose, CA) or a polyclonal goat anti-rabbit IgG covalently linked to FITC (GAR-FITC, dilution 1:100; Becton Dickinson), respectively, at 4°C for 30 minutes. Nonspecific fluorescence was assessed using irrelevant isotype-matched controls (IgM for anti-CD95 MoAb or normal rabbit IgG for anti-CD95L) followed by GAM-FITC or GAR-FITC.
To evaluate intracellular Nef expression, JH6.2, JBru.2, JBru.3, and JBru.mut.8 cell lines were fixed in PBS–2% paraformaldehyde for 20 minutes at room temperature, washed twice with PBS containing 10 mmol/L glycine, and permeabilized in PBS–Triton X 1% for 5 minutes at 4°C. After two washings with PBS, the cells were resuspended in PBS plus 10% normal goat serum for 10 minutes at room temperature, before addition of anti-Nef IgG1 MoAb (Transgene; dilution 1:100) for 30 minutes at 4°C. After two washings with PBS, GAM-FITC (dilution 1:100) was added to the cells and incubated for 30 minutes at 4°C. The negative controls consisted of an isotype-matched unreactive MoAb (anti-p66 IgG1 human cytomegalovirus, dilution 1:50; Du Pont Co, Wilmington, DE) followed by identical second-layer labeling as before.
Surface or intracellular fluorescence was analyzed by a FACScan flow cytometer (Becton Dickinson). Samples were assayed in duplicate (at least 10,000 events recorded), and the mean fluorescence intensity (MFI) was calculated for each sample by point-to-point subtraction of positive counts on the negative controls using Lysis II software (Becton Dickinson). MFI values are expressed in arbitrary units (AU).
Determination of apoptosis.
Apoptosis was evaluated as previously described34 by combining two independent methods: (1) propidium iodide (PI) staining followed by flow cytometry and (2) the TdT-mediated d-UTP-biotin nick end labeling (TUNEL) technique. For the first procedure, cells were harvested and fixed in 70% ethanol for at least 1 hour at 4°C. They were then treated with 0.5 μg RNase/mL (Type I-A; Sigma) and resuspended in PBS containing 50 μg/mL PI. Analysis was performed by FACScan with the FL2 detector in logarithmic mode, using Lysis II software (Becton Dickinson). The threshold of PI fluorescence was triggered on the Fl2 signal, where a clear-cut
distinction between cell debris and apoptotic cells was virtually always present. As previously demonstrated by electron microscopic analysis,35 cells sorted from the hypodiploid peak were virtually all apoptotic and cell debris was extremely rare.
The TUNEL technique was modified to stain the cells with 1 μg/mL FITC-avidin to monitor DNA fragmentation in situ. Fluorescent nuclei were scored using an MCR-1000 confocal microscope (Bio-Rad Microscience, Hemel, Hempstead, UK) equipped with a krypton/argon ion laser emitting at 488 nm. The signal was achieved through an Epidetector filter (passing band, 522/35 nm) (Bio-Rad Microscience), analyzed by CoMOS software (Bio-Rad Microscience), and printed on Ektachrome 64T Kodak film (Eastman Kodak, Rochester, NY) by a Focus Imagerecorder Plus (Focus Graphics Inc, Foster City, CA).
Statistical analysis.
The data are expressed as the mean ± SD for three or more experiments performed in duplicate. Statistical analysis was performed using the two-tailed Student’s t-test.
RESULTS
HIV-1 Nef induces apoptosis of Jurkat cells that is counterregulated by growth factors.
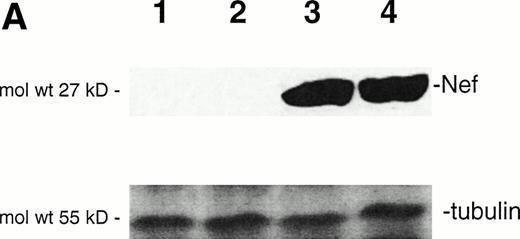
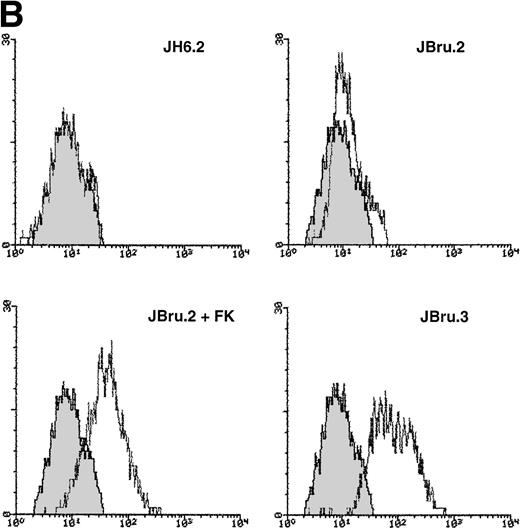
In this study, we aimed to characterize the mechanisms underlying in vitro cytopathic effects mediated by endogenous Nef.28-30 For this purpose, we used the HIV-1nef–transfected JBru.2 and JBru.3 CD4+lymphoblastoid T-cell clones and compared them with the control JH6.2 transfected with the backbone empty vector.21 Nef expression is constitutively high in JBru.3 cells, whereas it is almost undetectable in JBru.2 cells under basal conditions (Fig1). We have previously shown21that nef expression sharply increases in JBru.2 cells upon treatment with FK, phorbol esters, or Ca2+ ionophores, due to the presence of cAMP and NF-kB responsive elements in the human immediate early cytomegalovirus promoter regions driving nefexpression in these cells.33 As in preliminary experiments, FK alone did not modify the percentage of apoptosis in JH6.2 cells, at variance with phorbol esters and Ca2+ ionophores, and this agonist was chosen to induce Nef expression in JBru.2. The very weak or absent Nef expression in JBru.2 readily increased (P < .01) after stimulation of the cells for 5 hours with 10−5mol/L FK (Fig 1A and B). On the other hand, the constitutively expressed Nef protein was only minimally (P > .1) enhanced by the addition of 10−5 mol/L FK in JBru.3 (data not shown). Of note, we have previously reported that nefexpression levels in stimulated JBru.2 cells are comparable to levels detected in peripheral blood mononuclear cell cultures infected in vitro with HIV-1 NL43 isolate,36 hence indicating that physiologic levels of nef expression were achieved in our experimental system.
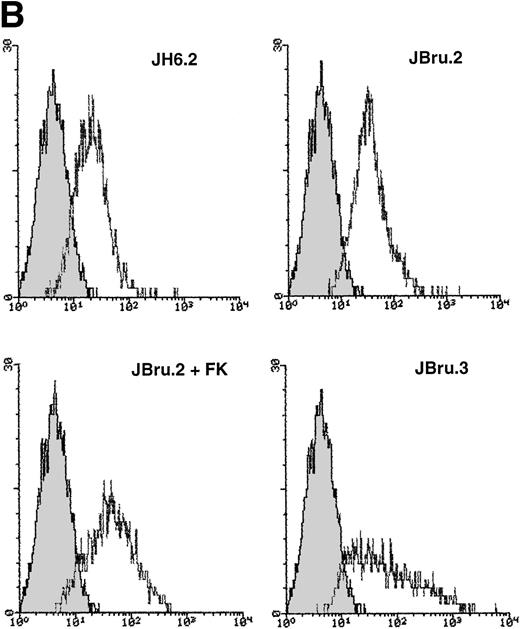
Analysis of intracellular Nef protein expression in untreated or FK-treated Nef transfectants by (A) Western blot and (B) flow cytometric analyses. (A) JH6.2 (lane 1), J.Bru.2 cultured in the absence (lane 2) or presence (lane 3) of FK, and J.Bru.3 (lane 4) were subjected to Western blot analysis for Nef and tubulin. (B) Nef protein levels were determined by indirect immunofluorescence revealed by flow cytometry. Unshadowed histograms represent cells treated with anti-Nef MoAb + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative Nef expression detected by fluorescence intensity (logarithmic scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI (mean ± SD) of the four experiments expressed in AU was 3 ± 1 (JH6.2), 21 ± 3.5 (JBru.2), 254 ± 38 (JBru.2 + FK), and 322 ± 55 (JBru.3).
Analysis of intracellular Nef protein expression in untreated or FK-treated Nef transfectants by (A) Western blot and (B) flow cytometric analyses. (A) JH6.2 (lane 1), J.Bru.2 cultured in the absence (lane 2) or presence (lane 3) of FK, and J.Bru.3 (lane 4) were subjected to Western blot analysis for Nef and tubulin. (B) Nef protein levels were determined by indirect immunofluorescence revealed by flow cytometry. Unshadowed histograms represent cells treated with anti-Nef MoAb + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative Nef expression detected by fluorescence intensity (logarithmic scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI (mean ± SD) of the four experiments expressed in AU was 3 ± 1 (JH6.2), 21 ± 3.5 (JBru.2), 254 ± 38 (JBru.2 + FK), and 322 ± 55 (JBru.3).
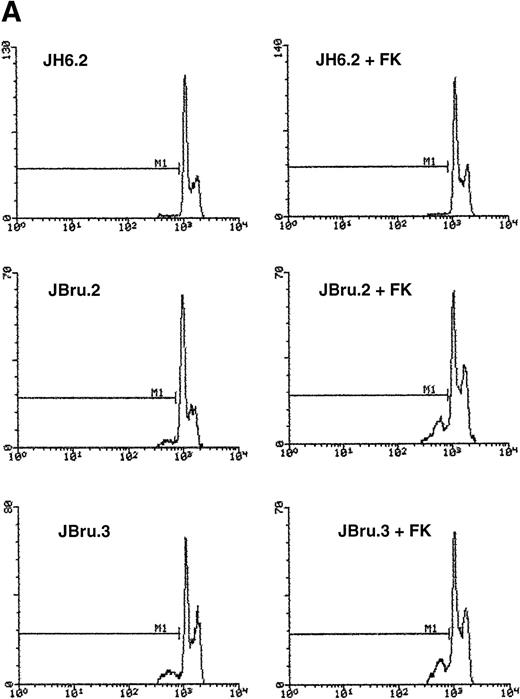
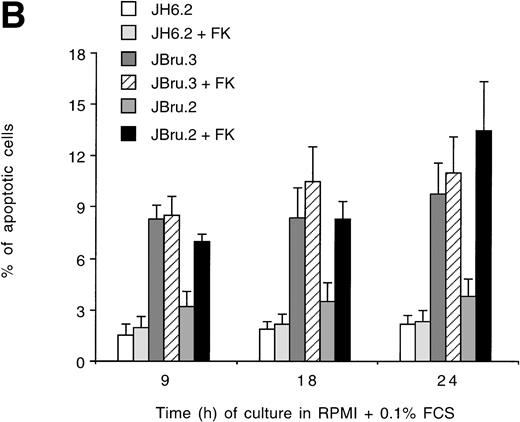
To evaluate the presence of apoptosis, we used two independent techniques: quantitative analysis by flow cytometry to measure the percentage of subdiploid DNA after PI staining (Fig 2A and B) and indirect immunofluorescence after application of the TUNEL technique revealed by confocal microscopy to monitor DNA fragmentation in situ (Fig 2C). Due to the use of strict criteria to exclude cell debris from the population of apoptotic cells after PI staining,35 the quantitative data obtained with both techniques were very similar.
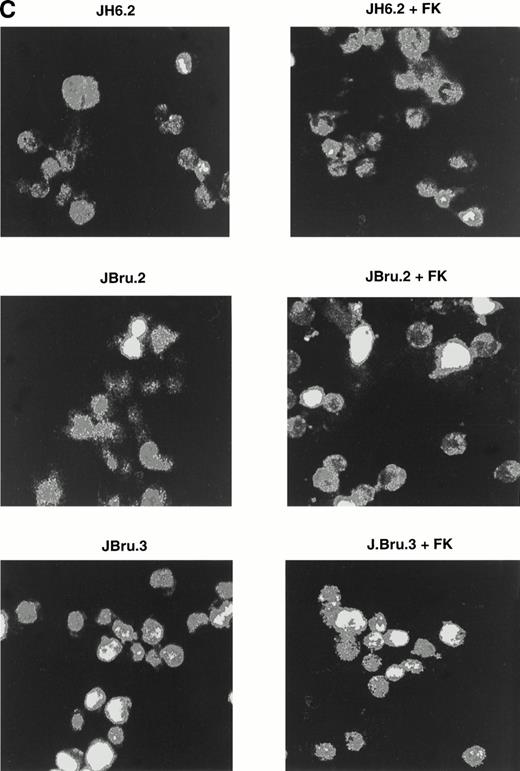
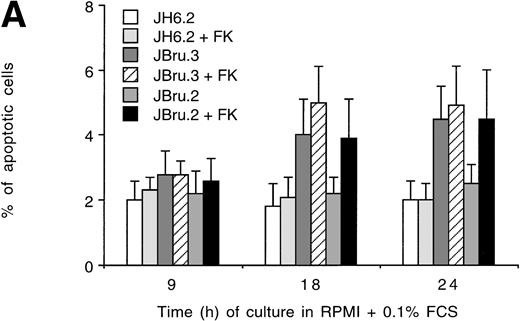
Evaluation of apoptosis by (A and B) flow cytometry after PI staining and (C) indirect immunofluorescence after TUNEL in untreated or FK-treated Nef transfectants cultured for up to 24 hours in 0.1% FCS. (A) Representative experiment shows the presence of apoptosis quantitatively evaluated by flow cytometry after PI staining in 24-hour serum-starved JH6.2, J.Bru2, and J.Bru.3 cells untreated (left) or treated with 10−5 mol/L FK (right). M1, apoptotic cells; x-axis, PI fluorescence (log scale); y-axis, relative number of cells. (B) Apoptosis was quantitatively evaluated by PI staining followed by flow cytometry at different culture times. Data are the mean ± SD of four-seven separate experiments in duplicate. (C) Representative of three separate experiments in which apoptosis was evaluated by TUNEL in confocal microscopy after 24 hours of serum starvation. Apoptotic cells were identified for the presence of intense fluorescent nuclei.
Evaluation of apoptosis by (A and B) flow cytometry after PI staining and (C) indirect immunofluorescence after TUNEL in untreated or FK-treated Nef transfectants cultured for up to 24 hours in 0.1% FCS. (A) Representative experiment shows the presence of apoptosis quantitatively evaluated by flow cytometry after PI staining in 24-hour serum-starved JH6.2, J.Bru2, and J.Bru.3 cells untreated (left) or treated with 10−5 mol/L FK (right). M1, apoptotic cells; x-axis, PI fluorescence (log scale); y-axis, relative number of cells. (B) Apoptosis was quantitatively evaluated by PI staining followed by flow cytometry at different culture times. Data are the mean ± SD of four-seven separate experiments in duplicate. (C) Representative of three separate experiments in which apoptosis was evaluated by TUNEL in confocal microscopy after 24 hours of serum starvation. Apoptotic cells were identified for the presence of intense fluorescent nuclei.
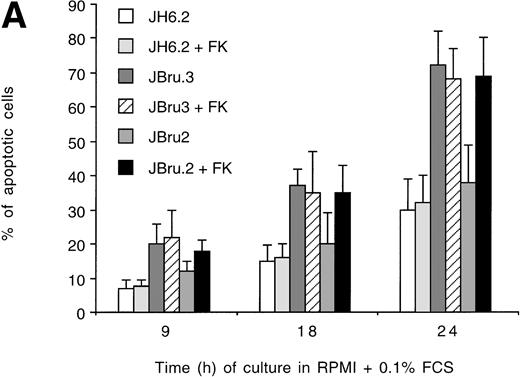
In the presence of low serum concentrations (0.1% FCS), only cell lines displaying clearly detectable Nef expression (FK-treated JBru.2 and untreated and FK-treated JBru.3; Fig 1) showed a progressive increase of apoptosis (Fig 2B). In particular, the percentage of apoptosis was significantly (P < .05) higher in Nef-expressing cells versus control JH6.2 and untreated JBru.2 cells from 9 hours of serum starvation onward.
HIV-1 nef-expressing cells are sensitized to CD95-induced apoptosis and express high levels of CD95.
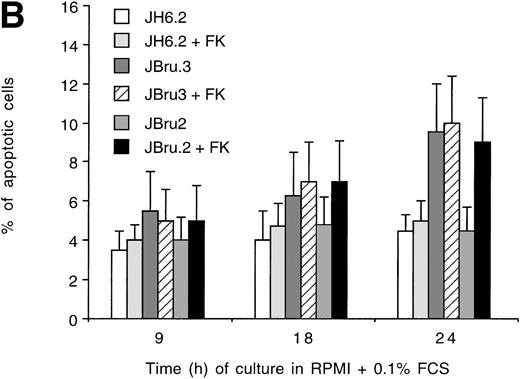
Fas is an important intermediate in T-cell apoptosis, and peripheral blood mononuclear cells from HIV-infected patients demonstrate enhanced CD95 expression that is correlated with an enhanced susceptibility to the induction of apoptosis with anti-CD95 antibodies.37 For these reasons, we investigated CD95-mediated apoptosis innef-transfected cells. JH6.2, JBru.2, and JBru.3 were cultured under low growth factor concentrations and in the presence of anti-CD95 IgM MoAb, which induces apoptosis in cells expressing functional CD95 (Fig 3A). The percentage of apoptotic cells showed a progressive increase in all cell lines following the addition of anti-CD95 IgM in culture. However, the kinetics of apoptosis was much faster and reached significantly (P < .05) higher levels in JBru.3 and FK-treated JBru.2 cell clones versus untreated JBru.2 and JH6.2.
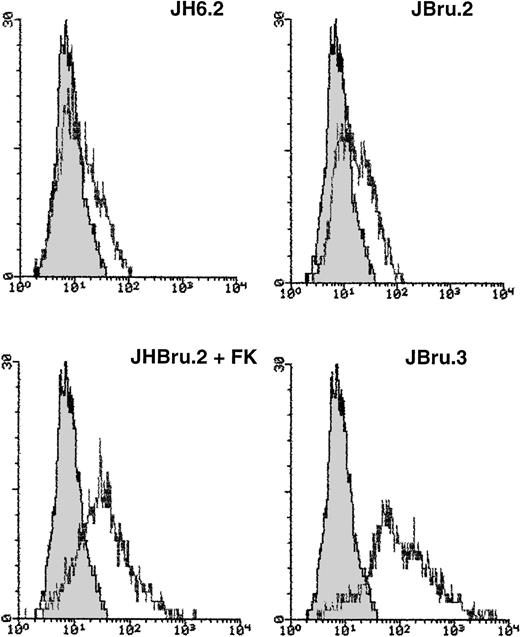
(A) Effect of addition of anti-CD95 IgM on the percentage of apoptosis in untreated or FK-treated Nef transfectants cultured in RPMI + 0.1% FCS for up to 24 hours. Apoptosis was quantified by PI staining and flow cytometry at different culture times after serum starvation. Data are the mean ± SD of four separate experiments in duplicate. (B) Surface expression of CD95 in untreated or FK-treated Nef transfectants cultured in RPMI 0.1% FCS for 24 hours. Unshadowed histograms represent cells treated with anti-CD95 + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative CD95 expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 189 ± 28 (JH6.2), 277 ± 51 (JBru.2), 325 ± 64 (JBru.2 + FK), and 394 ± 76 (JBru.3).
(A) Effect of addition of anti-CD95 IgM on the percentage of apoptosis in untreated or FK-treated Nef transfectants cultured in RPMI + 0.1% FCS for up to 24 hours. Apoptosis was quantified by PI staining and flow cytometry at different culture times after serum starvation. Data are the mean ± SD of four separate experiments in duplicate. (B) Surface expression of CD95 in untreated or FK-treated Nef transfectants cultured in RPMI 0.1% FCS for 24 hours. Unshadowed histograms represent cells treated with anti-CD95 + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative CD95 expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 189 ± 28 (JH6.2), 277 ± 51 (JBru.2), 325 ± 64 (JBru.2 + FK), and 394 ± 76 (JBru.3).
To explain this increased susceptibility to apoptosis, we next investigated the expression of CD95 (Fig 3B) on the cell surface of JH6.2, JBru.2, and JBru.3. CD95 expression was clearly detectable in all Jurkat cell clones, but it reached significantly higher (P < .01) MFI values in JBru.3 and FK-treated JBru.2 versus untreated J.Bru.2 or JH6.2. These results suggested that thenef-mediated susceptibility of transfected cells to CD95-triggered apoptosis could relate to enhanced membrane CD95 expression.
HIV-1 Nef induces CD95-dependent apoptosis through induction of CD95L membrane expression.
CD95 triggers apoptosis upon oligomerization by CD95L.38 We thus determined CD95L expression at the surface of JH6.2, JBru.2, and JBru.3 cells (Fig 4). In cells cultured in the presence of low serum (RPMI + 0.1% FCS) for 24 hours, membrane-bound CD95L was clearly detectable. However, the expression was significantly (P < .01) higher in JBru.3 and FK-treated JBru.2 versus untreated JBru.2 or control JH6.2 cells (Fig 4). These findings suggested that an upregulated expression of both CD95 and CD95L may be involved in the nef-dependent apoptosis observed innef-expressing Jurkat cells.
Surface expression of CD95L in untreated or FK-treated Nef transfectants cultured in RPMI + 0.1% FCS for 24 hours. Unshadowed histograms represent cells treated with anti-CD95L + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative CD95L expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 11 ± 4 (JH6.2), 63 ± 11 (JBru.2), 175 ± 46 (JBru.2 + FK), and 248 ± 70 (JBru.3).
Surface expression of CD95L in untreated or FK-treated Nef transfectants cultured in RPMI + 0.1% FCS for 24 hours. Unshadowed histograms represent cells treated with anti-CD95L + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative CD95L expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 11 ± 4 (JH6.2), 63 ± 11 (JBru.2), 175 ± 46 (JBru.2 + FK), and 248 ± 70 (JBru.3).
Because significant amounts of CD95L can be shed from the cell surface and released in the culture medium,39 we next investigated the role of nef-induced CD95L in nef-dependent apoptosis. Cells were cultured in the presence of an anti-CD95 Fab′ IgG, which blocks CD95/CD95L interactions and prevents apoptosis induced by this pathway (Fig 5A). When cells were cultured in the presence of an anti-CD95 Fab′ IgG, the degree of apoptosis remained very low over time, never exceeding 6% in all of the Jurkat transfectants cultured for up to 24 hours under low growth factor concentrations. Although nef-expressing cells showed slightly higher levels of apoptosis, no statistically significant (P > .1) differences were observed among the various Jurkat cell clones treated with anti-CD95 Fab′ IgG (Fig 5A). Moreover, when cells were pretreated for 1 hour with anti-CD95 Fab′ IgG and then with anti-CD95 IgM for an additional 23 hours, the fraction of apoptosis never exceeded 10% (Fig 5B), a much lower percentage (P < .01) than previously observed in cultures supplemented with anti-CD95 IgM alone (Fig 3A). Thus, the anti-CD95 Fab′ IgG was very efficient in blocking the CD95-mediated induction of apoptosis. Taken together, these data demonstrated that the CD95/CD95L pathway innef-expressing cells was not only upregulated but also functional, and fully accounted for the Nef-dependent apoptosis in lymphoid T-cell lines reported herein.
Effect of addition of (A) anti-CD95 Fab′ IgG alone or (B) followed by anti-CD95 IgM on the percentage of apoptosis in untreated or FK-treated Nef transfectants cultured in RPMI + 0.1% FCS for up to 24 hours. Apoptosis was quantified by PI staining and flow cytometry at different culture times after serum starvation. Data are the mean ± SD of three to four separate experiments in duplicate.
Effect of addition of (A) anti-CD95 Fab′ IgG alone or (B) followed by anti-CD95 IgM on the percentage of apoptosis in untreated or FK-treated Nef transfectants cultured in RPMI + 0.1% FCS for up to 24 hours. Apoptosis was quantified by PI staining and flow cytometry at different culture times after serum starvation. Data are the mean ± SD of three to four separate experiments in duplicate.
Mutation in the core sequence of Nef abrogates its ability to induce apoptosis and CD95/CD95L upregulation.
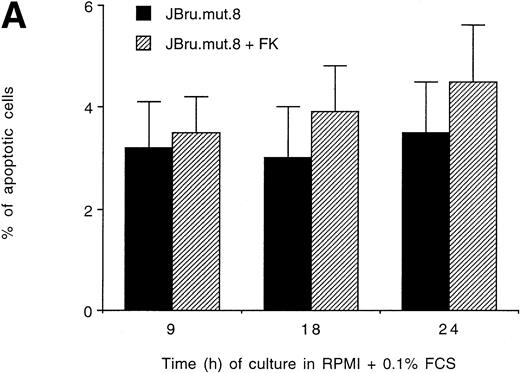
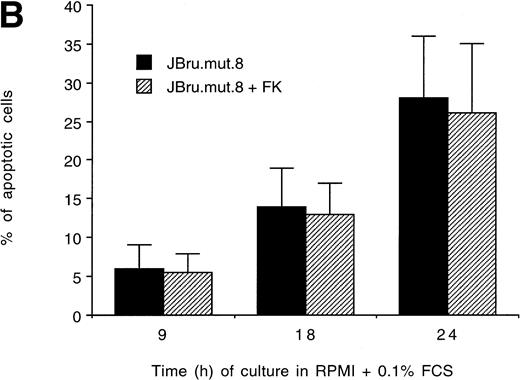
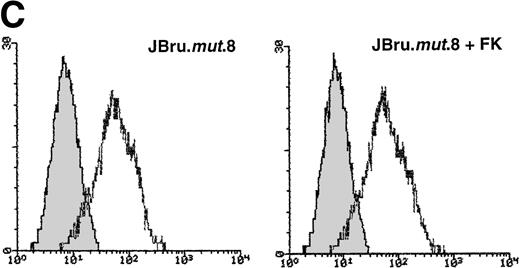
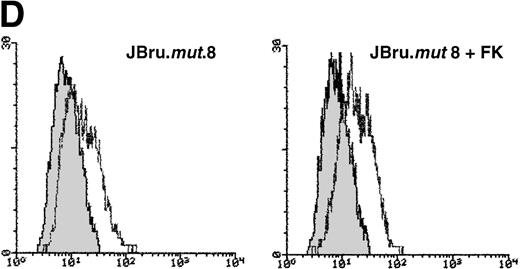
It has been previously shown that both HIV-1 and SIV Nef interact withsrc-like tyrosine kinase(s), and with a member of the p21-activated kinase (PAK) family of kinases.24 Moreover, it has been suggested that the association of SIV Nef with these kinases is important for the development of AIDS in rhesus macaques and may provide a novel target for clinical intervention.24,40 41 Therefore, in this group of experiments, the induction of apoptosis upon serum withdrawal was evaluated in a Jurkat cell clone stably transfected with anef-expressing plasmid in which the nef core region has been mutated by substitution of Pro residues 72 and 75 by Ala residues (JBru.mut.8). This mutant is unable to activatesrc-like tyrosine kinase(s) or PAK (Dutartre et al, manuscript submitted for publication). Nef expression in JBru.mut.8 was very low under basal conditions, whereas it rapidly and significantly (P < .01) increased upon treatment with FK, reaching levels similar to those observed in FK-treated JBru.2 (Fig 6A and B). The percentage of apoptosis in both untreated and FK-treated JBru.mut.8 cultured in RPMI + 0.1% FCS was very low (Fig7A). When anti-CD95 IgM was added to the culture, a progressive increase of apoptosis was noted (Fig 7B), reaching values similar to those previously observed in JH6.2 control cells (Fig 3A). Moreover, the surface expression of both CD95 (Fig 7C) and CD95L (Fig 7D) in JBru.mut.8 was significantly (P < .05) lower versus FK-treated JBru.2 or JBru.3 cells (Figs 3 and 4), while the CD4 surface expression was similar to that of JBru.2 and JBru.3 (data not shown). Thus, the Nef core domain responsible for the interaction with src-like tyrosine kinase(s) and PAK is critical for the ability of Nef to induce apoptosis and upregulate CD95/CD95L.
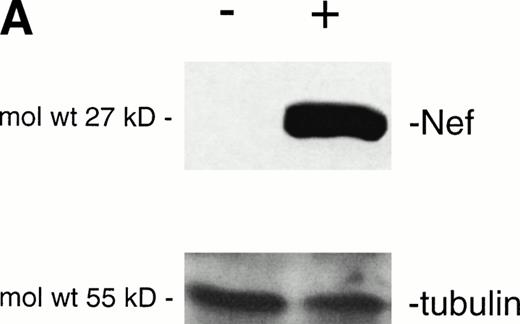
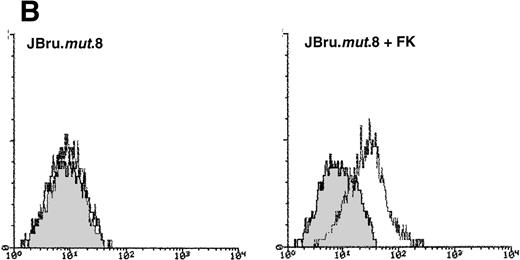
Analysis of intracellular Nef protein expression in untreated or FK-treated Nef transfectants by (A) Western blot and (B) flow cytometric analyses. (A) JBru.mut.8 cells were cultured in the absence (lane 1) or presence (lane 2) of FK and then subjected to Western blot analysis for Nef and tubulin. (B) Nef protein levels were determined by indirect immunofluorescence revealed by flow cytometry. Unshadowed histograms represent cells treated with anti-Nef MoAb + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative Nef expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 4 ± 1.5 (JBru.mut.8) and 151 ± 29 (JBru.mut.8 + FK).
Analysis of intracellular Nef protein expression in untreated or FK-treated Nef transfectants by (A) Western blot and (B) flow cytometric analyses. (A) JBru.mut.8 cells were cultured in the absence (lane 1) or presence (lane 2) of FK and then subjected to Western blot analysis for Nef and tubulin. (B) Nef protein levels were determined by indirect immunofluorescence revealed by flow cytometry. Unshadowed histograms represent cells treated with anti-Nef MoAb + GAM-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC). X-axis, relative Nef expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 4 ± 1.5 (JBru.mut.8) and 151 ± 29 (JBru.mut.8 + FK).
Evaluation of (A and B) apoptosis and surface expression of (C) CD95 and (D) CD95L in untreated and FK-treated JBru.mut.8. (A and B) Apoptosis was quantitatively evaluated by PI staining followed by flow cytometry at different culture times after serum starvation in the (A) absence or (B) presence of anti-CD95 IgM. Data are the mean ± SD of three separate experiments in duplicate. (C and D) Surface expression of CD95 and CD95L was detected by indirect staining in cells cultured for 24 hours in RPMI + 0.1% FCS. Unshadowed histograms represent cells treated with (C) anti-CD95 + GAM-FITC or (D) anti-CD95L + GAR-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC and cells treated with normal rabbit IgG + GAR-FITC). X-axis, relative (C) CD95 or (D) CD95L expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 233 ± 48 (JBru.mut.8) and 251 ± 62 (JBru.mut.8 + FK) in (C) and 43 ± 9 (JBru.mut.8) and 49 ± 17 (JBru.mut.8 + FK) in (D).
Evaluation of (A and B) apoptosis and surface expression of (C) CD95 and (D) CD95L in untreated and FK-treated JBru.mut.8. (A and B) Apoptosis was quantitatively evaluated by PI staining followed by flow cytometry at different culture times after serum starvation in the (A) absence or (B) presence of anti-CD95 IgM. Data are the mean ± SD of three separate experiments in duplicate. (C and D) Surface expression of CD95 and CD95L was detected by indirect staining in cells cultured for 24 hours in RPMI + 0.1% FCS. Unshadowed histograms represent cells treated with (C) anti-CD95 + GAM-FITC or (D) anti-CD95L + GAR-FITC, and shadowed histograms are negative controls (cells treated with anti-p66 of HCMV + GAM-FITC and cells treated with normal rabbit IgG + GAR-FITC). X-axis, relative (C) CD95 or (D) CD95L expression detected by fluorescence intensity (log scale); y-axis, relative number of cells. A representative of four separate experiments is shown. The MFI of the four experiments (AU) was 233 ± 48 (JBru.mut.8) and 251 ± 62 (JBru.mut.8 + FK) in (C) and 43 ± 9 (JBru.mut.8) and 49 ± 17 (JBru.mut.8 + FK) in (D).
DISCUSSION
The loss of functional immune cells is a hallmark of AIDS. Although the magnitude of the viral burden increases with disease progression42 and much emphasis has been placed on the direct cytopathic effect of a productive HIV-1 infection of CD4+ T lymphocytes,43 44 a considerable loss of uninfected or abortively infected bystander T lymphocytes occurs in HIV-infected individuals.
The induction of apoptosis following viral infection of a cell is generally viewed as an attempt by the host cell to limit virus replication. In fact, many viruses have their own antiapoptosis genes or upregulate antiapoptotic cellular genes, which can block the premature death of infected cells and thereby facilitate a persistent infection or prolong the survival of lytically infected cells to maximize the production of viral progeny.45 On the other hand, it has also been shown that apoptotic death may favor HIV-1 replication.46 Thus, like other animal viruses, HIV-1 may have developed strategies to modulate the apoptotic cell death of its target cells.
The regulatory HIV-1 Nef protein represents an interesting candidate for HIV-1–mediated induction of apoptosis. In fact, (1) it induces severe T-cell depletion in transgenic mice,16 (2) it is required for HIV pathogenicity in SCID-Hu mice independently of its impact on viral replication,15 (3) it is required for CTL killing in SIV-infected macaques31 and possibly also in humans,47 (4) it is abundantly expressed during acute HIV-1 infection,48 (5) it plays a relevant role in AIDS pathogenesis,7-11 (6) it shows cytotoxic/cytostatic activity in transfected cell lines,30 and (7) in its extracellular form, it binds to the cell surface and induces apoptosis of a variety of murine and human blood cells.49-51
In this study, we have established that endogenous expression of the HIV-1 Nef protein in cycling mature CD4+ T cells can regulate cell survival at two different levels. Firstly, it induces functional CD95L expression, which in turn triggers CD95-mediated T-cell death. This pathway correlates with the ability of Nef to interact with cellular kinases and T-cell signaling in vitro and relates to the role of nef in the evasion by infected cells of CTL responses in SIV-infected macaques.31 Secondly, Nef increases CD95-mediated T-cell death. Remarkably, Nef induced both effects in a dose-dependent fashion, as JBru.2 was unable to induce apoptosis unless stimulated to produce high levels of intracellular Nef by FK.
Okada et al49-51 have convincingly shown that extracellular Nef protein induces apoptosis in both lymphoid and myeloid cells of human and mouse origin through a CD95-independent pathway. The apparent discrepancy with our findings may be reconciled by taking into account that we explored the effect of intracellular Nef and Okada et al studied the effects of nonmyristylated recombinant Nef protein. It is possible that intracellular and extracellular Nef protein trigger apoptosis in their target cells through complementary (CD95-dependent and -independent, respectively) mechanisms, which can meet upstream to IL-1β converting enzyme (ICE).51
Several cell surface receptors including TNF-R1 and CD95 trigger apoptosis upon contact with their counterreceptors or ligands, TNF and CD95L for TNF-R1 and CD95, respectively.52 In particular, it has been demonstrated that the interaction between CD95 and CD95L plays an important role in the homeostatic regulation of the normal immune response. The expression of CD95 is diffuse, being found on a variety of extra-lymphoid tissues, whereas the expression of CD95L is much more tightly controlled, being restricted to activated lymphocytes53 and selected sites of immune privilege such as Sertoli cells, stromal cells of the anterior chamber of the eye, and neurons.54
The ability of Nef to upregulate CD95 and induce CD95L is twofold. It is particularly remarkable since CD95 expression per se does not lead to cell death, as ligation of CD95 by CD95L is required to trigger apoptosis. In addition, CD95 was also found to transduce activation signals in normal human T lymphocytes.53 Here, using anti-CD95 IgM antibodies, we found that Nef increased the sensitivity to CD95-mediated T-cell death. This may have resulted from the upregulation of CD95 membrane expression. Alternatively, Nef may operate by increasing CD95 apoptotic signaling.51 The increased CD95 expression and sensitivity to CD95-mediated cell death thus parallel those found in ex vivo T lymphocytes from HIV-infected patients.1-3
Furthermore, we demonstrated that nef can induce CD95L expression. Our data establish that the induction of CD95L membrane expression is instrumental in nef-induced cell death, since it was blocked by CD95 Fab′ antibodies, and the proline-mutated Nef protein failed to upregulate CD95L and to induce apoptosis. In this respect, it has been previously shown that Nef perturbs eye lens development in transgenic mice.55 It is likely thatnef-expressing cells bearing CD95L at the cell surface induce CD95-based cytotoxicity of CD95-expressing cells. Our results may thus provide a molecular basis for the severe nef-induced T-cell depletion found in nef-transgenic mice.16 However, in situ labeling of lymph nodes from HIV-infected children and SIV-infected macaques has indicated that apoptosis occurs predominantly in bystander cells and not in the productively infected cells themselves.3 Our study suggests that Nef-expressing CD4+ T cells with upregulated CD95L expression are a good candidate for the induction of apoptosis found in both CD4+and CD8+ uninfected cells.1-3,31 Our demonstration at the protein level of CD95L induction by Nef is also consistent with the demonstration of CD95L induction in HIV-infected macrophages and CD4+ T cells by RT-PCR.56 We propose that the nef-dependent regulation of CD95L may contribute in vivo to protect infected cells from CTL killing by inducing the cell death of Fas-bearing CTLs upon contact with thenef-expressing cells. Our current model is consistent with the recent observation of a nef-dependent evasion from CTL responses in SIV-infected macaques.31 The investigators suggested such a nef-dependent CD95L induction in PBMCs infected in vitro with SIV, yet the direct implication of nefcould not be demonstrated. It is remarkable that the various described functions of nef, including downregulation of Th1 cytokines,20 together with the herein-described function in the regulation of the CD95/CD95L pathway all may contribute in vivo to allow nef-expressing cells to escape from CTL responses and hence to increase viral replication.
Studies to characterize intracellular targets of Nef have shown that HIV-1 and SIV Nef associate with cellular serine/threonine kinases, as well as src-like tyrosine kinases.21-23 The serine/threonine kinase may represent a member of the PAK family,24 while tyrosine kinases comprise Hck in monocytes57 and Lck in T lymphocytes.21,23 A proline motif in HIV-1 Nef was found to be required for binding these various kinases to increase viral replication and infectivity and for impairment of CD3 signaling, but not for downregulation of membrane CD4.21,26,27 Here, we show that this motif was also required for induction of CD95L and apoptosis by Nef, suggesting that common cellular targets are implicated in these different pathways. Consistently, on the one hand, CD95L is known to be expressed following activation of the T-cell receptor. This expression is inhibited by cell treatment with immunosuppressive drugs including tyrosine kinase inhibitors58 and requires Lck in mature cycling T cells.59,60 Recently, using a reporter gene construct containing elements from the CD95L promoter, Latinis et al61 demonstrated that T-cell receptor–stimulated activation of the small G-protein Ras signaling pathway is also required for optimal activation of CD95L. Hence, both pathways may contribute to the transcriptional induction of CD95L, the PAK effectors being regulated by Ras and also by a Lck-Vav signaling pathway.62 Similarly, various viral infections are associated with increased CD95 expression, which was found to be inhibited by tyrosine kinase inhibitors.63 These results indicate a role for tyrosine kinases in common activation pathways initiated by different stimuli. On the other hand, CD95 ligation induces apoptosis and the small G-protein signaling cascade independently of Lck in human T cells.64 The relative contribution of these various signaling molecules in the Nef function awaits site-directed mutagenesis analysis to identify the specific regions of Nef implicated in Lck and/or PAK regulation.
Other HIV-1 gene products such as extracellular HIV-1 Tat and gp120 have been shown to increase the expression of CD95L on activated T cells.65 Together with Nef, these proteins may act in concert to induce CD95L and to increase CD95-based cytotoxicity. Because Nef is abundantly expressed early after infection,47 48 it may operate in early infection to induce CD95L, followed by Tat and then gp120 to further support it. Indeed, the concentrations (nanomolar to micromolar) of gp120 and Tat required to upregulate CD95L expression are very high and difficult to achieve in vivo also at sites of high viral replication such as lymph nodes.
The present report provides the first description of a twofold mechanism triggered by a viral protein to regulate cell death via the CD95-CD95L pathway. It also suggests cellular pathways regulating the expression of this cell death machinery.
Supported by the AIDS Project of the Italian Ministry of Health, the Association Nationale de Recherche sur le syndrome d’Immunodeficience Acquise, and the INSERM, and in part by a fellowship from the European Community (ERB-CHRX CT94-0537 to Y.C.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Giorgio Zauli, MD, PhD, Human Anatomy Section, Department of Morphology and Embryology, University of Ferrara, Via Fossato di Mortara 66, 44100 Ferrara, Italy; e-mail:zlg@dsn.unife.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal